-
PDF
- Split View
-
Views
-
Cite
Cite
Elissa Foster, Naim M Maalouf, Use of Cinacalcet for the Management of Primary Hyperparathyroidism in Pregnancy, JCEM Case Reports, Volume 2, Issue 7, July 2024, luae117, https://doi.org/10.1210/jcemcr/luae117
Close - Share Icon Share
Abstract
Primary hyperparathyroidism in pregnancy is uncommon. Consequently, there are no randomized controlled studies that address treatment of primary hyperparathyroidism in pregnancy, and the efficacy and safety of medical management with cinacalcet in this setting is unknown. We report a case of a 28-year-old woman with primary hyperparathyroidism and hypercalcemia that worsened during her third trimester of pregnancy. Cinacalcet led to achievement of normocalcemia, allowing the delay of parathyroidectomy until after delivery of the baby. We also review the published literature on cinacalcet use in the management of primary hyperparathyroidism during pregnancy. Cinacalcet is typically reserved for pregnant patients with severe and symptomatic hypercalcemia, primarily serving as a last resort to delay parathyroidectomy until either the second trimester or the postpartum period.
Introduction
The prevalence of primary hyperparathyroidism (PHPT) in pregnancy is low at around .03% to .05%, with a higher risk in women over the age of 45 years (1, 2). Parathyroid adenomas are the predominant pathophysiological cause of PHPT, like nonpregnancy-related cases (1). Physiologic changes of pregnancy including increased intestinal calcium absorption and enhanced bone resorption can worsen hypercalcemia in the setting of PHPT (1, 3). On the other hand, rapid fetal skeletal uptake of calcium across the placenta during the third trimester can be protective against hypercalcemia in the pregnant mother. At the same time, maternal hypercalcemia results in more calcium moving across the placenta and subsequent suppression of fetal parathyroid gland development and PTH secretion. Other fetal complications that may occur include tetany, miscarriage, and stillbirth (1, 3). For the mother, hypercalcemia in pregnancy has been associated with fatigue, hyperemesis, nephrolithiasis, pancreatitis, weight loss, preeclampsia, and seizures (1).
There are no systematic studies or guidelines regarding the management of PHPT in pregnancy. Historically, the preference is to perform parathyroidectomy during the second trimester (1). However, patients who present with severe or symptomatic hypercalcemia during the third trimester pose a therapeutic challenge. In 2012, the calcium-sensing receptor agonist cinacalcet was approved for medical management of PHPT for those not considered surgical candidates. While this offers a potentially new treatment modality in pregnant women who present with PHPT in the third trimester, there are no controlled studies on cinacalcet use in pregnancy. Its use in pregnancy is therefore only warranted “if the potential benefits outweigh the risks” (pregnancy category C) (4). We hereby present a unique case of cinacalcet use in a patient with symptomatic hypercalcemia due to PHPT during the third trimester of pregnancy and a review of the relevant published literature.
Case Presentation
In July 2020, a 28-year-old Jordanian woman was referred to our service for management of hypercalcemia during pregnancy. Upon reviewing records, it was determined that she had mild hypercalcemia dating back to 2013, with a calcium of 10.9 mg/dL (2.71 mmol/L) (normal reference range 8.6-10.2 mg/dL; 2.15-2.55 mmol/L) in 2015. She became pregnant in December 2019. Early in the patient's pregnancy, her hypercalcemia was mild and was managed with high fluid intake orally.
However, her hypercalcemia worsened in July 2020, at the beginning of her third trimester, which prompted the referral to our service. When seen in our clinic, her chief complaint was fatigue, but she also noted polydipsia and polyuria. She denied nausea, vomiting, excessive dairy intake, or use of calcium supplements. There was no history of nephrolithiasis, fracture, or prior neck radiation. Her family history was negative for calcium or parathyroid disorders. Her medications included vitamin D 5000 IU daily and a prenatal vitamin.
Diagnostic Assessment
During this initial evaluation performed at 31 weeks of gestation, serum calcium and PTH were found to be 12.2 mg/dL (3.04 mmol/L) and 126 pg/mL (13.4 pmol/L) (normal reference range 14-64 pg/mL; 1.5-6.8 pmol/L), respectively. Details of pertinent lab findings are shown in Table 1. Twenty-four-hour urinary studies were ordered, but the patient never completed the tests. However, an ultrasound of the neck was performed and showed an elongated hypoechoic nodule within the left parathyroid region appearing separate from the left thyroid lobe measuring 1.8 × .6 × 1.0 cm (Fig. 1). Thus, her hypercalcemia was likely caused by PHPT. It was thought that familial hypocalciuric hypercalcemia was less likely given the acquired and progressive hypercalcemia, and her neck imaging showed a single parathyroid adenoma. Furthermore, and despite her young age at presentation with PHPT, her personal and family history did not suggest an inherited syndrome.
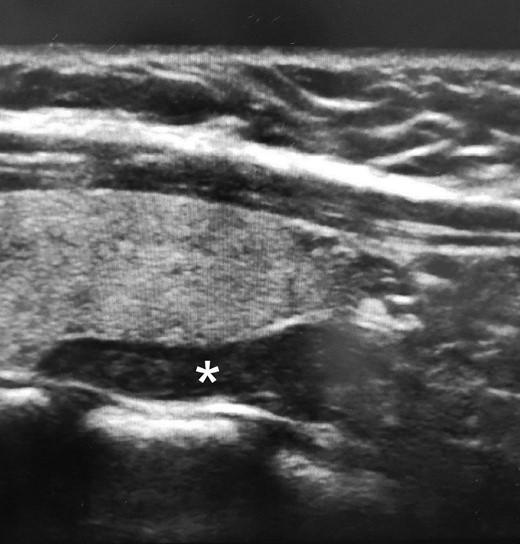
Neck ultrasound images showing an elongated hypoechoic nodule (*) measuring 1.8 × .6 × 1.0 cm posterior to and separate from the left thyroid lobe.
| Serum study . | Patient's value . | Reference range . |
|---|---|---|
| BUN | 4 mg/dL (1.43 mmol/L) | 7-25 mg/dL (2.50-8.92 mmol/L) |
| Creatinine | .33 mg/dL (29 μmol/L) | .5-1.1 mg/dL (44-97 μmol/L) |
| Calcium | 12.2 mg/dL (3.04 mmol/L) | 8.6-10.2 mg/dL (2.15-2.55 mmol/L) |
| Albumin | 3.5 g/dL (35 g/L) | 3.6-5.1 mg/dL (36-51 g/L) |
| Phosphorous | 2.3 mg/dL (.74 mmol/L) | 2.5-4.5 mg/dL (.81-1.45 mmol/L) |
| Alkaline phosphatase | 86 U/L (1.43 µkat/L) | 31-125 U/L (.52-2.09 µkat/L) |
| PTH intact | 127 pg/mL (13.5 pmol/L) | 14-64 pg/mL (1.5-6.8 pmol/L) |
| 25-OH-vitamin D | 20 ng/mL (49 nmol/L) | Deficiency if <20 ng/mL (< 49 nmol/L) |
| Serum study . | Patient's value . | Reference range . |
|---|---|---|
| BUN | 4 mg/dL (1.43 mmol/L) | 7-25 mg/dL (2.50-8.92 mmol/L) |
| Creatinine | .33 mg/dL (29 μmol/L) | .5-1.1 mg/dL (44-97 μmol/L) |
| Calcium | 12.2 mg/dL (3.04 mmol/L) | 8.6-10.2 mg/dL (2.15-2.55 mmol/L) |
| Albumin | 3.5 g/dL (35 g/L) | 3.6-5.1 mg/dL (36-51 g/L) |
| Phosphorous | 2.3 mg/dL (.74 mmol/L) | 2.5-4.5 mg/dL (.81-1.45 mmol/L) |
| Alkaline phosphatase | 86 U/L (1.43 µkat/L) | 31-125 U/L (.52-2.09 µkat/L) |
| PTH intact | 127 pg/mL (13.5 pmol/L) | 14-64 pg/mL (1.5-6.8 pmol/L) |
| 25-OH-vitamin D | 20 ng/mL (49 nmol/L) | Deficiency if <20 ng/mL (< 49 nmol/L) |
Abbreviations: BUN, blood urea nitrogen.
| Serum study . | Patient's value . | Reference range . |
|---|---|---|
| BUN | 4 mg/dL (1.43 mmol/L) | 7-25 mg/dL (2.50-8.92 mmol/L) |
| Creatinine | .33 mg/dL (29 μmol/L) | .5-1.1 mg/dL (44-97 μmol/L) |
| Calcium | 12.2 mg/dL (3.04 mmol/L) | 8.6-10.2 mg/dL (2.15-2.55 mmol/L) |
| Albumin | 3.5 g/dL (35 g/L) | 3.6-5.1 mg/dL (36-51 g/L) |
| Phosphorous | 2.3 mg/dL (.74 mmol/L) | 2.5-4.5 mg/dL (.81-1.45 mmol/L) |
| Alkaline phosphatase | 86 U/L (1.43 µkat/L) | 31-125 U/L (.52-2.09 µkat/L) |
| PTH intact | 127 pg/mL (13.5 pmol/L) | 14-64 pg/mL (1.5-6.8 pmol/L) |
| 25-OH-vitamin D | 20 ng/mL (49 nmol/L) | Deficiency if <20 ng/mL (< 49 nmol/L) |
| Serum study . | Patient's value . | Reference range . |
|---|---|---|
| BUN | 4 mg/dL (1.43 mmol/L) | 7-25 mg/dL (2.50-8.92 mmol/L) |
| Creatinine | .33 mg/dL (29 μmol/L) | .5-1.1 mg/dL (44-97 μmol/L) |
| Calcium | 12.2 mg/dL (3.04 mmol/L) | 8.6-10.2 mg/dL (2.15-2.55 mmol/L) |
| Albumin | 3.5 g/dL (35 g/L) | 3.6-5.1 mg/dL (36-51 g/L) |
| Phosphorous | 2.3 mg/dL (.74 mmol/L) | 2.5-4.5 mg/dL (.81-1.45 mmol/L) |
| Alkaline phosphatase | 86 U/L (1.43 µkat/L) | 31-125 U/L (.52-2.09 µkat/L) |
| PTH intact | 127 pg/mL (13.5 pmol/L) | 14-64 pg/mL (1.5-6.8 pmol/L) |
| 25-OH-vitamin D | 20 ng/mL (49 nmol/L) | Deficiency if <20 ng/mL (< 49 nmol/L) |
Abbreviations: BUN, blood urea nitrogen.
Treatment
In view of the significant hypercalcemia, a multidisciplinary conference between endocrinology, endocrine surgery, obstetrics, and maternal-fetal medicine was held, and it was felt that parathyroidectomy was not an appropriate treatment option due to concerns that anesthesia may induce premature labor. Due to the severity of hypercalcemia and its rate of progression, it was agreed to try cinacalcet to control serum calcium to attempt to delay the need for urgent parathyroidectomy. Concomitantly, betamethasone was started to accelerate fetal lung maturation with a goal to reach 37 weeks gestation, if possible.
Outcome and Follow-up
Cinacalcet 30 mg per day was initiated at 32 weeks and 2 days gestation. Weekly biochemical tests were performed thereafter and showed a prompt normalization of serum calcium within 1 week of therapy initiation. PTH levels, however, decreased at a slower rate (Fig. 2). Other chemistries such as liver enzymes remained normal. The patient remained clinically stable, and her fatigue improved. The patient underwent a scheduled cesarean section at 37 weeks gestation. The infant weighed 8 pounds and 9 ounces (3.88 kg) at birth. His Apgar scores at 1 and 5 minutes following delivery were 5 and 9, respectively. There was no significant hypocalcemia in the infant, and he did not require neonatal intensive care unit admission. Additional records on the child after discharge home were not available to us.
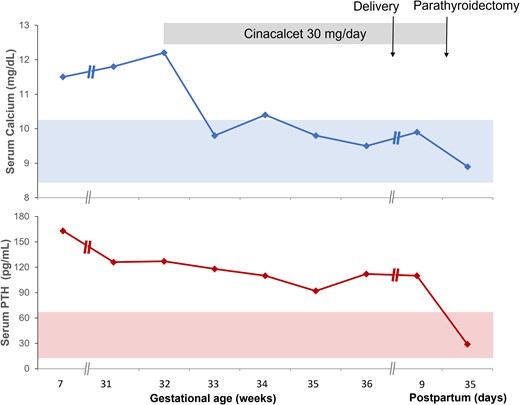
Temporal changes in serum calcium (top figure) and serum PTH (bottom figure) at various stages of the pregnancy and postpartum period. Note that her initial evaluation with endocrinology occurred at about 7 weeks gestation, although the pregnancy was unknown to the patient at that time. She was lost to follow-up until 31 weeks gestation when she was referred to endocrinology again.
Our patient continued cinacalcet up until she underwent a directed parathyroidectomy at about 3 weeks postpartum. Between delivery and parathyroidectomy, the patient desired to breastfeed and therefore would discard milk given that the safety of cinacalcet use in breastfeeding is unknown. At parathyroidectomy, she was found to have a normal-appearing left superior parathyroid gland and an enlarged left inferior parathyroid gland. This 3 cm and 1147 mg left inferior parathyroid gland was resected. Intraoperatively, her baseline PTH was 366.9 pg/mL (38.9 pmol/L) and the 5-, 10-, and 20-minute PTH levels were 33.7, 23.7, and 18.7 pg/mL (3.6, 2.5, 2.0 pmol/L), respectively. Histology was consistent with parathyroid adenoma. At about 2 weeks postoperatively, her serum calcium and PTH were 8.9 mg/dL (2.22 mmol/L) and 29 pg/mL (3.1 pmol/L), respectively.
Discussion
We hereby present a case of a pregnant woman with PHPT who was treated with cinacalcet during the third trimester, resulting in normalization of serum calcium, providing an opportunity to induce fetal lung maturation and delay parathyroidectomy until after delivery. Our case illustrates key management issues of PHPT in pregnancy, and we review the published literature on cinacalcet use in this setting.
During an average pregnancy, the mother delivers a cumulative of 30 g calcium, 20 g phosphorus, and .8 g magnesium on average to the baby carried to term (1). Approximately 80% of this transfer occurs during the third trimester of pregnancy. For an average-sized baby, the rate of calcium transfer from mother to baby can be upwards of 300 to 350 mg/day between the 35th and 40th week of gestation (1). To accommodate for this increasing fetal calcium demand, the pregnant mother's body responds primarily by increasing intestinal absorption of calcium by 2-fold. This is achieved partly by an increase in calcitriol levels but also through increased bone resorption during normal pregnancy (1). Counterintuitively, there is increased renal excretion of calcium during pregnancy, but this is explained by increased glomerular filtration in normal pregnancy. As calcitriol synthesis increases during pregnancy, PTH levels are suppressed in the early stage of normal pregnancy but later start to increase to low-normal levels. PTHrP and calcitonin increases, from production by the placenta and breast tissue. Elevated calcitonin plays a protective role in maternal skeleton health during pregnancy (1). Given the increased calcium absorption to meet the demands of the fetus, the placental transfer of calcium to the fetus protects against development of hypercalcemia in the pregnant mother. At the time of delivery, the protective factor is eliminated, putting the mother at risk for hypercalcemia.
PHPT is the most common cause of hypercalcemia in the general population (2, 5). Its prevalence in pregnancy is estimated to be .03% to .05% based on retrospective studies (1, 2). Symptoms of mild hypercalcemia can be nonspecific and often coincide with common symptoms of pregnancy including nausea/vomiting, fatigue, generalized weakness, and constipation. In more severe cases, it may present with nephrocalcinosis, nephrolithiasis, or pancreatitis (1, 5). Other complications include hypertension and preeclampsia/eclampsia (2). Neonatal complications in association with PHPT have been reported in several case studies, including neonatal hypoparathyroidism, hypocalcemia, tetany, and polyhydramnios (2).
The diagnosis is made by the findings of hypercalcemia and inappropriate increased PTH level. Given the dilutional effect seen in physiologic pregnancy, serum calcium may appear lower and mask the diagnosis of PHPT. Thus, it is important to measure ionized calcium or albumin-corrected calcium if the suspicion of PHPT is high (5). Neck imaging with computed tomography and Technetium 99 m sestamibi is avoided in pregnancy, and thus imaging of the parathyroid glands is usually obtained with ultrasound.
There are no randomized studies addressing the optimal treatment of PHPT in pregnancy. There is a consensus for managing PHPT in the second trimester with surgery. Therefore, medical management in the first or third trimester is generally required in symptomatic and/or severe hypercalcemia until parathyroidectomy is performed, either in the second trimester or postpartum.
Cinacalcet is the only Food and Drug Administration-approved medication for the treatment of PHPT, although there are no human studies done to evaluate its safety in pregnant or breastfeeding mothers. In fact, the Food and Drug Administration has classified cinacalcet as category C in pregnancy, meaning that animal studies have shown an adverse effect on the fetus but there are no adequate studies done in humans, although the benefits of its use may outweigh the potential risks (4). Cinacalcet has been shown to cross the placenta in rabbit studies, but it is unknown if it does so in humans (4).
We reviewed nine cases dating back to 2009 in which PHPT in pregnancy was managed medically with cinacalcet (Table 2). One case described a patient with parathyroid carcinoma diagnosed prior to pregnancy (6). In several cases, cinacalcet was not well tolerated due to nausea (a known side effect of cinacalcet) (6, 8, 9, 12). In other cases, cinacalcet lowered but did not normalize serum calcium, and parathyroidectomy was performed at gestational week 14 (8), 32 (10), 34 (13), or 37 (14), or postpartum (7, 9, 11, 12). In contrast, our patient had normalization in serum calcium with a lower cinacalcet dose, 30 mg/day, without side effects. Table 2 further summarizes key features of these case reports and our case.
| . | Our case . | Nadarasa (6)a . | Garcia (7) . | Horton (8) . | Vera (9) . | Latif (10) . | Eremkina (11) . | Ning (12) . | Horjus (13) . | Bashir (14) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Parathyroid pathology | Adenoma | Carcinoma | Adenoma | Ectopic Adenoma | Adenoma | Adenoma | Adenoma | Adenoma | Unknown | Unknown |
| Serum calcium prior to cinacalcet | 12.2 mg/dL (3.04 mmol/L) | a | 14.7 mg/dL (3.67 mmol/L) | 13.5 mg/dL (3.37 mmol/L) | 12.1 mg/dL (3.02 mmol/L) | >15 mg/dL (>3.74 mmol/L) | 11.4 mg/dL (2.86 mmol/L) | 12.3 mg/dL (3.07 mmol/L) | 14.3 mg/dL (3.57 mmol/L) | 12.7 mg/dL (3.17 mmol/L) |
| Serum PTH prior to cinacalcet | 126 pg/mL (13.4 pmol/L) | a | 288 pg/mL (30.5 pmol/L) | 254 pg/mL (27.0 pmol/L) | 109 pg/mL (11.6 pmol/L) | 350 pg/mL (37.1 pmol/L) | 169 pg/mL (17.9 pmol/L) | 135 pg/mL (14.3 pmol/L) | 152 pg/mL (16.1 pmol/L) | 168 pg/mL (17.8 pmol/L) |
| GA at Cinacalcet initiation (weeks) | 32 | a | 30 | 10 | 24 | 30 | 34 | 32 | 32 | 24 |
| Cinacalcet dose (mg/day) | 30 | 60-120 | 60 | 60 | 15-30 | 15 | 30 | 30-90 | 60-240 | 15-30 |
| sCalcium on Cinacalcet | 9.8 mg/dL (2.45 mmol/L) | a | No significant change | 10.7 mg/dL (2.67 mmol/L) | No significant change | No significant change | 10.6 mg/dL (2.64 mmol/L) | No significant change | 12.0 mg/dL (3.0 mmol/L) | 10.3 mg/dL (2.57 mmol/L) |
| Other therapies | IVF | a | IVF, calcitonin, furosemide, bisphosphonate | IVF | IVF | IVF, calcitonin | None | IVF, furosemide | IVF, calcitonin | IVF, calcitonin |
| GA at time of delivery (weeks) | 37 | Not reported | 33 | 37 | 32 | 38 | 37 | 34 | 34 | 37 |
| Obstetric complications | Postpartum hemorrhage | None reported | IUGR, preeclampsia | None reported | Nephrolithiasis premature membrane rupture | Pancreatitis | None reported | Pre-eclampsia | None reported | None reported |
| Neonatal effect | None reported | Hypocalcemia with second child | Late-onset hypocalcemia | None reported | Hypocalcemia with tetany | None reported | None reported | Hypocalcemia with respiratory distress | Transient hypocalcemia (mild) | None reported |
| Time of parathyroidectomy | Postpartum | a | Postpartum | 14 weeks gestation | Postpartum | 32 weeks gestation | Postpartum | Postpartum | 34 weeks gestation | Not discussed |
| . | Our case . | Nadarasa (6)a . | Garcia (7) . | Horton (8) . | Vera (9) . | Latif (10) . | Eremkina (11) . | Ning (12) . | Horjus (13) . | Bashir (14) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Parathyroid pathology | Adenoma | Carcinoma | Adenoma | Ectopic Adenoma | Adenoma | Adenoma | Adenoma | Adenoma | Unknown | Unknown |
| Serum calcium prior to cinacalcet | 12.2 mg/dL (3.04 mmol/L) | a | 14.7 mg/dL (3.67 mmol/L) | 13.5 mg/dL (3.37 mmol/L) | 12.1 mg/dL (3.02 mmol/L) | >15 mg/dL (>3.74 mmol/L) | 11.4 mg/dL (2.86 mmol/L) | 12.3 mg/dL (3.07 mmol/L) | 14.3 mg/dL (3.57 mmol/L) | 12.7 mg/dL (3.17 mmol/L) |
| Serum PTH prior to cinacalcet | 126 pg/mL (13.4 pmol/L) | a | 288 pg/mL (30.5 pmol/L) | 254 pg/mL (27.0 pmol/L) | 109 pg/mL (11.6 pmol/L) | 350 pg/mL (37.1 pmol/L) | 169 pg/mL (17.9 pmol/L) | 135 pg/mL (14.3 pmol/L) | 152 pg/mL (16.1 pmol/L) | 168 pg/mL (17.8 pmol/L) |
| GA at Cinacalcet initiation (weeks) | 32 | a | 30 | 10 | 24 | 30 | 34 | 32 | 32 | 24 |
| Cinacalcet dose (mg/day) | 30 | 60-120 | 60 | 60 | 15-30 | 15 | 30 | 30-90 | 60-240 | 15-30 |
| sCalcium on Cinacalcet | 9.8 mg/dL (2.45 mmol/L) | a | No significant change | 10.7 mg/dL (2.67 mmol/L) | No significant change | No significant change | 10.6 mg/dL (2.64 mmol/L) | No significant change | 12.0 mg/dL (3.0 mmol/L) | 10.3 mg/dL (2.57 mmol/L) |
| Other therapies | IVF | a | IVF, calcitonin, furosemide, bisphosphonate | IVF | IVF | IVF, calcitonin | None | IVF, furosemide | IVF, calcitonin | IVF, calcitonin |
| GA at time of delivery (weeks) | 37 | Not reported | 33 | 37 | 32 | 38 | 37 | 34 | 34 | 37 |
| Obstetric complications | Postpartum hemorrhage | None reported | IUGR, preeclampsia | None reported | Nephrolithiasis premature membrane rupture | Pancreatitis | None reported | Pre-eclampsia | None reported | None reported |
| Neonatal effect | None reported | Hypocalcemia with second child | Late-onset hypocalcemia | None reported | Hypocalcemia with tetany | None reported | None reported | Hypocalcemia with respiratory distress | Transient hypocalcemia (mild) | None reported |
| Time of parathyroidectomy | Postpartum | a | Postpartum | 14 weeks gestation | Postpartum | 32 weeks gestation | Postpartum | Postpartum | 34 weeks gestation | Not discussed |
Abbreviations: GA, gestational age; IUGR, intrauterine growth restriction; IVF, intravenous fluids.
aNedarasa et al (6) reports a case of a woman diagnosed with parathyroid carcinoma prior to pregnancy initially managed with parathyroidectomy and cinacalcet prior to and during 2 separate pregnancies
| . | Our case . | Nadarasa (6)a . | Garcia (7) . | Horton (8) . | Vera (9) . | Latif (10) . | Eremkina (11) . | Ning (12) . | Horjus (13) . | Bashir (14) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Parathyroid pathology | Adenoma | Carcinoma | Adenoma | Ectopic Adenoma | Adenoma | Adenoma | Adenoma | Adenoma | Unknown | Unknown |
| Serum calcium prior to cinacalcet | 12.2 mg/dL (3.04 mmol/L) | a | 14.7 mg/dL (3.67 mmol/L) | 13.5 mg/dL (3.37 mmol/L) | 12.1 mg/dL (3.02 mmol/L) | >15 mg/dL (>3.74 mmol/L) | 11.4 mg/dL (2.86 mmol/L) | 12.3 mg/dL (3.07 mmol/L) | 14.3 mg/dL (3.57 mmol/L) | 12.7 mg/dL (3.17 mmol/L) |
| Serum PTH prior to cinacalcet | 126 pg/mL (13.4 pmol/L) | a | 288 pg/mL (30.5 pmol/L) | 254 pg/mL (27.0 pmol/L) | 109 pg/mL (11.6 pmol/L) | 350 pg/mL (37.1 pmol/L) | 169 pg/mL (17.9 pmol/L) | 135 pg/mL (14.3 pmol/L) | 152 pg/mL (16.1 pmol/L) | 168 pg/mL (17.8 pmol/L) |
| GA at Cinacalcet initiation (weeks) | 32 | a | 30 | 10 | 24 | 30 | 34 | 32 | 32 | 24 |
| Cinacalcet dose (mg/day) | 30 | 60-120 | 60 | 60 | 15-30 | 15 | 30 | 30-90 | 60-240 | 15-30 |
| sCalcium on Cinacalcet | 9.8 mg/dL (2.45 mmol/L) | a | No significant change | 10.7 mg/dL (2.67 mmol/L) | No significant change | No significant change | 10.6 mg/dL (2.64 mmol/L) | No significant change | 12.0 mg/dL (3.0 mmol/L) | 10.3 mg/dL (2.57 mmol/L) |
| Other therapies | IVF | a | IVF, calcitonin, furosemide, bisphosphonate | IVF | IVF | IVF, calcitonin | None | IVF, furosemide | IVF, calcitonin | IVF, calcitonin |
| GA at time of delivery (weeks) | 37 | Not reported | 33 | 37 | 32 | 38 | 37 | 34 | 34 | 37 |
| Obstetric complications | Postpartum hemorrhage | None reported | IUGR, preeclampsia | None reported | Nephrolithiasis premature membrane rupture | Pancreatitis | None reported | Pre-eclampsia | None reported | None reported |
| Neonatal effect | None reported | Hypocalcemia with second child | Late-onset hypocalcemia | None reported | Hypocalcemia with tetany | None reported | None reported | Hypocalcemia with respiratory distress | Transient hypocalcemia (mild) | None reported |
| Time of parathyroidectomy | Postpartum | a | Postpartum | 14 weeks gestation | Postpartum | 32 weeks gestation | Postpartum | Postpartum | 34 weeks gestation | Not discussed |
| . | Our case . | Nadarasa (6)a . | Garcia (7) . | Horton (8) . | Vera (9) . | Latif (10) . | Eremkina (11) . | Ning (12) . | Horjus (13) . | Bashir (14) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Parathyroid pathology | Adenoma | Carcinoma | Adenoma | Ectopic Adenoma | Adenoma | Adenoma | Adenoma | Adenoma | Unknown | Unknown |
| Serum calcium prior to cinacalcet | 12.2 mg/dL (3.04 mmol/L) | a | 14.7 mg/dL (3.67 mmol/L) | 13.5 mg/dL (3.37 mmol/L) | 12.1 mg/dL (3.02 mmol/L) | >15 mg/dL (>3.74 mmol/L) | 11.4 mg/dL (2.86 mmol/L) | 12.3 mg/dL (3.07 mmol/L) | 14.3 mg/dL (3.57 mmol/L) | 12.7 mg/dL (3.17 mmol/L) |
| Serum PTH prior to cinacalcet | 126 pg/mL (13.4 pmol/L) | a | 288 pg/mL (30.5 pmol/L) | 254 pg/mL (27.0 pmol/L) | 109 pg/mL (11.6 pmol/L) | 350 pg/mL (37.1 pmol/L) | 169 pg/mL (17.9 pmol/L) | 135 pg/mL (14.3 pmol/L) | 152 pg/mL (16.1 pmol/L) | 168 pg/mL (17.8 pmol/L) |
| GA at Cinacalcet initiation (weeks) | 32 | a | 30 | 10 | 24 | 30 | 34 | 32 | 32 | 24 |
| Cinacalcet dose (mg/day) | 30 | 60-120 | 60 | 60 | 15-30 | 15 | 30 | 30-90 | 60-240 | 15-30 |
| sCalcium on Cinacalcet | 9.8 mg/dL (2.45 mmol/L) | a | No significant change | 10.7 mg/dL (2.67 mmol/L) | No significant change | No significant change | 10.6 mg/dL (2.64 mmol/L) | No significant change | 12.0 mg/dL (3.0 mmol/L) | 10.3 mg/dL (2.57 mmol/L) |
| Other therapies | IVF | a | IVF, calcitonin, furosemide, bisphosphonate | IVF | IVF | IVF, calcitonin | None | IVF, furosemide | IVF, calcitonin | IVF, calcitonin |
| GA at time of delivery (weeks) | 37 | Not reported | 33 | 37 | 32 | 38 | 37 | 34 | 34 | 37 |
| Obstetric complications | Postpartum hemorrhage | None reported | IUGR, preeclampsia | None reported | Nephrolithiasis premature membrane rupture | Pancreatitis | None reported | Pre-eclampsia | None reported | None reported |
| Neonatal effect | None reported | Hypocalcemia with second child | Late-onset hypocalcemia | None reported | Hypocalcemia with tetany | None reported | None reported | Hypocalcemia with respiratory distress | Transient hypocalcemia (mild) | None reported |
| Time of parathyroidectomy | Postpartum | a | Postpartum | 14 weeks gestation | Postpartum | 32 weeks gestation | Postpartum | Postpartum | 34 weeks gestation | Not discussed |
Abbreviations: GA, gestational age; IUGR, intrauterine growth restriction; IVF, intravenous fluids.
aNedarasa et al (6) reports a case of a woman diagnosed with parathyroid carcinoma prior to pregnancy initially managed with parathyroidectomy and cinacalcet prior to and during 2 separate pregnancies
Cinacalcet use in breastfeeding was addressed in 3 of these cases, and the mothers discontinued cinacalcet to breastfeed (9, 11). Our patient discarded pumped breastmilk up until 2 weeks after discontinuing the medication. Studies in rats showed cinacalcet was transferred to breast milk, but there is no data on whether it is transferred to human breast milk (4). Therefore, it is unclear if the child may develop hypocalcemia should the mother continue taking cinacalcet while breastfeeding.
In conclusion, our case highlights the role of medical treatment with cinacalcet in the setting of PHPT with severe hypercalcemia in pregnancy when it is necessary to wait to undergo parathyroidectomy, either in the second trimester or postpartum. There are no controlled studies done to guide treatment. We reviewed the use of cinacalcet in pregnancy in our case and other case reports. While our patient tolerated low-dose cinacalcet, which normalized her serum calcium, other reports have highlighted side effects such as nausea, which limited cinacalcet use. Although cinacalcet appears to lower serum calcium in pregnancy, little remains known about its safety/teratogenicity in humans and about its use in breastfeeding. These questions are important to address, and therefore more studies to evaluate cinacalcet use in pregnancy and breastfeeding are necessary.
Learning Points
There are no established guidelines for the management of PHPT in pregnancy. In symptomatic cases, parathyroidectomy is often preferred during the second trimester, while management is more challenging in patients in the first and third trimester.
Cinacalcet is approved for the medical management of primary hyperparathyroidism but is considered a category C medication in pregnancy.
Our patient had worsening symptomatic hypercalcemia during the third trimester and tolerated cinacalcet with improvement in serum calcium, allowing for delay of parathyroidectomy until after delivery.
It is unknown if cinacalcet is transferred to human breast milk and what its effects are on the breastfed infant.
Acknowledgments
The authors would like to thank Drs. Eleanor Lederer, Orson Moe, and Khashayar Sakhaee for their helpful discussions.
Contributors
All authors made individual contributions to the manuscript. N.M. was involved in the evaluation and management of the patient and manuscript revision. E.F. was involved in literature review and case write-up. All authors reviewed and approved the final draft.
Funding
No public or commercial funding.
Disclosures
None declared.
Informed Patient Consent for Publication
Signed informed consent could not be obtained from the patient or a proxy but has been approved by the treating institution.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.



