-
PDF
- Split View
-
Views
-
Cite
Cite
Maria Phylactou, Luke Dixon, Catherine Rennie, Thang Han, Jyotsana Gaur, Niamh M Martin, Ectopic Thyrotropin-Secreting Tumor in the Nasopharynx Causing Central Hyperthyroidism, JCEM Case Reports, Volume 3, Issue 1, January 2025, luae250, https://doi.org/10.1210/jcemcr/luae250
Close - Share Icon Share
Abstract
We report a 31-year-old man with diarrhea and tachycardia. Diagnostic workup confirmed raised free thyroid hormones with unsuppressed thyroid stimulating hormone (TSH). Laboratory assay and medication interference were excluded. Consistent with a high glycoprotein hormone α-subunit (α-GSU), the α-GSU:TSH molar ratio was increased. However, anterior pituitary panel testing also confirmed an isolated, raised follicle stimulating hormone (FSH) (17.3 IU/L; reference range, 1.7-8.0). Therefore, interpretation of α-GSU was limited given the co-existent elevated FSH.
There was no pituitary lesion on magnetic resonance imaging (MRI) and stimulated TSH was 232% of baseline levels following thyrotropin-releasing hormone (TRH) stimulation, making a diagnosis of TSH-oma less likely. Genetic analysis revealed no pathogenic variants in the thyroid hormone receptor β gene.
Due to the persistently elevated FSH, a follow-up pituitary MRI was arranged, which identified a nasopharyngeal mass on the floor of the sphenoid sinus, raising the possibility of ectopic pituitary tissue. The patient underwent endoscopic resection of this lesion, with subsequent normalization of free T4, TSH, and FSH within a few weeks. Histology confirmed a plurihormonal pituitary adenoma with staining for TSH, growth hormone, luteinizing hormone, and FSH. This case highlights the biochemical and radiological challenges of diagnosing ectopic TSH-secreting pituitary tumors.
Introduction
The differential diagnosis of elevated thyroid hormones in the context of nonsuppressed thyroid stimulating hormone (TSH), once laboratory and medication interference have been excluded, is a TSH-secreting pituitary adenoma (TSH-oma), or resistance to thyroid hormone β (RTHβ). However, differentiation between these 2 diagnoses may be challenging, even with thorough biochemical, genetic and radiological investigation.
TSH-secreting adenomas are rare, comprising less than 1% of all functioning pituitary adenomas, with an estimated incidence of 0.15 cases per million people per year [1]. Central hyperthyroidism caused by ectopic TSH-secreting tumors is even rarer, with only 16 cases reported in the literature to date [2-17]. The majority of these have been identified in the nasopharynx and 57% of cases affected women. More than two-thirds of TSH-omas secrete only TSH [18]. Although positive immunostaining with anti-gonadotropin antibodies is not uncommon, co-secretion of gonadotropins with TSH is unusual.
Here, we present a case of central hyperthyroidism caused by an extra-pituitary adenoma, located in the nasopharynx, co-secreting TSH and follicle-stimulating hormone (FSH). We evaluate the diagnostic challenges, particularly in reference to co-secretion of 2 glycoprotein hormones sharing a common alpha-subunit (α-GSU) and review the literature of similar cases to date.
Case Presentation
A 31-year-old man was referred to the endocrinology department with persistently raised free T3 (fT3) and free T4 (fT4) concentrations in the context of nonsuppressed TSH. Thyroid function was measured as part of a diagnostic workup for diarrhea, although this symptom quickly resolved following a change in diet. He did not report any other symptoms of hyperthyroidism, including weight loss, palpitations, tremor, heat intolerance, or sweating. His past medical history included childhood mumps. He did not take any regular or over-the-counter medications. He reported that his sister was recently diagnosed with hyperthyroidism abroad. On examination, he was tachycardic (heart rate 122 beats per minute, regular rhythm). There was no goiter.
Diagnostic Assessment
Free thyroid hormones were elevated: fT4 21.1-36.7 pmol/L (1.64-2.85 ng/dL) (reference range [RR] 9.0-23.0 pmol/L, 0.70-1.79 ng/dL) and fT3 8.4-9.5 pmol/L (5.47-6.18 pg/mL) (RR 2.4-6.0 pmol/L, 1.56-3.91 pg/mL), and TSH was not suppressed (4.62-9.0 mU/L [RR 0.3-4.2]) (Fig. 1A). Anterior pituitary panel testing confirmed a raised FSH of 17.3 IU/L (RR 1.7-8.0), with the remaining results within the reference range. His early morning total testosterone was normal (22.2 nmol/L, 6.4 ng/mL) (RR 10.0-30.0 nmol/L, 2.89-8.65 ng/mL) (Table 1).
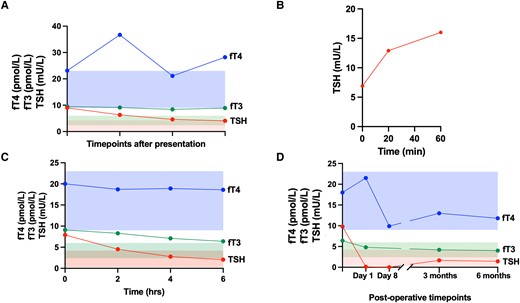
Thyroid function. (A) Free thyroid hormones (fT4 in blue, fT3 in green) and TSH (in red) concentrations at various timepoints following presentation. The shaded areas represent the normal reference range for each hormone (fT4 in blue, fT3 in green, TSH in red). (B) TSH concentrations at baseline and 20 and 60 minutes following stimulation with thyrotropin-releasing hormone (TRH, 200 μg, IV). C, Free thyroid hormones (fT4 in blue, fT3 in green) and TSH (in red) concentrations at baseline and at 2, 4, and 6 hours following administration of octreotide (100 μg, SC). The shaded areas represent the normal reference range for each hormone (fT4 in blue, fT3 in green, TSH in red). D, Free thyroid hormones (fT4 in blue, fT3 in green) and TSH (in red) concentrations following endoscopic resection of nasopharyngeal lesion. The shaded areas represent the normal reference range for each hormone (fT4 in blue, fT3 in green, TSH in red).
| Marker . | Result . | Reference range . |
|---|---|---|
| Total testosterone | 22.2 nmol/L (6.4 ng/mL) | 10-30 nmol/L (2.89-8.65 ng/mL) |
| LH | 2.3 IU/L | 2-12 IU/L |
| FSH | 17.3 IU/L | 1.7-8 IU/L |
| Cortisol | 464 nmol/L (16.8 μg/dL) | 160-550 nmol/L (5.8-19.9 μg/dL) |
| ACTH | 28.5 ng/L (6.3 pmol/L) | <30 ng/L (<6.6 pmol/L) |
| IGF-1 | 24.9 nmol/L (190.5 ng/mL) | 10.5-32.0 nmol/L (80.3-244.8 ng/mL) |
| Growth hormone | 0.15 μg/L | <2 μg/L |
| Prolactin | 301 mU/L (6404 mIU/L) | 60-300 mU/L (1277-6383 mIU/L) |
| α-GSU | 3.26 IU/L (3.26 μg/L) | <1.0 IU/L (<1.0 μg/L) |
| α-GSU:TSH molar ratio | 7.86 | 0.3-4.7 |
| SHBG | 47.3 nmol/L (4.49 μg/mL) | 14.6-94.6 nmol/L (1.39-8.99 μg/mL) |
| Marker . | Result . | Reference range . |
|---|---|---|
| Total testosterone | 22.2 nmol/L (6.4 ng/mL) | 10-30 nmol/L (2.89-8.65 ng/mL) |
| LH | 2.3 IU/L | 2-12 IU/L |
| FSH | 17.3 IU/L | 1.7-8 IU/L |
| Cortisol | 464 nmol/L (16.8 μg/dL) | 160-550 nmol/L (5.8-19.9 μg/dL) |
| ACTH | 28.5 ng/L (6.3 pmol/L) | <30 ng/L (<6.6 pmol/L) |
| IGF-1 | 24.9 nmol/L (190.5 ng/mL) | 10.5-32.0 nmol/L (80.3-244.8 ng/mL) |
| Growth hormone | 0.15 μg/L | <2 μg/L |
| Prolactin | 301 mU/L (6404 mIU/L) | 60-300 mU/L (1277-6383 mIU/L) |
| α-GSU | 3.26 IU/L (3.26 μg/L) | <1.0 IU/L (<1.0 μg/L) |
| α-GSU:TSH molar ratio | 7.86 | 0.3-4.7 |
| SHBG | 47.3 nmol/L (4.49 μg/mL) | 14.6-94.6 nmol/L (1.39-8.99 μg/mL) |
Abbreviations: α-GSU, glycoprotein hormone α-subunit; ACTH, adrenocorticotropic hormone; FSH, follicle stimulating hormone; IGF-1, insulin-like growth factor 1; LH, luteinizing hormone; SHBG, sex hormone binding globulin; TSH, thyrotropin (thyroid-stimulating hormone).
| Marker . | Result . | Reference range . |
|---|---|---|
| Total testosterone | 22.2 nmol/L (6.4 ng/mL) | 10-30 nmol/L (2.89-8.65 ng/mL) |
| LH | 2.3 IU/L | 2-12 IU/L |
| FSH | 17.3 IU/L | 1.7-8 IU/L |
| Cortisol | 464 nmol/L (16.8 μg/dL) | 160-550 nmol/L (5.8-19.9 μg/dL) |
| ACTH | 28.5 ng/L (6.3 pmol/L) | <30 ng/L (<6.6 pmol/L) |
| IGF-1 | 24.9 nmol/L (190.5 ng/mL) | 10.5-32.0 nmol/L (80.3-244.8 ng/mL) |
| Growth hormone | 0.15 μg/L | <2 μg/L |
| Prolactin | 301 mU/L (6404 mIU/L) | 60-300 mU/L (1277-6383 mIU/L) |
| α-GSU | 3.26 IU/L (3.26 μg/L) | <1.0 IU/L (<1.0 μg/L) |
| α-GSU:TSH molar ratio | 7.86 | 0.3-4.7 |
| SHBG | 47.3 nmol/L (4.49 μg/mL) | 14.6-94.6 nmol/L (1.39-8.99 μg/mL) |
| Marker . | Result . | Reference range . |
|---|---|---|
| Total testosterone | 22.2 nmol/L (6.4 ng/mL) | 10-30 nmol/L (2.89-8.65 ng/mL) |
| LH | 2.3 IU/L | 2-12 IU/L |
| FSH | 17.3 IU/L | 1.7-8 IU/L |
| Cortisol | 464 nmol/L (16.8 μg/dL) | 160-550 nmol/L (5.8-19.9 μg/dL) |
| ACTH | 28.5 ng/L (6.3 pmol/L) | <30 ng/L (<6.6 pmol/L) |
| IGF-1 | 24.9 nmol/L (190.5 ng/mL) | 10.5-32.0 nmol/L (80.3-244.8 ng/mL) |
| Growth hormone | 0.15 μg/L | <2 μg/L |
| Prolactin | 301 mU/L (6404 mIU/L) | 60-300 mU/L (1277-6383 mIU/L) |
| α-GSU | 3.26 IU/L (3.26 μg/L) | <1.0 IU/L (<1.0 μg/L) |
| α-GSU:TSH molar ratio | 7.86 | 0.3-4.7 |
| SHBG | 47.3 nmol/L (4.49 μg/mL) | 14.6-94.6 nmol/L (1.39-8.99 μg/mL) |
Abbreviations: α-GSU, glycoprotein hormone α-subunit; ACTH, adrenocorticotropic hormone; FSH, follicle stimulating hormone; IGF-1, insulin-like growth factor 1; LH, luteinizing hormone; SHBG, sex hormone binding globulin; TSH, thyrotropin (thyroid-stimulating hormone).
Laboratory assay interference was excluded in the first instance, with similar thyroid function tests confirmed using the Alinity (Abbott), Access TSH (Beckman Coulter), DELFIA (Revvity) and ADVIA Centaur αTgII Assay (Siemens) platforms. Glycoprotein hormone alpha-subunit (α-GSU) was raised (3.26 IU/L, 3.26 μg/L) (RR < 1.0 IU/L, <1 μg/L); however, SHBG was normal (47.3 nmol/L, 4.49 μg/mL) (RR 14.6-94.6 nmol/L, 1.39-8.99 μg/mL). The α-GSU:TSH molar ratio ([(α-GSU (μg/L)/TSH (mU/L)] × 10) was also raised (7.86 [RR 0.3-4.7] [19]) (Table 1). Review of his sister's thyroid function tests confirmed primary hyperthyroidism with raised fT4 4.38 ng/dL (56.38 pmol/L) (RR 0.7-1.48 ng/dL, 9.01-19.05 pmol/L)), fT3 16.35 pg/mL (25 pmol/L) (RR 1.71-3.71 pg/mL, 2.63-5.70 pmol/L) and suppressed TSH (< 0.01 mU/L, RR 0.35-4). A pituitary magnetic resonance imaging (MRI) with contrast and a subsequent dynamic MRI did not reveal a pituitary lesion (Fig. 2A and 2D).
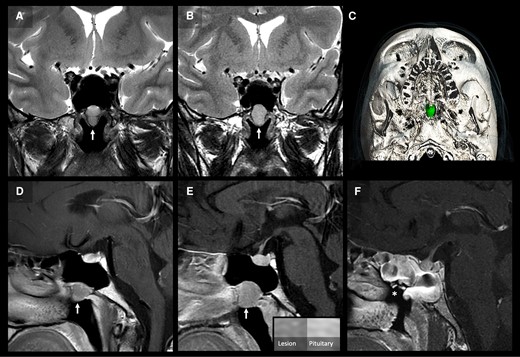
Pituitary imaging. Two serial coronal T2-weighted (A, B) and postcontrast sagittal T1-weighted (D, E) MRI images of the pituitary at presentation (A, D), and 4 years later (B, E) demonstrate a slow-growing ovoid lesion in the bony floor of the paranasal sphenoid sinus projecting into the nasopharynx. The lesion exhibits homogenous, moderately high, T2-weighted signal and adenoma-like enhancement, less than the normal pituitary (E). 3D-reconstruction of the operative planning CT (C) with segmentation of the lesion (green) shows smooth remodeling and scalloping of the surrounding bone. Postoperative postcontrast sagittal T1-weighted MRI (F) shows complete resection of the lesion (asterisk in surgical cavity) and expected postsurgical mucosal edema in the adjacent sphenoid sinus.
A thyrotropin-releasing hormone (TRH) stimulation test showed a 232%-fold-change in TSH from baseline: TSH at t = 0 6.9 mU/L, at t = 20 minutes 12.9 mU/L, and at t = 60 minutes 16 mU/L (Fig. 1B), suggestive of RTHβ. However, fluorescent sequencing analysis of the hormone binding domain (exons 710) of the thyroid hormone receptor β gene did not detect any pathogenic variants. A 10-day T3 suppression test was offered to the patient, but he declined this due to the intensive testing schedule.
In view of the absence of a pituitary lesion on MRI and the increase in TSH in response to TRH stimulation, he was managed as a presumed case of RTHβ. During follow-up, he reported palpitations and was started on propranolol with good effect. To further investigate the consistently elevated FSH, always with normal testosterone, further investigations were organized to exclude primary testicular dysfunction as a cause. Interestingly, serum inhibin B was normal (176.5 ng/L, [RR 25-325]), as was seminal fluid analysis. Indeed, the patient welcomed his first child during this period.
In light of this, further pituitary imaging was organized, which once again showed a normal pituitary gland appearance but identified a well-defined, enhancing mass in the floor of the sphenoid sinus projecting into the nasopharynx that had grown in size compared to previous MRIs (Fig. 2B and 2D).
This new radiological finding raised the possibility of ectopic pituitary tissue within the nasopharynx being the source of TSH and FSH. Consistent with a TSH-oma, TSH fell following octreotide administration (TSH at T0 = 7.90 mU/L, T2 hours = 4.53 mU/L, T4 hours = 2.78 mU/L, T6 hours = 2.08 mU/L) although FSH did not change (Fig. 1C). Ear, nose, and throat (ENT) examination with a nasal endoscope confirmed the presence of a lesion protruding through the floor of the sphenoid sinus (Fig. 2E).
Treatment
The patient underwent endoscopic resection, through the right nasal cavity, of the sphenoid sinus lesion.
Histology confirmed a pituitary adenoma (hematoxylin and eosin [H&E]) showing cells in acinar arrangement with perivascular pseudorosettes. Immunohistochemistry revealed that the cells showed bimodal expression of the transcription factors Pit-1 and SF-1 and absence of immunostaining for T-Pit. There was patchy cytoplasmic labeling for TSH, growth hormone (GH), FSH, and luteinizing hormone (LH), with rare cells positive for prolactin. The Ki67 index was approximately 2% and focally up to 5% (Fig. 3).
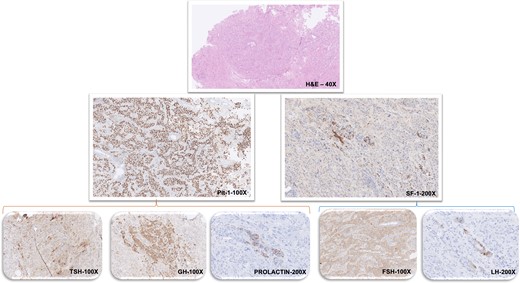
Pathological and immunohistochemical staining results of nasopharyngeal tissue. Histopathological findings (stains and magnification as labeled): Hematoxylin and eosin staining shows pituitary adenoma with tumor cells arranged predominantly in an acinar pattern with focal perivascular pseudorosettes. On immunohistochemistry the tumor cells show widespread strong nuclear labeling for Pit-1 along with focal nuclear staining for SF-1. Tumor shows patchy cytoplasmic expression for TSH, GH, and prolactin. There is widespread cytoplasmic staining for FSH while LH expression is seen in a few tumor cells.
Outcome and Follow-Up
Within 8 days postoperatively, TSH fell and was transiently undetectable, associated with a low normal fT4 (fT4 9.9 pmol/L, 0.77 ng/dL, TSH <0.01 mU/L). However, thyroid function spontaneously normalized within a few weeks (Fig. 1D). Interestingly, serum FSH also normalized following the removal of the nasopharyngeal lesion. A postoperative MRI scan showed complete resection of the lesion (Fig. 2F).
Discussion
We hereby present the case of a 31-year-old man with an ectopic TSH-secreting pituitary tumor located in the nasopharynx, causing central hyperthyroidism.
Brucker-Davis and colleagues described the classical diagnostic criteria for TSH-secreting adenomas, in a case series of 25 patients with the condition. These comprise an unresponsive TRH test (ie, blunted TSH response to TRH stimulation), high α-GSU, and a high α-GSU:TSH ratio [20]. In this case series, responses to TRH were defined as: absent response if TSH rise was < 2 mU/L, decreased response if stimulated TSH was < 5 mU/L or < 200% of baseline, and normal response if stimulated TSH was > 5 mU/L or > 200% of baseline [20]. Patients with TSH-omas had significantly lower stimulated TSH compared to patients with RTHβ [20]. Overall, a flat or reduced response to TRH stimulation was found to have a 71% sensitivity and 96% specificity for TSH-oma [20].
Interestingly, our patient's stimulated TSH was 232% of his baseline level, following the TRH stimulation test. According to the Brucker-Davis diagnostic criteria, this is considered a positive response to TRH, and thus not in keeping with a TSH-secreting tumor. Furthermore, on review of the 16 other published case reports of ectopic TSH-secreting pituitary adenomas, 5 included details of a TRH stimulation test. Of these, one patient had an absent response to TRH (stimulated TSH < 2 μU/mL) [6], 2 had a decreased response to TRH (stimulated TSH < 200% of baseline) [7, 14], whereas the other 2 had a positive response (stimulated TSH > 200% of baseline) [2, 12]. Hence, it is unclear whether the recommended diagnostic cutoffs for the TSH response to TRH in TSH-omas can be extrapolated to ectopic pituitary tissue secreting TSH.
The majority of TSH-omas secrete only TSH (72%), with the remainder of tumors usually co-secreting GH or prolactin, since these pituitary hormones all share the Pit1 transcription factor. Co-secretion of gonadotropins with TSH is extremely rare. Of the 16 case reports of ectopic TSH-secreting pituitary adenomas in the literature, 4 reported a raised FSH; however, 3 of these involved postmenopausal women, thus only one involved co-secretion of FSH [6]. Traditionally, the α-GSU/TSH molar ratio can be used in the biochemical distinction between a TSH-oma and RTHβ. A high α-GSU/TSH molar ratio is present in 80% of TSH-omas [21]. However, no validated cutoffs exist, as the molar ratio must take the circulating levels of other pituitary glycoprotein hormones (such as LH and FSH) into account. Molar ratios in eugonadal men are usually 0.3 whereas the ratio can be as high as 29.1 in postmenopausal women [22]. The co-secretion of TSH and FSH in this case limited the utility of α-GSU and the α-GSU/TSH molar ratio in the diagnostic workup.
It is well established that in up to 10% of individuals with RTHβ, a pathogenic variant cannot be identified [23]. Thus, during the initial diagnostic workup for this patient, particularly the TSH response to TRH stimulation, the challenging interpretation of the elevated α-GSU/TSH molar ratio, and a normal pituitary gland on imaging, the presumed initial diagnosis was RTHβ, even in the absence of a confirmatory genetic analysis.
In 13 out of the 16 patients with ectopic TSH-secreting pituitary tissue reported in the literature, the tumor was localized in the nasopharynx. Interestingly, this area is considered a radiological “blind spot” as the paranasal sinuses oftentimes contain obscuring mucosal edema that might lead to ectopic tissue being missed. On review of our patient's imaging, the nasopharyngeal lesion was present from the outset, but was much smaller in size at first presentation and the enlargement over time enabled us to focus on this area.
In summary, we present a case of an ectopic pituitary tumor located in the nasopharynx, co-secreting TSH and FSH. We describe the biochemical challenges to achieve the correct diagnosis, evaluating the limitations of diagnostic tests in TSH-omas, particularly in the context of tumors co-secreting glycoprotein hormones. Finally, this case highlights the need for careful radiological review in cases where TSH-oma is suspected yet the pituitary gland is normal on imaging, to explore the possibility of ectopic pituitary tissue.
Learning Points
The most sensitive diagnostic test for distinguishing a TSH-oma from RTHβ is calculating the α-GSU to TSH molar ratio. However, the ratio also reflects the presence of other glycoprotein hormones and has limited use when FSH is raised due to a shared α-GSU.
A blunted TSH response to TRH is the most specific investigation for TSH-omas. However, the diagnostic accuracy of TRH stimulation testing in patients with ectopic TSH-secreting lesions remains uncertain. Assessing biochemical response to octreotide may be useful in cases where a TSH-oma is suspected but there is no pituitary adenoma on imaging.
The nasopharynx and sphenoid sinus are important review areas for ectopic pituitary tissue. They can represent an imaging “blind spot” as the paranasal sinuses often contain distracting and obscuring mucosal edema. These should be reviewed carefully when biochemistry is suggestive of a functioning pituitary tumor, but the pituitary gland has a normal appearance on MRI.
Acknowledgments
We thank Dr. Risheka Walls for her invaluable input in the diagnostic assessment of this patient.
Contributors
All authors made individual contributions to authorship. L.D.: imaging section and preparation of the MRI images, J.G.: histopathology section and preparation of histology images. M.P., C.R., T.H., N.M.: manuscript preparation. All authors reviewed and approved the final draft.
Funding
This work was supported by the National Institute for Health Research (NIHR), the NIHR Imperial Clinical Research Facility, and NIHR Imperial Biomedical Research Centre at Imperial College Healthcare NHS Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. The Section of Endocrinology and Investigative Medicine is funded by grants from the UK Medical Research Council (MRC), Biotechnology and Biological Sciences Research Council, and NIHR. M.P. is supported by an NIHR Academic Clinical Lectureship.
Disclosures
None declared
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient.
Data Availability Statement
Original data generated during this study are included in this published article.
References
Abbreviations
- α-GSU
glycoprotein hormone α-subunit
- FSH
follicle-stimulating hormone
- fT3
free triiodothyronine
- fT4
free thyroxine
- GH
growth hormone
- H&E
hematoxylin and eosin
- LH
luteinizing hormone
- MRI
magnetic resonance imaging
- RR
reference range
- RTHβ
resistance to thyroid hormone β
- THR
thyrotropin-releasing hormone
- TSH
thyrotropin (thyroid-stimulating hormone)



