-
PDF
- Split View
-
Views
-
Cite
Cite
Bronwyn G A Stuckey, Deila Dedic, Rui Zhang, Amira Rabbah, Adina F Turcu, Richard J Auchus, Abiraterone in Classic Congenital Adrenal Hyperplasia: Results of Medical Therapy Before Adrenalectomy, JCEM Case Reports, Volume 2, Issue 6, June 2024, luae077, https://doi.org/10.1210/jcemcr/luae077
Close - Share Icon Share
Abstract
We present the case of a 20-year-old woman with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency, with uncontrolled hyperandrogenemia despite supraphysiological glucocorticoid therapy. We used abiraterone acetate, an inhibitor of the 17-hydroxylase/17,20-lyase enzyme, to suppress adrenal androgen synthesis and allow physiological glucocorticoid and mineralocorticoid therapy, as a proof-of-concept, before proceeding to bilateral adrenalectomy. We report the patient's clinical course, the changes in adrenal steroids, and the immunohistochemistry of the adrenals.
Introduction
Classic congenital adrenal hyperplasia (CAH) is an autosomal recessive condition resulting from reduced enzyme activity within the adrenal biosynthesis pathway. More than 90% of CAH cases result from deficiency of the enzyme 21-hydroxylase, a product of the CYP21A2 gene [1]. In 21-hydroxylase deficiency (21OHD), the enzyme block impairs synthesis of glucocorticoid and mineralocorticoid and shunts steroidogenesis into pathways leading to excess androgen production and, in females, ambiguous genitalia at birth. Management aims to restore sodium balance, deliver adequate glucocorticoid therapy, and reduce androgenization. Depending upon the gene mutations and degree of residual enzyme activity, control of hyperandrogenemia may be difficult without supraphysiological doses of glucocorticoids, causing iatrogenic cushingoid side effects.
Abiraterone is a potent inhibitor of cytochrome P450 17A1 (CYP17A1) and blocks androgen biosynthesis in the adrenals and gonads. Abiraterone acetate is approved for use in the treatment of metastatic prostate cancer and, by blocking the initial steps in androgen production, could be a glucocorticoid-sparing agent for patients with 21OHD [2]. In 2014 a pilot study of 6 patients with 21OHD demonstrated that, in the presence of physiological glucocorticoid therapy, abiraterone acetate normalized adrenal androgen production [3, 4].
We report the management of a female patient with classic 21OHD in whom abiraterone was used to control androgen excess before proceeding to bilateral adrenalectomy.
Case Presentation
A 20-year-old woman was diagnosed at birth with classic salt-wasting 21OHD after features of ambiguous genitalia were noted. Genetics confirmed compound heterozygous mutations of CYP21A2—c.952C > T [p.Gln318X] on 1 allele and a complete gene deletion on the other.
During childhood, management was difficult. Adrenal androgen production was resistant to suppression without supraphysiological glucocorticoid doses, with side effects of weight gain and cushingoid features. Better control was attempted with continuous subcutaneous hydrocortisone infusion 30 to 40 mg/day with diurnal variation mimicking circadian rhythm. However, this was complicated by infections at the site of needle insertion and abandoned. She had salt craving despite fludrocortisone acetate 0.15 mg twice daily.
She had menses once at age 11 years, none until the age of 13 years when she regained menses for 3 months, and none thereafter. She had surgery for clitoral reduction, labioplasty, and vaginoplasty. She had no history of adrenal crises. She took an oral contraceptive briefly, but this led to weight gain. She did not need contraception. She has longstanding anxiety and depression.
Diagnostic Assessment
She was 152.8 cm tall and weighed 94.7 kg, with a body mass index of 41 kg/m2. Blood pressure was 116/80 mmHg seated and 117/80 mmHg standing. She had hirsutism over the face and abdomen, cushingoid features of centripetal weight, dorsocervical fat, and violaceous striae.
On a dose of hydrocortisone 10 mg with breakfast, 4 mg with lunch and dinner, and 2.5 mg prednisolone at bedtime (28 mg glucocorticoid equivalents daily), baseline 17-hydroxyprogesterone (17OHP) was 1040 nmol/L (34 368 ng/dL) (reference range 0.2-2.0 nmol/L; 6.6-66.0 ng/dL), androstenedione 125 nmol/L (3580 ng/dL) (reference range 0.9-7.5 nmol/L; 25.8-214.8 ng/dL), testosterone 18 nmol/L (519 ng/dL) (reference range <2.0 nmol/L; < 58 ng/dL), and dihydrotestosterone 1.10 nmol/L (31.9 ng/dL) (reference range 0.15-0.60 nmol/L; 4.36-17.42 ng/dL).
Patient serum samples were analyzed at PathWest Laboratory, Nedlands, by liquid chromatography-tandem mass spectrometry as published [5].
Treatment
We considered bilateral adrenalectomy with physiological adrenal hormone replacement to control adrenal androgen production, reduce glucocorticoid dose, restore menses, and facilitate weight loss. We trialed abiraterone acetate therapy prior to adrenalectomy as “proof-of-concept” that proceeding to surgery would not be associated with adverse effects or adrenal crises. Abiraterone acetate (Zytiga®) was obtained with approval from Sir Charles Gairdner Hospital Drug and Therapeutics Committee and supplied by Janssen-Cilag Pty Ltd on “compassionate supply.” The patient signed informed consent. Abiraterone was started at 125 mg daily for 1 week and increased to 250 mg daily thereafter. Glucocorticoid treatment was reduced to hydrocortisone monotherapy 18 mg daily in divided doses.
After 7 months of medical therapy, she had bilateral adrenalectomy by laparoscopic approach with perioperative glucocorticoid stress doses.
Outcome and Follow-up
Table 1 shows the changes in adrenal and ovarian steroids during abiraterone treatment and after adrenalectomy. Glucocorticoid therapy continued with hydrocortisone 18 mg daily in divided doses and fludrocortisone 0.15 mg in the morning and 0.1 mg at night. Abiraterone decreased androstenedione and testosterone to within normal reference intervals. Progesterone and 17OHP remained high, although the latter decreased from baseline. Blood pressure remained normal. During abiraterone therapy, her mood improved and no episodes of adrenal crisis occurred; however, amenorrhea persisted, without substantial weight loss.
Adrenal and ovarian steroids pre- and post-abiraterone therapy and pre- and postbilateral adrenalectomy
| Day . | −18 . | 0 . | +36 . | +210 . | +236 . | +238 . | . |
|---|---|---|---|---|---|---|---|
| Hormone . | Pre-abiraterone . | . | Post-abiraterone . | Pre-adrenalectomy . | . | Post-adrenalectomy . | Reference range . |
| LH | <0.1 U/L | Abiraterone | 0.1 U/L | 0.3 U/L | Bilateral adrenalectomy | 1.3 U/L | 2-10 U/L |
| <0.1 mU/mL | 0.1 mU/mL | 0.3 mU/mL | 1.3 mU/mL | 2-10 mU/mL | |||
| FSH | <1 U/L | <1 U/L | 1 U/L | 5 U/L | 2-10 U/L | ||
| <1 mU/mL | <1 mU/mL | 1 mU/mL | 5 mU/mL | 2-10 mU/mL | |||
| E2 | 310 pmol/L | 180 pmol/L | 160 pmol/L | 110 pmol/L | 11-450 pmol/L | ||
| 84 pg/mL | 49 pg/mL | 44 pg/mL | 30 pg/mL | 3-122 pg/mL | |||
| Prog | 66 nmol/L | 32 nmol/L | 87 nmol/L | 3 nmol/L | <6 nmol/L | ||
| 21 ng/mL | 10 ng/mL | 27 ng/mL | 0.9 ng/mL | <2 ng/mL | |||
| 17OHP | 1040 nmol/L | 378 nmol/L | 328 nmol/L | 0.2 nmol/L | 0.2-2.0 nmol/L | ||
| 34 368 ng/dL | 12 492 ng/dL | 10 839 ng/dL | 6.6 ng/dL | 6.6-66.0 ng/dL | |||
| 17OHPreg | 6.8 nmol/L | 3.2 nmol/L | 4.8 nmol/L | <0.1 nmol/L | <6.8 nmol/L | ||
| 226 ng/dL | 106 ng/dL | 159 ng/dL | <3 ng/dL | <226 ng/dL | |||
| Andro | 125 nmol/L | 13.3 nmol/L | 5.1 nmol/L | 0.6 nmol/L | 0.9-7.5 nmol/L | ||
| 3580 ng/dL | 383.8 ng/dL | 146.0 ng/dL | 17.2 ng/dL | 25.8-214.8 ng/dL | |||
| Testo | 18 nmol/L | 3.5 nmol/L | 1.1 nmol/L | 0.2 nmol/L | <2 nmol/L | ||
| 519 ng/dL | 152.9 ng/dL | 31.7 ng/dL | 5.8 ng/dL | <58 ng/dL | |||
| DHT | 1.1 nmol/L | 0.3 nmol/L | <0.08 nmol/L | <0.08 nmol/L | 0.15-0.60 nmol/L | ||
| 31.9 ng/dL | 8.7 ng/dL | <2.3 ng/dL | <2.3 ng/dL | 4.4-17.4 ng/dL | |||
| DHEA | 0.46 nmol/L | <0.01 nmol/L | <0.01 nmol/L | <0.01 nmol/L | 4.6-27 nmol/L | ||
| 13.3 ng/dL | <0.3 ng/dL | <0.3 ng/dL | <0.3 ng/dL | 133-778 ng/dL | |||
| 11DF | 1.5 nmol/L | 0.7 nmol/L | 0.7 nmol/L | <0.1 nmol/L | <1.0 nmol/L | ||
| 52 ng/dL | 24 ng/dL | 24 ng/dL | <3 ng/dL | <3 ng/dL | |||
| SHBG | 38 nmol/L | 37 nmol/L | 38 nmol/L | 38 nmol/L | 30-120 nmol/L | ||
| 361 ug/dL | 352 ug/dL | 361 ug/dL | 361 ug/dL | 285-1140 ug/dL |
| Day . | −18 . | 0 . | +36 . | +210 . | +236 . | +238 . | . |
|---|---|---|---|---|---|---|---|
| Hormone . | Pre-abiraterone . | . | Post-abiraterone . | Pre-adrenalectomy . | . | Post-adrenalectomy . | Reference range . |
| LH | <0.1 U/L | Abiraterone | 0.1 U/L | 0.3 U/L | Bilateral adrenalectomy | 1.3 U/L | 2-10 U/L |
| <0.1 mU/mL | 0.1 mU/mL | 0.3 mU/mL | 1.3 mU/mL | 2-10 mU/mL | |||
| FSH | <1 U/L | <1 U/L | 1 U/L | 5 U/L | 2-10 U/L | ||
| <1 mU/mL | <1 mU/mL | 1 mU/mL | 5 mU/mL | 2-10 mU/mL | |||
| E2 | 310 pmol/L | 180 pmol/L | 160 pmol/L | 110 pmol/L | 11-450 pmol/L | ||
| 84 pg/mL | 49 pg/mL | 44 pg/mL | 30 pg/mL | 3-122 pg/mL | |||
| Prog | 66 nmol/L | 32 nmol/L | 87 nmol/L | 3 nmol/L | <6 nmol/L | ||
| 21 ng/mL | 10 ng/mL | 27 ng/mL | 0.9 ng/mL | <2 ng/mL | |||
| 17OHP | 1040 nmol/L | 378 nmol/L | 328 nmol/L | 0.2 nmol/L | 0.2-2.0 nmol/L | ||
| 34 368 ng/dL | 12 492 ng/dL | 10 839 ng/dL | 6.6 ng/dL | 6.6-66.0 ng/dL | |||
| 17OHPreg | 6.8 nmol/L | 3.2 nmol/L | 4.8 nmol/L | <0.1 nmol/L | <6.8 nmol/L | ||
| 226 ng/dL | 106 ng/dL | 159 ng/dL | <3 ng/dL | <226 ng/dL | |||
| Andro | 125 nmol/L | 13.3 nmol/L | 5.1 nmol/L | 0.6 nmol/L | 0.9-7.5 nmol/L | ||
| 3580 ng/dL | 383.8 ng/dL | 146.0 ng/dL | 17.2 ng/dL | 25.8-214.8 ng/dL | |||
| Testo | 18 nmol/L | 3.5 nmol/L | 1.1 nmol/L | 0.2 nmol/L | <2 nmol/L | ||
| 519 ng/dL | 152.9 ng/dL | 31.7 ng/dL | 5.8 ng/dL | <58 ng/dL | |||
| DHT | 1.1 nmol/L | 0.3 nmol/L | <0.08 nmol/L | <0.08 nmol/L | 0.15-0.60 nmol/L | ||
| 31.9 ng/dL | 8.7 ng/dL | <2.3 ng/dL | <2.3 ng/dL | 4.4-17.4 ng/dL | |||
| DHEA | 0.46 nmol/L | <0.01 nmol/L | <0.01 nmol/L | <0.01 nmol/L | 4.6-27 nmol/L | ||
| 13.3 ng/dL | <0.3 ng/dL | <0.3 ng/dL | <0.3 ng/dL | 133-778 ng/dL | |||
| 11DF | 1.5 nmol/L | 0.7 nmol/L | 0.7 nmol/L | <0.1 nmol/L | <1.0 nmol/L | ||
| 52 ng/dL | 24 ng/dL | 24 ng/dL | <3 ng/dL | <3 ng/dL | |||
| SHBG | 38 nmol/L | 37 nmol/L | 38 nmol/L | 38 nmol/L | 30-120 nmol/L | ||
| 361 ug/dL | 352 ug/dL | 361 ug/dL | 361 ug/dL | 285-1140 ug/dL |
Follicular phase reference ranges are quoted for all steroids.
Abbreviations: 11DF, 11-deoxycortisol; 17OHP, 17-hydroxyprogesterone; 17OHPreg, 17-hydroxypregnenolone; andro, androstenedione; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; prog, progesterone; SHBG, sex hormone binding globulins; testo, testosterone.
Adrenal and ovarian steroids pre- and post-abiraterone therapy and pre- and postbilateral adrenalectomy
| Day . | −18 . | 0 . | +36 . | +210 . | +236 . | +238 . | . |
|---|---|---|---|---|---|---|---|
| Hormone . | Pre-abiraterone . | . | Post-abiraterone . | Pre-adrenalectomy . | . | Post-adrenalectomy . | Reference range . |
| LH | <0.1 U/L | Abiraterone | 0.1 U/L | 0.3 U/L | Bilateral adrenalectomy | 1.3 U/L | 2-10 U/L |
| <0.1 mU/mL | 0.1 mU/mL | 0.3 mU/mL | 1.3 mU/mL | 2-10 mU/mL | |||
| FSH | <1 U/L | <1 U/L | 1 U/L | 5 U/L | 2-10 U/L | ||
| <1 mU/mL | <1 mU/mL | 1 mU/mL | 5 mU/mL | 2-10 mU/mL | |||
| E2 | 310 pmol/L | 180 pmol/L | 160 pmol/L | 110 pmol/L | 11-450 pmol/L | ||
| 84 pg/mL | 49 pg/mL | 44 pg/mL | 30 pg/mL | 3-122 pg/mL | |||
| Prog | 66 nmol/L | 32 nmol/L | 87 nmol/L | 3 nmol/L | <6 nmol/L | ||
| 21 ng/mL | 10 ng/mL | 27 ng/mL | 0.9 ng/mL | <2 ng/mL | |||
| 17OHP | 1040 nmol/L | 378 nmol/L | 328 nmol/L | 0.2 nmol/L | 0.2-2.0 nmol/L | ||
| 34 368 ng/dL | 12 492 ng/dL | 10 839 ng/dL | 6.6 ng/dL | 6.6-66.0 ng/dL | |||
| 17OHPreg | 6.8 nmol/L | 3.2 nmol/L | 4.8 nmol/L | <0.1 nmol/L | <6.8 nmol/L | ||
| 226 ng/dL | 106 ng/dL | 159 ng/dL | <3 ng/dL | <226 ng/dL | |||
| Andro | 125 nmol/L | 13.3 nmol/L | 5.1 nmol/L | 0.6 nmol/L | 0.9-7.5 nmol/L | ||
| 3580 ng/dL | 383.8 ng/dL | 146.0 ng/dL | 17.2 ng/dL | 25.8-214.8 ng/dL | |||
| Testo | 18 nmol/L | 3.5 nmol/L | 1.1 nmol/L | 0.2 nmol/L | <2 nmol/L | ||
| 519 ng/dL | 152.9 ng/dL | 31.7 ng/dL | 5.8 ng/dL | <58 ng/dL | |||
| DHT | 1.1 nmol/L | 0.3 nmol/L | <0.08 nmol/L | <0.08 nmol/L | 0.15-0.60 nmol/L | ||
| 31.9 ng/dL | 8.7 ng/dL | <2.3 ng/dL | <2.3 ng/dL | 4.4-17.4 ng/dL | |||
| DHEA | 0.46 nmol/L | <0.01 nmol/L | <0.01 nmol/L | <0.01 nmol/L | 4.6-27 nmol/L | ||
| 13.3 ng/dL | <0.3 ng/dL | <0.3 ng/dL | <0.3 ng/dL | 133-778 ng/dL | |||
| 11DF | 1.5 nmol/L | 0.7 nmol/L | 0.7 nmol/L | <0.1 nmol/L | <1.0 nmol/L | ||
| 52 ng/dL | 24 ng/dL | 24 ng/dL | <3 ng/dL | <3 ng/dL | |||
| SHBG | 38 nmol/L | 37 nmol/L | 38 nmol/L | 38 nmol/L | 30-120 nmol/L | ||
| 361 ug/dL | 352 ug/dL | 361 ug/dL | 361 ug/dL | 285-1140 ug/dL |
| Day . | −18 . | 0 . | +36 . | +210 . | +236 . | +238 . | . |
|---|---|---|---|---|---|---|---|
| Hormone . | Pre-abiraterone . | . | Post-abiraterone . | Pre-adrenalectomy . | . | Post-adrenalectomy . | Reference range . |
| LH | <0.1 U/L | Abiraterone | 0.1 U/L | 0.3 U/L | Bilateral adrenalectomy | 1.3 U/L | 2-10 U/L |
| <0.1 mU/mL | 0.1 mU/mL | 0.3 mU/mL | 1.3 mU/mL | 2-10 mU/mL | |||
| FSH | <1 U/L | <1 U/L | 1 U/L | 5 U/L | 2-10 U/L | ||
| <1 mU/mL | <1 mU/mL | 1 mU/mL | 5 mU/mL | 2-10 mU/mL | |||
| E2 | 310 pmol/L | 180 pmol/L | 160 pmol/L | 110 pmol/L | 11-450 pmol/L | ||
| 84 pg/mL | 49 pg/mL | 44 pg/mL | 30 pg/mL | 3-122 pg/mL | |||
| Prog | 66 nmol/L | 32 nmol/L | 87 nmol/L | 3 nmol/L | <6 nmol/L | ||
| 21 ng/mL | 10 ng/mL | 27 ng/mL | 0.9 ng/mL | <2 ng/mL | |||
| 17OHP | 1040 nmol/L | 378 nmol/L | 328 nmol/L | 0.2 nmol/L | 0.2-2.0 nmol/L | ||
| 34 368 ng/dL | 12 492 ng/dL | 10 839 ng/dL | 6.6 ng/dL | 6.6-66.0 ng/dL | |||
| 17OHPreg | 6.8 nmol/L | 3.2 nmol/L | 4.8 nmol/L | <0.1 nmol/L | <6.8 nmol/L | ||
| 226 ng/dL | 106 ng/dL | 159 ng/dL | <3 ng/dL | <226 ng/dL | |||
| Andro | 125 nmol/L | 13.3 nmol/L | 5.1 nmol/L | 0.6 nmol/L | 0.9-7.5 nmol/L | ||
| 3580 ng/dL | 383.8 ng/dL | 146.0 ng/dL | 17.2 ng/dL | 25.8-214.8 ng/dL | |||
| Testo | 18 nmol/L | 3.5 nmol/L | 1.1 nmol/L | 0.2 nmol/L | <2 nmol/L | ||
| 519 ng/dL | 152.9 ng/dL | 31.7 ng/dL | 5.8 ng/dL | <58 ng/dL | |||
| DHT | 1.1 nmol/L | 0.3 nmol/L | <0.08 nmol/L | <0.08 nmol/L | 0.15-0.60 nmol/L | ||
| 31.9 ng/dL | 8.7 ng/dL | <2.3 ng/dL | <2.3 ng/dL | 4.4-17.4 ng/dL | |||
| DHEA | 0.46 nmol/L | <0.01 nmol/L | <0.01 nmol/L | <0.01 nmol/L | 4.6-27 nmol/L | ||
| 13.3 ng/dL | <0.3 ng/dL | <0.3 ng/dL | <0.3 ng/dL | 133-778 ng/dL | |||
| 11DF | 1.5 nmol/L | 0.7 nmol/L | 0.7 nmol/L | <0.1 nmol/L | <1.0 nmol/L | ||
| 52 ng/dL | 24 ng/dL | 24 ng/dL | <3 ng/dL | <3 ng/dL | |||
| SHBG | 38 nmol/L | 37 nmol/L | 38 nmol/L | 38 nmol/L | 30-120 nmol/L | ||
| 361 ug/dL | 352 ug/dL | 361 ug/dL | 361 ug/dL | 285-1140 ug/dL |
Follicular phase reference ranges are quoted for all steroids.
Abbreviations: 11DF, 11-deoxycortisol; 17OHP, 17-hydroxyprogesterone; 17OHPreg, 17-hydroxypregnenolone; andro, androstenedione; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; prog, progesterone; SHBG, sex hormone binding globulins; testo, testosterone.
At adrenalectomy, the right and left adrenals weighed 36 g and 36.2 g, respectively. Histology showed diffuse expansion of the cortex, composed of mainly large polygonal cells with bland, round nuclei surrounded by pale eosinophilic to microvacuolar cytoplasm. All the tissue had marked lobularity but no distinct nodules. Medullary tissue was mainly obscured but where present showed no histological abnormality.
After antigen retrieval and peroxidase blocking, 5 μm sections were incubated with primary antibody at room temperature (Table 2). Detection used the FLEX HRP Envision System (Dako) with diaminobenzidine chromogen application for 10 minutes as described [6]. Slides were counterstained with Harris hematoxylin for 5 seconds and then dehydrated and coverslipped for visualization.
| Antibodies used for immunohistochemistry . | |||||
|---|---|---|---|---|---|
| Antigen retrieval . | |||||
| Antigen . | Dilution . | Buffer . | Time . | Antibody type . | RRID . |
| CYP11B2 | 1:100 | Tris•HCl | Overnight | Mouse monoclonal | AB_2650562 |
| CYP11B1 | 1:100 | Tris•HCl | Overnight | Rat monoclonal | AB_2650563 |
| CYP17A1 | 1:1000 | Tris•HCl | I hour | Rabbit polyclonal | AB_2857939 |
| Antibodies used for immunohistochemistry . | |||||
|---|---|---|---|---|---|
| Antigen retrieval . | |||||
| Antigen . | Dilution . | Buffer . | Time . | Antibody type . | RRID . |
| CYP11B2 | 1:100 | Tris•HCl | Overnight | Mouse monoclonal | AB_2650562 |
| CYP11B1 | 1:100 | Tris•HCl | Overnight | Rat monoclonal | AB_2650563 |
| CYP17A1 | 1:1000 | Tris•HCl | I hour | Rabbit polyclonal | AB_2857939 |
Abbreviations: RRID, research resource identifier.
| Antibodies used for immunohistochemistry . | |||||
|---|---|---|---|---|---|
| Antigen retrieval . | |||||
| Antigen . | Dilution . | Buffer . | Time . | Antibody type . | RRID . |
| CYP11B2 | 1:100 | Tris•HCl | Overnight | Mouse monoclonal | AB_2650562 |
| CYP11B1 | 1:100 | Tris•HCl | Overnight | Rat monoclonal | AB_2650563 |
| CYP17A1 | 1:1000 | Tris•HCl | I hour | Rabbit polyclonal | AB_2857939 |
| Antibodies used for immunohistochemistry . | |||||
|---|---|---|---|---|---|
| Antigen retrieval . | |||||
| Antigen . | Dilution . | Buffer . | Time . | Antibody type . | RRID . |
| CYP11B2 | 1:100 | Tris•HCl | Overnight | Mouse monoclonal | AB_2650562 |
| CYP11B1 | 1:100 | Tris•HCl | Overnight | Rat monoclonal | AB_2650563 |
| CYP17A1 | 1:1000 | Tris•HCl | I hour | Rabbit polyclonal | AB_2857939 |
Abbreviations: RRID, research resource identifier.
Light microscopy and immunohistochemistry demonstrated a continuous, substantial zona glomerulosa marked by CYP11B2 (aldosterone synthase) expression (Fig. 1A), consistent with salt craving and chronically elevated plasma renin. However, aldosterone cannot be synthesized in cells lacking CYP21A2 activity despite robust CYP11B2 expression. CYP17A1 (the target of abiraterone) (Fig. 1B) and CYP11B1 (11-hydroxylase) (Fig. 1C) were expressed throughout the remainder of the adrenal cortex down to the medulla.
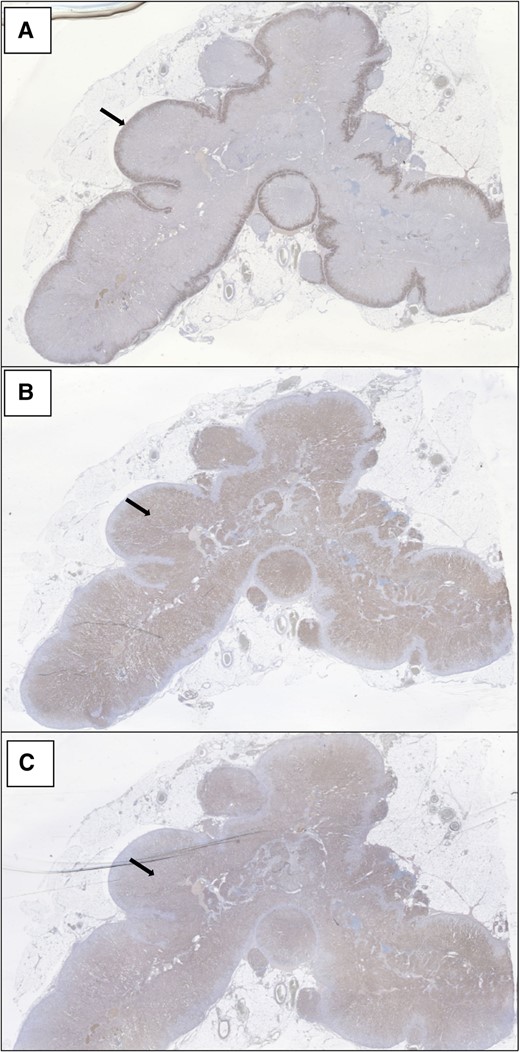
Demonstrates continuous, substantial CYP11B2 (aldosterone synthase) expression in the zona glomerulosa of the adrenal (panel A). CYP17A1 (17-hydroxylase/17,20-lyase, the target of Abiraterone) (Panel B) and CYP11B1 (11-hydroxylase) (Panel C) are expressed throughout the remainder of the adrenal cortex down to the medulla.
Following adrenalectomy, menses returned, hirsutism improved, and cushingoid features faded. In the 2 years postadrenalectomy, she was hospitalized 3 times for evolving adrenal crises, precipitated by a missed glucocorticoid dose, gastrointestinal infection, and excess alcohol consumption, respectively. Although she had no initial weight loss, she has subsequently lost about 30 kg since surgery.
Discussion
Management of classic CAH is a juggling act between supraphysiological glucocorticoid doses and uncontrolled androgens. Therapeutic targets are upper normal to mildly elevated 17OHP and androstenedione levels [7]. The ease or difficulty of achieving this depends upon the CYP21A2 gene mutation and thereby the degree of residual 21-hydroxylase activity. The side effects of high glucocorticoid doses may be manifest as growth impairment, obesity, skin thinning, striae, and bone loss. Conversely, failure to control androgenization leads to amenorrhea, hirsutism, infertility, and low self-esteem [8]. Various methods of controlling androgens have been trialed and were trialed in our patient over the years, including multiple daily doses of glucocorticoid throughout the 24 hours, subcutaneous infusion of glucocorticoid, and the addition of antiandrogens and an oral contraceptive.
Abiraterone is a potent inhibitor of the CYP17A1 enzyme and blocks androgen biosynthesis in adrenal glands [9]. Use in patients with normal adrenal metabolism leads to shunting of steroid production via 21-hydroxylase to increase 11-deoxycorticosterone, resulting in low renin hypertension [10]. In 21OHD, this pathway is deficient, and hypertension and hypokalaemia do not occur. Abiraterone, therefore, might be effective as a glucocorticoid-sparing agent in patients with 21OHD where hyperandrogenemia is difficult to control.
Abiraterone acetate was studied in 6 women with classic 21OHD whose serum androstenedione concentration was greater than 1.5 times above the upper limit of normal [3]. All participants received hydrocortisone 20 mg daily in divided doses, fludrocortisone acetate, and an oral contraceptive. Abiraterone acetate 250 mg daily for 6 days lowered androstenedione and testosterone to normal in all women without the need for supraphysiological glucocorticoid therapy. Progesterone, upstream of both the iatrogenic block at 17-hydroxylase and the endogenous block at 21-hydroxylase, rose markedly during abiraterone therapy, as it did in our patient [3, 4]. The greater inhibition of androgens over 17OHP was seen in this trial [3] and also in studies of abiraterone monotherapy in prostate cancer [10], due to similar if not equal inhibition of 2 sequential steps to androgens vs 1 step to 17OHP. Unlike the women in the previous study, our patient was not taking an oral contraceptive and had continued amenorrhea while taking abiraterone, consistent with an effect of the elevated progesterone, suppressing gonadotropins and endometrial development. If abiraterone were used long term in 21OHD, undesirable effects of the persistently raised progesterone might be offset by the addition of estradiol therapy. Rather than use in adults, where the rise in progesterone might adversely affect reproductive, bone, and cardiometabolic health, the prolonged use of abiraterone in prepubertal children, in whom sex steroids are physiologically low, should pose little risk and might allow more physiological glucocorticoid dosing, avoiding the growth retardation seen with high doses.
Wright et al showed that abiraterone acetate also lowered 11-oxygenated androgens, including 11β-hydroxyandrostenedione and 11- ketotestosterone [4]. Since 11-ketotestosterone has been shown to be the dominant androgen in adrenarche as well as most patients with 21OHD, this finding raises the possibility of the usefulness of abiraterone in both 21OHD and premature adrenarche [11, 12].
The decision for bilateral adrenalectomy was made only after failure of all available treatment options. The patient's positive response, both biochemically and psychologically, to androgen blockade with abiraterone influenced the decision to proceed with surgery.
Endocrine Society guidelines advise against adrenalectomy for reasons of surgical risk; possible increased risk of adrenal crisis due to loss of residual adrenal function; loss of regulatory hormones, particularly adrenal medullary hormones; and the possible risk of long-term development of adrenal rest tumors [7]. In reviewing 48 cases of bilateral adrenalectomy, Mackay et al reported that 74% of patients reported symptomatic improvement postoperatively in menstrual function, weight loss, and reduction in androgenic symptoms [13]. Six patients had postoperative adrenal crises in the setting of infection, operation, or noncompliance. It is not clear how many had had previous adrenal crises before adrenalectomy. The increase in risk of adrenal crisis postadrenalectomy may have several contributing factors. Supraphysiological glucocorticoid doses, particularly long-acting glucocorticoids, may be protective in the setting of physiological stress. The intact adrenal in 21OHD directly and indirectly (via liver metabolism) produces several steroids with mineralocorticoid and glucocorticoid activity [14]. The presence of the adrenal medulla and epinephrine may have a crucial role in mitigating symptoms of adrenal crisis, such as hypoglycemia. However, even in 21OHD patients with intact adrenals, there is evidence of hypofunction of the adrenal medulla with impaired epinephrine responses to stress and exercise [15, 16].
The development of adrenal rest tumors in the ovaries, uterine ligaments, and surrounding retroperitoneum following adrenalectomy with return of hyperandrogenemia has also been reported [17, 18].
As in finding the balance between hyperandrogenemia and hypercortisolism in medical therapy, the decision for adrenalectomy balances the possible postoperative complications and the known preoperative side effects of therapy. The patient's response to 7 months of abiraterone therapy reinforced our decision to proceed. Despite the initial episodes of adrenal crises, our patient has reaffirmed her decision to proceed to adrenalectomy, rather than continue with abiraterone. It has resulted in a return of menses with a reduction in the circulating progestogens, a reduction in hyperandrogenism, and continuing weight loss with the reduction of glucocorticoid dose.
In the future, effective nonglucocorticoid treatments of the androgen excess in 21OHD may be available, potentially mitigating the consequences of glucocorticoid therapy while maintaining adequate control of androgens [19].
Depending on the gene mutation and degree of residual enzyme activity, hyperandrogenemia in 21OHD can be difficult to treat. Abiraterone blocks adrenal androgen production allowing glucocorticoid-sparing treatment of classic 21OHD. However, progesterone rises, and the treatment does not restore menstrual regularity or fertility. In difficult cases, bilateral adrenalectomy might be considered. Surgery has been shown to reduce androgen production, allow physiological glucocorticoid doses, and lead to a return of menses. These benefits should be weighed against a possible increase in adrenal crises and development of adrenal rest tumors. Better nonglucocorticoid treatments are needed for the long-term management of classic 21OHD.
Learning Points
The management of classic 21-hydroxylase deficiency with glucocorticoid therapy is a balance between control of hyperandrogenemia and avoidance of cushingoid side effects.
Abiraterone inhibits 17-hydroxylase/17,20-lyase, blocks androgen biosynthesis in the adrenal glands, and allows physiological glucocorticoid therapy.
In classic 21-hydroxylase deficiency, abiraterone therapy leads to a rise in progesterone resulting in amenorrhea.
Bilateral adrenalectomy can be considered, but with recognition of the possible adverse consequences of adrenal rest tumors and increased susceptibility to adrenal crisis.
Acknowledgments
We acknowledge the contribution of our colleagues Hieu Nguyen and Simon Ryan who performed the adrenalectomy.
Contributors
B.G.A.S. provided patient care and wrote the manuscript. D.D. was involved in patient care and review of the literature. R.Z. performed the liquid chromatography-mass spectrometry assays. A.R. and A.F.T. performed the immunohistochemistry. R.J.A. provided advice on patient management and contributed to the writing and editing of the manuscript.
Funding
Janssen-Cilag Pty Ltd provided abiraterone acetate for the patient as “compassionate supply.” There was no other public or commercial funding.
Disclosures
A.T. is an editor for JCEM Case Reports and played no role in the journal's evaluation of the manuscript. The authors have, otherwise, nothing to disclose and no perceived conflicts of interest.
Informed Patient Consent for Publication
Signed informed consent was obtained directly from the patient.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.



