-
PDF
- Split View
-
Views
-
Cite
Cite
Bin Guan, Sunita K Agarwal, James M Welch, Smita Jha, Lee S Weinstein, William F Simonds, A Germline ZFX Missense Variant in a Patient With Primary Hyperparathyroidism, JCEM Case Reports, Volume 2, Issue 8, August 2024, luae115, https://doi.org/10.1210/jcemcr/luae115
Close - Share Icon Share
Abstract
A 51-year-old woman with a history of primary hyperparathyroidism (PHPT) with prior parathyroidectomy, osteoporosis, and learning disability was referred for hypercalcemia discovered after a fall. Family history was negative for PHPT, pituitary, enteropancreatic neuroendocrine, or jaw tumors. Dysmorphic facies, multiple cutaneous melanocytic nevi, café au lait macules, long fingers, and scoliosis were observed. Laboratory evaluation showed an elevated parathyroid hormone (PTH) level, hypercalcemia, and hypophosphatemia, all consistent with PHPT. Preoperative imaging revealed a right inferior candidate parathyroid lesion. The patient underwent right inferior parathyroidectomy with normalization of PTH, calcium, and phosphorus. Genetic testing showed a likely pathogenic de novo heterozygous germline missense variant p.R764W in the ZFX gene that encodes a zinc-finger transcription factor previously shown to harbor somatic missense variants in a subset of sporadic parathyroid tumors. Germline variants in ZFX have been reported in patients with an X-linked intellectual disability syndrome with an increased risk for congenital anomalies and PHPT. Further research may determine if genetic testing for ZFX could be of potential benefit for patients with PHPT and developmental anomalies, even in the absence of a family history of parathyroid disease.
Introduction
Primary hyperparathyroidism (PHPT) is a disorder of mineral metabolism resulting from the inappropriate or excessive secretion of parathyroid hormone (PTH) from one or several abnormal parathyroid glands (1). Most cases of PHPT are sporadic and occur randomly with no apparent familial predisposition (∼85%). The majority of the remaining approximately 15% of patients with a heritable predisposition to develop PHPT carry a germline mutation of a gene known to confer susceptibility to parathyroid neoplasia, a set of genes that includes MEN1, GCM2, CDC73, and CASR (2). We present a case of PHPT associated with learning disability, dysmorphic facial features, scoliosis, and multiple dermatologic abnormalities, but lacking a family history of PHPT. Genetic analysis revealed a likely pathogenic de novo heterozygous germline missense variant p.R764W in the ZFX gene that encodes a Krüppel C2H2-type zinc-finger protein on the X chromosome. Somatic missense variants in ZFX had previously been detected in a subset of sporadic parathyroid tumors (3). This case illustrates that genetic testing for ZFX may be indicated in patients with PHPT associated with developmental anomalies, and is consonant with recent reports of germline ZFX variants found in patients with an X-linked syndrome of developmental delay/intellectual disability and dysmorphic facial features, a syndrome that may also include PHPT (4).
Case Presentation
The patient is a 51-year-old woman with a past medical history of PHPT status post parathyroid surgery 14 years earlier, hypothyroidism on levothyroxine replacement, scoliosis since childhood, osteoporosis with multiple fragility fractures, psoriasis, migraines, chronic hematuria without evidence of nephrolithiasis, status post hysterectomy at age 30 for uterine fibroids and menorrhagia, and learning disability since childhood (characterized by memory and word-finding issues, and academic difficulties but not requiring special education). Her parathyroid surgery was performed outside the United States and no operative or surgical pathology reports are available. How many (if any) parathyroid glands were removed during this operation is therefore unknown. Furthermore, no documentation was available demonstrating postoperative normalization of calcium. She was referred to our specialty clinic for further evaluation of hypercalcemia discovered incidentally on presentation with a fall and bone pain 8 months earlier. With the fall, the patient sustained no bone fractures but suffered a small subdural hematoma. Outside laboratory studies at the time of the fall demonstrated hypercalcemia with a total calcium of 12.9 mg/dL (reference interval [RI] 8.6-10.2 mg/dL; 3.23 mmol/L), ionized calcium of 7.3 mg/dL (RI 4.48-5.28 mg/dL; 1.82 mmol/L), elevated PTH of 162 pg/mL (RI 10-65 pg/mL; 162 ng/L), 25-hydroxyvitamin D of 16 ng/mL (RI 33-100 ng/mL; 39.94 nmol/L), albumin of 3.9 gm/dL(RI 3.5-5.2 g/dL; 39 g/L), and 24-hour urinary calcium excretion of 204 mg (RI 50-250 mg/24 h; 5.1 mmol) with a corresponding creatinine excretion of 0.68 g (RI 0.8-1.80 g/24 h; 5.19 mmol). Outside Tc99m-sestamibi parathyroid scan demonstrated a possible right inferior lesion suspicious for parathyroid adenoma. Subsequent ultrasound-guided fine-needle aspiration of the corresponding 0.8 cm, hypoechoic right lower pole lesion, however, found an aspirate PTH value of 123 pg/mL, not significantly different from her serum level. Review of systems revealed a several-month history of unintentional weight loss, cold intolerance, and episodic bony pain in her legs lasting up to days at a time. In addition to the medical history detailed earlier, the patient had 1 pregnancy leading to a stillborn child at 28 weeks with a cleft lip and palate. The patient had 2 older sisters and a younger brother. The patient's oldest sister had osteopenia and their mother had osteoporosis. Family history was negative for PHPT, kidney stones, pituitary, enteropancreatic neuroendocrine, or jaw tumors. The patient was referred for further evaluation of hypercalcemia due to PHPT, and additional parathyroid tumor localization studies. Whether this PHPT was persistent or recurrent was unknown because of the lack of available biochemical documentation following her prior parathyroid surgery.
Diagnostic Assessment
At the time of physical examination, vital signs were unremarkable. Distinctive physical features were observed that included a thin, long, oval-shaped face, thick eyebrows, long philtrum, long tubular nose, pointed chin, long ear length with large earlobes, and full lips (Fig. 1A-1E), as well as a high-arched palate. The patient exhibited multiple cutaneous abnormalities. The lower vermilion lip had numerous brown macules (see Fig. 1A-1C). A cobblestone appearance of the gingiva was noted. The superior portion of the right ear helix had a skin-colored nodule (see Fig. 1D). She had numerous pigmented lesions (see Fig. 1A-1H), including medium to dark brown macules, elevated dark brown papules, and melanocytic nevi. These were distributed diffusely with no areas of sparing and involved the palms and soles. Multiple café au lait macules (Fig. 1I) and intertriginous freckling were also noted. The thyroid was full, symmetric, nontender, and with a well-healed overlying midline surgical scar. The patient had notable scoliosis (see Fig. 1F).
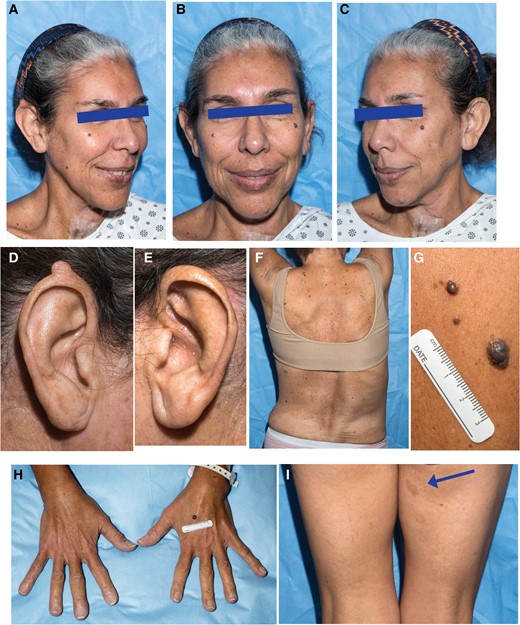
Facial features and other physical findings in a patient with primary hyperparathyroidism and germline missense p.R764W ZFX variant. A to C, Facial features demonstrating long, oval-shaped face, thick eyebrows, long philtrum, long tubular nose, pointed chin, long ears, melanocytic facial nevi, and macules involving the lower vermilion lip. D and E, Close-up of ears showing skin-colored nodule on superior helix of the right ear. F, Posterior view of thorax demonstrating spinal scoliosis. G, Close-up view of multiple raised melanocytic nevi. H, Dorsal view of hands showing elongated fingers. I. Posterior view of thighs, showing café-au-lait macule on right medial thigh (arrow).
Diagnostic studies included blood and urine biochemical analysis, cervical and renal ultrasound, magnetic resonance imaging and computed tomography of the neck, Tc99m-sestamibi parathyroid scan, and bone mineral density testing by dual-energy x-ray absorptiometry. Laboratory testing at the time of referral confirmed the metabolic abnormalities originally noted, consistent with PHPT and vitamin D deficiency (Table 1). Prolactin and fasting gastrin were normal. Renal ultrasound showed bilateral medullary nephrocalcinosis. Dual-energy x-ray absorptiometry scan showed osteoporosis in the anteroposterior spine (L2, L3, L4) with a T-score −3.4, and osteopenia in the femoral neck, total hip, and 1/3 forearm. Magnetic resonance imaging of the neck showed enlargement of the thyroid gland with multiple nodules, with portions of the gland extending into the superior mediastinum, as well as a 1.8 × 0.7-cm suspected enlarged right inferior parathyroid gland. The same candidate lesion was identified on computed tomography and ultrasound of the neck. Tc99m-sestamibi scan was negative.
Patient laboratory values (and corresponding laboratory reference range) from samples obtained at time of referral, immediately prior to, and 14 months following, parathyroid surgery
| Biochemical parameter . | Time of referral . | Immediately preop . | 14 mo postop . | Normal range . |
|---|---|---|---|---|
| Intact PTH | 234.3 pg/mL | 144.3 pg/mL | 53.4 pg/mL | 15 to 65 pg/mL |
| (234.3 ng/L) | (144.3 ng/L) | (53.4 ng/L) | (15 to 65 ng/L) | |
| Calcium, total | 13.0 mg/dL | 12.6 mg/dL | 9.2 mg/dL | 8.6 to 10.2 mg/dL |
| (3.26 mmol/L) | (3.14 mmol/L) | (2.30 mmol/L) | (2.15 to 2.55 mmol/L) | |
| Calcium, ionized | 7.12 mg/dL | 6.68 mg/dL | 4.92 mg/dL | 4.48 to 5.28 mg/dL |
| (1.78 mmol/L) | (1.67 mmol/L) | (1.23 mmol/L) | (1.12 to 1.32 mmol/L) | |
| Phosphorus | 2.3 mg/dL | 1.6 mg/dL | 3.2 mg/dL | 2.5 to 4.5 mg/dL |
| (0.74 mmol/L) | (0.51 mmol/L) | (1.03 mmol/L) | (0.81 to 1.45 mmol/L) | |
| Magnesium | 1.80 mg/dL | 1.80 mg/dL | 1.80 mg/dL | 1.60 to 2.60 mg/dL |
| (0.83 mmol/L) | (0.83 mmol/L) | (0.83 mmol/L) | (0.66 to 1.07 mmol/L) | |
| Albumin | 4.4 g/dL | 4.2 g/dL | 4.0 g/dL | 3.5 to 5.2 g/dL |
| (44 g/L) | (42 g/L) | (40 g/L) | (35 to 52 g/L) | |
| 25-hydroxy vitamin D | 15 ng/mL | 48 ng/mL | 42 ng/mL | 33 to 100 ng/mL |
| (37 nmol/L) | (120 nmol/L) | (105 nmol/L) | (82 to 250 nmol/L) | |
| Creatinine | 0.66 mg/dL | 0.73 mg/dL | 0.88 mg/dL | 0.51 to 0.95 mg/dL |
| (58.33 μmol/L) | (65 μmol/L) | (78 μmol/L) | (45 to 84 μmol/L) | |
| eGFR | 102 mL/min/1.73 m2 | 95 mL/min/1.73 m2 | 75 mL/min/1.73 m2 | > 60 mL/min/1.73 m2 |
| (1.70 mL/sec/m2) | (1.58 mL/sec/m2) | (1.25 mL/sec/m2) | (> 1 mL/sec/m2) | |
| 24-hour urine Calcium excretion | 269 mg/24 hr | 50 to 250 mg/24 hr | ||
| (6.72s mmol/24 hr) | (1.25 to 6.25 mmol/24 hr) | |||
| 24-hour urine Creatinine excretion | 0.5 g/24 hr | 0.8 to 1.80 g/24 hr | ||
| (4 mmol/24 hr) | (7 to 16 mmol/24 hr) | |||
| CCCR | 0.027 | < 0.01 suggests FHH > 0.02 excludes FHH |
| Biochemical parameter . | Time of referral . | Immediately preop . | 14 mo postop . | Normal range . |
|---|---|---|---|---|
| Intact PTH | 234.3 pg/mL | 144.3 pg/mL | 53.4 pg/mL | 15 to 65 pg/mL |
| (234.3 ng/L) | (144.3 ng/L) | (53.4 ng/L) | (15 to 65 ng/L) | |
| Calcium, total | 13.0 mg/dL | 12.6 mg/dL | 9.2 mg/dL | 8.6 to 10.2 mg/dL |
| (3.26 mmol/L) | (3.14 mmol/L) | (2.30 mmol/L) | (2.15 to 2.55 mmol/L) | |
| Calcium, ionized | 7.12 mg/dL | 6.68 mg/dL | 4.92 mg/dL | 4.48 to 5.28 mg/dL |
| (1.78 mmol/L) | (1.67 mmol/L) | (1.23 mmol/L) | (1.12 to 1.32 mmol/L) | |
| Phosphorus | 2.3 mg/dL | 1.6 mg/dL | 3.2 mg/dL | 2.5 to 4.5 mg/dL |
| (0.74 mmol/L) | (0.51 mmol/L) | (1.03 mmol/L) | (0.81 to 1.45 mmol/L) | |
| Magnesium | 1.80 mg/dL | 1.80 mg/dL | 1.80 mg/dL | 1.60 to 2.60 mg/dL |
| (0.83 mmol/L) | (0.83 mmol/L) | (0.83 mmol/L) | (0.66 to 1.07 mmol/L) | |
| Albumin | 4.4 g/dL | 4.2 g/dL | 4.0 g/dL | 3.5 to 5.2 g/dL |
| (44 g/L) | (42 g/L) | (40 g/L) | (35 to 52 g/L) | |
| 25-hydroxy vitamin D | 15 ng/mL | 48 ng/mL | 42 ng/mL | 33 to 100 ng/mL |
| (37 nmol/L) | (120 nmol/L) | (105 nmol/L) | (82 to 250 nmol/L) | |
| Creatinine | 0.66 mg/dL | 0.73 mg/dL | 0.88 mg/dL | 0.51 to 0.95 mg/dL |
| (58.33 μmol/L) | (65 μmol/L) | (78 μmol/L) | (45 to 84 μmol/L) | |
| eGFR | 102 mL/min/1.73 m2 | 95 mL/min/1.73 m2 | 75 mL/min/1.73 m2 | > 60 mL/min/1.73 m2 |
| (1.70 mL/sec/m2) | (1.58 mL/sec/m2) | (1.25 mL/sec/m2) | (> 1 mL/sec/m2) | |
| 24-hour urine Calcium excretion | 269 mg/24 hr | 50 to 250 mg/24 hr | ||
| (6.72s mmol/24 hr) | (1.25 to 6.25 mmol/24 hr) | |||
| 24-hour urine Creatinine excretion | 0.5 g/24 hr | 0.8 to 1.80 g/24 hr | ||
| (4 mmol/24 hr) | (7 to 16 mmol/24 hr) | |||
| CCCR | 0.027 | < 0.01 suggests FHH > 0.02 excludes FHH |
Abnormal values are shown in bold font. Values in parenthesis are International System of Units (SI). Abbreviations: PTH, parathyroid hormone; eGFR, estimated glomerular filtration rate; CCCR, calcium-to-creatinine urinary clearance ratio.
Patient laboratory values (and corresponding laboratory reference range) from samples obtained at time of referral, immediately prior to, and 14 months following, parathyroid surgery
| Biochemical parameter . | Time of referral . | Immediately preop . | 14 mo postop . | Normal range . |
|---|---|---|---|---|
| Intact PTH | 234.3 pg/mL | 144.3 pg/mL | 53.4 pg/mL | 15 to 65 pg/mL |
| (234.3 ng/L) | (144.3 ng/L) | (53.4 ng/L) | (15 to 65 ng/L) | |
| Calcium, total | 13.0 mg/dL | 12.6 mg/dL | 9.2 mg/dL | 8.6 to 10.2 mg/dL |
| (3.26 mmol/L) | (3.14 mmol/L) | (2.30 mmol/L) | (2.15 to 2.55 mmol/L) | |
| Calcium, ionized | 7.12 mg/dL | 6.68 mg/dL | 4.92 mg/dL | 4.48 to 5.28 mg/dL |
| (1.78 mmol/L) | (1.67 mmol/L) | (1.23 mmol/L) | (1.12 to 1.32 mmol/L) | |
| Phosphorus | 2.3 mg/dL | 1.6 mg/dL | 3.2 mg/dL | 2.5 to 4.5 mg/dL |
| (0.74 mmol/L) | (0.51 mmol/L) | (1.03 mmol/L) | (0.81 to 1.45 mmol/L) | |
| Magnesium | 1.80 mg/dL | 1.80 mg/dL | 1.80 mg/dL | 1.60 to 2.60 mg/dL |
| (0.83 mmol/L) | (0.83 mmol/L) | (0.83 mmol/L) | (0.66 to 1.07 mmol/L) | |
| Albumin | 4.4 g/dL | 4.2 g/dL | 4.0 g/dL | 3.5 to 5.2 g/dL |
| (44 g/L) | (42 g/L) | (40 g/L) | (35 to 52 g/L) | |
| 25-hydroxy vitamin D | 15 ng/mL | 48 ng/mL | 42 ng/mL | 33 to 100 ng/mL |
| (37 nmol/L) | (120 nmol/L) | (105 nmol/L) | (82 to 250 nmol/L) | |
| Creatinine | 0.66 mg/dL | 0.73 mg/dL | 0.88 mg/dL | 0.51 to 0.95 mg/dL |
| (58.33 μmol/L) | (65 μmol/L) | (78 μmol/L) | (45 to 84 μmol/L) | |
| eGFR | 102 mL/min/1.73 m2 | 95 mL/min/1.73 m2 | 75 mL/min/1.73 m2 | > 60 mL/min/1.73 m2 |
| (1.70 mL/sec/m2) | (1.58 mL/sec/m2) | (1.25 mL/sec/m2) | (> 1 mL/sec/m2) | |
| 24-hour urine Calcium excretion | 269 mg/24 hr | 50 to 250 mg/24 hr | ||
| (6.72s mmol/24 hr) | (1.25 to 6.25 mmol/24 hr) | |||
| 24-hour urine Creatinine excretion | 0.5 g/24 hr | 0.8 to 1.80 g/24 hr | ||
| (4 mmol/24 hr) | (7 to 16 mmol/24 hr) | |||
| CCCR | 0.027 | < 0.01 suggests FHH > 0.02 excludes FHH |
| Biochemical parameter . | Time of referral . | Immediately preop . | 14 mo postop . | Normal range . |
|---|---|---|---|---|
| Intact PTH | 234.3 pg/mL | 144.3 pg/mL | 53.4 pg/mL | 15 to 65 pg/mL |
| (234.3 ng/L) | (144.3 ng/L) | (53.4 ng/L) | (15 to 65 ng/L) | |
| Calcium, total | 13.0 mg/dL | 12.6 mg/dL | 9.2 mg/dL | 8.6 to 10.2 mg/dL |
| (3.26 mmol/L) | (3.14 mmol/L) | (2.30 mmol/L) | (2.15 to 2.55 mmol/L) | |
| Calcium, ionized | 7.12 mg/dL | 6.68 mg/dL | 4.92 mg/dL | 4.48 to 5.28 mg/dL |
| (1.78 mmol/L) | (1.67 mmol/L) | (1.23 mmol/L) | (1.12 to 1.32 mmol/L) | |
| Phosphorus | 2.3 mg/dL | 1.6 mg/dL | 3.2 mg/dL | 2.5 to 4.5 mg/dL |
| (0.74 mmol/L) | (0.51 mmol/L) | (1.03 mmol/L) | (0.81 to 1.45 mmol/L) | |
| Magnesium | 1.80 mg/dL | 1.80 mg/dL | 1.80 mg/dL | 1.60 to 2.60 mg/dL |
| (0.83 mmol/L) | (0.83 mmol/L) | (0.83 mmol/L) | (0.66 to 1.07 mmol/L) | |
| Albumin | 4.4 g/dL | 4.2 g/dL | 4.0 g/dL | 3.5 to 5.2 g/dL |
| (44 g/L) | (42 g/L) | (40 g/L) | (35 to 52 g/L) | |
| 25-hydroxy vitamin D | 15 ng/mL | 48 ng/mL | 42 ng/mL | 33 to 100 ng/mL |
| (37 nmol/L) | (120 nmol/L) | (105 nmol/L) | (82 to 250 nmol/L) | |
| Creatinine | 0.66 mg/dL | 0.73 mg/dL | 0.88 mg/dL | 0.51 to 0.95 mg/dL |
| (58.33 μmol/L) | (65 μmol/L) | (78 μmol/L) | (45 to 84 μmol/L) | |
| eGFR | 102 mL/min/1.73 m2 | 95 mL/min/1.73 m2 | 75 mL/min/1.73 m2 | > 60 mL/min/1.73 m2 |
| (1.70 mL/sec/m2) | (1.58 mL/sec/m2) | (1.25 mL/sec/m2) | (> 1 mL/sec/m2) | |
| 24-hour urine Calcium excretion | 269 mg/24 hr | 50 to 250 mg/24 hr | ||
| (6.72s mmol/24 hr) | (1.25 to 6.25 mmol/24 hr) | |||
| 24-hour urine Creatinine excretion | 0.5 g/24 hr | 0.8 to 1.80 g/24 hr | ||
| (4 mmol/24 hr) | (7 to 16 mmol/24 hr) | |||
| CCCR | 0.027 | < 0.01 suggests FHH > 0.02 excludes FHH |
Abnormal values are shown in bold font. Values in parenthesis are International System of Units (SI). Abbreviations: PTH, parathyroid hormone; eGFR, estimated glomerular filtration rate; CCCR, calcium-to-creatinine urinary clearance ratio.
Treatment
The patient was treated preoperatively for vitamin D deficiency with high-dose ergocalciferol (50 000 IU weekly) and referred for parathyroidectomy after reconfirmation of the diagnosis. (see Table 1).
The patient underwent unilateral right neck exploration with excision of a right lower parathyroid and right hemithyroidectomy. Intraoperative PTH monitoring showed preincision PTH was 170.2 pg/mL (170.2 ng/L), preexcision level was 154.2 pg/mL (154.2 ng/L); 5 and 10 minutes post excision PTH values were 80 and 70 pg/mL (80 and 70 ng/L), respectively. The procedure was terminated after unilateral exploration since greater than 50% reduction from the highest PTH values was achieved. The excised right lower parathyroid gland measured 1.5 × 1.6 × 0.9 cm. Histologic review both of frozen and permanent parathyroid specimens showed hypercellular parathyroid tissue consistent with a benign parathyroid adenoma. Pathologic analysis of the excised thyroid tissue showed lymphocytic thyroiditis with nodular hyperplasia.
Outcome and Follow-up
The patient did well postoperatively with no new episodes of skeletal pain. Biochemical testing at the time of 2-week, 2-month, and 14-month (see Table 1) follow-up visits demonstrated complete resolution of her PHPT and maintenance of replete vitamin D status.
A portion of the patient's excised parathyroid tumor was analyzed by RNA sequencing, which revealed the presence of transcript encoding a missense variant in the ZFX gene (ZFX: NM_003410.4: exon10: c.2290C > T: p.R764W) not present in a reference parathyroid tumor sample (Fig. 2A). The ZFX p.R764W variant allele frequency was 62% in the tumor RNA (Fig. 2B). No candidate pathogenic variant in other genes associated with PHPT, including MEN1, CDC73, AP2S1, CASR, GNA11, RET, CDKN1B, FLCN, and GCM2, was identified in the patient's germline DNA on mutational analysis (Invitae).
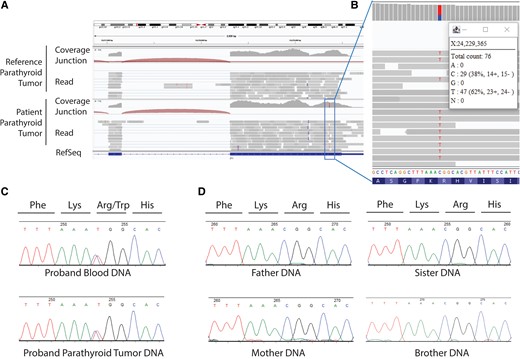
Results of RNA sequencing (RNAseq) analysis of parathyroid tumor and DNA sequencing of patient and family samples. A, The Integrative Genomics Viewer (IGV) view of RNAseq analysis of parathyroid tumor transcripts. Binary alignment map files were viewed in the IGV browser showing parathyroid tumor samples with reference sequence (parathyroid tumor from unrelated patient) or variant sequence (the patient's excised parathyroid tumor) in the ZFX gene. RNAseq analysis employed polyA selection, a total of 22.9 million 150-bp paired-end reads, and 22.0 million reads aligned, with Illumina sequencing by Quick Biology. B, Expanded detail from IGV view of the RNAseq analysis that reveals a missense variant in the ZFX gene (ZFX: NM_003410.4:exon10: c.2290C > T:p.R764W) with a variant allele frequency of 62% in the RNA. C, Sanger sequencing of proband (patient) leukocyte (upper) and parathyroid tumor (lower) DNA samples as chromatographic traces with signal intensity, in relative fluorescence units, and bases assigned as shown. Both tracings show presence of heterozygous missense p.R764W ZFX variant. D, Results of Sanger sequencing of parent and sibling blood DNA showing absence of the p.R764 ZFX variant.
The finding of the ZFX p.R764W variant transcript was of interest since somatic missense mutations in ZFX had been reported previously in a subset of sporadic parathyroid tumors (3). Subsequent DNA sequencing revealed a heterozygous ZFX p.R764W missense variant both in the patient's parathyroid tumor and leukocyte DNA (Fig. 2C), demonstrating that the ZFX variant was likely not somatic, but in the germline. This variant was rare and not represented among approximately 180 000 alleles in the Genome Aggregation Database (gnomAD exome v2.1.1). Additional germline testing was performed on salivary DNA collected from the patient's parents, brother, and a sister. The variant was not identified in the parents and siblings, suggesting the patient's missense variant arose de novo (Fig. 2D). Despite not finding the variant in the parents’ salivary DNA, however, because their serum calcium and PTH values were not determined, we cannot completely exclude germline mosaicism in a parent as an explanation for the proband's germline DNA testing result.
Two years after parathyroid surgery at our center, the patient was incidentally discovered to have a retroperitoneal mass at the L2 level revealed to be a schwannoma by core biopsy.
Discussion
Genetic testing of our patient demonstrated a heterozygous germline missense variant p.R764W in the ZFX gene. ZFX maps to the X chromosome and encodes an 805-residue protein predicted to contain both an N-terminal Zfx/Zfy transactivation domain (5) and a C-terminal region containing an array of 13 individual Krüppel C2H2 (Cys2His2)-type zinc-finger protein domains (6) (Fig. 3A). The ZFX gene product is a ubiquitously expressed transcription factor that is highly evolutionarily conserved among vertebrates. ZFX is essential for survival of hematopoietic and embryonic stem cells (7, 8) and when overexpressed in various human cancers has been associated with a poor prognosis (9-11).
![Predicted protein consequence of germline and somatic ZFX missense variants in the present work and related studies, and regional missense constraint analysis. A, Schematic of protein domain structure of ZFX transcription factor (Uniprot P17010) (not to scale). N-terminal Zfx/Zfy transcriptional activator domain (InterPro IPR006794) encompasses residues 70 to 410, shown in blue (not to scale). Individual C2H2 zinc finger domains (InterPro IPR006794), 13 in total, shown in yellow. ZFX residues 747 to 775 comprise zinc finger 12, and residues 776 to 803 comprise zinc finger 13. Individual residues shown in red font altered by germline and/or somatic ZFX missense variants indicated below by black arrows (3, 4) (see also Table 2). B, GnomAD v2.1.1 regional missense constraint analysis with legend indicating color coding of missense observed/expected (o/e) ratio from 0.0 to 1.0+ (from gnomAD browser [Broad Institute]). Gray (ZFX residues Met1 to Val161), o/e ratio 0.9261, P value = not significant; orange (ZFX residues Val161 to Ser 646), o/e ratio 0.4850, P value = 1.000e-12; dark red (ZFX residues Ser646 to Pro805 includes the C-terminal portion of zinc finger 8 (residues 633-661) through the C-terminus of ZFX), o/e ratio 0.1561, P value = 1.435e-11.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcemcr/2/8/10.1210_jcemcr_luae115/10/m_luae115f3.jpeg?Expires=1750308018&Signature=AKid8HBTRFdUx9dxX-Tk9qDaV5zc6m6QUDPA5ONclVjgk4zf3abApgVmxX96malj9IWSTysp~YGwYFkoeSvnMbnIC-fBq~Ww029sGjSIZ-L3QYPPBVr9P9bnTEzPehztt3ks9ytfICl1Pmo1LXKh7pmO9BJWtOGsQGyJ6IsfXFVJ9wLtOveT7ShM6ZX09NkgUJlxB5-kd8ftjpCXRehq4HQxLfQElKaTR91jWaaEq9mEhG~LfFjsKqzxgkNdTjKi2J~GLQgp8LCKq~WE10SpfMlz9MAIWDoN-r9f8nIFeB-UJbMuGiMRH96iqn29dz-uBFUhNZ9irq06p9r-iyZY8w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Predicted protein consequence of germline and somatic ZFX missense variants in the present work and related studies, and regional missense constraint analysis. A, Schematic of protein domain structure of ZFX transcription factor (Uniprot P17010) (not to scale). N-terminal Zfx/Zfy transcriptional activator domain (InterPro IPR006794) encompasses residues 70 to 410, shown in blue (not to scale). Individual C2H2 zinc finger domains (InterPro IPR006794), 13 in total, shown in yellow. ZFX residues 747 to 775 comprise zinc finger 12, and residues 776 to 803 comprise zinc finger 13. Individual residues shown in red font altered by germline and/or somatic ZFX missense variants indicated below by black arrows (3, 4) (see also Table 2). B, GnomAD v2.1.1 regional missense constraint analysis with legend indicating color coding of missense observed/expected (o/e) ratio from 0.0 to 1.0+ (from gnomAD browser [Broad Institute]). Gray (ZFX residues Met1 to Val161), o/e ratio 0.9261, P value = not significant; orange (ZFX residues Val161 to Ser 646), o/e ratio 0.4850, P value = 1.000e-12; dark red (ZFX residues Ser646 to Pro805 includes the C-terminal portion of zinc finger 8 (residues 633-661) through the C-terminus of ZFX), o/e ratio 0.1561, P value = 1.435e-11.
ZFX has been previously linked to parathyroid disease in 2 other contexts. Somatic missense variants in ZFX involving 2 adjacent arginine residues (R786/R787) present in the most C-terminal (13th) zinc-finger domain were detected in a subset of sporadic parathyroid tumors by Soong and Arnold (3) (Fig. 3A) (Table 2). More recently, germline ZFX variants were identified in patients with an X-linked syndrome of developmental delay/intellectual disability and dysmorphic facial features (4). Included among these patients was a subset with missense ZFX variants who also manifest PHPT (4). The subset of these patients with PHPT all had missense variants located within or between zinc-finger domains 12 or 13 (4). Such mutations in ZFX are likely a rare cause of PHPT, since we screened the leukocyte DNA of 327 patients seen at our center with seemingly sporadic PHPT by Sanger sequencing that covered zinc-finger domains 12 and 13 of ZFX, as well as the DNA from 37 parathyroid tumors, and found no such pathogenic variants (data not shown).
| ZFX variant . | Variant type . | Sex at birth . | PHPT (Y, N, ND) . | Age of onset of PHPT, y . | Age at parathyroid surgery, y . | S or MG parathyroid disease . | Syndromic features present (Y, N, ND) . | Family history of PHPT (Y, N, ND) . | Recurrent or persistent PHPT (Y, N, ND) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|
| R764W | Germline | F | Y | 37 | 37, 51 | S | Y | N | Y | This report |
| R764W | Germline | M | N | NA | NA | NA | Y | ND | NA | Patient 1 (4) |
| R764W | Germline | M | ND | NA | NA | NA | Y | ND | ND | Patient 2 (4) |
| T771M | Germline | M | ND | NA | NA | NA | Y | ND | ND | Patient 3 (4) |
| T771M | Germline | F | Y | 19 | NA | NA | Y | ND | ND | Patient 4 (4) |
| T771M | Germline | M | ND | NA | NA | NA | Y | ND | ND | Patient 5 (4) |
| Y774C | Germline | M | N | NA | NA | NA | Y | Y | NA | Patient 6A (4) |
| Y774C | Germline | M | N | NA | NA | NA | Y | Y | NA | Patient 6B (4) |
| Y774C | Germline | F | N | NA | NA | NA | Y | Y | NA | Patient 6C (4) |
| Y774C | Germline | M | N | NA | NA | NA | Y | ND | NA | Patient 7 (4) |
| R786Q | Germline | M | Y | ND | 13 | ND | Y | Y | ND | Patient 8 (4) |
| R786Q | Germline | F | Y | 12 | 15 | S | Y | ND | N | Patient 9 (4) |
| R786Q | Somatic | (3) | ||||||||
| R786L | Somatic | (3) | ||||||||
| R787T | Somatic | (3) | ||||||||
| ZFX variant . | Variant type . | Sex at birth . | PHPT (Y, N, ND) . | Age of onset of PHPT, y . | Age at parathyroid surgery, y . | S or MG parathyroid disease . | Syndromic features present (Y, N, ND) . | Family history of PHPT (Y, N, ND) . | Recurrent or persistent PHPT (Y, N, ND) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|
| R764W | Germline | F | Y | 37 | 37, 51 | S | Y | N | Y | This report |
| R764W | Germline | M | N | NA | NA | NA | Y | ND | NA | Patient 1 (4) |
| R764W | Germline | M | ND | NA | NA | NA | Y | ND | ND | Patient 2 (4) |
| T771M | Germline | M | ND | NA | NA | NA | Y | ND | ND | Patient 3 (4) |
| T771M | Germline | F | Y | 19 | NA | NA | Y | ND | ND | Patient 4 (4) |
| T771M | Germline | M | ND | NA | NA | NA | Y | ND | ND | Patient 5 (4) |
| Y774C | Germline | M | N | NA | NA | NA | Y | Y | NA | Patient 6A (4) |
| Y774C | Germline | M | N | NA | NA | NA | Y | Y | NA | Patient 6B (4) |
| Y774C | Germline | F | N | NA | NA | NA | Y | Y | NA | Patient 6C (4) |
| Y774C | Germline | M | N | NA | NA | NA | Y | ND | NA | Patient 7 (4) |
| R786Q | Germline | M | Y | ND | 13 | ND | Y | Y | ND | Patient 8 (4) |
| R786Q | Germline | F | Y | 12 | 15 | S | Y | ND | N | Patient 9 (4) |
| R786Q | Somatic | (3) | ||||||||
| R786L | Somatic | (3) | ||||||||
| R787T | Somatic | (3) | ||||||||
Abbreviations: F, female; M, male; MG, multigland; N, no; ND, not determined or not reported; NA, not applicable; PHPT, primary hyperparathyroidism; S, single; Y, yes.
| ZFX variant . | Variant type . | Sex at birth . | PHPT (Y, N, ND) . | Age of onset of PHPT, y . | Age at parathyroid surgery, y . | S or MG parathyroid disease . | Syndromic features present (Y, N, ND) . | Family history of PHPT (Y, N, ND) . | Recurrent or persistent PHPT (Y, N, ND) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|
| R764W | Germline | F | Y | 37 | 37, 51 | S | Y | N | Y | This report |
| R764W | Germline | M | N | NA | NA | NA | Y | ND | NA | Patient 1 (4) |
| R764W | Germline | M | ND | NA | NA | NA | Y | ND | ND | Patient 2 (4) |
| T771M | Germline | M | ND | NA | NA | NA | Y | ND | ND | Patient 3 (4) |
| T771M | Germline | F | Y | 19 | NA | NA | Y | ND | ND | Patient 4 (4) |
| T771M | Germline | M | ND | NA | NA | NA | Y | ND | ND | Patient 5 (4) |
| Y774C | Germline | M | N | NA | NA | NA | Y | Y | NA | Patient 6A (4) |
| Y774C | Germline | M | N | NA | NA | NA | Y | Y | NA | Patient 6B (4) |
| Y774C | Germline | F | N | NA | NA | NA | Y | Y | NA | Patient 6C (4) |
| Y774C | Germline | M | N | NA | NA | NA | Y | ND | NA | Patient 7 (4) |
| R786Q | Germline | M | Y | ND | 13 | ND | Y | Y | ND | Patient 8 (4) |
| R786Q | Germline | F | Y | 12 | 15 | S | Y | ND | N | Patient 9 (4) |
| R786Q | Somatic | (3) | ||||||||
| R786L | Somatic | (3) | ||||||||
| R787T | Somatic | (3) | ||||||||
| ZFX variant . | Variant type . | Sex at birth . | PHPT (Y, N, ND) . | Age of onset of PHPT, y . | Age at parathyroid surgery, y . | S or MG parathyroid disease . | Syndromic features present (Y, N, ND) . | Family history of PHPT (Y, N, ND) . | Recurrent or persistent PHPT (Y, N, ND) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|
| R764W | Germline | F | Y | 37 | 37, 51 | S | Y | N | Y | This report |
| R764W | Germline | M | N | NA | NA | NA | Y | ND | NA | Patient 1 (4) |
| R764W | Germline | M | ND | NA | NA | NA | Y | ND | ND | Patient 2 (4) |
| T771M | Germline | M | ND | NA | NA | NA | Y | ND | ND | Patient 3 (4) |
| T771M | Germline | F | Y | 19 | NA | NA | Y | ND | ND | Patient 4 (4) |
| T771M | Germline | M | ND | NA | NA | NA | Y | ND | ND | Patient 5 (4) |
| Y774C | Germline | M | N | NA | NA | NA | Y | Y | NA | Patient 6A (4) |
| Y774C | Germline | M | N | NA | NA | NA | Y | Y | NA | Patient 6B (4) |
| Y774C | Germline | F | N | NA | NA | NA | Y | Y | NA | Patient 6C (4) |
| Y774C | Germline | M | N | NA | NA | NA | Y | ND | NA | Patient 7 (4) |
| R786Q | Germline | M | Y | ND | 13 | ND | Y | Y | ND | Patient 8 (4) |
| R786Q | Germline | F | Y | 12 | 15 | S | Y | ND | N | Patient 9 (4) |
| R786Q | Somatic | (3) | ||||||||
| R786L | Somatic | (3) | ||||||||
| R787T | Somatic | (3) | ||||||||
Abbreviations: F, female; M, male; MG, multigland; N, no; ND, not determined or not reported; NA, not applicable; PHPT, primary hyperparathyroidism; S, single; Y, yes.
Like the patient presented here, about half of the syndromic patients characterized by Shepherdson et al demonstrated a de novo inheritance pattern (4). Including the present work, 6 distinct germline or somatic ZFX missense variants, involving 5 residues present in the twelfth or thirteenth ZFX zinc finger domains, have been implicated in PHPT (see Table 2). In agreement with previous studies (3), our RNA sequencing data showed that the ZFX gene escaped X inactivation with the variant allele expressed in 62% of patient tumor transcripts, supporting the oncogenic roles of ZFX pathogenic missense variants in parathyroid tumorigenesis (3) (see Fig. 2B). Substantially skewed X inactivation was observed in leukocyte DNA samples among female ZFX variant carriers (4). According to the 2015 American College of Medical Genetics and Genomics and the Association for Molecular Pathology variant interpretation criteria, the variant c.2290C > T (p.R764W) can be considered pathogenic for the dominant ZFX-related intellectual disability syndrome (12).
The exact pathophysiologic mechanism by which missense variants in the penultimate and ultimate ZFX zinc-finger domains may result in parathyroid neoplasia remains unclear. Interestingly, the C-terminal region of ZFX encompassing residues 646 to 805, a region that includes zinc-finger domains 12 and 13, is highly intolerant of missense variation, suggesting that this region is functionally important for human fitness (Fig. 3B). The region of the ZFX protein containing zinc-finger domains 11 to 13 was previously shown to be necessary and sufficient for DNA binding (13). Shepherdson and colleagues showed that PHPT-associated ZFX missense variants mapping to the last 2 zinc-finger domains may result in either gain or loss of transcriptional function at particular gene targets (4).
Beyond the dysmorphic facies, intellectual delay, scoliosis, and disorder of mineral metabolism, similarities in cutaneous findings between this patient and the patient cohort with PHPT described by Shepherdson et al (4) reinforce the syndromic aspects of this disorder. These include melanocytic nevi (often multiple) and café au lait macules seen both in our patient (Fig. 1) and the patients evaluated in Shepherdson et al (4) (Fig. 4).
This case lends support to the argument for further study to determine if genetic testing for ZFX may be of potential benefit for patients with PHPT associated with developmental anomalies, even in the absence of a family history. This case report is in line with the recent identification of germline ZFX variants in patients with an X-linked syndrome of developmental delay/intellectual disability and dysmorphic facial features that may include PHPT (4). This case furthermore augments and reinforces our understanding of the clinical features that may be associated with the syndrome associated with germline missense variants involving the C-terminal zinc-finger domains 12 and 13 of ZFX and PHPT.
Learning Points
Germline missense variants in ZFX are a rare cause of syndromic PHPT.
Features often include developmental delay/intellectual disability, dysmorphic facies, and cutaneous abnormalities, including melanocytic nevi and café au lait macules.
Germline ZFX variants associated with PHPT may result from X-linked inheritance or arise de novo and, with reference to Shepherdson et al (4), can manifest both in males and females.
Skewed inactivation of the mutant ZFX allele was not observed in the parathyroid tumor, suggesting that tumor predisposition may result from a gain-of-function effect of missense variants involving ZFX C-terminal zinc finger domains 12 and 13 in parathyroid cells.
Further study may determine if germline genetic screening for ZFX variants should be recommended in patients who present with PHPT younger than age 40 with one or several of the characteristic syndromic features, even in the absence of a positive family history.
Acknowledgments
We are grateful to the fellows of the NIH Inter-Institute Endocrinology Training Program; Mr Craig Cochran, Ms Lynn Bliss, and the 5NW patient care staff; the specialty consultants of the NIH Clinical Center; and the NIDDK Clinical Core.
Contributors
All authors made individual contributions to authorship. J.M.W, L.S.W., and W.F.S. were involved in the diagnosis and management of the patient; B.G. was involved in genetic analysis; and S.K.A. and S.J. were involved in data interpretation. All authors were involved in manuscript writing. All authors reviewed and approved the final draft.
Funding
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (grant Nos. 1ZIADK075035, 1ZIADK043006, and 1ZIADK043012).
Disclosures
None declared.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.
References
Abbreviations
- CCCR
calcium-to-creatinine urinary clearance ratio
- eGFR
estimated glomerular filtration rate
- FHH
familial hypocalciuric hypercalcemia
- PHPT
primary hyperparathyroidism
- PTH
parathyroid hormone
- RI
reference interval




