-
PDF
- Split View
-
Views
-
Cite
Cite
Lourdes Ibáñez, Marta Díaz, Cristina García-Beltrán, Rita Malpique, Edurne Garde, Abel López-Bermejo, Francis de Zegher, Toward a Treatment Normalizing Ovulation Rate in Adolescent Girls With Polycystic Ovary Syndrome, Journal of the Endocrine Society, Volume 4, Issue 5, May 2020, bvaa032, https://doi.org/10.1210/jendso/bvaa032
Close - Share Icon Share
Abstract
Adolescent polycystic ovary syndrome (PCOS) is characterized by androgen excess and oligomenorrhea, and commonly driven by hepato-visceral fat excess (“central obesity”) ensuing from a mismatch between prenatal and postnatal nutrition, on a background of genetic susceptibility. There is no approved treatment for adolescent PCOS.
We report the pooled results of 2 pilot studies in nonobese girls with PCOS (N = 62, age 15.8 years) that compared the effects of randomized treatment for 1 year, either with an oral estro-progestogen contraceptive (OC), or with a low-dose combination of spironolactone-pioglitazone-metformin (SPIOMET, targeting the excess of ectopic fat).
Auxological and endocrine-metabolic variables (including fasting insulin, androgens, high-molecular-weight adiponectin [HMW-adiponectin], and microRNA [miR]-451a), body composition (dual x-ray absorptiometry) and hepato-visceral fat (magnetic resonance imaging) were assessed on- and posttreatment. Data from menstrual diaries were combined with weekly salivary progesterone measurements to infer ovulation rates during the second and fourth quarter of the posttreatment year.
OC and SPIOMET treatment reduced the androgen excess comparably, and had no differential effects on total-body lean or fat mass. However, SPIOMET was accompanied by more broadly normalizing effects, including on hepato-visceral fat and on circulating insulin, HMW-adiponectin, and miR-451a. On average, there were 3-fold more ovulations post-SPIOMET than post-OC; normovulation was only observed after SPIOMET; anovulation was >10-fold more prevalent post-OC.
Pooled results of randomized studies in nonobese adolescent girls with PCOS indicate that SPIOMET treatment leads to an overall healthier, more insulin-sensitive condition—with less ectopic fat—than OC treatment, and to a more normal posttreatment ovulation rate.
There is no approved treatment for polycystic ovary syndrome (PCOS), a prevalent condition in adolescent girls and young women [1, 2]. Many of these patients are guided into a trajectory that starts with oral contraceptive (OC) treatment, leads into oligo-anovulatory subfertility, then into the use of assisted reproductive techniques, and ultimately into pregnancies with a double-to-triple risk for complications (such as gestational diabetes, preeclampsia, and preterm birth) potentially with lifelong sequelae in the offspring [2].
Evidence is converging toward the insight that adolescent PCOS is frequently driven by hepato-visceral fat excess (“central obesity”) ensuing from a mismatch between (rather restrictive) prenatal and (rather abundant) postnatal nutrition, on a background of (epi)genetic susceptibility [3, 4, 5]. This insight has prompted the exploration of an alternative treatment for PCOS consisting of the intake of a low-dose combination of spironolactone (a mixed anti-androgen and anti-mineralocorticoid, also activating brown adipose tissue) [6] with pioglitazone and metformin (2 insulin sensitizers acting through different mechanisms) (SPIOMET) for 1 year. This combination proved to have more normalizing effects than OC treatment, in particular, on ectopic fat excess, insulin sensitivity, and posttreatment ovulation rate [7]. The limited power of the first study (N = 34) prompted the launch of a second study with virtually identical design. Here we report the pooled results of both studies in nonobese girls with PCOS (N = 62).
1. Materials and Methods
A. Study Population & Design
Both pilot studies (ISRCTN29234515 and ISRCTN11062950) had an open-label, randomized, controlled design, and were conducted in the Adolescent Endocrinology Unit of Sant Joan de Déu University Hospital, Barcelona, Spain. Recruitment was biased against overweight/obesity because, in our setting, overweight/obese adolescent girls are primarily referred to the Adolescent Obesity Unit rather than to the Adolescent Endocrinology Unit. In each study, the on-treatment year was followed by a posttreatment year. Study completion rate was 89% (62/71) (Fig. 1, flow chart).
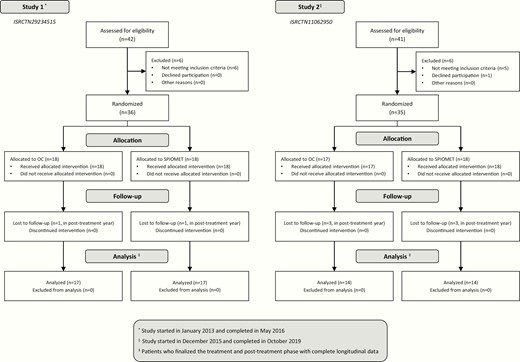
The inclusion criteria were hirsutism (score > 8 on modified Ferriman-Gallwey scale), oligomenorrhea (menstrual intervals > 45 days), gynecological age > 2.0 years, and absence of sexual activity (no need for contraception). Exclusion criteria were 21‐hydroxylase deficiency; glucose intolerance or diabetes; evidence of thyroid, liver, or kidney dysfunction; hyperprolactinemia; and prior use of medications affecting gonadal/adrenal function, or carbohydrate/lipid metabolism [7, 8]. Mediterranean diet and regular exercise were recommended to all participating girls; OC treatment consisted of 20 μg ethinylestradiol plus 100 mg levonorgestrel for 21/28 days, and placebo for 7/28 days; SPIOMET treatment consisted of a low‐dose combination of spironolactone 50 mg/day, pioglitazone 7.5 mg/day, and metformin 850 mg/day [7].
Age‐matched, healthy girls (N = 52; mean age 16.3 years) recruited from nearby schools served as controls. All had regular menstrual cycles, and none was hirsute or taking medication.
The primary endpoint was posttreatment ovulation rate; secondary outcomes included hirsutism score, fasting insulin, androgens, lipids, high-molecular-weight (HMW) adiponectin, C-reactive protein (CRP), carotid intima-media thickness (cIMT), body composition, and hepato-visceral fat [7]; circulating microRNA (miR)-451a could only be measured in a subset of the participating girls (footnote below Table 1).
Data From Adolescent Girls With Polycystic Ovary Syndrome (PCOS) Who Were Randomized to Receive Ethinylestradiol-Levonorgestrel (N = 31) or Low-Dose Spironolactone-Pioglitazone-Metformin (N = 31) for 12 Months, and who remained subsequently untreated for 12 months
| . | . | . | Ethinylestradiol-Levonorgestrel (N = 31) . | SPIOMET (N=31) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Controls (N = 52) . | PCOS (N = 62) . | Starta . | 12 mo . | 24 mo . | Δ 0–12 mo . | Δ 12–24 mo . | Starta . | 12 mo . | 24 mo . | Δ 0–12 mo . | Δ 12–24 mo . |
| Birthweight Z-score | 0.2 ± 0.1 | -0.6 ± 0.1*** | -0.6 ± 0.2 | -- | -- | -- | -- | -0.6 ± 0.1 | -- | -- | -- | -- |
| Age at Menarche (yr) | 12.4 ± 0.1 | 11.6 ± 0.1*** | 11.6 ± 0.1 | -- | -- | -- | -- | 11.6 ± 0.2 | -- | -- | -- | -- |
| Age (yr) | 16.3 ± 0.2 | 15.8 ± 0.2 | 15.9 ± 0.2 | -- | -- | -- | -- | 15.7 ± 0.2 | -- | -- | -- | -- |
| BMI (kg/m2) | 21.3 ± 0.3 | 24.2 ± 0.5*** | 24.2 ± 0.7 | 24.9 ± 0.8c | 25.1 ± 0.8 | 0.7 ± 0.3 | 0.2 ± 0.3 | 24.2 ± 0.7 | 23.9 ± 0.7 | 23.9 ± 0.7 | -0.2 ± 0.3e | 0.0 ± 0.2 |
| BMI Z-score | 0.0 ± 0.1 | 0.8 ± 0.1*** | 0.9 ± 0.2 | 1.1 ± 0.2b | 1.2 ± 0.2 | 0.2 ± 0.1 | 0.1 ± 0.3 | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.2 | -0.1 ± 0.1e | 0.1 ± 0.3 |
| Δ Z-score Birthweight to BMI | -0.2 ± 0.2 | 1.4 ± 0.2*** | 1.5 ± 0.3 | 1.7 ± 0.3b | 1.8 ± 0.3 | 0.2 ± 0.1 | 0.1 ± 0.1 | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.3 | 0.0 ± 0.1e | 0.0 ± 0.1 |
| Waist Circumference (cm) | 74 ± 1 | 77 ± 1 | 76 ± 2 | 78 ± 2b | 78 ± 2 | 2 ± 1 | 0 ± 1 | 77 ± 2 | 74 ± 1d | 74 ± 1 | -3 ± 0.8g | 0 ± 1 |
| Hirsutism score | -- | 17 ± 1 | 17 ± 1 | 14 ± 1d | 14 ± 1 | -3 ± 1 | 0 ± 1 | 16 ± 1 | 11 ± 1d | 9 ± 1c | -5 ± 1g | -2 ± 1 |
| SHBG (nmol/L) | 63 ± 3 | 30 ± 2*** | 31 ± 2 | 61 ± 5d | 32 ± 3d | 30 ± 4 | -29 ± 5 | 30 ± 2 | 32 ± 2 | 39 ± 3c | 2 ± 2g | 7 ± 2g |
| Testosterone (nmol/L) | 0.7 ± 0.1 | 1.4 ± 0.1*** | 1.3 ± 0.1 | 0.7 ± 0.1d | 1.6 ± 0.2d | -0.6 ± 0.1 | 0.9 ± 0.2 | 1.5 ± 0.2 | 0.8 ± 0.1c | 1.2 ± 0.2c | -0.7 ± 0.2 | 0.4 ± 0.2 |
| Androstenedione (nmol/L) | 3.5 ± 0.2 | 5.3 ± 0.3*** | 4.8 ± 0.3 | 2.5 ± 0.2d | 5.7 ± 0.6d | -2.3 ± 0.3 | 3.2 ± 0.5 | 5.7 ± 0.4 | 3.5 ± 0.3d | 5.3 ± 0.6c | -2.2 ± 0.4 | 1.8 ± 0.6 |
| Free Testosterone Z-score | 0.0 ± 0.2 | 2.9 ± 0.5*** | 2.3 ± 0.5 | 0.3 ± 0.3c | 3.6 ± 0.8d | -2.0 ± 0.6 | 3.3 ± 0.7 | 3.2 ± 0.9 | 0.5 ± 0.3c | 2.0 ± 0.7c | -2.7 ± 0.9 | 1.5 ± 0.7 |
| Free Androstenedione Z-score | 0.0 ± 0.2 | 1.8 ± 0.3*** | 1.1 ± 0.3 | -0.9 ± 0.2d | 2.2 ± 0.5d | -2.0 ± 0.3 | 3.1 ± 0.5 | 2.2 ± 0.4 | 0.1 ± 0.3d | 1.8 ± 0.6c | -2.1 ± 0.4 | 1.7 ± 0.6 |
| Fasting Insulin (pmol/L) | 49 ± 7 | 76 ± 7*** | 83 ± 7 | 104 ± 7b | 76 ± 7c | 21 ± 7 | -28 ± 7 | 69 ± 7 | 42 ± 7d | 49 ± 7 | -27 ± 7g | 7 ± 7f |
| HOMA-IR | 1.5 ± 0.1 | 2.3 ± 0.2*** | 2.6 ± 0.3 | 3.0 ± 0.3 | 2.2 ± 0.2c | 0.4 ± 0.2 | -0.8 ± 0.3 | 2.1 ± 0.2 | 1.2 ± 0.1d | 1.3 ± 0.2 | -0.9 ± 0.3f | 0.1 ± 0.2f |
| OGTT Mean Glycemia Z-score | -- | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | -0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1c | 0.1 ± 0.1 | -0.1 ± 0.1f | 0.0 ± 0.1 |
| Mean Insulinemia Z-score | -- | 3.2 ± 0.3 | 3.5 ± 0.4 | 3.7 ± 0.5 | 3.1 ± 0.5 | 0.2 ± 0.5 | -0.6 ± 0.5 | 2.8 ± 0.4 | 0.6 ± 0.2d | 0.6 ± 0.2 | -2.2 ± 0.3g | 0.0 ± 0.2 |
| ALT (µkat/L) | 0.30 ± 0.02 | 0.23 ± 0.02*** | 0.23 ± 0.02 | 0.32 ± 0.03c | 0.27 ± 0.02 | 0.09 ± 0.02 | -0.05 ± 0.03 | 0.23 ± 0.02 | 0.23 ± 0.02 | 0.23 ± 0.02 | -0.00 ± 0.02e | -0.00 ± 0.02 |
| AST (µkat/L) | 0.25 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.00 ± 0.02 | 0.00 ± 0.02 | 0.28 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | -0.01 ± 0.02 | 0.00 ± 0.02 |
| GGT (µkat/L) | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.30 ± 0.02d | 0.25 ± 0.02b | 0.08 ± 0.02 | -0.05 ± 0.02 | 0.22 ± 0.02 | 0.18 ± 0.02 c | 0.22 ± 0.02c | -0.04 ± 0.02g | 0.04 ± 0.02 g |
| Triacylglycerol (mmol/L) | 0.60 ± 0.03 | 0.68 ± 0.03 | 0.66 ± 0.03 | 0.75 ± 0.05b | 0.64 ± 0.03c | 0.09 ± 0.03 | -0.11 ± 0.03 | 0.70 ± 0.05 | 0.67 ± 0.05 | 0.63 ± 0.05 | -0.03 ± 0.05e | -0.04 ± 0.03 |
| LDL-cholesterol (mmol/L) | 2.2 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.7 ± 0.1d | 2.2 ± 0.1d | 0.4 ± 0.1 | -0.5 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.0 ± 0.1d | 0.0 ± 0.1f | -0.2 ± 0.1e |
| HDL-cholesterol (mmol/L) | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1 | 0.0 ± 0.1 | 0.1 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1c | 1.3 ± 0.1b | 0.1 ± 0.1e | -0.1 ± 0.1f |
| HMW-adiponectin (mg/L) | 9.3 ± 0.8 | 6.8 ± 0.6* | 6.5 ± 0.6 | 8.9 ± 1.3b | 8.6 ± 0.8 | 2.6 ± 1.1 | -0.3 ± 1.5 | 7.1 ± 0.9 | 17.1 ± 2.6d | 10.3 ± 1.5c | 10.0 ± 2.1f | -7 ± 2e |
| C-Reactive Protein (nmol/L) | 6.7 ± 0.9 | 14.3 ± 1.9*** | 11.4 ± 1.9 | 24.8 ± 3.8c | 18.1 ± 3.8 | 13.4 ± 3.8 | -6.7 ± 5.7 | 17.1 ± 3.8 | 6.7 ± 0.9c | 6.7 ± 0.9 | -10.4 ± 3.8g | 0.0 ± 0.9 |
| Carotid IMT (mm) | -- | .37 ± .00 | .37 ± .01 | .37 ± .01 | .36 ± .01b | .00 ± .00 | -.01 ± .01 | .37 ± 0.01 | .35 ± 0.01d | .35 ± 0.01 | -.02 ± 0.01e | .00 ± .01 |
| Systolic Blood Pressure (mmHg) | 113 ± 1 | 115 ± 1 | 113 ± 2 | 115 ± 1 | 112 ± 2 | 2 ± 2 | -3 ± 2 | 116 ± 1 | 112 ± 1b | 114 ± 2 | -4 ± 2e | 2 ± 2 |
| Diastolic Blood Pressure (mmHg) | 70 ± 1 | 72 ± 1 | 71 ± 1 | 74 ± 1b | 73 ± 1 | 3 ± 1 | -1 ± 1 | 73 ± 1 | 71 ± 1 | 70 ± 1 | -2 ± 1e | -1 ± 1 |
| miR-451a Z-score | 0.00 ± 0.28 | -3.57 ± 0.11*** | -3.75 ± 0.12 | -3.31 ± 0.12 | -3.59 ± 0.16 | -- | -- | -3.32 ± 0.19 | 0.37 ± 0.31 d | -1.05 ± 0.43c | -- | -- |
| DXA BMD (g/cm2) | -- | 1.19 ± 0.01 | 1.18 ± 0.02 | 1.19 ± 0.02 | 1.20 ± 0.02b | 0.01 ± 0.01 | 0.01 ± 0.01 | 1.19 ± 0.02 | 1.19 ± 0.02 | 1.21 ± 0.02b | 0.00 ± 0.01 | 0.02 ± 0.01 |
| Lean Mass (Kg) | -- | 35.6 ± 0.6 | 35.7 ± 0.8 | 36.4 ± 0.9 | 36.5 ± 0.9 | 0.7 ± 0.4 | 0.1 ± 0.2 | 35.5 ± 0.9 | 35.6 ± 0.8 | 36.1 ± 0.8 | 0.1 ± 0.3 | 0.5 ± 0.3 |
| Fat Mass (Kg) | -- | 22.1 ± 1.0 | 21.8 ± 1.4 | 23.2 ± 1.5c | 23.4 ± 1.6 | 1.4 ± 0.5 | 0.2 ± 0.6 | 22.4 ± 1.6 | 22.5 ± 1.4 | 22.1 ± 1.7 | 0.1 ± 0.8 | -0.4 ± 0.6 |
| Abd MRI Subc Fat (cm2) | 94 ± 9 | 174 ± 14*** | 169 ± 18 | 184 ± 19 | 180 ± 20 | 15 ± 9 | -4 ± 13 | 179 ± 21 | 171 ± 19 | 167 ± 23 | -8 ± 11 | -4 ± 9 |
| Visceral Fat (cm2) | 28 ± 1 | 43 ± 2*** | 41 ± 3 | 45 ± 4 | 39 ± 3 | 4 ± 3 | -6 ± 3 | 44 ± 3 | 35 ± 2b | 36 ± 3 | -9 ± 4f | 1 ± 2 |
| Liver Fat (%) | 10 ± 1 | 17 ± 1*** | 17 ± 1 | 19 ± 1 | 17 ± 1b | 2 ± 1 | -2 ± 2 | 18 ± 1 | 10 ± 1d | 10 ± 1 | -8 ± 1g | 0 ± 1 |
| . | . | . | Ethinylestradiol-Levonorgestrel (N = 31) . | SPIOMET (N=31) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Controls (N = 52) . | PCOS (N = 62) . | Starta . | 12 mo . | 24 mo . | Δ 0–12 mo . | Δ 12–24 mo . | Starta . | 12 mo . | 24 mo . | Δ 0–12 mo . | Δ 12–24 mo . |
| Birthweight Z-score | 0.2 ± 0.1 | -0.6 ± 0.1*** | -0.6 ± 0.2 | -- | -- | -- | -- | -0.6 ± 0.1 | -- | -- | -- | -- |
| Age at Menarche (yr) | 12.4 ± 0.1 | 11.6 ± 0.1*** | 11.6 ± 0.1 | -- | -- | -- | -- | 11.6 ± 0.2 | -- | -- | -- | -- |
| Age (yr) | 16.3 ± 0.2 | 15.8 ± 0.2 | 15.9 ± 0.2 | -- | -- | -- | -- | 15.7 ± 0.2 | -- | -- | -- | -- |
| BMI (kg/m2) | 21.3 ± 0.3 | 24.2 ± 0.5*** | 24.2 ± 0.7 | 24.9 ± 0.8c | 25.1 ± 0.8 | 0.7 ± 0.3 | 0.2 ± 0.3 | 24.2 ± 0.7 | 23.9 ± 0.7 | 23.9 ± 0.7 | -0.2 ± 0.3e | 0.0 ± 0.2 |
| BMI Z-score | 0.0 ± 0.1 | 0.8 ± 0.1*** | 0.9 ± 0.2 | 1.1 ± 0.2b | 1.2 ± 0.2 | 0.2 ± 0.1 | 0.1 ± 0.3 | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.2 | -0.1 ± 0.1e | 0.1 ± 0.3 |
| Δ Z-score Birthweight to BMI | -0.2 ± 0.2 | 1.4 ± 0.2*** | 1.5 ± 0.3 | 1.7 ± 0.3b | 1.8 ± 0.3 | 0.2 ± 0.1 | 0.1 ± 0.1 | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.3 | 0.0 ± 0.1e | 0.0 ± 0.1 |
| Waist Circumference (cm) | 74 ± 1 | 77 ± 1 | 76 ± 2 | 78 ± 2b | 78 ± 2 | 2 ± 1 | 0 ± 1 | 77 ± 2 | 74 ± 1d | 74 ± 1 | -3 ± 0.8g | 0 ± 1 |
| Hirsutism score | -- | 17 ± 1 | 17 ± 1 | 14 ± 1d | 14 ± 1 | -3 ± 1 | 0 ± 1 | 16 ± 1 | 11 ± 1d | 9 ± 1c | -5 ± 1g | -2 ± 1 |
| SHBG (nmol/L) | 63 ± 3 | 30 ± 2*** | 31 ± 2 | 61 ± 5d | 32 ± 3d | 30 ± 4 | -29 ± 5 | 30 ± 2 | 32 ± 2 | 39 ± 3c | 2 ± 2g | 7 ± 2g |
| Testosterone (nmol/L) | 0.7 ± 0.1 | 1.4 ± 0.1*** | 1.3 ± 0.1 | 0.7 ± 0.1d | 1.6 ± 0.2d | -0.6 ± 0.1 | 0.9 ± 0.2 | 1.5 ± 0.2 | 0.8 ± 0.1c | 1.2 ± 0.2c | -0.7 ± 0.2 | 0.4 ± 0.2 |
| Androstenedione (nmol/L) | 3.5 ± 0.2 | 5.3 ± 0.3*** | 4.8 ± 0.3 | 2.5 ± 0.2d | 5.7 ± 0.6d | -2.3 ± 0.3 | 3.2 ± 0.5 | 5.7 ± 0.4 | 3.5 ± 0.3d | 5.3 ± 0.6c | -2.2 ± 0.4 | 1.8 ± 0.6 |
| Free Testosterone Z-score | 0.0 ± 0.2 | 2.9 ± 0.5*** | 2.3 ± 0.5 | 0.3 ± 0.3c | 3.6 ± 0.8d | -2.0 ± 0.6 | 3.3 ± 0.7 | 3.2 ± 0.9 | 0.5 ± 0.3c | 2.0 ± 0.7c | -2.7 ± 0.9 | 1.5 ± 0.7 |
| Free Androstenedione Z-score | 0.0 ± 0.2 | 1.8 ± 0.3*** | 1.1 ± 0.3 | -0.9 ± 0.2d | 2.2 ± 0.5d | -2.0 ± 0.3 | 3.1 ± 0.5 | 2.2 ± 0.4 | 0.1 ± 0.3d | 1.8 ± 0.6c | -2.1 ± 0.4 | 1.7 ± 0.6 |
| Fasting Insulin (pmol/L) | 49 ± 7 | 76 ± 7*** | 83 ± 7 | 104 ± 7b | 76 ± 7c | 21 ± 7 | -28 ± 7 | 69 ± 7 | 42 ± 7d | 49 ± 7 | -27 ± 7g | 7 ± 7f |
| HOMA-IR | 1.5 ± 0.1 | 2.3 ± 0.2*** | 2.6 ± 0.3 | 3.0 ± 0.3 | 2.2 ± 0.2c | 0.4 ± 0.2 | -0.8 ± 0.3 | 2.1 ± 0.2 | 1.2 ± 0.1d | 1.3 ± 0.2 | -0.9 ± 0.3f | 0.1 ± 0.2f |
| OGTT Mean Glycemia Z-score | -- | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | -0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1c | 0.1 ± 0.1 | -0.1 ± 0.1f | 0.0 ± 0.1 |
| Mean Insulinemia Z-score | -- | 3.2 ± 0.3 | 3.5 ± 0.4 | 3.7 ± 0.5 | 3.1 ± 0.5 | 0.2 ± 0.5 | -0.6 ± 0.5 | 2.8 ± 0.4 | 0.6 ± 0.2d | 0.6 ± 0.2 | -2.2 ± 0.3g | 0.0 ± 0.2 |
| ALT (µkat/L) | 0.30 ± 0.02 | 0.23 ± 0.02*** | 0.23 ± 0.02 | 0.32 ± 0.03c | 0.27 ± 0.02 | 0.09 ± 0.02 | -0.05 ± 0.03 | 0.23 ± 0.02 | 0.23 ± 0.02 | 0.23 ± 0.02 | -0.00 ± 0.02e | -0.00 ± 0.02 |
| AST (µkat/L) | 0.25 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.00 ± 0.02 | 0.00 ± 0.02 | 0.28 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | -0.01 ± 0.02 | 0.00 ± 0.02 |
| GGT (µkat/L) | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.30 ± 0.02d | 0.25 ± 0.02b | 0.08 ± 0.02 | -0.05 ± 0.02 | 0.22 ± 0.02 | 0.18 ± 0.02 c | 0.22 ± 0.02c | -0.04 ± 0.02g | 0.04 ± 0.02 g |
| Triacylglycerol (mmol/L) | 0.60 ± 0.03 | 0.68 ± 0.03 | 0.66 ± 0.03 | 0.75 ± 0.05b | 0.64 ± 0.03c | 0.09 ± 0.03 | -0.11 ± 0.03 | 0.70 ± 0.05 | 0.67 ± 0.05 | 0.63 ± 0.05 | -0.03 ± 0.05e | -0.04 ± 0.03 |
| LDL-cholesterol (mmol/L) | 2.2 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.7 ± 0.1d | 2.2 ± 0.1d | 0.4 ± 0.1 | -0.5 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.0 ± 0.1d | 0.0 ± 0.1f | -0.2 ± 0.1e |
| HDL-cholesterol (mmol/L) | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1 | 0.0 ± 0.1 | 0.1 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1c | 1.3 ± 0.1b | 0.1 ± 0.1e | -0.1 ± 0.1f |
| HMW-adiponectin (mg/L) | 9.3 ± 0.8 | 6.8 ± 0.6* | 6.5 ± 0.6 | 8.9 ± 1.3b | 8.6 ± 0.8 | 2.6 ± 1.1 | -0.3 ± 1.5 | 7.1 ± 0.9 | 17.1 ± 2.6d | 10.3 ± 1.5c | 10.0 ± 2.1f | -7 ± 2e |
| C-Reactive Protein (nmol/L) | 6.7 ± 0.9 | 14.3 ± 1.9*** | 11.4 ± 1.9 | 24.8 ± 3.8c | 18.1 ± 3.8 | 13.4 ± 3.8 | -6.7 ± 5.7 | 17.1 ± 3.8 | 6.7 ± 0.9c | 6.7 ± 0.9 | -10.4 ± 3.8g | 0.0 ± 0.9 |
| Carotid IMT (mm) | -- | .37 ± .00 | .37 ± .01 | .37 ± .01 | .36 ± .01b | .00 ± .00 | -.01 ± .01 | .37 ± 0.01 | .35 ± 0.01d | .35 ± 0.01 | -.02 ± 0.01e | .00 ± .01 |
| Systolic Blood Pressure (mmHg) | 113 ± 1 | 115 ± 1 | 113 ± 2 | 115 ± 1 | 112 ± 2 | 2 ± 2 | -3 ± 2 | 116 ± 1 | 112 ± 1b | 114 ± 2 | -4 ± 2e | 2 ± 2 |
| Diastolic Blood Pressure (mmHg) | 70 ± 1 | 72 ± 1 | 71 ± 1 | 74 ± 1b | 73 ± 1 | 3 ± 1 | -1 ± 1 | 73 ± 1 | 71 ± 1 | 70 ± 1 | -2 ± 1e | -1 ± 1 |
| miR-451a Z-score | 0.00 ± 0.28 | -3.57 ± 0.11*** | -3.75 ± 0.12 | -3.31 ± 0.12 | -3.59 ± 0.16 | -- | -- | -3.32 ± 0.19 | 0.37 ± 0.31 d | -1.05 ± 0.43c | -- | -- |
| DXA BMD (g/cm2) | -- | 1.19 ± 0.01 | 1.18 ± 0.02 | 1.19 ± 0.02 | 1.20 ± 0.02b | 0.01 ± 0.01 | 0.01 ± 0.01 | 1.19 ± 0.02 | 1.19 ± 0.02 | 1.21 ± 0.02b | 0.00 ± 0.01 | 0.02 ± 0.01 |
| Lean Mass (Kg) | -- | 35.6 ± 0.6 | 35.7 ± 0.8 | 36.4 ± 0.9 | 36.5 ± 0.9 | 0.7 ± 0.4 | 0.1 ± 0.2 | 35.5 ± 0.9 | 35.6 ± 0.8 | 36.1 ± 0.8 | 0.1 ± 0.3 | 0.5 ± 0.3 |
| Fat Mass (Kg) | -- | 22.1 ± 1.0 | 21.8 ± 1.4 | 23.2 ± 1.5c | 23.4 ± 1.6 | 1.4 ± 0.5 | 0.2 ± 0.6 | 22.4 ± 1.6 | 22.5 ± 1.4 | 22.1 ± 1.7 | 0.1 ± 0.8 | -0.4 ± 0.6 |
| Abd MRI Subc Fat (cm2) | 94 ± 9 | 174 ± 14*** | 169 ± 18 | 184 ± 19 | 180 ± 20 | 15 ± 9 | -4 ± 13 | 179 ± 21 | 171 ± 19 | 167 ± 23 | -8 ± 11 | -4 ± 9 |
| Visceral Fat (cm2) | 28 ± 1 | 43 ± 2*** | 41 ± 3 | 45 ± 4 | 39 ± 3 | 4 ± 3 | -6 ± 3 | 44 ± 3 | 35 ± 2b | 36 ± 3 | -9 ± 4f | 1 ± 2 |
| Liver Fat (%) | 10 ± 1 | 17 ± 1*** | 17 ± 1 | 19 ± 1 | 17 ± 1b | 2 ± 1 | -2 ± 2 | 18 ± 1 | 10 ± 1d | 10 ± 1 | -8 ± 1g | 0 ± 1 |
Values are mean ± SEM.
Abbreviations: Abd MRI, abdominal magnetic resonance imaging; BMD, bone mineral density; BMI, body mass index; DXA, dual x-ray absorptiometry; HMW adiponectin, high-molecular-weight adiponectin; HOMA-IR, homeostasis model assessment - insulin resistance; IMT, intima-media thickness; miR-451a, microRNA-451a; OGTT, oral glucose tolerance test; SHBG, sex hormone-binding globulin;
#miR-451a (controls, n = 13; OC at start, n = 12; SPIOMET at start, n = 9; OC at 12 mo, n = 25; SPIOMET at 12 mo, n = 24; OC at 24 mo, n = 15; SPIOMET at 24 mo, n = 16)
ano significant differences between randomized subgroups at start
P < 0.05, cP ≤ 0.01 and dP ≤ 0.001 within subgroups for 0-12 mo & 12-24 mo change (Δ)
P < 0.05, fP ≤ 0.01, gP ≤ 0.001 between subgroups for 0-12 mo & 12-24 change (Δ)
*P < 0.05, **P ≤ 0.01 and ***P ≤ 0.001 between all PCOS at start and control group
Data From Adolescent Girls With Polycystic Ovary Syndrome (PCOS) Who Were Randomized to Receive Ethinylestradiol-Levonorgestrel (N = 31) or Low-Dose Spironolactone-Pioglitazone-Metformin (N = 31) for 12 Months, and who remained subsequently untreated for 12 months
| . | . | . | Ethinylestradiol-Levonorgestrel (N = 31) . | SPIOMET (N=31) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Controls (N = 52) . | PCOS (N = 62) . | Starta . | 12 mo . | 24 mo . | Δ 0–12 mo . | Δ 12–24 mo . | Starta . | 12 mo . | 24 mo . | Δ 0–12 mo . | Δ 12–24 mo . |
| Birthweight Z-score | 0.2 ± 0.1 | -0.6 ± 0.1*** | -0.6 ± 0.2 | -- | -- | -- | -- | -0.6 ± 0.1 | -- | -- | -- | -- |
| Age at Menarche (yr) | 12.4 ± 0.1 | 11.6 ± 0.1*** | 11.6 ± 0.1 | -- | -- | -- | -- | 11.6 ± 0.2 | -- | -- | -- | -- |
| Age (yr) | 16.3 ± 0.2 | 15.8 ± 0.2 | 15.9 ± 0.2 | -- | -- | -- | -- | 15.7 ± 0.2 | -- | -- | -- | -- |
| BMI (kg/m2) | 21.3 ± 0.3 | 24.2 ± 0.5*** | 24.2 ± 0.7 | 24.9 ± 0.8c | 25.1 ± 0.8 | 0.7 ± 0.3 | 0.2 ± 0.3 | 24.2 ± 0.7 | 23.9 ± 0.7 | 23.9 ± 0.7 | -0.2 ± 0.3e | 0.0 ± 0.2 |
| BMI Z-score | 0.0 ± 0.1 | 0.8 ± 0.1*** | 0.9 ± 0.2 | 1.1 ± 0.2b | 1.2 ± 0.2 | 0.2 ± 0.1 | 0.1 ± 0.3 | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.2 | -0.1 ± 0.1e | 0.1 ± 0.3 |
| Δ Z-score Birthweight to BMI | -0.2 ± 0.2 | 1.4 ± 0.2*** | 1.5 ± 0.3 | 1.7 ± 0.3b | 1.8 ± 0.3 | 0.2 ± 0.1 | 0.1 ± 0.1 | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.3 | 0.0 ± 0.1e | 0.0 ± 0.1 |
| Waist Circumference (cm) | 74 ± 1 | 77 ± 1 | 76 ± 2 | 78 ± 2b | 78 ± 2 | 2 ± 1 | 0 ± 1 | 77 ± 2 | 74 ± 1d | 74 ± 1 | -3 ± 0.8g | 0 ± 1 |
| Hirsutism score | -- | 17 ± 1 | 17 ± 1 | 14 ± 1d | 14 ± 1 | -3 ± 1 | 0 ± 1 | 16 ± 1 | 11 ± 1d | 9 ± 1c | -5 ± 1g | -2 ± 1 |
| SHBG (nmol/L) | 63 ± 3 | 30 ± 2*** | 31 ± 2 | 61 ± 5d | 32 ± 3d | 30 ± 4 | -29 ± 5 | 30 ± 2 | 32 ± 2 | 39 ± 3c | 2 ± 2g | 7 ± 2g |
| Testosterone (nmol/L) | 0.7 ± 0.1 | 1.4 ± 0.1*** | 1.3 ± 0.1 | 0.7 ± 0.1d | 1.6 ± 0.2d | -0.6 ± 0.1 | 0.9 ± 0.2 | 1.5 ± 0.2 | 0.8 ± 0.1c | 1.2 ± 0.2c | -0.7 ± 0.2 | 0.4 ± 0.2 |
| Androstenedione (nmol/L) | 3.5 ± 0.2 | 5.3 ± 0.3*** | 4.8 ± 0.3 | 2.5 ± 0.2d | 5.7 ± 0.6d | -2.3 ± 0.3 | 3.2 ± 0.5 | 5.7 ± 0.4 | 3.5 ± 0.3d | 5.3 ± 0.6c | -2.2 ± 0.4 | 1.8 ± 0.6 |
| Free Testosterone Z-score | 0.0 ± 0.2 | 2.9 ± 0.5*** | 2.3 ± 0.5 | 0.3 ± 0.3c | 3.6 ± 0.8d | -2.0 ± 0.6 | 3.3 ± 0.7 | 3.2 ± 0.9 | 0.5 ± 0.3c | 2.0 ± 0.7c | -2.7 ± 0.9 | 1.5 ± 0.7 |
| Free Androstenedione Z-score | 0.0 ± 0.2 | 1.8 ± 0.3*** | 1.1 ± 0.3 | -0.9 ± 0.2d | 2.2 ± 0.5d | -2.0 ± 0.3 | 3.1 ± 0.5 | 2.2 ± 0.4 | 0.1 ± 0.3d | 1.8 ± 0.6c | -2.1 ± 0.4 | 1.7 ± 0.6 |
| Fasting Insulin (pmol/L) | 49 ± 7 | 76 ± 7*** | 83 ± 7 | 104 ± 7b | 76 ± 7c | 21 ± 7 | -28 ± 7 | 69 ± 7 | 42 ± 7d | 49 ± 7 | -27 ± 7g | 7 ± 7f |
| HOMA-IR | 1.5 ± 0.1 | 2.3 ± 0.2*** | 2.6 ± 0.3 | 3.0 ± 0.3 | 2.2 ± 0.2c | 0.4 ± 0.2 | -0.8 ± 0.3 | 2.1 ± 0.2 | 1.2 ± 0.1d | 1.3 ± 0.2 | -0.9 ± 0.3f | 0.1 ± 0.2f |
| OGTT Mean Glycemia Z-score | -- | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | -0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1c | 0.1 ± 0.1 | -0.1 ± 0.1f | 0.0 ± 0.1 |
| Mean Insulinemia Z-score | -- | 3.2 ± 0.3 | 3.5 ± 0.4 | 3.7 ± 0.5 | 3.1 ± 0.5 | 0.2 ± 0.5 | -0.6 ± 0.5 | 2.8 ± 0.4 | 0.6 ± 0.2d | 0.6 ± 0.2 | -2.2 ± 0.3g | 0.0 ± 0.2 |
| ALT (µkat/L) | 0.30 ± 0.02 | 0.23 ± 0.02*** | 0.23 ± 0.02 | 0.32 ± 0.03c | 0.27 ± 0.02 | 0.09 ± 0.02 | -0.05 ± 0.03 | 0.23 ± 0.02 | 0.23 ± 0.02 | 0.23 ± 0.02 | -0.00 ± 0.02e | -0.00 ± 0.02 |
| AST (µkat/L) | 0.25 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.00 ± 0.02 | 0.00 ± 0.02 | 0.28 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | -0.01 ± 0.02 | 0.00 ± 0.02 |
| GGT (µkat/L) | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.30 ± 0.02d | 0.25 ± 0.02b | 0.08 ± 0.02 | -0.05 ± 0.02 | 0.22 ± 0.02 | 0.18 ± 0.02 c | 0.22 ± 0.02c | -0.04 ± 0.02g | 0.04 ± 0.02 g |
| Triacylglycerol (mmol/L) | 0.60 ± 0.03 | 0.68 ± 0.03 | 0.66 ± 0.03 | 0.75 ± 0.05b | 0.64 ± 0.03c | 0.09 ± 0.03 | -0.11 ± 0.03 | 0.70 ± 0.05 | 0.67 ± 0.05 | 0.63 ± 0.05 | -0.03 ± 0.05e | -0.04 ± 0.03 |
| LDL-cholesterol (mmol/L) | 2.2 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.7 ± 0.1d | 2.2 ± 0.1d | 0.4 ± 0.1 | -0.5 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.0 ± 0.1d | 0.0 ± 0.1f | -0.2 ± 0.1e |
| HDL-cholesterol (mmol/L) | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1 | 0.0 ± 0.1 | 0.1 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1c | 1.3 ± 0.1b | 0.1 ± 0.1e | -0.1 ± 0.1f |
| HMW-adiponectin (mg/L) | 9.3 ± 0.8 | 6.8 ± 0.6* | 6.5 ± 0.6 | 8.9 ± 1.3b | 8.6 ± 0.8 | 2.6 ± 1.1 | -0.3 ± 1.5 | 7.1 ± 0.9 | 17.1 ± 2.6d | 10.3 ± 1.5c | 10.0 ± 2.1f | -7 ± 2e |
| C-Reactive Protein (nmol/L) | 6.7 ± 0.9 | 14.3 ± 1.9*** | 11.4 ± 1.9 | 24.8 ± 3.8c | 18.1 ± 3.8 | 13.4 ± 3.8 | -6.7 ± 5.7 | 17.1 ± 3.8 | 6.7 ± 0.9c | 6.7 ± 0.9 | -10.4 ± 3.8g | 0.0 ± 0.9 |
| Carotid IMT (mm) | -- | .37 ± .00 | .37 ± .01 | .37 ± .01 | .36 ± .01b | .00 ± .00 | -.01 ± .01 | .37 ± 0.01 | .35 ± 0.01d | .35 ± 0.01 | -.02 ± 0.01e | .00 ± .01 |
| Systolic Blood Pressure (mmHg) | 113 ± 1 | 115 ± 1 | 113 ± 2 | 115 ± 1 | 112 ± 2 | 2 ± 2 | -3 ± 2 | 116 ± 1 | 112 ± 1b | 114 ± 2 | -4 ± 2e | 2 ± 2 |
| Diastolic Blood Pressure (mmHg) | 70 ± 1 | 72 ± 1 | 71 ± 1 | 74 ± 1b | 73 ± 1 | 3 ± 1 | -1 ± 1 | 73 ± 1 | 71 ± 1 | 70 ± 1 | -2 ± 1e | -1 ± 1 |
| miR-451a Z-score | 0.00 ± 0.28 | -3.57 ± 0.11*** | -3.75 ± 0.12 | -3.31 ± 0.12 | -3.59 ± 0.16 | -- | -- | -3.32 ± 0.19 | 0.37 ± 0.31 d | -1.05 ± 0.43c | -- | -- |
| DXA BMD (g/cm2) | -- | 1.19 ± 0.01 | 1.18 ± 0.02 | 1.19 ± 0.02 | 1.20 ± 0.02b | 0.01 ± 0.01 | 0.01 ± 0.01 | 1.19 ± 0.02 | 1.19 ± 0.02 | 1.21 ± 0.02b | 0.00 ± 0.01 | 0.02 ± 0.01 |
| Lean Mass (Kg) | -- | 35.6 ± 0.6 | 35.7 ± 0.8 | 36.4 ± 0.9 | 36.5 ± 0.9 | 0.7 ± 0.4 | 0.1 ± 0.2 | 35.5 ± 0.9 | 35.6 ± 0.8 | 36.1 ± 0.8 | 0.1 ± 0.3 | 0.5 ± 0.3 |
| Fat Mass (Kg) | -- | 22.1 ± 1.0 | 21.8 ± 1.4 | 23.2 ± 1.5c | 23.4 ± 1.6 | 1.4 ± 0.5 | 0.2 ± 0.6 | 22.4 ± 1.6 | 22.5 ± 1.4 | 22.1 ± 1.7 | 0.1 ± 0.8 | -0.4 ± 0.6 |
| Abd MRI Subc Fat (cm2) | 94 ± 9 | 174 ± 14*** | 169 ± 18 | 184 ± 19 | 180 ± 20 | 15 ± 9 | -4 ± 13 | 179 ± 21 | 171 ± 19 | 167 ± 23 | -8 ± 11 | -4 ± 9 |
| Visceral Fat (cm2) | 28 ± 1 | 43 ± 2*** | 41 ± 3 | 45 ± 4 | 39 ± 3 | 4 ± 3 | -6 ± 3 | 44 ± 3 | 35 ± 2b | 36 ± 3 | -9 ± 4f | 1 ± 2 |
| Liver Fat (%) | 10 ± 1 | 17 ± 1*** | 17 ± 1 | 19 ± 1 | 17 ± 1b | 2 ± 1 | -2 ± 2 | 18 ± 1 | 10 ± 1d | 10 ± 1 | -8 ± 1g | 0 ± 1 |
| . | . | . | Ethinylestradiol-Levonorgestrel (N = 31) . | SPIOMET (N=31) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Controls (N = 52) . | PCOS (N = 62) . | Starta . | 12 mo . | 24 mo . | Δ 0–12 mo . | Δ 12–24 mo . | Starta . | 12 mo . | 24 mo . | Δ 0–12 mo . | Δ 12–24 mo . |
| Birthweight Z-score | 0.2 ± 0.1 | -0.6 ± 0.1*** | -0.6 ± 0.2 | -- | -- | -- | -- | -0.6 ± 0.1 | -- | -- | -- | -- |
| Age at Menarche (yr) | 12.4 ± 0.1 | 11.6 ± 0.1*** | 11.6 ± 0.1 | -- | -- | -- | -- | 11.6 ± 0.2 | -- | -- | -- | -- |
| Age (yr) | 16.3 ± 0.2 | 15.8 ± 0.2 | 15.9 ± 0.2 | -- | -- | -- | -- | 15.7 ± 0.2 | -- | -- | -- | -- |
| BMI (kg/m2) | 21.3 ± 0.3 | 24.2 ± 0.5*** | 24.2 ± 0.7 | 24.9 ± 0.8c | 25.1 ± 0.8 | 0.7 ± 0.3 | 0.2 ± 0.3 | 24.2 ± 0.7 | 23.9 ± 0.7 | 23.9 ± 0.7 | -0.2 ± 0.3e | 0.0 ± 0.2 |
| BMI Z-score | 0.0 ± 0.1 | 0.8 ± 0.1*** | 0.9 ± 0.2 | 1.1 ± 0.2b | 1.2 ± 0.2 | 0.2 ± 0.1 | 0.1 ± 0.3 | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.2 | -0.1 ± 0.1e | 0.1 ± 0.3 |
| Δ Z-score Birthweight to BMI | -0.2 ± 0.2 | 1.4 ± 0.2*** | 1.5 ± 0.3 | 1.7 ± 0.3b | 1.8 ± 0.3 | 0.2 ± 0.1 | 0.1 ± 0.1 | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.3 | 0.0 ± 0.1e | 0.0 ± 0.1 |
| Waist Circumference (cm) | 74 ± 1 | 77 ± 1 | 76 ± 2 | 78 ± 2b | 78 ± 2 | 2 ± 1 | 0 ± 1 | 77 ± 2 | 74 ± 1d | 74 ± 1 | -3 ± 0.8g | 0 ± 1 |
| Hirsutism score | -- | 17 ± 1 | 17 ± 1 | 14 ± 1d | 14 ± 1 | -3 ± 1 | 0 ± 1 | 16 ± 1 | 11 ± 1d | 9 ± 1c | -5 ± 1g | -2 ± 1 |
| SHBG (nmol/L) | 63 ± 3 | 30 ± 2*** | 31 ± 2 | 61 ± 5d | 32 ± 3d | 30 ± 4 | -29 ± 5 | 30 ± 2 | 32 ± 2 | 39 ± 3c | 2 ± 2g | 7 ± 2g |
| Testosterone (nmol/L) | 0.7 ± 0.1 | 1.4 ± 0.1*** | 1.3 ± 0.1 | 0.7 ± 0.1d | 1.6 ± 0.2d | -0.6 ± 0.1 | 0.9 ± 0.2 | 1.5 ± 0.2 | 0.8 ± 0.1c | 1.2 ± 0.2c | -0.7 ± 0.2 | 0.4 ± 0.2 |
| Androstenedione (nmol/L) | 3.5 ± 0.2 | 5.3 ± 0.3*** | 4.8 ± 0.3 | 2.5 ± 0.2d | 5.7 ± 0.6d | -2.3 ± 0.3 | 3.2 ± 0.5 | 5.7 ± 0.4 | 3.5 ± 0.3d | 5.3 ± 0.6c | -2.2 ± 0.4 | 1.8 ± 0.6 |
| Free Testosterone Z-score | 0.0 ± 0.2 | 2.9 ± 0.5*** | 2.3 ± 0.5 | 0.3 ± 0.3c | 3.6 ± 0.8d | -2.0 ± 0.6 | 3.3 ± 0.7 | 3.2 ± 0.9 | 0.5 ± 0.3c | 2.0 ± 0.7c | -2.7 ± 0.9 | 1.5 ± 0.7 |
| Free Androstenedione Z-score | 0.0 ± 0.2 | 1.8 ± 0.3*** | 1.1 ± 0.3 | -0.9 ± 0.2d | 2.2 ± 0.5d | -2.0 ± 0.3 | 3.1 ± 0.5 | 2.2 ± 0.4 | 0.1 ± 0.3d | 1.8 ± 0.6c | -2.1 ± 0.4 | 1.7 ± 0.6 |
| Fasting Insulin (pmol/L) | 49 ± 7 | 76 ± 7*** | 83 ± 7 | 104 ± 7b | 76 ± 7c | 21 ± 7 | -28 ± 7 | 69 ± 7 | 42 ± 7d | 49 ± 7 | -27 ± 7g | 7 ± 7f |
| HOMA-IR | 1.5 ± 0.1 | 2.3 ± 0.2*** | 2.6 ± 0.3 | 3.0 ± 0.3 | 2.2 ± 0.2c | 0.4 ± 0.2 | -0.8 ± 0.3 | 2.1 ± 0.2 | 1.2 ± 0.1d | 1.3 ± 0.2 | -0.9 ± 0.3f | 0.1 ± 0.2f |
| OGTT Mean Glycemia Z-score | -- | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | -0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1c | 0.1 ± 0.1 | -0.1 ± 0.1f | 0.0 ± 0.1 |
| Mean Insulinemia Z-score | -- | 3.2 ± 0.3 | 3.5 ± 0.4 | 3.7 ± 0.5 | 3.1 ± 0.5 | 0.2 ± 0.5 | -0.6 ± 0.5 | 2.8 ± 0.4 | 0.6 ± 0.2d | 0.6 ± 0.2 | -2.2 ± 0.3g | 0.0 ± 0.2 |
| ALT (µkat/L) | 0.30 ± 0.02 | 0.23 ± 0.02*** | 0.23 ± 0.02 | 0.32 ± 0.03c | 0.27 ± 0.02 | 0.09 ± 0.02 | -0.05 ± 0.03 | 0.23 ± 0.02 | 0.23 ± 0.02 | 0.23 ± 0.02 | -0.00 ± 0.02e | -0.00 ± 0.02 |
| AST (µkat/L) | 0.25 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.00 ± 0.02 | 0.00 ± 0.02 | 0.28 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | -0.01 ± 0.02 | 0.00 ± 0.02 |
| GGT (µkat/L) | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.30 ± 0.02d | 0.25 ± 0.02b | 0.08 ± 0.02 | -0.05 ± 0.02 | 0.22 ± 0.02 | 0.18 ± 0.02 c | 0.22 ± 0.02c | -0.04 ± 0.02g | 0.04 ± 0.02 g |
| Triacylglycerol (mmol/L) | 0.60 ± 0.03 | 0.68 ± 0.03 | 0.66 ± 0.03 | 0.75 ± 0.05b | 0.64 ± 0.03c | 0.09 ± 0.03 | -0.11 ± 0.03 | 0.70 ± 0.05 | 0.67 ± 0.05 | 0.63 ± 0.05 | -0.03 ± 0.05e | -0.04 ± 0.03 |
| LDL-cholesterol (mmol/L) | 2.2 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.7 ± 0.1d | 2.2 ± 0.1d | 0.4 ± 0.1 | -0.5 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.0 ± 0.1d | 0.0 ± 0.1f | -0.2 ± 0.1e |
| HDL-cholesterol (mmol/L) | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1 | 0.0 ± 0.1 | 0.1 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.1c | 1.3 ± 0.1b | 0.1 ± 0.1e | -0.1 ± 0.1f |
| HMW-adiponectin (mg/L) | 9.3 ± 0.8 | 6.8 ± 0.6* | 6.5 ± 0.6 | 8.9 ± 1.3b | 8.6 ± 0.8 | 2.6 ± 1.1 | -0.3 ± 1.5 | 7.1 ± 0.9 | 17.1 ± 2.6d | 10.3 ± 1.5c | 10.0 ± 2.1f | -7 ± 2e |
| C-Reactive Protein (nmol/L) | 6.7 ± 0.9 | 14.3 ± 1.9*** | 11.4 ± 1.9 | 24.8 ± 3.8c | 18.1 ± 3.8 | 13.4 ± 3.8 | -6.7 ± 5.7 | 17.1 ± 3.8 | 6.7 ± 0.9c | 6.7 ± 0.9 | -10.4 ± 3.8g | 0.0 ± 0.9 |
| Carotid IMT (mm) | -- | .37 ± .00 | .37 ± .01 | .37 ± .01 | .36 ± .01b | .00 ± .00 | -.01 ± .01 | .37 ± 0.01 | .35 ± 0.01d | .35 ± 0.01 | -.02 ± 0.01e | .00 ± .01 |
| Systolic Blood Pressure (mmHg) | 113 ± 1 | 115 ± 1 | 113 ± 2 | 115 ± 1 | 112 ± 2 | 2 ± 2 | -3 ± 2 | 116 ± 1 | 112 ± 1b | 114 ± 2 | -4 ± 2e | 2 ± 2 |
| Diastolic Blood Pressure (mmHg) | 70 ± 1 | 72 ± 1 | 71 ± 1 | 74 ± 1b | 73 ± 1 | 3 ± 1 | -1 ± 1 | 73 ± 1 | 71 ± 1 | 70 ± 1 | -2 ± 1e | -1 ± 1 |
| miR-451a Z-score | 0.00 ± 0.28 | -3.57 ± 0.11*** | -3.75 ± 0.12 | -3.31 ± 0.12 | -3.59 ± 0.16 | -- | -- | -3.32 ± 0.19 | 0.37 ± 0.31 d | -1.05 ± 0.43c | -- | -- |
| DXA BMD (g/cm2) | -- | 1.19 ± 0.01 | 1.18 ± 0.02 | 1.19 ± 0.02 | 1.20 ± 0.02b | 0.01 ± 0.01 | 0.01 ± 0.01 | 1.19 ± 0.02 | 1.19 ± 0.02 | 1.21 ± 0.02b | 0.00 ± 0.01 | 0.02 ± 0.01 |
| Lean Mass (Kg) | -- | 35.6 ± 0.6 | 35.7 ± 0.8 | 36.4 ± 0.9 | 36.5 ± 0.9 | 0.7 ± 0.4 | 0.1 ± 0.2 | 35.5 ± 0.9 | 35.6 ± 0.8 | 36.1 ± 0.8 | 0.1 ± 0.3 | 0.5 ± 0.3 |
| Fat Mass (Kg) | -- | 22.1 ± 1.0 | 21.8 ± 1.4 | 23.2 ± 1.5c | 23.4 ± 1.6 | 1.4 ± 0.5 | 0.2 ± 0.6 | 22.4 ± 1.6 | 22.5 ± 1.4 | 22.1 ± 1.7 | 0.1 ± 0.8 | -0.4 ± 0.6 |
| Abd MRI Subc Fat (cm2) | 94 ± 9 | 174 ± 14*** | 169 ± 18 | 184 ± 19 | 180 ± 20 | 15 ± 9 | -4 ± 13 | 179 ± 21 | 171 ± 19 | 167 ± 23 | -8 ± 11 | -4 ± 9 |
| Visceral Fat (cm2) | 28 ± 1 | 43 ± 2*** | 41 ± 3 | 45 ± 4 | 39 ± 3 | 4 ± 3 | -6 ± 3 | 44 ± 3 | 35 ± 2b | 36 ± 3 | -9 ± 4f | 1 ± 2 |
| Liver Fat (%) | 10 ± 1 | 17 ± 1*** | 17 ± 1 | 19 ± 1 | 17 ± 1b | 2 ± 1 | -2 ± 2 | 18 ± 1 | 10 ± 1d | 10 ± 1 | -8 ± 1g | 0 ± 1 |
Values are mean ± SEM.
Abbreviations: Abd MRI, abdominal magnetic resonance imaging; BMD, bone mineral density; BMI, body mass index; DXA, dual x-ray absorptiometry; HMW adiponectin, high-molecular-weight adiponectin; HOMA-IR, homeostasis model assessment - insulin resistance; IMT, intima-media thickness; miR-451a, microRNA-451a; OGTT, oral glucose tolerance test; SHBG, sex hormone-binding globulin;
#miR-451a (controls, n = 13; OC at start, n = 12; SPIOMET at start, n = 9; OC at 12 mo, n = 25; SPIOMET at 12 mo, n = 24; OC at 24 mo, n = 15; SPIOMET at 24 mo, n = 16)
ano significant differences between randomized subgroups at start
P < 0.05, cP ≤ 0.01 and dP ≤ 0.001 within subgroups for 0-12 mo & 12-24 mo change (Δ)
P < 0.05, fP ≤ 0.01, gP ≤ 0.001 between subgroups for 0-12 mo & 12-24 change (Δ)
*P < 0.05, **P ≤ 0.01 and ***P ≤ 0.001 between all PCOS at start and control group
Blood sampling in both patients and controls was at all time points performed either in the follicular phase of the menstrual cycle (days 3-7) or after 2 months of amenorrhea; at study start, the ratio of amenorrheic to oligomenorrheic girls was 1 to 7.
B. Assessments
Birth weight, birth length, and body mass index (BMI) (and their Z‐scores) were retrieved from medical records. Endocrine‐metabolic variables and cIMT were assessed as described [7, 8]. Homeostatic model assessment–insulin resistance (HOMA-IR) was calculated as [fasting insulin in mU/L] x [fasting glucose in mg/dL]/405. Ovulation rates were inferred by combining data from menstrual diaries and from progesterone concentrations assessed in weekly saliva samples, obtained over 12 weeks in the second quarter and then 12 weeks in the fourth quarter of the posttreatment year [7]. Progesterone was measured by enzyme-linked immunosorbent assay (ELISA) (Novatec, Inmundiagnostica, cat# DSNOV25, RRID:AB_2827743) [9]. Circulating miR-451a was measured as described [5], with results expressed in Z-scores, using the data of healthy control girls as reference; circulating miR-451a concentrations are known to be low in adolescent girls with PCOS (average Z-scores between −3 and −4), and to associate negatively with the degree of androgen excess (as judged by circulating testosterone or free androgen index, when the gonadotropic axis is not silenced), with HOMA-IR, and with hepatic and visceral fat; a normalizing rise of circulating miR-451a concentrations in adolescent girls with PCOS can thus point to a normalizing course toward metabolic health, including toward a normal ovulation rate [5]. In a search for a noninvasive, cycle-independent, on-treatment set of markers that allows to anticipate the posttreatment ovulation rate, we tested whether a “metabolic health Z-score”, which combines the Z-scores of fasting insulinemia and circulating miR-451a, associated to posttreatment ovulation rate.
Body composition was assessed by dual x‐ray absorptiometry with a Lunar Prodigy and Lunar software (version 3.4/3.5, Lunar Corp, Madison, Wisconsin); abdominal fat (subcutaneous and visceral) and hepatic fat were assessed by magnetic resonance imaging (MRI) using a multiple‐slice MRI 1.5 Tesla scan (Signa LX Echo Speed Plus Excite, General Electric, Milwaukee, Wisconsin), as described [7, 8].
C. Statistics & Ethics
Statistical analyses were performed with SPSS 23.0 (IBM, Armonk, New York). Longitudinal changes in quantitative variables between groups were compared by repeated‐measures general linear model. Differences in longitudinal changes between groups were tested by the interaction term among between‐ and within‐subject effects. P < 0.05 was considered significant. Data are presented as mean ± standard error of the mean (SEM).
The studies were conducted after approval by the Institutional Review Board of Sant Joan de Déu Hospital, after written informed consent by the parents, and after assent by each participating girl.
2. Results
Table 1 summarizes the pooled results, which indicate that SPIOMET treatment was accompanied by more broadly normalizing effects than OC, including for waist circumference, circulating insulin, HMW-adiponectin and CRP, cIMT, as well as on visceral and hepatic fat (Fig. 2).
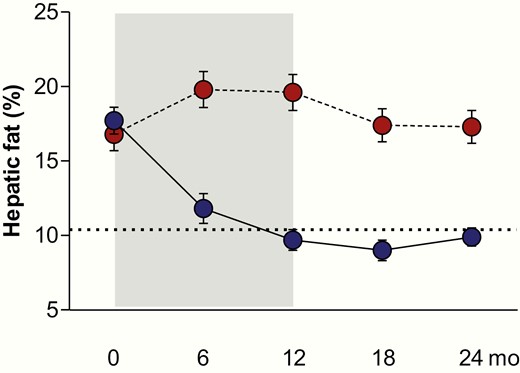
Hepatic fat content (by magnetic resonance imaging) in nonobese adolescent girls with PCOS who were randomized to receive either an oral contraceptive (OC; N = 31; red circles) for 12 months, or a low-dose combination of spironolactone-pioglitazone-metformin (SPIOMET; N = 31; blue circles) for 12 months; subsequently, both subgroups were untreated for 12 months. Body weight did not change in either subgroup. The dotted line indicates the average level in healthy control girls of similar age. Results are expressed as mean ± SEM. P < 0.0001 for on-treatment change between subgroups.
Table 2 shows that there were a mean 3-fold and a median 5-fold more ovulations after SPIOMET than OC; normovulation (as judged by 5 or 6 ovulations over 24 weeks) was only observed after SPIOMET; anovulation (as judged by 0 or 1 ovulation over 24 weeks) was > 10-fold more frequent after OC. Menstrual regularity after SPIOMET (90%) was only 2-fold more prevalent than after OC (42%), thus underestimated the difference in ovulation rates.
Posttreatment Ovulation Results in Adolescent Girls With Polycystic Ovary Syndrome Who Were Randomized to Receive an Oral Contraceptive (OC) or Low-Dose Spironolactone + Pioglitazone + Metformin (SPIOMET) for 12 Months, and Were Subsequently Followed for 12 Months Without Treatment. Ovulations Were Assessed Twice Over 12 Weeks, for a Total of 24 Weeks: Between the Study Timepoints of 15 to 18 months (posttreatment months 3-6) and 21 to 24 months (posttreatment months 9-12)
| . | OC N = 31 . | SPIOMET N = 31 . | ||||
|---|---|---|---|---|---|---|
| . | 15-18 mo (12 wk) . | 21-24 mo (12 wk) . | Total (24 wk) . | 15-18 mo (12 wk) . | 21-24 mo (12 wk) . | Total (24 wk) . |
| Mean number of ovulations± SEM | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.6 ± 0.2 | 2.3 ± 0.2# | 2.2 ± 0.2# | 4.5 ± 0.3# |
| Median number of ovulations(interquartile range) | 1 (0-1) | 1 (0-1) | 1 (1-3) | 3 (2-3)# | 2 (2-3)# | 5 (3-6)# |
| Normo-ovulatory fraction (%)5 or 6 ovulations /24 wk | -- | -- | 0 | -- | -- | 62# |
| Oligo-ovulatory fraction (%)2, 3, or 4 ovulations /24 wk | -- | -- | 47 | -- | -- | 35 |
| An-ovulatory fraction (%)0 or 1 ovulation /24 wk | -- | -- | 53 | -- | -- | 3# |
| . | OC N = 31 . | SPIOMET N = 31 . | ||||
|---|---|---|---|---|---|---|
| . | 15-18 mo (12 wk) . | 21-24 mo (12 wk) . | Total (24 wk) . | 15-18 mo (12 wk) . | 21-24 mo (12 wk) . | Total (24 wk) . |
| Mean number of ovulations± SEM | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.6 ± 0.2 | 2.3 ± 0.2# | 2.2 ± 0.2# | 4.5 ± 0.3# |
| Median number of ovulations(interquartile range) | 1 (0-1) | 1 (0-1) | 1 (1-3) | 3 (2-3)# | 2 (2-3)# | 5 (3-6)# |
| Normo-ovulatory fraction (%)5 or 6 ovulations /24 wk | -- | -- | 0 | -- | -- | 62# |
| Oligo-ovulatory fraction (%)2, 3, or 4 ovulations /24 wk | -- | -- | 47 | -- | -- | 35 |
| An-ovulatory fraction (%)0 or 1 ovulation /24 wk | -- | -- | 53 | -- | -- | 3# |
#P < 0.0001 between subgroups
Posttreatment Ovulation Results in Adolescent Girls With Polycystic Ovary Syndrome Who Were Randomized to Receive an Oral Contraceptive (OC) or Low-Dose Spironolactone + Pioglitazone + Metformin (SPIOMET) for 12 Months, and Were Subsequently Followed for 12 Months Without Treatment. Ovulations Were Assessed Twice Over 12 Weeks, for a Total of 24 Weeks: Between the Study Timepoints of 15 to 18 months (posttreatment months 3-6) and 21 to 24 months (posttreatment months 9-12)
| . | OC N = 31 . | SPIOMET N = 31 . | ||||
|---|---|---|---|---|---|---|
| . | 15-18 mo (12 wk) . | 21-24 mo (12 wk) . | Total (24 wk) . | 15-18 mo (12 wk) . | 21-24 mo (12 wk) . | Total (24 wk) . |
| Mean number of ovulations± SEM | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.6 ± 0.2 | 2.3 ± 0.2# | 2.2 ± 0.2# | 4.5 ± 0.3# |
| Median number of ovulations(interquartile range) | 1 (0-1) | 1 (0-1) | 1 (1-3) | 3 (2-3)# | 2 (2-3)# | 5 (3-6)# |
| Normo-ovulatory fraction (%)5 or 6 ovulations /24 wk | -- | -- | 0 | -- | -- | 62# |
| Oligo-ovulatory fraction (%)2, 3, or 4 ovulations /24 wk | -- | -- | 47 | -- | -- | 35 |
| An-ovulatory fraction (%)0 or 1 ovulation /24 wk | -- | -- | 53 | -- | -- | 3# |
| . | OC N = 31 . | SPIOMET N = 31 . | ||||
|---|---|---|---|---|---|---|
| . | 15-18 mo (12 wk) . | 21-24 mo (12 wk) . | Total (24 wk) . | 15-18 mo (12 wk) . | 21-24 mo (12 wk) . | Total (24 wk) . |
| Mean number of ovulations± SEM | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.6 ± 0.2 | 2.3 ± 0.2# | 2.2 ± 0.2# | 4.5 ± 0.3# |
| Median number of ovulations(interquartile range) | 1 (0-1) | 1 (0-1) | 1 (1-3) | 3 (2-3)# | 2 (2-3)# | 5 (3-6)# |
| Normo-ovulatory fraction (%)5 or 6 ovulations /24 wk | -- | -- | 0 | -- | -- | 62# |
| Oligo-ovulatory fraction (%)2, 3, or 4 ovulations /24 wk | -- | -- | 47 | -- | -- | 35 |
| An-ovulatory fraction (%)0 or 1 ovulation /24 wk | -- | -- | 53 | -- | -- | 3# |
#P < 0.0001 between subgroups
Fig. 3 illustrates that the randomized treatments led to marked differences in on-treatment metabolic health (as judged by combined Z-scores of fasting insulin and miR-451a) and in posttreatment ovulation rate, both of which were more normalized after SPIOMET.
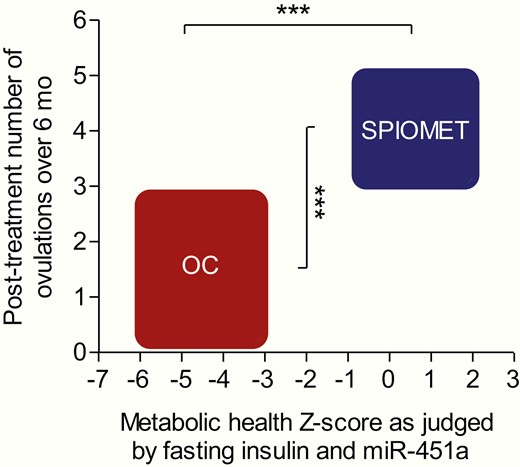
Randomized treatment of adolescent girls with PCOS, either with an oral contraceptive (OC) or with a low-dose combination of spironolactone-pioglitazone-metformin (SPIOMET) for 12 months, results in an on-treatment difference of metabolic health (N = 22 vs 24) and in a posttreatment difference of ovulation rate (N = 30 vs 29), so that this combined metabolic-reproductive outcome is markedly to the advantage of SPIOMET. Metabolic health Z-score was calculated by subtracting the Z-score of fasting insulin from the Z-score of circulating miR-451a after 12 months on treatment. Posttreatment number of ovulations over 6 months was inferred by combining data from menstrual diaries and weekly progesterone measurements in saliva over 12 + 12 weeks, between posttreatment months 3 to 6 and 9 to 12. Body weight did not change in either subgroup. The breadth and height of the boxes represent the ranges from −1 SD to +1 SD, respectively, for metabolic health Z-score and ovulation number. *** P < 0.0001.
3. Discussion
Pooled data corroborated SPIOMET as a combination treatment that is accompanied by more normalization of the endocrine-metabolic status, and is followed by markedly more ovulations than OC in nonobese adolescent girls with PCOS. The consistency of the ovulation rates across the posttreatment year suggests that the lower ovulation rates after OC are attributable to persistence of the underpinning PCOS pathophysiology rather than to residual inhibition of the gonadotropic axis. In healthy young women, ovulatory function is known to recover within 3 months after stopping OC treatment [10, 11].
Ectopic adiposity and insulin resistance failed to improve during standard treatment with OC. In contrast, SPIOMET treatment was accompanied by a loss of hepato-visceral fat excess and by a normalization of insulin sensitivity (as judged by HOMA-IR, and by the insulin response to an oral glucose load), both of which were maintained during the posttreatment year, via mechanisms that remain to be identified. The downward normalization of liver fat on SPIOMET may partly relate to the upward normalization of circulating miR-451a, which reduces the expression of thyroid hormone responsive spot 14 (THRSP), the key gene driving liver steatosis [12, 13].
The present findings corroborate the concept that insulin resistance reflects ectopic lipid accumulation, particularly in the liver, and that it precedes the development of disorders such as type 2 diabetes and nonalcoholic fatty liver disease [14]. Increased hepatic fat and insulin resistance are prevalent findings in both nonobese and obese adolescents with PCOS, and seem to relate to the underpinning PCOS pathophysiology rather than to testosterone concentrations [15, 16]. Targeting a reduction in androgen levels may thus not be the best choice to normalize the entire PCOS phenotype and to address subsequent comorbidities. The diverging effects of OC and SPIOMET on insulin resistance and ectopic fat (Fig. 3) may herald diverging influences on subsequent risk for PCOS-associated disorders such as anovulatory subfertility, gestational diabetes, and/or type 2 diabetes.
The present results remain to be further confirmed in larger and more diverse PCOS populations, including in girls with obesity, with different ethnic and developmental backgrounds, and with other environmental exposures. In addition, SPIOMET’s capacity to reduce an excess of liver fat while total body weight remains virtually unchanged (Fig. 2), remains to be tested beyond PCOS settings, in older age ranges, and in a cascade of fatty liver diseases, including nonalcoholic steatohepatitis.
In conclusion, pooled results in nonobese adolescent girls with PCOS confirmed SPIOMET as a treatment that attenuates insulin resistance, reduces ectopic adiposity, and is followed by a more normal ovulation rate than OC.
Abbreviations
- BMI
body mass index
- cIMT
carotid intima-media thickness
- CRP
C-reactive protein
- HMW
high-molecular-weight
- HOMA-IR
homeostatic model assessment–insulin resistance
- miR
microRNA
- OC
oral contraceptive
- MRI
magnetic resonance imaging
- PCOS
polycystic ovary syndrome
- SEM
standard error of the mean
- SPIOMET
spironolactone-pioglitazone-metformin
Acknowledgments
L.I., M.D., R.M., C.G.B., and E.G. are Clinical Investigators of CIBERDEM (Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas, Instituto de Salud Carlos III, Madrid, Spain). A.L.B. is a Clinical Investigator of the I3 Fund for Scientific Research (Ministry of Science and Innovation, Spain).
Financial Support: this study was supported by the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III, and by the Fondo Europeo de Desarrollo Regional (FEDER) (PI18/00109).
Clinical Trial Information: ISRCTN29234515 & ISRCTN11062950
Author Contributions: L.I. contributed to study design and data interpretation, wrote and reviewed/edited manuscript. M.D. researched data, contributed to data interpretation, and reviewed/edited manuscript. C.G.B. researched data, contributed to data interpretation, and reviewed/edited manuscript. R.M. contributed to data interpretation, wrote the manuscript, and reviewed/edited manuscript. E.G. researched data, and reviewed/edited manuscript. A.L.B. reviewed/edited manuscript. F.d.Z. contributed to study design and data interpretation, wrote and reviewed/edited manuscript.
L.I. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References



