-
PDF
- Split View
-
Views
-
Cite
Cite
Yoshinari Obata, Yukari Fujita, Ayaka Korechika, Rieko Nakatani, Junji Kozawa, Iichiro Shimomura, Marked Increase in Thyrotropin Receptor Antibodies With the Development of Pretibial Myxedema After Total Thyroidectomy, JCEM Case Reports, Volume 3, Issue 7, July 2025, luaf107, https://doi.org/10.1210/jcemcr/luaf107
Close - Share Icon Share
Abstract
A recent study revealed that the complex of thyrotropin receptors (TSHRs) and human leukocyte antigen (HLA) class II molecules within the thyroid gland, rather than TSHR alone, induces anti-TSHR autoantibody production by disrupting self-tolerance in Graves disease (GD). However, this complex has not yet been identified outside the thyroid gland. Complete removal of the thyroid gland typically reduces autoantibody titers by eliminating the antigen source. We present the case of a 51-year-old woman with GD and Graves ophthalmopathy who exhibited a marked increase in anti-TSHR autoantibodies concurrent with the development of pretibial myxedema (PTM) despite having undergone a total thyroidectomy. Immunohistological analysis of PTM tissues revealed the coexpression of TSHR and HLA class II molecules, forming a complex similar to that previously identified in the thyroid gland. These findings suggest that TSHR complexed with HLA class II molecules in PTM tissues may contribute to autoantibody production in this patient after total thyroidectomy, although the involvement of potential residual thyroid tissue cannot be entirely excluded. This case suggests that autoantibodies can be induced outside the thyroid gland and provides novel insights into the pathogenesis and progression of GD and related disorders.
Introduction
Graves disease (GD) is an autoimmune disorder caused by autoantibodies that stimulate thyrotropin receptors (TSHR), resulting in excessive thyroid hormone production [1]. Patients with GD may also exhibit extrathyroidal manifestations [1-3], including pretibial myxedema (PTM), a rare dermopathy associated with GD, characterized by the accumulation of glycosaminoglycans and lymphocyte infiltration within the dermis [1, 2]. Another common complication associated with GD, Graves ophthalmopathy (GO), is an autoimmune inflammatory orbital condition that presents with various ocular manifestations and may be potentially sight-threatening [1, 3]. TSHR is expressed in normal skin and orbital fibrocytes/fibroblasts, and activation of these cells by anti-TSHR autoantibodies is believed to play a key role in the development both of PTM and GO [3-5].
The primary antigen triggering anti-TSHR autoantibody production has traditionally been considered to be TSHR within the thyroid gland. However, the exact mechanisms underlying autoantibody production remain unclear. Recent research has highlighted the role of human leukocyte antigen (HLA) class II molecules, which are typically absent in healthy thyroid tissue but are aberrantly expressed in the thyroid tissues of patients with GD, where they form complexes with TSHR [6]. Notably, TSHR complexed with HLA class II molecules—but not TSHR alone—disrupts self-tolerance due to altered antigenic properties, ultimately leading to the production of anti-TSHR autoantibodies [6]. Beyond GD, there is growing recognition of self-antigens complexed with HLA class II molecules in various autoimmune diseases, including rheumatoid arthritis and vasculitis [7-9].
Complete removal of the thyroid gland typically reduces autoantibody titers by eliminating the antigen source [10, 11]. However, rare cases of GO or PTM development accompanied by elevated anti-TSHR autoantibody titers following total thyroidectomy have been reported, although the underlying mechanism remains unclear [12-15]. Here, we present the case of a patient with GD and GO who exhibited a significant increase in anti-TSHR autoantibodies along with the development of PTM, despite a previous total thyroidectomy. Immunohistological analysis revealed, for the first time, that HLA class II molecules and TSHR were coexpressed in PTM tissues, forming a complex similar to that previously identified in the thyroid gland. We discuss a potential pathogenic mechanism for GD and related disorders, in which autoantibody production may be induced outside the thyroid gland, such as in PTM tissues, as observed in this case.
Case Presentation
A 51-year-old woman with GD complicated by GO and PTM was admitted to our hospital for evaluation. She was initially diagnosed with GD at age 49 years based on hyperthyroidism and elevated TSHR antibody (TRAb) levels. Treatment with thiamazole was initiated, which promptly restored her thyroid function. However, 6 months later, she developed diplopia and eyelid swelling, leading to a diagnosis of GO (Fig. 1). Despite undergoing 2 courses of steroid pulse therapy, external radiation therapy, and subsequent treatment with oral prednisolone and immunosuppressive drugs, her GO showed insufficient improvement. At age 50, she underwent orbital decompression surgery and total thyroidectomy due to worsening optic neuropathy. However, her TRAb levels did not decrease, and GO remained active. At age 51 years, she developed edema on the dorsum of both feet, which was diagnosed as PTM. Concurrently, her TRAb levels significantly increased from 20.6 IU/L to 70.4 IU/L, as measured using an electrochemiluminescence immunoassay (AIA-PACK CL TRAb kit; Tosoh Co; reference range: <1.3 IU/L), despite a prior total thyroidectomy. TRAb levels were also markedly elevated to 48 700%, as assessed using a bioassay kit (Biosensor TSAb Yamasa; Yamasa Co; reference range: <110%), indicating the presence of TSHR-stimulating but not -blocking antibodies. The patient was subsequently admitted to our hospital for further evaluation. The clinical course and TRAb trends on admission are shown in Fig. 1.

The patient's clinical course and trends in thyrotropin receptor antibody (TRAb) titers leading up to her admission. Zero months on the horizontal axis represents the onset of Graves ophthalmopathy. Arrows indicate the administration of methylprednisolone 750 mg/day for 3 days per arrow.
Abbreviations: CAS, Clinical Activity Score; CyA, cyclosporine; MMF, mycophenolate mofetil; PSL, prednisolone.
Diagnostic Assessment
On admission, the patient's height, weight, and body mass index were 154.5 cm, 89.4 kg, and 37.5 kg/m2, respectively. She had no family history of thyroid disorders. Her current medications included oral hypoglycemic agents for type 2 diabetes, along with 5 mg prednisolone and 1000 mg mycophenolate mofetil for GO. The patient exhibited ocular protrusion, spontaneous periorbital pain, conjunctival hyperemia and swelling, eyelid redness and swelling, and lacrimal papule swelling, with a high Clinical Activity Score of 6/7 (Fig. 2A) [16]. Magnetic resonance imaging revealed diffuse enlargement of the external ocular muscles with high signal intensities on T2-weighted images (Fig. 2B). These physical and imaging findings indicated high activity of GO. In addition, she presented with painful, dark-purple sclerotic lesions and nonpitting edema on the dorsum of both feet (Fig. 3A). Skin biopsy revealed edematous changes and abundant mucin deposition in the dermis and subcutaneous tissues (Fig. 3B), along with infiltration of CD20+ B and CD3+ T cells (Fig. 3C), consistent with PTM. Her thyroid function was stabilized with 100 μg of levothyroxine sodium: thyrotropin 4.69 μU/mL (4.69 mU/L; reference: 0.61-4.23 μU/mL [0.61-4.23 mU/L]), free thyroxine 1.6 ng/dL (20.6 pmol/L; reference: 0.8-1.7 ng/dL [10.3-21.9 pmol/L]), and free 3,5,3′-triiodothyronine 2.1 pg/mL (3.23 pmol/L; reference: 2.1-3.1 pg/mL [3.23-4.76 pmol/L]). Her hemoglobin A1c was 10.0%. HLA typing indicated that the patient carried HLA-DP5 (DRA1*02:02/DPB1*05:01), a genotype strongly associated with GD in the Japanese population [17, 18]. Although neck ultrasonography showed no residual thyroid tissue, the serum thyroglobulin level was detectable at 1.0 ng/mL (reference: 3.7-35.1 ng/mL), suggesting the presence of residual tissue. However, this alone seems unlikely to account for the marked increase in TRAb levels as late as 6 months postoperatively, since thyroid antigen levels would be expected to decline substantially after surgery.
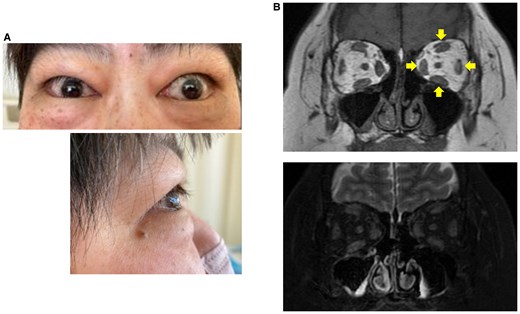
Graves ophthalmopathy in the patient. A, Frontal and lateral views of the patient's eyes. B, Orbital magnetic resonance imaging in T1-weighted (upper panel) and fat-suppressed T2-weighted (lower panel) imaging. Arrows show diffuse enlargement of the external ocular muscles.
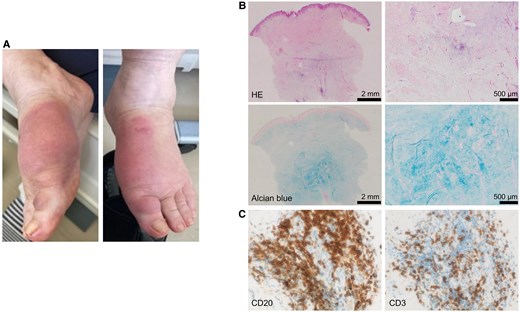
Pretibial myxedema (PTM) in the patient. A, Physical findings on the dorsum of both feet. B, Hematoxylin and eosin (HE) staining (upper panels) and alcian blue staining (lower panels) of PTM tissue at low magnification (left panels) and high magnification (right panels). C, CD20 staining (left panel) and CD3 staining (right panel) of PTM tissue.
To investigate the source of the markedly elevated TRAb levels even after total thyroidectomy, we focused on PTM tissues that developed concurrently with the increase in TRAb levels. In the thyroid gland, TSHR complexed with ectopically expressed HLA class II molecules—but not TSHR alone—has been suggested to contribute to autoantibody production by disrupting self-tolerance [6]. Immunohistological analysis of the PTM tissues in our patient confirmed the expression of TSHR, consistent with previous findings (Fig. 4A) [4, 5]. Notably, HLA class II molecules were also aberrantly expressed in PTM tissues and colocalized with TSHR (see Fig. 4A). Furthermore, we conducted a proximity ligation assay (PLA) using the Duolink in situ PLA kit (Sigma-Aldrich), which detects protein-protein interactions within a 40-nm range. Positive signals from PLA indicated the formation of a complex between TSHR and HLA class II molecules (Fig. 4B). These findings, combined with the clinical course, suggest that TSHR complexed with HLA class II molecules in PTM tissues may have contributed to autoantibody production in our patient following total thyroidectomy.
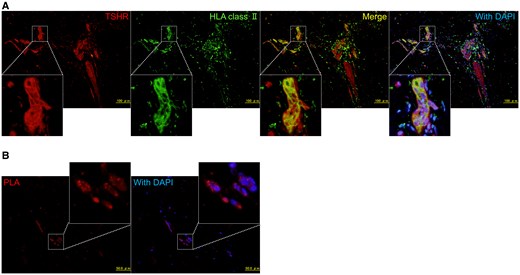
Immunohistological analysis of the pretibial myxedema (PTM) tissues in the patient. A, Immunostaining of thyrotropin receptors (TSHRs) and human leukocyte antigen (HLA) class II (DR + DQ + DP) in PTM tissues. Formaldehyde-fixed and paraffin-embedded sections of the PTM biopsy tissues were incubated with rabbit anti-TSHR polyclonal (HPA026680, Sigma-Aldrich) and mouse anti-HLA class II (DR + DQ + DP) monoclonal (ab7856, Abcam) antibodies as primary antibodies. These were followed by Alexa Fluor 594–conjugated anti-rabbit immunoglobulin G (IgG) antibodies (Molecular Probes) and biotinylated anti-mouse IgG antibodies (Vector Laboratories) as secondary antibodies, with Alexa Fluor 488–conjugated streptavidin (Life Technologies). B, Proximity ligation assay (PLA) using a Duolink in situ PLA kit (Sigma-Aldrich) in the PTM tissues. Sections of PTM biopsy tissues were incubated with rabbit anti-TSHR and mouse anti-HLA class II (DR + DQ + DP) antibodies. PLA signals were visualized as red fluorescent protein signals using anti-rabbit PLUS and anti-mouse MINUS PLA probes.
Treatment
In addition to ongoing oral prednisolone and mycophenolate mofetil, intralesional injections of triamcinolone acetonide were initiated for PTM.
Outcome and Follow-up
The local treatment was less effective and the PTM lesions have persisted. The patient’s TRAb levels have remained elevated.
Discussion
Here, we present a patient with GD and GO who exhibited a marked increase in TRAb levels concurrent with the onset of PTM despite a previous total thyroidectomy. Notably, this is the first reported case demonstrating that TSHR complexes with HLA class II molecules, which may trigger autoantibody production, are also expressed in PTM tissues.
Several cases of new-onset GO or PTM with elevated TRAb levels, even after total thyroidectomy, have been reported, although the mechanisms remain unclear [12-15]. In some of these cases, persistent antibody production triggered by residual thyroid tissue, such as unresected fragments of the pyramidal lobe, may be involved. On the other hand, given that TSHR is expressed on fibrocytes/fibroblasts both in GO and PTM tissues [3-5], it has been postulated that these tissues may contribute to autoantibody production. However, definitive evidence supporting this hypothesis is currently lacking. Our novel in vivo findings suggest that TSHR complexes with HLA class II molecules in PTM tissues, similar to those observed in the thyroid gland, act as pathogenic antigens, initiating an immune response that may lead to autoantibody production, possibly in the surrounding lymphoid tissues. A recent study identified ectopic lymphoid-like structures in PTM tissues containing CD138+ plasma cells, along with adjacent CD3+ T cells and CD20+ B cells. Furthermore, this study demonstrated that these plasma cells produced TRAb when cultured in vitro [19]. Therefore, autoantibodies may also be produced within PTM tissues by infiltrating immune cells. Such autoantibody production may partially explain the considerable increase in TRAb levels concurrent with the onset of PTM in our patient.
GO and PTM share many pathophysiological features, including TSHR expression and lymphocyte infiltration [3-5]. Therefore, it is plausible that autoantibody production may also be triggered in the orbital tissues of GO through a mechanism similar to that observed in PTM tissues and the thyroid gland, although orbital tissues were not examined in our patient. TRAb levels are reported to be higher in GD patients with GO compared to those without it, and elevated TRAb levels are associated with greater severity of GO [20]. The decrease in TRAb levels after total thyroidectomy has been shown to be significantly delayed in patients with GO [10]. Furthermore, in our patient, TRAb levels remained elevated even after total thyroidectomy. This evidence supports the possibility that GO tissues also contribute to autoantibody production. Specifically, TSHR complexed with HLA class II molecules in the thyroid gland acts as a pathogenic antigen, leading to the production of anti-TSHR autoantibodies and the development of GD [6]. These autoantibodies stimulate TSHR in orbital and cutaneous tissues, contributing to the development of GO and PTM, respectively. Furthermore, autoantibody production may also be induced by the TSHR-HLA class II complex in GO and PTM tissues, potentially exacerbating GD, GO, and PTM either additively or synergistically.
The patient's autoantibodies increased despite the immunosuppressive treatment with prednisolone and mycophenolate mofetil. Furthermore, her GO and PTM remained refractory. Novel therapeutic agents currently under development, including other immunomodulators or TSHR-specific modalities [21, 22], are warranted.
This case report had several limitations. First, we cannot entirely exclude the possibility that the elevated antibodies observed after total thyroidectomy in our patient were produced by residual thyroid tissue, given the detectable thyroglobulin levels. Further evaluation for residual thyroid tissue using 123I scintigraphy has not been yet performed. Second, our patient carried the disease-susceptibility allele HLA-DP5, consistent with a previous study that demonstrated the pathogenic role of TSHR complexed with HLA class II molecules [6]. However, it remains unclear whether other HLA alleles are involved in autoantibody production through a mechanism similar to that of HLA-DP5. Third, we were unable to conduct immunohistological analysis of the resected thyroid tissues from our patient. Finally, this report describes a single case, highlighting the need for further accumulation of cases to achieve a more comprehensive understanding of this phenomenon.
In conclusion, we present a patient with GD and GO who exhibited a substantial increase in anti-TSHR autoantibodies concurrent with the development of PTM despite a prior total thyroidectomy. Immunohistological analysis revealed, for the first time, that TSHR forms a complex with HLA class II molecules in PTM tissues, similar to that previously observed in the thyroid gland. This complex may have contributed to autoantibody production in the surrounding lymphoid tissues or within PTM tissues, leading to a significant increase in anti-TSHR autoantibody levels. This case suggests that autoantibodies can be induced outside the thyroid gland and provides novel insights into the pathogenesis and progression of GD and related disorders.
Learning Points
In the thyroid gland, TSHR complexed with ectopically expressed HLA class II molecules has been suggested to contribute to autoantibody production by disrupting self-tolerance.
TSHR complexed with HLA class II molecules can also be expressed in PTM tissues.
Autoantibody production may be induced outside the thyroid gland, such as in PTM tissues, through TSHR-HLA class II complexes.
Acknowledgments
The authors thank Dr Takashi Katsura (Department of Diabetes and Endocrinology, Japan Community Health Care Organization Osaka Hospital), Dr Kosuke Mukai, Dr Kazuyuki Miyashita, and Dr Hitoshi Nishizawa (Department of Metabolic Medicine, Graduate School of Medicine, Osaka University) for their valuable support. They also thank Prof Hisashi Arase (Laboratory of Immunochemistry, WPI Immunology Frontier Research Center, and Department of Immunochemistry, Research Institute for Microbial Diseases, Osaka University) for providing anti-TSHR antibodies and technical support for immunohistological analysis. Additionally, the authors thank Dr Yukinobu Nakagawa (Department of Dermatology, Graduate School of Medicine, Osaka University) for performing the skin biopsy of the patient.
Contributors
All authors made individual contributions to authorship. Y.O. was involved in conceptualization of the study and preparation of the original draft. Y.F. was involved in histopathology section and preparation of histology images. A.K. and R.N. were involved in the diagnosis and management of the patient. J.K. and I.S. provided supervision and revised the original draft. All authors reviewed and approved the final draft.
Funding
Not applicable.
Disclosures
None declared.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Abbreviations
- GD
Graves disease
- GO
Graves ophthalmopathy
- HLA
human leukocyte antigen
- PLA
proximity ligation assay
- PTM
pretibial myxedema
- TRAb
thyrotropin receptor antibody
- TSHR
thyrotropin receptor



