-
PDF
- Split View
-
Views
-
Cite
Cite
Ayse Nurcan Cebeci, Steven Hebert, Heiko Reutter, Oliver Rompel, Joachim Woelfle, SIADH as a Rare Complication of Foramen Magnum Stenosis in an Infant With Achondroplasia, JCEM Case Reports, Volume 2, Issue 8, August 2024, luae144, https://doi.org/10.1210/jcemcr/luae144
Close - Share Icon Share
Abstract
Achondroplasia is the most common skeletal dysplasia and is associated with serious complications such as foramen magnum stenosis (FMS). This case report describes an infant with achondroplasia who presented with a syndrome of inappropriate antidiuretic hormone secretion (SIADH), secondary to significant FMS and myelocompression. A 2-month-old boy with prenatally diagnosed achondroplasia was referred due to disordered breathing and altered consciousness. On admission, apathy, hypotonus, and hypothermia with typical features of achondroplasia were noticed. Laboratory investigations revealed severe hyponatremia and hypochloridaemia with normal glucose and urea levels. The diagnosis of SIADH was made based on low serum osmolality in the presence of high urine osmolality, along with an elevated copeptin level. An emergency computerized tomography showed a high-grade stenosis at the cranio-cervical junction; subsequent magnetic resonance imaging demonstrated myelocompression. The patient underwent decompression surgery the next day; serum osmolality increased after the operation. Spontaneous breathing after extubation was sufficient whereas tetraplegia persisted despite intensive physiotherapy. Clinicians should be aware of SIADH as a presenting sign of FMS in children with achondroplasia. Further discussion is warranted regarding improving parental education and timing of screening recommendations.
Introduction
Achondroplasia is the most common skeletal dysplasia associated with disproportionate short stature (1). In patients with achondroplasia, the foramen magnum is dysplastic due to abnormal endochondral ossification caused by gain-of-function mutations in the fibroblast growth factor receptor-3 (FGFR3) gene (2). Although rare, foramen magnum and cervical spinal stenosis are the most severe complications in achondroplasia as these complications are often associated with cervicomedullary compression and sudden infant death (3). Hence, all children with achondroplasia should be screened for foramen magnum stenosis (FMS). If detected early, neurosurgical decompression is an effective treatment of FMS (4). However, there is no worldwide consensus regarding the timing and method of screening.
Since cranio-cervical compression might lead to severe respiratory problems, a comprehensive neurologic evaluation and sleep studies investigating centrally caused respiratory abnormalities in infants with achondroplasia are essential (5). In Germany, current recommendations advise polysomnography at 2 to 3 months and cranial imaging at 6 to 9 months of age for screening of FMS (6). On the other hand, the American Academy of Pediatrics recommends evaluation of every infant with achondroplasia for cranio-cervical junction risks via neurologic examination, polysomnography, and neuroimaging either with computerized tomography (CT) or magnetic resonance imaging (MRI) as soon as the diagnosis is recognised (1).
More recently, the European Society of Paediatric Radiology and European Society of Neuroradiology recommended MRI as a preferred imaging modality (7). They concluded that MRI would be more precise than neurological examination and polysomnography in identifying cervicomedullary compression due to FMS.
Here we present a very young infant with achondroplasia and FMS who also exhibited syndrome of inappropriate antidiuretic hormone secretion (SIADH) secondary to significant FMS and myelocompression. Our aim is to draw attention to the early presentation of a life-threatening complication of achondroplasia and to discuss the optimal timing of cranial MRI.
Case Presentation
A 2-month-old boy, prenatally diagnosed with achondroplasia due to FGFR3 mutation with no family history of achondroplasia, was referred to our hospital due to disordered breathing and altered consciousness. Fourteen days earlier the patient had been admitted to an external hospital due to weight loss; at that time mild hyponatremia was detected. Intravenous fluid administration was started by the treating physicians, with a maximal Na+- replacement dose of 17 mmol/kg/d (17 mEq/kg/day). Sodium substitution was gradually reduced, and oral therapy was initiated with a dose of 3 mmol per day (3 mEq/day). Sodium substitution was terminated 6 days later after normalization of serum sodium concentration. After discharge, the patient was fed formula prepared with still water 8 × 60 mL/day. During the following days he seemed more agitated; due to altered breathing, the parents presented the patient in our hospital.
On physical examination, the patient was apathic, pale, hypotonic, and hypothermic (34.8 °C) with typical features of achondroplasia (frontal bossing with small nasal bridge, short limbs, and brachydactyly). Weight was 3500 g, pulse was 104/minute, and capillary refill time was 2 to 3 seconds. Shallow breathing with nasal flaring and suprasternal retractions and deep sighing was observed, compatible with central apnoea. Blood gas analysis revealed respiratory acidosis, necessitating intubation of the patient.
Diagnostic Assessment
Initial laboratory investigations revealed severe hyponatremia (114 mmol/L, 114 mEq/L; normal reference range: 131-141 mmol/L, 131-141 mEq/L), hypochloridaemia (82 mmol/L, 82 mEq/L; normal reference range: 98-109 mmol/L, 98-109 mmol/L), normal glucose and urea levels, and a high urinary Na+ concentration (131.8 mol/mol Cr, 131.8 mEq/L; normal reference range 40-115 mmol/L, 40-115 mmol/L) (Table 1). The patient had normal free T3 and free T4 serum concentrations and appropriately elevated cortisol levels (188 ng/mL, 518.6 nmol/L; normal reference range 10-150 ng/mL, 82.3-579.3 nmol/L). Urinary output was low (98 mL in 24 hours; corresponding to 1.1 mL/kg/hour). A diagnosis of SIADH was made based on low serum osmolality (237 mOsm/kg; normal reference range: 275-290 mOsm/kg) in the presence of high urine osmolality (431 mOsm/kg; normal reference range: <1000 mOsm/kg; may vary depending on serum osmolality and hydration status) along with an elevated copeptin level (202 pmol/L; normal reference range: 2.6-20 pmol/L). An emergency CT revealed a high-grade stenosis at the cranio-cervical junction; subsequent MRI demonstrated myelocompression (shown in Fig. 1A). The degree of FMS was compatible with an extended achondroplasia foramen magnum score (eAFMS) 4b (8). The eAFMS is a 9-point scale (0-4b) where 0 represents normal appearance of the craniocervical junction and 4b represents myelopathic T2 signal change and effaced cerebrospinal fluid signal.
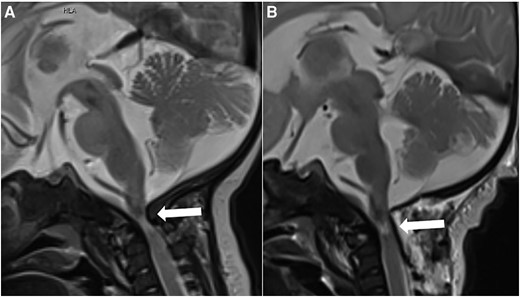
(A) Preoperative brain MRI at the age of 2 months. Sagittal T2-weighted image demonstrates high-grade stenosis of the foramen magnum with compression and myelopathy of the upper cervical spinal cord (arrow). (B) Brain MRI 3 days after decompression surgery. Sagittal T2-weighted image demonstrates a moderately enlarged foramen magnum with a partially unfolded upper cervical spinal cord but remaining signs of short segment cervical myelopathy (arrow). Abbreviations: MRI, magnetic resonance imaging.
| Parameter tested . | On admission . | After surgery . | Normal range . |
|---|---|---|---|
| Serum sodium | 114 mmol/L (114 mEq/L) | 140 mmol/L (140 mEq/L) | 131-141 mmol/L (131-141 mEq/L) |
| Serum potassium | 4.2 mmol/L (4.2 mEq/L) | 3.8 mmol/L (3.8 mEq/L) | 3.7-5.6 mmol/L (3.7-5.6 mEq/L) |
| Serum chloride | 82 mmol/L 82 mEq/L | 109 mmol/L 109 mEq/L | 98-109 mmol/L (98-109 mEq/L) |
| Serum blood urea nitrogen | 7.5 mmol/L 21 mg/dL | 8.9 mmol/L 25 mg/dL | 1.8-7.1 mmol/L (4-28 mg/dL) |
| Serum creatinin | 11.5 µmol/L .13 mg/dL | 35.4 µmol/L .4 mg/dL | 15-37 µmol/L (.15-.33 mg/dL) |
| Serum osmolality | 237 mOsm/kg | 290 mOsm/kg | 275-290 mOsm/kg |
| Urine sodium | 131.8 mol/mol Cr 131.8 mEq/L | 40.2 mol/mol Cr 40.2 mEq/L | 40-115 mol/mol Cr (40-115 mEq/L) |
| Urine osmolality | 431 mOsm/kg | 202 mOsm/kg | <1000 mOsm/kg |
| Serum copeptin | 202 pmol/L | 4.1 pmol/L | 2.6-20 pmol/L |
| Parameter tested . | On admission . | After surgery . | Normal range . |
|---|---|---|---|
| Serum sodium | 114 mmol/L (114 mEq/L) | 140 mmol/L (140 mEq/L) | 131-141 mmol/L (131-141 mEq/L) |
| Serum potassium | 4.2 mmol/L (4.2 mEq/L) | 3.8 mmol/L (3.8 mEq/L) | 3.7-5.6 mmol/L (3.7-5.6 mEq/L) |
| Serum chloride | 82 mmol/L 82 mEq/L | 109 mmol/L 109 mEq/L | 98-109 mmol/L (98-109 mEq/L) |
| Serum blood urea nitrogen | 7.5 mmol/L 21 mg/dL | 8.9 mmol/L 25 mg/dL | 1.8-7.1 mmol/L (4-28 mg/dL) |
| Serum creatinin | 11.5 µmol/L .13 mg/dL | 35.4 µmol/L .4 mg/dL | 15-37 µmol/L (.15-.33 mg/dL) |
| Serum osmolality | 237 mOsm/kg | 290 mOsm/kg | 275-290 mOsm/kg |
| Urine sodium | 131.8 mol/mol Cr 131.8 mEq/L | 40.2 mol/mol Cr 40.2 mEq/L | 40-115 mol/mol Cr (40-115 mEq/L) |
| Urine osmolality | 431 mOsm/kg | 202 mOsm/kg | <1000 mOsm/kg |
| Serum copeptin | 202 pmol/L | 4.1 pmol/L | 2.6-20 pmol/L |
| Parameter tested . | On admission . | After surgery . | Normal range . |
|---|---|---|---|
| Serum sodium | 114 mmol/L (114 mEq/L) | 140 mmol/L (140 mEq/L) | 131-141 mmol/L (131-141 mEq/L) |
| Serum potassium | 4.2 mmol/L (4.2 mEq/L) | 3.8 mmol/L (3.8 mEq/L) | 3.7-5.6 mmol/L (3.7-5.6 mEq/L) |
| Serum chloride | 82 mmol/L 82 mEq/L | 109 mmol/L 109 mEq/L | 98-109 mmol/L (98-109 mEq/L) |
| Serum blood urea nitrogen | 7.5 mmol/L 21 mg/dL | 8.9 mmol/L 25 mg/dL | 1.8-7.1 mmol/L (4-28 mg/dL) |
| Serum creatinin | 11.5 µmol/L .13 mg/dL | 35.4 µmol/L .4 mg/dL | 15-37 µmol/L (.15-.33 mg/dL) |
| Serum osmolality | 237 mOsm/kg | 290 mOsm/kg | 275-290 mOsm/kg |
| Urine sodium | 131.8 mol/mol Cr 131.8 mEq/L | 40.2 mol/mol Cr 40.2 mEq/L | 40-115 mol/mol Cr (40-115 mEq/L) |
| Urine osmolality | 431 mOsm/kg | 202 mOsm/kg | <1000 mOsm/kg |
| Serum copeptin | 202 pmol/L | 4.1 pmol/L | 2.6-20 pmol/L |
| Parameter tested . | On admission . | After surgery . | Normal range . |
|---|---|---|---|
| Serum sodium | 114 mmol/L (114 mEq/L) | 140 mmol/L (140 mEq/L) | 131-141 mmol/L (131-141 mEq/L) |
| Serum potassium | 4.2 mmol/L (4.2 mEq/L) | 3.8 mmol/L (3.8 mEq/L) | 3.7-5.6 mmol/L (3.7-5.6 mEq/L) |
| Serum chloride | 82 mmol/L 82 mEq/L | 109 mmol/L 109 mEq/L | 98-109 mmol/L (98-109 mEq/L) |
| Serum blood urea nitrogen | 7.5 mmol/L 21 mg/dL | 8.9 mmol/L 25 mg/dL | 1.8-7.1 mmol/L (4-28 mg/dL) |
| Serum creatinin | 11.5 µmol/L .13 mg/dL | 35.4 µmol/L .4 mg/dL | 15-37 µmol/L (.15-.33 mg/dL) |
| Serum osmolality | 237 mOsm/kg | 290 mOsm/kg | 275-290 mOsm/kg |
| Urine sodium | 131.8 mol/mol Cr 131.8 mEq/L | 40.2 mol/mol Cr 40.2 mEq/L | 40-115 mol/mol Cr (40-115 mEq/L) |
| Urine osmolality | 431 mOsm/kg | 202 mOsm/kg | <1000 mOsm/kg |
| Serum copeptin | 202 pmol/L | 4.1 pmol/L | 2.6-20 pmol/L |
In addition, postnatal genetic analysis confirmed the prenatally diagnosed heterozygous mutation of FGFR c.1138G > A p.(Gly380Arg).
Treatment
Before surgery, severe hyponatremia was treated with fluid restriction, hypertonic saline (3%), and furosemide. Under that regimen, serum sodium concentration increased to 120 mmol/L (120 mEq/L) within the first 5 hours, with a further rise in serum sodium concentration during 36 hours to a Na concentration of 130 mmol/L (130 mEq/L), thus slightly exceeding the goal of maximally increasing serum Na concentration of 8 mmol/L (8 mEq/L) in 24 hours. The patient underwent decompression surgery the next day. A midline craniectomy with partial resection of the first and second vertebrae with decompression was performed. Prompt improvement of laboratory parameters was observed after surgery, with an increase in serum osmolality and a decrease in urinary sodium excretion (Table 1). An MRI on the third postoperative day revealed an unfolded spinal cord with postoperative hematoma without a space occupying effect (Fig. 1B).
Outcome and Follow-up
After successful extubation on the ninth day of hospitalization, only reduced spontaneous motor activity of the extremities consistent with tetraplegia was observed, so that a high-level spinal injury was assumed. During follow-up, significant residual neurological impairment persisted despite intensive physiotherapy. At the last follow-up at an age of 22 months, the child exhibited tetraplegia, with moderate spasticity of both arms, only little spontaneous movements of the lower extremities (R > L), and impaired sensory perception, consistent with incomplete tetraplegia due to partial severing of the spinal cord.
Discussion
Although spinal cord compression due to FMS is rare, decompression surgery has been required in 6.7% to 13.3% of children with achondroplasia by the age of 2 (9). Moreover, sudden infant death related to central apnoea in these patients might be as high as 7.5% (10). While screening for FMS is recommended in all infants with achondroplasia, not all patients receive proper screening. Nadel et al (4) reported that only 13.9% of 3577 children aged 19 years or younger were fully and appropriately screened according to the 2005 American Academy of Pediatrics guidelines. They also reported that the percentage of children with achondroplasia undergoing cervicomedullary decompression was highest in infancy (28 per 1000 patient-years) and that this decreased with age (5 per 1000 patient-years for all other ages combined).
In the pediatric population, a common cause of hyponatremia is SIADH, which is caused by impaired free water excretion due to inability to suppress antidiuretic hormone secretion (11). Pulmonary diseases and surgical procedures as well as central nervous system disorders including hemorrhage, infection, trauma, stroke, and certain medications might lead to SIADH in children. Noninflammatory obstruction of the fourth ventricular outlet due to malformations as seen in achondroplasia may result in hydrocephalus, which in rare cases has been reported to cause SIADH (12). Thus, SIADH should be suspected in hyponatremic patients with low serum osmolality in the presence of high urine osmolality. Clinicians should be aware that SIADH in infants with achondroplasia might be caused due to compression of central structures. Although our patient had already developed hyponatremia 2 weeks earlier, this finding was attributed to dehydration, and infusion therapy was initiated in the external hospital. In order to avoid devastating pontine myelinolysis, infusion therapy should be closely monitored and potentially adapted in order to increase serum Na concentration not more than 8 mmol/L (8 mEq/L) in 24 hours.
In our patient, neurologic symptoms were overlooked until he developed central apnoea. Careful neurological examination and parental education seem to be key factors during follow-up of achondroplasia. A recent expert panel recommended a comprehensive physical examination, including an age-appropriate neurological examination every 2 months for the first year of life (2). Craniocervical imaging is suggested in case of abnormalities in physical findings along with polysomnography. Abnormalities such as feeding difficulties, respiratory distress, sleep disturbances, poor muscle tone, weakness, seizures, apnoea, and movement asymmetry should be considered signs of FMS in infants with achondroplasia. Decompression surgery might lead to an improvement of neurologic symptoms and breathing problems; however, it has poor efficacy when the intervention is delayed (9). Our patient had severe cervicomedullary compression and has persistent neurologic sequelae despite decompression surgery. We are convinced that parental education regarding specific neurologic features is crucial and might prevent severe complications in such patients.
As pointed out earlier, every screening method has its difficulties. Polysomnography requires overnight sleep, and there is a lack of age-appropriate norms (2). While CT does not provide adequate images of the brainstem and upper cervical cord, MRI frequently requires general anesthesia (1). The recent international consensus statement recommends MRI as the preferred imaging modality to investigate cervicomedullary compression in achondroplasia. In asymptomatic infants with achondroplasia, MRI scanning should be considered during the first months of life to evaluate the cervicomedullary junction and foramen magnum size (13).
Flexion-extension MRI has been proposed in order to better detect dynamic FMS. The European Society of Paediatric Radiology stated that this method might be challenging and associated with further risks without substantial benefits (7). The European Achondroplasia Forum recommended in 2023 that MRI should be carried out as routine monitoring for FMS at 3 to 6 months of age and repeated in accordance with findings in other routine assessments (14). Our case underlines that early cranial imaging via MRI is crucial, since cervicomedullary compression due to FMS might be seen in patients younger than 3 months of age.
The eAFM score was first developed by Cheung et al (15) to standardize MRI assessment of foramen magnum. Jenko et al (8) later showed that eAFMS has a good observer reliability. In our case, an emergency CT followed by a noncontrast MRI revealed a significant stenosis corresponding to eAFMS 4b. Neurosurgical decompression resolved breathing problems and hyponatremia due to SIADH. Thus, early cranial imaging of very young infants with achondroplasia might be life-saving.
In conclusion, clinicians should be aware of SIADH as a potential presenting sign of FMS in children with achondroplasia. Further discussion is needed regarding improving parental education and timing of screening recommendations including neuroimaging especially in children who did not receive polysomnography.
Learning Points
Foramen magnum and high cervical spinal stenosis are the most severe complications in achondroplasia, often associated with cervicomedullary compression, paresis, paralysis, and sudden infant death.
The incidence of cervicomedullary compression is highest in infancy and decreases with age.
Although current guidelines recommend that all children with achondroplasia should be screened for foramen magnum stenosis, there is no worldwide consensus regarding the timing and the method of screening.
SIADH due to cervicomedullary compression might be a presenting symptom in very young infants with achondroplasia.
Parental education regarding the specific features of cervicomedullary compression and early cranial imaging are important and might be live-saving.
Contributors
All authors made individual contributions to authorship. A.N.C. and J.W.: concept, data interpretation, writing, final draft, revising of the manuscript; S.H., H.R., and O.R.: data acquisition, data interpretation, revising of the manuscript. All authors reviewed and approved the final draft.
Funding
No public or commercial funding.
Disclosures
None declared.
Informed Patient Consent for Publication
Signed informed consent was obtained directly from the patient's relatives or guardians.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.



