-
PDF
- Split View
-
Views
-
Cite
Cite
Yoshihiro Takahashi, Yukio Horikawa, Yumi Matsuyama, Kimiko Asai, Junki Endo, Daisuke Yabe, A Novel Multiple Endocrine Neoplasia Type 1 Gene Variant Found in Scalp Pulmonary Neuroendocrine Tumor Metastasis, JCEM Case Reports, Volume 3, Issue 4, April 2025, luaf047, https://doi.org/10.1210/jcemcr/luaf047
Close - Share Icon Share
Abstract
Multiple endocrine neoplasia type 1 (MEN1) is a genetic disorder usually diagnosed following hyperparathyroidism or pancreatic and gastrointestinal neuroendocrine neoplasm (NEN). We report here a case of MEN1 that was diagnosed following cancer multigene panel testing of a scalp metastasis of small cell lung carcinoma (SCLC). A 45-year-old male had noticed weight loss 20 months before admission to our department. He was identified with multiple nodules in the lungs, and bronchoscopy permitted diagnosis of SCLC at another hospital. He was then relocated to our hospital, where he began receiving chemotherapy and radiation therapy. A metastatic lesion had appeared on his scalp 3 months before admission, which had been diagnosed as a neuroendocrine tumor (NET, corresponding to grade 2) based on histopathological examination. Cancer multigene panel testing was performed and a MEN1 variant (c.266T > G; p.Leu89Arg) was discovered; the patient was then referred to our department. Germline genetic testing revealed the same, novel germline variant in MEN1, leading to his diagnosis of MEN1 and lung NEN metastases. In this case, the stage of NENs can vary between the primary tumor (SCLC) and its metastases (NET), potentially involving second-hit mutations or tumor suppressor genes.
Introduction
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant disorder caused by germline variants in the MEN1 tumor-suppressor gene. MEN1 comprises 10 exons and encodes the protein menin [1]. This syndrome is associated with an increased risk of neuroendocrine neoplasms (NENs) in various organs. The prevalence of duodenopancreatic NENs reaches around 50% by age 50 years and 90% by the age of 80 years or older [2]. NENs are categorized as well-differentiated neuroendocrine tumors (NETs) or poorly differentiated neuroendocrine carcinomas (NECs).
Lung NEN is rare (1%-2% of lung cancers), with a prevalence of about 5% in MEN1 [3]. Lung NECs are classified as small cell lung carcinoma (SCLC) or large cell neuroendocrine carcinoma. Among bronchial NENs in MEN1, typical NENs account for 53% and SCLC for 6% [4]. The 5-year survival rate after curative surgery for typical pulmonary NENs is 87% to 100%, but SCLC prognosis remains poor (5-year survival 16.4%) [3].
Cancer multigene panel testing is increasingly used for lung tumors, including SCLC. The primary role is to examine somatic variants in tumors but it can also detect germline variants that are involved in hereditary diseases such as MEN1. Identifying latent hereditary diseases can lead to the proposal of the most appropriate treatment method.
Here, we present a case in which MEN1 and lung NEN were diagnosed by cancer multigene panel testing for metastatic lesions.
Case Presentation
A 45-year-old man with no medical or smoking history was initially evaluated at a different hospital because of weight loss. His family history revealed that his mother had been diagnosed with a carcinoid tumor located near the pancreatic lymph nodes.
Twenty months before admission to our department, the patient became aware of weight loss. A chest computed tomography scan conducted 13 months prior at another hospital identified multiple pulmonary nodules (Fig. 1). Bronchoscopy permitted a diagnosis of SCLC (CD56-positive, synaptophysin-positive, chromogranin A-positive, Ki-67 index 50%, fission image >20 cells/10 high power field) (Fig. 2). Metastatic spread was observed in the right parietal lobe, liver, right adrenal gland, spine, and pelvis (Fig. 3). Concurrently, the patient exhibited a mild elevation in serum calcium levels (10.5-11.2 mg/dL [reference range, 8.8-10.1]; 2.62-2.79 mmol/L [2.20-2.52]). On admission to our institution 12 months ago, chemoradiotherapy was begun, including 4 cycles of cisplatin, etoposide, and durvalumab followed by 7 maintenance courses of durvalumab and targeted radiation therapy for brain metastases. Three months before his admission to our department, the scalp lesion was resected. On histological examination, it was identified as a metastasis of NEN, corresponding to grade 2 (CD56-positive, synaptophysin-positive, chromogranin A-positive, Ki-67 index 11%, fission image 12 cells/10 high power field) [Fig. 2]). In addition, cancer multigene panel testing of the lesion identified a MEN1 variant (c.266T > G; p.Leu89Arg [NM_130799.2]) with an allele frequency (variant allele frequency) of 95.9%. The patient was then transferred to our department for germline genetic testing and endocrine function assessment.
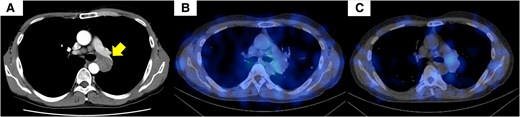
Contrast-enhanced computed tomography (CT) and somatostatin receptor scintigraphy of the chest. (A) Contrast-enhanced CT of the chest showed a 28-mm irregular nodule in the left S6 hilar region. (B, C) Somatostatin receptor scintigraphy showed no accumulation within the lung tumor. Permission for the use of the CT image has already been obtained from another hospital.
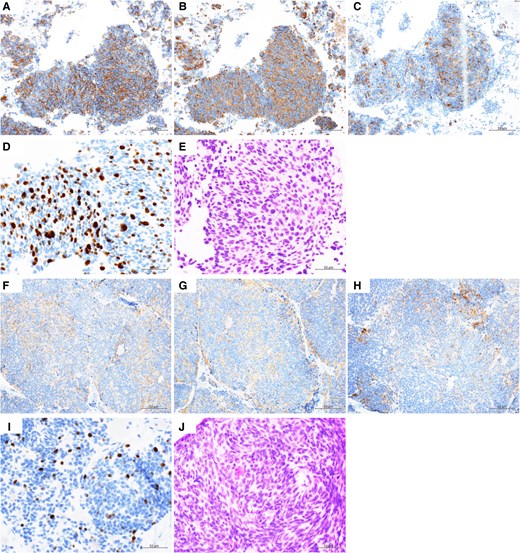
Immunohistochemical staining of the mediastinal lymph node and the scalp. The mediastinal lymph node (A-E) and the scalp (F-J) were investigated by immunohistochemical staining: CD56 (A, F), synaptophysin (B, G), and chromogranin A (C, H). The Ki-67 proliferation index in hot spots was 50% in the mediastinal lymph node and 11% in the scalp (D, I). The mitotic rate was >20/10 high power field in the mediastinal lymph node and 12/10 high power field in the scalp (E, J).
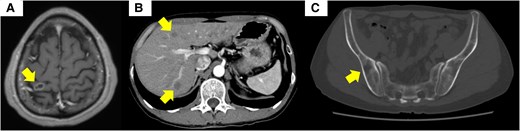
Imaging studies of metastasis in the brain, the lung, and the bone. (A) Magnetic resonance imaging of the brain showed a 12-mm ring-shaped contrast-enhanced mass in the right parietal lobe. (B) Contrast-enhanced computed tomography (CT) of the abdomen showed multiple masses within the liver. (C) CT of the iliac bone showed osteolytic changes. Permission for the use of the CT image has already been obtained from another hospital.
Diagnostic Assessment
On examination, his height was 173.6 cm, weight was 61.1 kg (body mass index, 20.3 kg/m2), and vital signs were within normal range (blood pressure: 114/78 mm Hg; pulse rate: 98 beats per minute). A 2-cm surgical incision was observed in the parietal region. Other physical findings were normal. Laboratory blood tests indicated the presence of mild hypercalcemia (10.5 mg/dL; 2.62 mmol/L) and hyperparathyroidism (intact PTH 67 pg/mL [10-65]; 7.11 pmol/L [1.06-6.90]), as in Table 1. An ultrasound of the neck revealed hypertrophy of the parathyroid glands (Fig. 4). Genetic analysis was conducted on DNA extracted from his peripheral blood, focusing on germ cell lines. This assessment confirmed the presence of the novel heterozygous MEN1 p.Leu89Arg variant (Fig. 5).
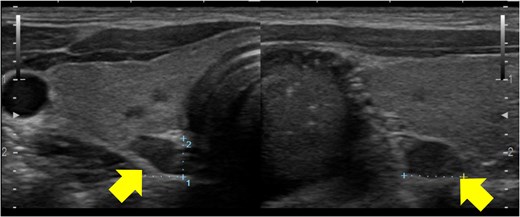
Ultrasonography of the parathyroid glands. Ultrasonography showed enlargement of all parathyroid glands.
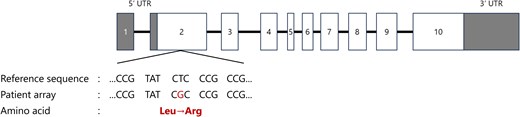
Structure of the MEN1 gene and the variant in this case. White boxes indicate exons, horizontal bars indicate introns, gray boxes represent untranslated regions (UTRs). In this case, a missense variant was found in exon 2 (c.266T > G; p.Leu89Arg). *The figure was drawn with reference to reference 4.
| Biochemistry . | Value (SI units) . | Reference range (SI units) . |
|---|---|---|
| TP | 6.9 g/dL (69 g/L) | 6.6-8.1 g/dL (66-81 g/L) |
| Alb | 4.4 g/dL (44 g/L) | 4.1-5.1 g/dL (41-51 g/L) |
| T-Bil | 0.5 mg/dL (8.55 μmol/L) | 0.4-1.5 mg/dL (3.42-20.52 μmol/L) |
| AST | 22 IU/L | 13-30 IU/L |
| ALT | 27 IU/L | 10-42 IU/L |
| γ-GTP | 40 IU/L | 13-64 IU/L |
| Cre | 0.83 mg/dL (73.4 μmol/L) | 0.65-1.07 mg/dL (57.5-94.6 μmol/L) |
| BUN | 13.9 mg/dL (4.96 mmol/L) | 8.0-20.0 mg/dL (2.86-7.14 mmol/L) |
| eGFR | 79.8 mL/min/1.73 m2 | >60 mL/min/1.73 m2 |
| AMY | 58 IU/L | 44-132 IU/L |
| UA | 4.8 mg/dL (285.5 μmol/L) | 3.7-7.8 mg/dL (220.1-463.9 μmol/L) |
| Sodium | 141 mEq/L (141 mmol/L) | 138-145 mEq/L (138-145 mmol/L) |
| Potassium | 5.2 mEq/L (5.2 mmol/L) | 3.6-4.8 mEq/L (3.6-4.8 mmol/L) |
| Chloride | 107 mEq/L (107 mmol/L) | 101-108 mEq/L (101-108 mmol/L) |
| Calcium | 10.5 mg/dL (2.62 mmol/L) | 8.8-10.1 mg/dL (2.20-2.52 mmol/L) |
| Phosphorus | 2.8 mg/dL (0.90 mmol/L) | 2.7-4.6 mg/dL (0.87-1.49 mmol/L) |
| Magnesium | 2.0 mg/dL (0.82 mmol/L) | 1.8-2.6 mg/dL (0.74-1.07 mmol/L) |
| Plasma glucose | 94 mg/dL (5.2 mmol/L) | 70-110 mg/dL (3.9-6.1 mmol/L) |
| Blood count | ||
| WBC | 7670/μL | 3300-8600/μL |
| RBC | 429 × 104/μL | 435-555 × 104/μL |
| Hb | 13.8 g/dL (138 g/L) | 13.7-16.8 g/dL (137-168 g/L) |
| Hct | 42.3% | 40.7-50.1% |
| Plt | 28.9 × 104/μL | 15.8-34.8 × 104/μL |
| Urinalysis | ||
| Protein | Negative | |
| Glucose | Negative | |
| Blood | Negative | |
| Calcium | 2.7 mg/dL (0.67 mmol/L) | |
| Cre | 32.6 mg/dL (2881 μmol/L) | |
| Endocrinological evaluation | ||
| PRL | 17.1 ng/mL (743.5 pmol/L) | 3.6-12.8 ng/mL (156.5-556.5 pmol/L) |
| TSH | 1.99 μIU/mL (1.99 mIU/L) | 0.61-4.23 μIU/mL (0.61-4.23 mIU/L) |
| Free T3 | 3.01 pg/mL (4.62 pmol/L) | 2.3-4.0 pg/mL (3.53-6.14 pmol/L) |
| Free T4 | 1.32 ng/dL (0.17 pmol/L) | 0.90-1.70 ng/dL (0.12-0.22 pmol/L) |
| Intact PTH | 67 pg/mL (7.11 pmol/L) | 10-65 pg/mL (1.06-6.90 pmol/L) |
| PTHrP | <1.0 pmol/L | <1.1 pmol/L |
| Insulin | 16.90 μIU/mL (117.4 pmol/L) | ≤18.7 μIU/mL (<129.9 pmol/L) |
| Glucagon | 34.1 pg/mL (34.1 ng/L) | 5.4-55.0 pg/mL (5.4-55.0 ng/L) |
| Tumor marker | ||
| NSE | 17.9 ng/mL | ≤16.3 ng/mL |
| proGRP | 5630 pg/mL | <74.7 pg/mL |
| Biochemistry . | Value (SI units) . | Reference range (SI units) . |
|---|---|---|
| TP | 6.9 g/dL (69 g/L) | 6.6-8.1 g/dL (66-81 g/L) |
| Alb | 4.4 g/dL (44 g/L) | 4.1-5.1 g/dL (41-51 g/L) |
| T-Bil | 0.5 mg/dL (8.55 μmol/L) | 0.4-1.5 mg/dL (3.42-20.52 μmol/L) |
| AST | 22 IU/L | 13-30 IU/L |
| ALT | 27 IU/L | 10-42 IU/L |
| γ-GTP | 40 IU/L | 13-64 IU/L |
| Cre | 0.83 mg/dL (73.4 μmol/L) | 0.65-1.07 mg/dL (57.5-94.6 μmol/L) |
| BUN | 13.9 mg/dL (4.96 mmol/L) | 8.0-20.0 mg/dL (2.86-7.14 mmol/L) |
| eGFR | 79.8 mL/min/1.73 m2 | >60 mL/min/1.73 m2 |
| AMY | 58 IU/L | 44-132 IU/L |
| UA | 4.8 mg/dL (285.5 μmol/L) | 3.7-7.8 mg/dL (220.1-463.9 μmol/L) |
| Sodium | 141 mEq/L (141 mmol/L) | 138-145 mEq/L (138-145 mmol/L) |
| Potassium | 5.2 mEq/L (5.2 mmol/L) | 3.6-4.8 mEq/L (3.6-4.8 mmol/L) |
| Chloride | 107 mEq/L (107 mmol/L) | 101-108 mEq/L (101-108 mmol/L) |
| Calcium | 10.5 mg/dL (2.62 mmol/L) | 8.8-10.1 mg/dL (2.20-2.52 mmol/L) |
| Phosphorus | 2.8 mg/dL (0.90 mmol/L) | 2.7-4.6 mg/dL (0.87-1.49 mmol/L) |
| Magnesium | 2.0 mg/dL (0.82 mmol/L) | 1.8-2.6 mg/dL (0.74-1.07 mmol/L) |
| Plasma glucose | 94 mg/dL (5.2 mmol/L) | 70-110 mg/dL (3.9-6.1 mmol/L) |
| Blood count | ||
| WBC | 7670/μL | 3300-8600/μL |
| RBC | 429 × 104/μL | 435-555 × 104/μL |
| Hb | 13.8 g/dL (138 g/L) | 13.7-16.8 g/dL (137-168 g/L) |
| Hct | 42.3% | 40.7-50.1% |
| Plt | 28.9 × 104/μL | 15.8-34.8 × 104/μL |
| Urinalysis | ||
| Protein | Negative | |
| Glucose | Negative | |
| Blood | Negative | |
| Calcium | 2.7 mg/dL (0.67 mmol/L) | |
| Cre | 32.6 mg/dL (2881 μmol/L) | |
| Endocrinological evaluation | ||
| PRL | 17.1 ng/mL (743.5 pmol/L) | 3.6-12.8 ng/mL (156.5-556.5 pmol/L) |
| TSH | 1.99 μIU/mL (1.99 mIU/L) | 0.61-4.23 μIU/mL (0.61-4.23 mIU/L) |
| Free T3 | 3.01 pg/mL (4.62 pmol/L) | 2.3-4.0 pg/mL (3.53-6.14 pmol/L) |
| Free T4 | 1.32 ng/dL (0.17 pmol/L) | 0.90-1.70 ng/dL (0.12-0.22 pmol/L) |
| Intact PTH | 67 pg/mL (7.11 pmol/L) | 10-65 pg/mL (1.06-6.90 pmol/L) |
| PTHrP | <1.0 pmol/L | <1.1 pmol/L |
| Insulin | 16.90 μIU/mL (117.4 pmol/L) | ≤18.7 μIU/mL (<129.9 pmol/L) |
| Glucagon | 34.1 pg/mL (34.1 ng/L) | 5.4-55.0 pg/mL (5.4-55.0 ng/L) |
| Tumor marker | ||
| NSE | 17.9 ng/mL | ≤16.3 ng/mL |
| proGRP | 5630 pg/mL | <74.7 pg/mL |
Values in parentheses are International System of Units.
Abbreviations: Alb, albumin; ALT, alanine aminotransferase; AMY, amylase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cre, creatinine; eGFR, estimated glomerular filtration rate; γ-GTP, γ-glutamyl transpeptidase; Hb, hemoglobin; Hct, hematocrit; NSE, neuron-specific enolase; Plt, platelet; PRL, prolactin; proGRP, pro-gastorin releasing peptide; PTHrP, PTH-related peptide; RBC, red blood cells; T-Bil, total bilirubin; TP, total protein; UA, uric acid; WBC, white blood cells.
| Biochemistry . | Value (SI units) . | Reference range (SI units) . |
|---|---|---|
| TP | 6.9 g/dL (69 g/L) | 6.6-8.1 g/dL (66-81 g/L) |
| Alb | 4.4 g/dL (44 g/L) | 4.1-5.1 g/dL (41-51 g/L) |
| T-Bil | 0.5 mg/dL (8.55 μmol/L) | 0.4-1.5 mg/dL (3.42-20.52 μmol/L) |
| AST | 22 IU/L | 13-30 IU/L |
| ALT | 27 IU/L | 10-42 IU/L |
| γ-GTP | 40 IU/L | 13-64 IU/L |
| Cre | 0.83 mg/dL (73.4 μmol/L) | 0.65-1.07 mg/dL (57.5-94.6 μmol/L) |
| BUN | 13.9 mg/dL (4.96 mmol/L) | 8.0-20.0 mg/dL (2.86-7.14 mmol/L) |
| eGFR | 79.8 mL/min/1.73 m2 | >60 mL/min/1.73 m2 |
| AMY | 58 IU/L | 44-132 IU/L |
| UA | 4.8 mg/dL (285.5 μmol/L) | 3.7-7.8 mg/dL (220.1-463.9 μmol/L) |
| Sodium | 141 mEq/L (141 mmol/L) | 138-145 mEq/L (138-145 mmol/L) |
| Potassium | 5.2 mEq/L (5.2 mmol/L) | 3.6-4.8 mEq/L (3.6-4.8 mmol/L) |
| Chloride | 107 mEq/L (107 mmol/L) | 101-108 mEq/L (101-108 mmol/L) |
| Calcium | 10.5 mg/dL (2.62 mmol/L) | 8.8-10.1 mg/dL (2.20-2.52 mmol/L) |
| Phosphorus | 2.8 mg/dL (0.90 mmol/L) | 2.7-4.6 mg/dL (0.87-1.49 mmol/L) |
| Magnesium | 2.0 mg/dL (0.82 mmol/L) | 1.8-2.6 mg/dL (0.74-1.07 mmol/L) |
| Plasma glucose | 94 mg/dL (5.2 mmol/L) | 70-110 mg/dL (3.9-6.1 mmol/L) |
| Blood count | ||
| WBC | 7670/μL | 3300-8600/μL |
| RBC | 429 × 104/μL | 435-555 × 104/μL |
| Hb | 13.8 g/dL (138 g/L) | 13.7-16.8 g/dL (137-168 g/L) |
| Hct | 42.3% | 40.7-50.1% |
| Plt | 28.9 × 104/μL | 15.8-34.8 × 104/μL |
| Urinalysis | ||
| Protein | Negative | |
| Glucose | Negative | |
| Blood | Negative | |
| Calcium | 2.7 mg/dL (0.67 mmol/L) | |
| Cre | 32.6 mg/dL (2881 μmol/L) | |
| Endocrinological evaluation | ||
| PRL | 17.1 ng/mL (743.5 pmol/L) | 3.6-12.8 ng/mL (156.5-556.5 pmol/L) |
| TSH | 1.99 μIU/mL (1.99 mIU/L) | 0.61-4.23 μIU/mL (0.61-4.23 mIU/L) |
| Free T3 | 3.01 pg/mL (4.62 pmol/L) | 2.3-4.0 pg/mL (3.53-6.14 pmol/L) |
| Free T4 | 1.32 ng/dL (0.17 pmol/L) | 0.90-1.70 ng/dL (0.12-0.22 pmol/L) |
| Intact PTH | 67 pg/mL (7.11 pmol/L) | 10-65 pg/mL (1.06-6.90 pmol/L) |
| PTHrP | <1.0 pmol/L | <1.1 pmol/L |
| Insulin | 16.90 μIU/mL (117.4 pmol/L) | ≤18.7 μIU/mL (<129.9 pmol/L) |
| Glucagon | 34.1 pg/mL (34.1 ng/L) | 5.4-55.0 pg/mL (5.4-55.0 ng/L) |
| Tumor marker | ||
| NSE | 17.9 ng/mL | ≤16.3 ng/mL |
| proGRP | 5630 pg/mL | <74.7 pg/mL |
| Biochemistry . | Value (SI units) . | Reference range (SI units) . |
|---|---|---|
| TP | 6.9 g/dL (69 g/L) | 6.6-8.1 g/dL (66-81 g/L) |
| Alb | 4.4 g/dL (44 g/L) | 4.1-5.1 g/dL (41-51 g/L) |
| T-Bil | 0.5 mg/dL (8.55 μmol/L) | 0.4-1.5 mg/dL (3.42-20.52 μmol/L) |
| AST | 22 IU/L | 13-30 IU/L |
| ALT | 27 IU/L | 10-42 IU/L |
| γ-GTP | 40 IU/L | 13-64 IU/L |
| Cre | 0.83 mg/dL (73.4 μmol/L) | 0.65-1.07 mg/dL (57.5-94.6 μmol/L) |
| BUN | 13.9 mg/dL (4.96 mmol/L) | 8.0-20.0 mg/dL (2.86-7.14 mmol/L) |
| eGFR | 79.8 mL/min/1.73 m2 | >60 mL/min/1.73 m2 |
| AMY | 58 IU/L | 44-132 IU/L |
| UA | 4.8 mg/dL (285.5 μmol/L) | 3.7-7.8 mg/dL (220.1-463.9 μmol/L) |
| Sodium | 141 mEq/L (141 mmol/L) | 138-145 mEq/L (138-145 mmol/L) |
| Potassium | 5.2 mEq/L (5.2 mmol/L) | 3.6-4.8 mEq/L (3.6-4.8 mmol/L) |
| Chloride | 107 mEq/L (107 mmol/L) | 101-108 mEq/L (101-108 mmol/L) |
| Calcium | 10.5 mg/dL (2.62 mmol/L) | 8.8-10.1 mg/dL (2.20-2.52 mmol/L) |
| Phosphorus | 2.8 mg/dL (0.90 mmol/L) | 2.7-4.6 mg/dL (0.87-1.49 mmol/L) |
| Magnesium | 2.0 mg/dL (0.82 mmol/L) | 1.8-2.6 mg/dL (0.74-1.07 mmol/L) |
| Plasma glucose | 94 mg/dL (5.2 mmol/L) | 70-110 mg/dL (3.9-6.1 mmol/L) |
| Blood count | ||
| WBC | 7670/μL | 3300-8600/μL |
| RBC | 429 × 104/μL | 435-555 × 104/μL |
| Hb | 13.8 g/dL (138 g/L) | 13.7-16.8 g/dL (137-168 g/L) |
| Hct | 42.3% | 40.7-50.1% |
| Plt | 28.9 × 104/μL | 15.8-34.8 × 104/μL |
| Urinalysis | ||
| Protein | Negative | |
| Glucose | Negative | |
| Blood | Negative | |
| Calcium | 2.7 mg/dL (0.67 mmol/L) | |
| Cre | 32.6 mg/dL (2881 μmol/L) | |
| Endocrinological evaluation | ||
| PRL | 17.1 ng/mL (743.5 pmol/L) | 3.6-12.8 ng/mL (156.5-556.5 pmol/L) |
| TSH | 1.99 μIU/mL (1.99 mIU/L) | 0.61-4.23 μIU/mL (0.61-4.23 mIU/L) |
| Free T3 | 3.01 pg/mL (4.62 pmol/L) | 2.3-4.0 pg/mL (3.53-6.14 pmol/L) |
| Free T4 | 1.32 ng/dL (0.17 pmol/L) | 0.90-1.70 ng/dL (0.12-0.22 pmol/L) |
| Intact PTH | 67 pg/mL (7.11 pmol/L) | 10-65 pg/mL (1.06-6.90 pmol/L) |
| PTHrP | <1.0 pmol/L | <1.1 pmol/L |
| Insulin | 16.90 μIU/mL (117.4 pmol/L) | ≤18.7 μIU/mL (<129.9 pmol/L) |
| Glucagon | 34.1 pg/mL (34.1 ng/L) | 5.4-55.0 pg/mL (5.4-55.0 ng/L) |
| Tumor marker | ||
| NSE | 17.9 ng/mL | ≤16.3 ng/mL |
| proGRP | 5630 pg/mL | <74.7 pg/mL |
Values in parentheses are International System of Units.
Abbreviations: Alb, albumin; ALT, alanine aminotransferase; AMY, amylase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cre, creatinine; eGFR, estimated glomerular filtration rate; γ-GTP, γ-glutamyl transpeptidase; Hb, hemoglobin; Hct, hematocrit; NSE, neuron-specific enolase; Plt, platelet; PRL, prolactin; proGRP, pro-gastorin releasing peptide; PTHrP, PTH-related peptide; RBC, red blood cells; T-Bil, total bilirubin; TP, total protein; UA, uric acid; WBC, white blood cells.
Based on the American College of Medical Genetics and Genomics criteria [5], the variant was determined to be likely pathogenic: PM1 (located in a variant hot spot and/or critical and well-established functional domain without benign variation), PM2 (extremely low frequency in Exome Sequencing Project, 1000 Genomes, or Exome Aggregation Consortium), PP3 (multiple lines of computational evidence support a deleterious effect on the gene or gene product [SIFT [https://sift.bii.a-star.edu.sg/ accessed on October 1, 2023] [6], PolyPhen-2 [http://genetics.bwh.harvard.edu/pph2/index.shtml accessed on October 1, 2023] [7]), PP4 (patient's phenotype or family history is highly specific for a disease with a single genetic etiology). Thus, a diagnosis of MEN1 was established.
The patient then underwent somatostatin receptor scintigraphy, which did not reveal any accumulation within the lung tumor (Fig. 1). No indications of pituitary adenomas, including prolactinoma, were found.
Treatment
After the germline genetic testing, everolimus (10 mg/day) was initiated following the established protocols.
Outcome and Follow-up
At 6 months after admission to our department, the patient developed drug-induced interstitial pneumonia (grade 2) with no observable alteration in the primary tumor or its metastases. We reduced the dose of everolimus to 5 mg/day and started prednisolone at 15 mg/day. Subsequently, because of progressive brain metastases, γ knife radiosurgery was administered. Nine months after admission to our department, the patient transitioned to palliative care and passed away 2 months later, 11 months after the initial admission.
Discussion
The cancer multigene panel testing of the scalp metastasis identified the MEN1 p.Leu89Arg variant in this case. Subsequently, the same variant was identified by germline testing. The MEN1 p.Leu89Arg variant is thought to underlie the structural integrity and functional capacity of the menin protein, as inferred from multiple in silico analysis programs. Although these findings led to diagnosis of MEN1 with lung NEN, several salient features distinguish this case: (1) identification of a novel MEN1 mutation as a germline mutation, (2) the stage of NEN differed between the primary and metastatic sites, and (3) mutations in the MEN1 gene in SCLC may present an opportunity to trial mTOR inhibitors as a potential chemotherapeutic approach.
The evolving landscape of genetic diagnostics, particularly advancements in cancer multigene panel testing, is anticipated to encourage germline genetic evaluations. Indeed, germline assessment encompassing 26 NENs unveiled germline variants in 50% of the cases (13 cases involving the RET, KIT, SDHB, SDHC, SDHD, and PDGFRA genes) [8]. As in this case, germline assessment may lead to detection of novel variants of hereditary diseases. However, it is imperative that such findings are meticulously cross-referenced with the specific carcinoma types and familial medical histories to ensure comprehensive and accurate results.
It is important to emphasize that lung NETs and NECs manifest distinct genetic profiles. Although MEN1 variants predominantly characterize lung NETs, lung NECs frequently exhibit variants associated with cell-cycle regulation genes such as TP53 and RB1 as well as genes within the PI3K/AKT/mTOR signaling pathway [9]. In fact, MEN1 variants are most frequently observed in lung NETs, with a prevalence of about 10% [3]. Moreover, germline variants within the MEN1 gene can precipitate MEN1. A recent investigation sourced from the Dutch MEN1 Study Group database revealed that 22.9% of lung patients with NEN underwent germline genetic testing [10]. Indeed, 5 somatic mutations of MEN1 p.Leu89Arg have been reported in tumors and hyperplasia (Table 2) [11-15]; 1 of these cases was a lung NET, as in the present case [13]. On the other hand, there are no previous reports of the germline missense mutation p.Leu89Arg of MEN1 in MEN1.
Interestingly, in the present case, the stage of the NENs differed between the mediastinal lymph nodes (NEC) and the scalp metastases (NET). It was reported previously that different organs showed different stages of tumors and hyperplasia of MEN1 metastases [16]. In this report, only MEN1 c.496C > T was found in parathyroid tumors, whereas 2 different mutations (c.496C > T, c.784-9G > A?) were found in MEN1 in thymic and pancreatic NETs (grade 3). MEN1 is caused by heterozygous mutations in the MEN1 gene; thus, MEN1 as a tumor suppressor gene is latent at the somatic cell level and requires a somatic second hit for tumor development. In addition, in our case, the metastasis on the scalp was of a lower grade than the primary tumor. In general, metastases are at the same or more advanced stage than the primary tumor. However, in a previous report of 10 cases of pancreatic NEN with metastatic lesions [17], 1 case was found in which there were fewer genetic mutations in the metastatic lesions than in the primary lesion. It has been suggested that this phenomenon may have occurred before the metastatic lesion was identified clinically.
In this case, after the diagnosis of MEN1 accompanied by NEN, we initiated everolimus, an mTOR inhibitor. Although this drug is approved for the treatment of NEN, it has not been incorporated into treatment regimens for SCLC in any country [18, 19]. However, everolimus administration in patients with lung and gastrointestinal NET was reported to prolong progression-free survival with high tolerability. In RADIANT-4, the effectiveness of everolimus was verified in subjects with nonfunctional NENs. In the subgroup analysis of lung NEN in this study, the everolimus group significantly prolonged progression-free survival (everolimus group: 9.2 months vs placebo group: 3.6 months) [20]. Currently, there is no molecularly targeted drug indicated for SCLC, but confirming germline mutations may offer an opportunity to switch to more specific chemotherapy.
Learning Points
It is important to consider the possibility that patients with NENs having somatic variants may also harbor underlying germline variants.
In MEN1, metastatic foci can be present before clinical identification, necessitating routine whole-body screening even if no metastases are detected at the initial diagnosis.
Mutations in the MEN1 gene in small cell lung carcinoma may present an opportunity to trial mTOR inhibitors as a potential chemotherapeutic approach.
Acknowledgments
The authors are grateful to the patient for his contribution to this study. The authors also thank J. Kawada and H. Tsuchida for their technical assistance, and M. Yato, Y. Ogiso, and M. Nozu for secretarial assistance.
Contributors
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published. Y.T., Y.H., and D.Y. contributed to the analysis, collection, and interpretation of data and writing of the manuscript. Y.M., K.A., and J.E. contributed to the analysis, collection, and interpretation of data and critical revisions of the manuscript for important intellectual content. All authors approved the version to be published.
Funding
No public or commercial funding.
Disclosures
Y.H. has received speaker fees from Sumitomo Pharma Co., Ltd. D.Y. has received consulting or speaker fees from Sumitomo Pharma Co., Ltd, Eli Lilly Japan K.K., MSD K.K., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co. Ltd, and Tanabe Mitsubishi Pharma, Co. Ltd, and clinically commissioned/joint research grants from Taisho Pharmaceutical Co. Ltd, Novo Nordisk Pharma Ltd, Arklay Co. Ltd and Nippon Boehringer Ingelheim Co. Ltd. The remaining authors have no conflicts of interest to disclose.
Informed Patient Consent for Publication
Signed informed consent obtained directly from patient.
Data Availability Statement
Original data generated and analyzed for this case report is included in this published article.



