-
PDF
- Split View
-
Views
-
Cite
Cite
Michael Tang, Chanyanuch Nakapakorn, Shumei Meng, A Journey of Diagnosing a Case of Immune Checkpoint Inhibitor Pembrolizumab-Induced Diabetes Mellitus, JCEM Case Reports, Volume 2, Issue 1, January 2024, luad126, https://doi.org/10.1210/jcemcr/luad126
Close - Share Icon Share
Abstract
Autoimmune diabetes mellitus (DM) due to pembrolizumab is a rare but reported complication of immune checkpoint inhibitors (ICIs). It is often missed for a long initial period, leading to unnecessary admissions and poor glucose management. We report a case of a 72-year-old woman with prior history of gastrointestinal stromal tumor (GIST) and current diagnosis of squamous cell carcinoma (SCC) of the lung, who upon presentation at the emergency department with symptoms of encephalopathy, was diagnosed as having sodium glucose cotransporter 2 inhibitor (SGLT-2i)–induced diabetic ketoacidosis (DKA). Upon further investigation, we learned that this patient had multiple hospitalizations for recurrent DKA over 2 years after being managed on metformin and SGLT-2i. Biochemical testing helped confirm pembrolizumab-induced autoimmune diabetes with significantly elevated glutamic acid decarboxylase-65 (GAD65) autoantibodies and an undetectable C-peptide level. The patient has had clinical improvement with insulin therapy without further DKA episodes. She continued to be managed by outpatient endocrinology with improved glucose control. Altogether, this case demonstrates the importance of keeping a high vigilance for possible new onset of autoimmune endocrine diseases, such as diabetes, in patients treated with ICIs, to enable earlier diagnosis and prompt initiation of correct therapy.
Introduction
Diabetes mellitus (DM) is a group of endocrine diseases characterized by sustained high blood glucose levels through different mechanisms. There are many types of DM; the most common are type 1 or 2, but additional types exist, including monogenic, latent autoimmune diabetes of adults (LADA), and ketosis-prone diabetes (KPD). Correct classification of types of diabetes are important, as different treatments may be needed.
Recently, the use of immunotherapies such as immune checkpoint inhibitors (ICIs) has made great progress in cancer treatment. These ICIs are monoclonal antibodies developed to target cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein (PD-1), and PD ligand 1 (PD-L1). PD-1 is expressed on activated T cells to limit the activity of T cells at the time of an inflammatory response to suppress autoimmunity. It also binds to its ligand PD-L1 expressed on tumor cells to cause inhibition of T cell receptor-mediated programmed cell death. In addition, PD-1 also exists on regulatory T cells, where it may enhance their proliferation after binding to the ligands. Pembrolizumab, a PD-1 inhibitor, is designed to enhance immune-mediated antitumor activity but may also pose risk for a wide spectrum of immune-related adverse events (IAEs) [1].
While approved for melanoma, non-small cell lung cancer (NSCLC), head and neck squamous cell cancer (SCC) and many other malignancies, pembrolizumab has also been used more broadly as an important part of anticancer therapies. It prevents downregulation of T-cell proliferation and survival allowing for a more efficient antitumor activity [1]. However, type 1 DM in patients treated with pembrolizumab has been documented in 0.2% to 2% of patients [1, 2].
PD-1 inhibitor–related IAEs include hypophysitis, thyroiditis, adrenalitis, and DM. There is no standardization protocol or practical guidelines on surveillance and patient monitoring for the development of IAEs. Thus, it is important to consider the time of onset, clinical course, and possible reversibility of ICI-induced immune problems to further guide how immunotherapies like PD-1 inhibitors are used to better understand the consequences and management of new-onset endocrine conditions with concurrent tumor treatment.
Case Presentation
Our patient is a 72-year-old female with an extensive history of chronic illnesses, including hypertension, smoking, chronic bronchitis, primary hypothyroidism, and chronic pain. Home medications include amlodipine, albuterol, budesonide inhalers, cyclobenzaprine, hydrocodone, methocarbamol, and levothyroxine. She has a history of stage IV gastrointestinal stromal tumor (GIST) that was resected in 2011 and she was previously on long-term imatinib until 2019. At the time, she had also been diagnosed with stage 1b SCC of the right lung and underwent a right lower lobectomy in 2011. In 2015, she had a recurrence of stage 1a SCC treated with a partial right upper lobectomy of her lung. Her cancer unfortunately came back as an unresectable stage IVa SCC which was then treated with chemotherapy. She completed 4 cycles of carboplatin, taxol, and pembrolizumab from August through November of 2019. Since then, she has been on maintenance with pembrolizumab. Of note, the patient has a family history of stomach cancer in her father, breast cancer in her paternal grandmother, and lung cancer in her paternal aunt.
In January 2020, she presented at the hospital with fatigue, mild hypoxia, and hyperglycemia and she was admitted for influenza that progressed to pneumonia. During this admission, she was reported to have a new diagnosis of DM type 2. At that time, her body mass index was 23.49. Her glycated hemoglobin (A1c) was 9.5% (reference range, 4-5.6%) and her blood glucose level was greater than 400 mg/dL (22.2 mmol/L) (reference range, 70-99 mg/dL [3.9-5.5 mmol/L]) at admission, which normalized to around 200 mg/dL (11.1 mmol/L) with insulin. She was discharged on metformin 1000 mg twice a day. Over the years, she was on many different doses of insulin and sodium glucose cotransporter 2 inhibitor (SGLT-2i) without sufficient control of her glucose levels. Except for when she was admitted, pembrolizumab was continued from August 2019 to February 2023 and stopped then due to her admissions and weakness. In April 2023, an endocrinologist was consulted during another hospitalization for SGLT-2i-induced diabetic ketoacidosis (DKA), initially thought to be from her empagliflozin. She was labeled with brittle diabetes due to her labile glucose and frequent severe hypoglycemia. Her A1c was 7.8%. Due to the fact that her new diagnosis of DM occurred after being on pembrolizumab and her frequent DKA with labile glucose, autoimmune DM from pembrolizumab was considered. These chronological events along with key labs and glucose trends over time are summarized in Fig. 1.
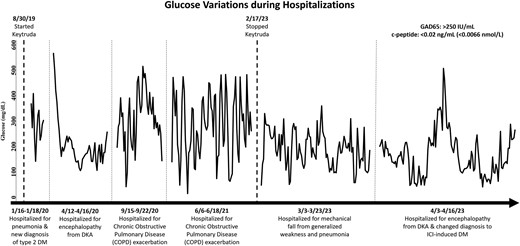
Timeline of glucose control during hospitalizations in relation to the use of pembrolizumab and labs leading to the diagnosis of ICI-induced DM.
Diagnostic Assessment
She has had frequent admissions for either DKA or hypoglycemia which was thought to be due to nonadherence to medications, infections, and other medications, such as steroids. Labs from August 2022 showed a C-peptide level less than 0.02 ng/mL (0.0066 nmol/L), but the patient did not follow up with the endocrinologist for the results due to new hospital admissions. Her A1c continued to fluctuate around 7% from 2020 to 2023. Her last dose of pembrolizumab was in February 2023. During the recent admission in April 2023, we ordered a test of her glutamic acid decarboxylase-65 (GAD65) autoantibodies, which was found to be positive for GAD65 above 250 [IU]/mL. Adrenal function and thyroid dysfunction were both checked. She had normal adrenal function and stable primary hypothyroidism with a morning cortisol level of 15.3 µg/dL (422.127 nmol/L) (reference range, 3.7-19.4 µg/dL [102.083-535.246 nmol/L]) and fT4 of 1.08 ng/dL (13.899 pmol/L) (reference range, 0.93-1.70 ng/dL [11.969-21.879 pmol/L]).
Treatment
She was discharged to a skilled nursing facility on glargine 6 units every morning with sliding scale lispro insulin (SSI) of 1 unit for every 50 above 200 mg/dL (11.1 mmol/L) of glucose 3 times a day before meals and at bedtime while she still had poor oral intake.
Outcome and Follow-Up
The patient followed up in the outpatient endocrinology clinic in early June 2023. She reports some difficulties managing her DM due to some mild hypoglycemic episodes and lack of appetite from her other chronic conditions; however, she rarely had hyperglycemic episodes greater than 300 mg/dL (16.65 mmol/L) as before. The current regimen is now glargine 12 units every morning, lispro 1 unit for every 15 g of carbohydrates, and SSI 1 unit for every 50 above 150 mg/dL (8.3 mmol/L) of glucose before every meal and 1 unit for every 50 above 200 mg/dL (11.1 mmol/L) of glucose at bedtime. Due to her inconsistent oral intake, her total daily insulin dose was not able to be obtained. Her A1c has decreased to 6.9% from 7.8% since April 2023 before Dexcom G6 was ordered to assist her with diabetes management at home in June 2023.
Discussion
Pembrolizumab is a PD-1 inhibitor that is used in the treatment of non-small cell lung cancer (NSCLC) among other indications, such as for melanoma and Hodgkin lymphoma [3]. Clinically, pembrolizumab has been known to reduce tumor progression and improve survival by enhancing immune surveillance; however, the risk of IAEs has been previously reported.
One study noted that the median time interval for the development of DM after initiation of an ICI was around 5 months. Out of 1444 patients treated with PD-1 inhibitors, 12 patients developed new-onset insulin-dependent diabetes, of whom, 6 patients have measured C-peptide, 7 patients have measured pancreatic enzymes, and 7 patients have been tested for type 1 diabetes antibodies. Notably, 57% of the tested patients had an antibody against GAD65 and 83% of those tested had low or undetectable C-peptide levels [1]. Likewise, our patient had an elevated GAD65 autoantibody of above 250 and a low C-peptide level of less than 0.02 ng/mL (0.0066 nmol/L). The lack of detectable C-peptide suggests that the patient has deficient insulin production. Although negative autoantibodies do not exclude autoimmune diabetes, the presence of these markers is correlated to a higher positive predictive value [4]. Taking into consideration the presence of antibodies to GAD65, the etiology of this patient's diabetes is most likely from deficient insulin production from impaired function of islet beta cells, like autoimmune type 1 diabetes.
Notably, the incidence of most cancers, like that of type 2 diabetes, increases with age. Thus, ICI-induced DM can be missed as type 2 DM and can present at any age when the medications are started [5]. ICI-induced DM arises from immune system dysregulation and destruction of the pancreatic islet beta cells. In ICI-induced DM, the proposed pathogenesis is a combination of genetically predisposed individuals who develop autoreactive T cells against beta cells in the pancreas in response to an environmental trigger such as anti-PD-1/PD-L1 therapy. This allows the innate immunity to target and cause beta-cell destruction [6].
One of the most severe, life-threatening complications of insulin deficiency is DKA. Patients generally present with a clinical triad of acidosis, ketosis, and hyperglycemia greater than 250 mg/dL (13.875 mmol/L) [7]. Of all precipitants, some of the most common culprits are surgery, trauma, dehydration, or infection [8]. The patient in our case study was initially evaluated to have multiple DKA episodes with various possible precipitating factors including medication noncompliance and infection. Despite trials of diabetic medications, she continued to have poor glycemic control and DKA until the right diagnosis was made and the correct therapy was applied.
Given the broader utilization of pembrolizumab in anticancer regimens for multiple cancer types, the associated IAEs, including autoimmune DM, are expected to be encountered more often. Although ICI-induced DM is rare, it can be life-threatening without early recognition. Currently, there is a lack of standard screening protocol. In addition, the onset of this unique type of DM is often acute, which means that the A1c test is not a good screening tool. As a result, this leads to incorrect diagnosis, delayed proper treatment, and possible interruption of effective cancer therapy with ICIs due to uncontrolled DM and its complications like in this case. This patient has been taking imatinib, a tyrosine kinase inhibitor, and chemotherapy carboplatin and taxol, but whether these medications have any direct interaction with ICIs to further enhance the development of autoimmune DM in this case is unclear. Nevertheless, raising more awareness, having multidisciplinary teamwork, and advocating earlier screening are strongly recommended.
Learning Points
New-onset DM in patients with cancer carries a broad differential diagnosis.
Clinicians should be aware of the risk of ICIs causing autoimmune DM to prompt early identification with appropriate testing (eg, GAD antibodies, hemoglobin A1c, C-peptide) to reduce the risk of recurrent DKA and facilitate prompt initiation of insulin treatment. Earlier screening protocol development and multidisciplinary collaboration are strongly recommended.
ICIs can not only cause problems with the pancreas, but also impacts on other endocrine glands should be considered, such as on the thyroid, adrenal, and pituitary.
Contributors
All authors made individual contributions to authorship. S.M. and M.T. were involved in the diagnosis and management of this patient. M.T., C.N., and S.M. were involved in the manuscript preparation and submission. All authors reviewed and approved the final draft.
Funding
No public or commercial funding.
Disclosures
None declared.
Informed Patient Consent for Publication
Signed informed consent obtained directly from the patient.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.
References
Abbreviations
- A1c
glycated hemoglobin
- DKA
diabetic ketoacidosis
- DM
diabetes mellitus
- GAD65
glutamic acid decarboxylase 65-kilodalton isoform
- A1c
glycated hemoglobin
- IAE
immune-related adverse event
- ICI
immune checkpoint inhibitor
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death ligand 1
- SCC
squamous cell carcinoma
- SGLT-2i
sodium glucose cotransporter 2 inhibitor
- SSI
sliding scale lispro insulin



