-
PDF
- Split View
-
Views
-
Cite
Cite
Erin R Okawa, Roxanne Gardner, Colleen Feltmate, Michelle S Hirsch, Jeffrey W Craig, Yee-Ming Chan, An Unusual Cause of Secondary Amenorrhea in an Adolescent: Expanding the Differential, Journal of the Endocrine Society, Volume 4, Issue 12, December 2020, bvaa159, https://doi.org/10.1210/jendso/bvaa159
Close - Share Icon Share
Abstract
Secondary amenorrhea is not uncommon in the adolescent female population. There are multiple etiologies to consider, and a comprehensive evaluation is often pursued. Sometimes, however, despite a thorough workup, the diagnosis remains unclear. Here, we report an unusual cause of secondary amenorrhea in a 15-year-old girl. Our patient presented with secondary amenorrhea after a 4-year history of regular menstrual cycles. Her evaluation was notable for very low FSH and low estradiol but normal LH; pregnancy, adrenal, thyroid, prolactin studies, and brain magnetic resonance imaging scan did not reveal a cause of her amenorrhea. Her transabdominal ultrasound showed an enlarged right ovary, initially suggestive of a hemorrhagic cyst. Inhibin A and B were measured because of the persistently low FSH; these were found to be very elevated, concerning for an inhibin-producing tumor. The patient had surgical removal of her right ovary; pathology revealed a juvenile granulosa-cell tumor. Postoperatively, the patient had normalization of serum inhibin A and B and resumption of normal menstrual cycles. This report illustrates that careful consideration of laboratory findings and other studies is essential for correctly identifying the underlying cause of secondary amenorrhea, particularly when the results are not consistent with common causes of this condition.
Secondary amenorrhea occurs in up to 7.6% of adolescent females [1]. The differential in this population is broad and includes pregnancy, anovulatory cycles, hypo- and hypergonadotropic hypogonadism, polycystic ovarian syndrome, thyroid disease, and acquired uterine conditions. Here, we report a case of an adolescent girl who presented with this chief complaint and findings that were not consistent with these more common etiologies, leading to the discovery of a rare cause of secondary amenorrhea.
Case Presentation
A 15-year, 10-month-old girl presented to an adolescent gynecology clinic for evaluation of secondary amenorrhea. Her menstrual periods had been “like clockwork” since age 11 years. However, at age 15 years, 4 months, she had a 3-month period of metrorrhagia; thereafter, she had complete cessation of menstrual bleeding. She was an honor-roll student, denied new psychosocial stressors, and denied past or current sexual activity or restrictive eating. She had been an avid dancer but had elected to take time off from dance 7 months prior. She took no medications and had no allergies. On physical examination her height was 169.3 cm (+1.0 SD score [SDS]), her weight was 59.7 kg (+0.6 SDS), and her body mass index was 20.6 kg/m2 (+0.1 SDS). She had Tanner V breast development and no acne, hirsutism, thyromegaly, or galactorrhea.
Initial laboratory testing showed low FSH (<2 IU/L, reference range for follicular phase 3.0-11.3 IU/L) and normal range LH (9.1 IU/L, reference range for follicular phase 2.1-12.2 IU/L). A pregnancy test was negative. Dehydroepiandrosterone sulfate, 17-hydroxyprogesterone, prolactin, TSH, and free thyroxine were all normal.
The patient was advised to return to clinic in 2 to 4 weeks but did not return until 11 months later at age 16 years, 9 months. Her amenorrhea had persisted and she had new complaints of right lower quadrant discomfort and temporal headaches. She denied changes in activity level or diet. Repeat laboratory studies again showed low FSH (0.2 IU/L), high normal LH (7.0 IU/L), and normal prolactin (2.4 ng/mL). Estradiol was low at 21 pg/mL (reference range for follicular phase 30-500 pg/mL), and total and free testosterone were normal. A transabdominal ultrasound was notable for an enlarged right ovary (5.0 × 4.6 × 3.6 cm with a calculated volume of 42.6 mL) with a central area of increased echogenicity and absent color Doppler flow, suggestive of a hemorrhagic cyst. The left ovary appeared normal and measured 1.9 cm × 1.8 cm × 1.2 cm with a calculated volume of 2.0 mL. The uterus appeared normal with an endometrial thickness of 2.3 mm. The visualization of a potential hemorrhagic cyst (which usually results from bleeding into a corpus luteum) suggested that ovulation may have occurred recently; however, no menstrual bleeding ensued. The patient was given a course of medroxyprogesterone to induce withdrawal bleeding; no bleeding or spotting occurred. A repeat ultrasound 6 weeks later continued to show an enlarged right ovary containing a cyst.
Given concern for idiopathic hypogonadotropic hypogonadism, a brain magnetic resonance imaging scan was performed and did not reveal any intracranial pathology. The patient was referred to a pediatric endocrinologist who, because of the specific suppression of FSH, measured inhibin A and B levels; inhibin A was 49 pg/mL (reference range for early follicular phase, 1.8-17.3 pg/mL), and inhibin B was 2220 pg/mL (reference range for follicular phase, 10-290 pg/mL). The patient was promptly referred to a gynecologic oncologist and underwent resection of her right ovary and fallopian tube; a 4.8-cm tumor was found. Pathology revealed a juvenile granulosa-cell tumor with no evidence of local spread (Fig. 1). Postoperatively, the patient had resumption of cyclic menstrual periods. Three months later, her late-follicular phase inhibin A was 81 pg/mL (reference range, < 97.5 pg/mL) and her inhibin B was 34 pg/mL (reference range, < 36 pg/mL).
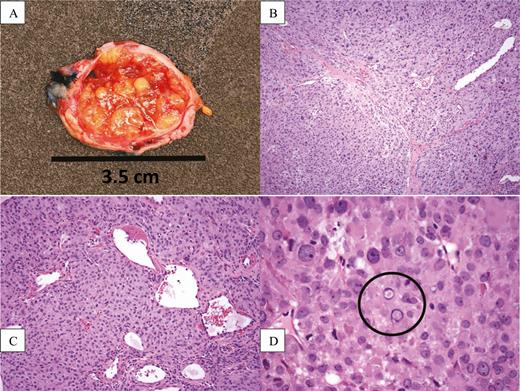
(A) Gross cross-sectional appearance of the resected ovary, showing a smooth outer capsule surrounding a yellow, fleshy, lobulated, and partially cystic/hemorrhagic mass replacing the normal ovarian parenchyma. (B) 100× magnification showing large nodules of tumor separated by distinct fibrous bands. (C) 200× magnification showing that the tumor nodules are composed of solid sheets of epithelioid tumor cells resembling primitive granulosa cells. (D) 600× magnification showing that the tumor cells contain round to ovoid nuclei with prominent nucleoli and occasional nuclear pseudoinclusions (circled).
Discussion
In adolescents and reproductive-age adults, the most common cause of secondary amenorrhea is pregnancy. The expanded differential includes anovulatory cycles, hypo- and hypergonadotropic hypogonadism, thyroid disease, polycystic ovarian syndrome (PCOS), uterine pathology (Asherman syndrome) and, rarely, ovarian tumors. The history, patterns of gonadotropin secretion, and, often, imaging studies and/or provocative testing are helpful in determining the etiology.
Anovulatory cycles in adolescents are secondary to the immaturity of the hypothalamic-pituitary-gonadal axis. During the first 2 years after menarche, ovulation occurs in about 50% of all menstrual cycles; by 5 years postmenarche, about 75% of cycles are ovulatory [2]. Demonstration of withdrawal bleeding after a progesterone challenge can be helpful in determining whether there is unopposed estrogen stimulation leading to buildup of the uterine lining. The lack of withdrawal bleeding in our patient, her low estradiol levels, and her 4-year history of normal menstrual periods made this explanation for her amenorrhea unlikely.
In hypogonadotropic hypogonadism, GnRH and/or gonadotropin secretion is impaired, typically resulting in low levels of both FSH and LH. This pattern of laboratory findings may be secondary to structural defects of the pituitary, including mass lesions or adenomas, physiological stressors (termed functional amenorrhea), or congenital defects in GnRH secretion or action. Hyperprolactinemia, which may occur from a pituitary adenoma, pituitary stalk compression, or medications that lower dopamine levels, is the most common pituitary cause of secondary amenorrhea, responsible for up to 14% of cases [3]. Women with hyperprolactinemia have reduced LH-pulse frequency and reduced LH responsiveness to estrogen, suggestive of GnRH suppression [4]. Very rarely, hypogonadotropic hypogonadism may be secondary to glucocorticoid and/or androgen-secreting adrenal tumors. In our patient, the robust LH was incongruent with this diagnosis, and a brain magnetic resonance imaging scan did not identify any pituitary pathology.
In hypergonadotropic hypogonadism, LH and FSH are elevated because of lack of negative feedback from dysfunctional ovaries. Hypergonadotropic hypogonadism occurring in an adolescent indicates primary ovarian insufficiency, which can be caused by autoimmune diseases, metabolic disorders such as galactosemia, surgical oophorectomy, genetic abnormalities such as Turner syndrome or premutation of the gene for fragile-X syndrome (FMR1), and exposure to cytotoxic chemotherapies or radiation. Our patient did not have elevated FSH and LH to indicate primary ovarian insufficiency.
Either hyperthyroidism or severe primary hypothyroidism can lead to secondary amenorrhea. In hyperthyroidism, increased SHBG secretion from the liver leads to decreased free estradiol, and LH and FSH are both elevated, LH to a greater degree [5]. In severe hypothyroidism, uninhibited TRH stimulates prolactin production, resulting in elevated prolactin levels and hypogonadotropic hypogonadism. Our patient had no thyromegaly on examination, and normal TSH and free thyroxine levels.
PCOS is a heterogeneous disorder that presents with oligo- or anovulation, hyperandrogenism, and metabolic derangements including insulin resistance and dyslipidemia. Serum LH may be elevated relative to serum FSH, which may contribute to increased production of androgens by the theca cells of the ovary. This in turn leads to further dysregulation of the hypothalamic-pituitary-ovarian axis [6]. Pelvic ultrasound may show multiple follicles and an enlarged ovarian volume (> 10-12 mL) [7]. Although this patient’s elevated LH:FSH ratio may seem similar to that seen with PCOS, in PCOS the LH is high but the FSH is typically normal, not frankly low. This patient’s lack of hirsutism, low normal testosterone, and lack of polycystic ovarian morphology on ultrasound findings were also incongruent with PCOS.
Asherman syndrome occurs as a result of trauma to the uterine cavity that leads to intrauterine adhesions. Our patient denied any history of uterine surgery and had no history of uterine infections.
In this case, the specific suppression of FSH led to the suspicion of an inhibin-secreting tumor. Inhibins are members of the transforming growth factor-β superfamily and are secreted in response to FSH. The inhibins in turn inhibit FSH secretion without affecting LH secretion. Inhibin A is normally secreted by the corpus luteum; inhibin B is normally secreted by the granulosa cells of antral follicles.
Ovarian tumors are rare neoplasms in children and adolescents, with an annual incidence of 2.2 cases/100 000 females [8]. They can be divided into 3 categories: germ-cell tumors (by far the most common), epithelial tumors, and sex cord-stromal tumors. Juvenile granulosa-cell tumors fall into the third category and account for up to 12% of ovarian tumors found in childhood [9]. The secretory activity of juvenile granulosa-cell tumors can lead to early detection: secretion of estrogens can lead to precocious puberty, secretion of androgens can lead to virilization, and secretion of inhibins can lead to primary or secondary amenorrhea [10, 11]. Juvenile granulosa-cell tumors may also present with abdominal pain or swelling. Tumor stage is the most important prognostic factor in girls with this type of tumor. Good prognostic factors include low stage, smaller size (< 10 cm), and the absence of tumor rupture [10].
Secondary amenorrhea in an adolescent is not uncommon. This case demonstrates the importance of pursuing a definitive diagnosis if the data do not correspond with common disease patterns. Had this patient been placed empirically on oral contraceptive pills, a frequently prescribed treatment for secondary amenorrhea, there likely would have been further delays in treatment. Instead, careful consideration of her unique findings allowed detection and proper treatment of her ovarian tumor.
Abbreviations
Acknowledgments
Written consent to publication was provided by the patient.
E.R.O.’s current affiliation is the Division of Pediatric Endocrinology, Department of Pediatrics, Mattel Children’s Hospital, Los Angeles, California 90095. J.W.C.’s current affiliation is the Department of Pathology and Laboratory Medicine, BC Cancer, Vancouver, British Columbia, Canada V5Z 4E6.
Author Contributions: Y.-M.C. acquired data and conceptualized the report. E.R.O. drafted the initial manuscript. R.G., C.F., M.S.H., and J.W.C. acquired, analyzed, and/or interpreted data. All authors reviewed and revised the manuscript, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work. Written consent to publication was provided by the patient.
Additional Information
Disclosure Summary: The authors have no conflicts of interest to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.



