-
PDF
- Split View
-
Views
-
Cite
Cite
John W. Funder, Robert M. Carey, Carlos Fardella, Celso E. Gomez-Sanchez, Franco Mantero, Michael Stowasser, William F. Young, Victor M. Montori, Case Detection, Diagnosis, and Treatment of Patients with Primary Aldosteronism: An Endocrine Society Clinical Practice Guideline, The Journal of Clinical Endocrinology & Metabolism, Volume 93, Issue 9, 1 September 2008, Pages 3266–3281, https://doi.org/10.1210/jc.2008-0104
Close - Share Icon Share
Objective: Our objective was to develop clinical practice guidelines for the diagnosis and treatment of patients with primary aldosteronism.
Participants: The Task Force comprised a chair, selected by the Clinical Guidelines Subcommittee (CGS) of The Endocrine Society, six additional experts, one methodologist, and a medical writer. The Task Force received no corporate funding or remuneration.
Evidence: Systematic reviews of available evidence were used to formulate the key treatment and prevention recommendations. We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) group criteria to describe both the quality of evidence and the strength of recommendations. We used “recommend” for strong recommendations and “suggest” for weak recommendations.
Consensus Process: Consensus was guided by systematic reviews of evidence and discussions during one group meeting, several conference calls, and multiple e-mail communications. The drafts prepared by the task force with the help of a medical writer were reviewed successively by The Endocrine Society’s CGS, Clinical Affairs Core Committee (CACC), and Council. The version approved by the CGS and CACC was placed on The Endocrine Society’s Web site for comments by members. At each stage of review, the Task Force received written comments and incorporated needed changes.
Conclusions: We recommend case detection of primary aldosteronism be sought in higher risk groups of hypertensive patients and those with hypokalemia by determining the aldosterone-renin ratio under standard conditions and that the condition be confirmed/excluded by one of four commonly used confirmatory tests. We recommend that all patients with primary aldosteronism undergo adrenal computed tomography as the initial study in subtype testing and to exclude adrenocortical carcinoma. We recommend the presence of a unilateral form of primary aldosteronism should be established/excluded by bilateral adrenal venous sampling by an experienced radiologist and, where present, optimally treated by laparoscopic adrenalectomy. We recommend that patients with bilateral adrenal hyperplasia, or those unsuitable for surgery, optimally be treated medically by mineralocorticoid receptor antagonists.
Summary of Recommendations
1.0 Case detection
1.1 We recommend the case detection of primary aldosteronism (PA) in patient groups with relatively high prevalence of PA. (1|⊕⊕OO) These include patients with Joint National Commission stage 2 (>160–179/100–109 mm Hg), stage 3 (>180/110 mm Hg), or drug-resistant hypertension; hypertension and spontaneous or diuretic-induced hypokalemia; hypertension with adrenal incidentaloma; or hypertension and a family history of early-onset hypertension or cerebrovascular accident at a young age (<40 yr). We also recommend case detection for all hypertensive first-degree relatives of patients with PA. (1|⊕OOO)
1.2 We recommend use of the plasma aldosterone to renin ratio (ARR) to detect cases of PA in these patient groups. (1|⊕⊕OO)
2.0 Case confirmation
2.1 Instead of proceeding directly to subtype classification, we recommend that patients with a positive ARR undergo testing, by any of four confirmatory tests, to definitively confirm or exclude the diagnosis. (1|⊕⊕OO)
3.0 Subtype classification
3.1 We recommend that all patients with PA undergo an adrenal computed tomography (CT) scan as the initial study in subtype testing and to exclude large masses that may represent adrenocortical carcinoma. (1|⊕⊕OO)
3.2 We recommend that, when surgical treatment is practicable and desired by the patient, the distinction between unilateral and bilateral adrenal disease be made by adrenal venous sampling (AVS) by an experienced radiologist. (1|⊕⊕⊕O)
3.3 In patients with onset of confirmed PA earlier than at 20 yr of age and in those who have a family history of PA or of strokes at young age (<40 yr), we suggest genetic testing for glucocorticoid-remediable aldosteronism (GRA). (2|⊕OOO)
4.0 Treatment
4.1 We recommend that treatment by unilateral laparoscopic adrenalectomy be offered to patients with documented unilateral PA [i.e. aldosterone-producing adenoma (APA) or unilateral adrenal hyperplasia (UAH)]. (1|⊕⊕OO) If a patient is unable or unwilling to undergo surgery, we recommend medical treatment with a mineralocorticoid receptor (MR) antagonist. (1|⊕⊕OO)
4.2 In patients with PA due to bilateral adrenal disease, we recommend medical treatment with an MR antagonist (1|⊕⊕ OO); we suggest spironolactone as the primary agent with eplerenone as an alternative. (2|⊕OOO)
4.3 In patients with GRA, we recommend the use of the lowest dose of glucocorticoid that can normalize blood pressure and serum potassium levels rather than first-line treatment with an MR antagonist. (1|⊕OOO)
Method of Development of Evidence-Based Guidelines
The Clinical Guidelines Subcommittee of The Endocrine Society deemed detection, diagnosis, and treatment of patients with PA a priority area in need of practice guidelines and appointed a seven-member Task Force to formulate evidence-based recommendations. The Task Force followed the approach recommended by the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) group, an international group with expertise in development and implementation of evidence-based guidelines (1).
The Task Force used the best available research evidence that members identified to inform the recommendations and consistent language and graphical descriptions of both the strength of a recommendation and the quality of evidence. In terms of the strength of the recommendation, strong recommendations use the phrase “we recommend” and the number 1, and weak recommendations use the phrase “we suggest” and the number 2. Cross-filled circles indicate the quality of the evidence, such that ⊕OOO denotes very low quality evidence; ⊕⊕OO, low quality; ⊕⊕⊕O, moderate quality; and ⊕⊕⊕⊕, high quality. The Task Force has confidence that patients who receive care according to the strong recommendations will derive, on average, more good than harm. Weak recommendations require more careful consideration of the patient’s circumstances, values, and preferences to determine the best course of action. A detailed description of this grading scheme has been published elsewhere (2).
Linked to each recommendation is a description of the evidence, values that panelists considered in making the recommendation (when making these explicit was necessary), and remarks, a section in which panelists offer technical suggestions for testing conditions, dosing, and monitoring. These technical comments reflect the best available evidence applied to a typical patient. Often, this evidence comes from the unsystematic observations of the panelists and should, therefore, be considered suggestions.
Definition and Clinical Significance of PA
What is PA?
PA is a group of disorders in which aldosterone production is inappropriately high, relatively autonomous from the renin-angiotensin system, and nonsuppressible by sodium loading. Such inappropriate production of aldosterone causes cardiovascular damage, suppression of plasma renin, hypertension, sodium retention, and potassium excretion that if prolonged and severe may lead to hypokalemia. PA is commonly caused by an adrenal adenoma, by unilateral or bilateral adrenal hyperplasia, or in rare cases by the inherited condition of GRA.
How common is PA?
Most experts previously described PA in less than 1% of patients with mild-to-moderate essential hypertension and had assumed hypokalemia was a sine qua non for diagnosis (3–9). Accumulating evidence has challenged these assumptions. Cross-sectional and prospective studies report PA in more than 10% of hypertensive patients, both in general and in specialty settings (10–18).
How frequent is hypokalemia in PA?
In recent studies, only a minority of patients with PA (9–37%) had hypokalemia (19). Thus, normokalemic hypertension constitutes the most common presentation of the disease, with hypokalemia probably present in only the more severe cases. Half the patients with an APA and 17% of those with idiopathic hyperaldosteronism (IHA) had serum potassium concentrations less than 3.5 mmol/liter (17, 20). Thus, the presence of hypokalemia has low sensitivity and specificity and a low positive predictive value for the diagnosis of PA.
Why is PA important?
This condition is important not only because of its prevalence but also because PA patients have higher cardiovascular morbidity and mortality than age- and sex-matched patients with essential hypertension and the same degree of blood pressure elevation (21, 22). Furthermore, specific treatments are available that ameliorate the impact of this condition on patient-important outcomes.
1.0 Case Detection
1.1 We recommend the case detection of PA in patient groups with relatively high prevalence of PA (listed in Table 1) (Fig. 1). (1|⊕⊕OO) These include patients with Joint National Commission stage 2 (>160–179/100–109 mm Hg), stage 3 (>180/110 mm Hg), or drug-resistant hypertension; hypertension and spontaneous or diuretic-induced hypokalemia; hypertension with adrenal incidentaloma; or hypertension and a family history of early-onset hypertension or cerebrovascular accident at a young age (<40 yr). We also recommend case detection for all hypertensive first-degree relatives of patients with PA. (1|⊕OOO)
| Patient group . | Prevalence . |
|---|---|
| Moderate/severe hypertension. The prevalence rates cited here are from Mosso et al. (16). Others have reported similar estimates (18, 23–25). The classification of blood pressure for adults (aged 18 yr and older) was based on the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, which establishes three different stages: stage 1, SBP 140–159, DBP 90–99; stage 2, SBP 160–179, DBP 100–109; stage 3, SBP > 180, DBP > 110 (10). When SBP and DBP were in different categories, the higher category was selected to classify the individual’s blood pressure status. | Overall, 6.1%; stage 1 (mild), 2%; stage 2 (moderate), 8%; stage 3 (severe), 13% |
| Resistant hypertension, defined as SBP > 140 and DBP > 90 despite treatment with three hypertensive medications. The prevalence rates cited here are from Refs. 26–31. | 17–23% |
| Hypertensive patients with spontaneous or diuretic-induced hypokalemia. | Specific prevalence figures are not available, but PA is more frequently found in this group. |
| Hypertension with adrenal incidentaloma (32–37), defined as an adrenal mass detected incidentally during imaging performed for extraadrenal reasons. | Median, 2% (range, 1.1–10%) One retrospective study that excluded patients with hypokalemia and severe hypertension found APA in 16 of 1004 subjects (37). |
| Patient group . | Prevalence . |
|---|---|
| Moderate/severe hypertension. The prevalence rates cited here are from Mosso et al. (16). Others have reported similar estimates (18, 23–25). The classification of blood pressure for adults (aged 18 yr and older) was based on the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, which establishes three different stages: stage 1, SBP 140–159, DBP 90–99; stage 2, SBP 160–179, DBP 100–109; stage 3, SBP > 180, DBP > 110 (10). When SBP and DBP were in different categories, the higher category was selected to classify the individual’s blood pressure status. | Overall, 6.1%; stage 1 (mild), 2%; stage 2 (moderate), 8%; stage 3 (severe), 13% |
| Resistant hypertension, defined as SBP > 140 and DBP > 90 despite treatment with three hypertensive medications. The prevalence rates cited here are from Refs. 26–31. | 17–23% |
| Hypertensive patients with spontaneous or diuretic-induced hypokalemia. | Specific prevalence figures are not available, but PA is more frequently found in this group. |
| Hypertension with adrenal incidentaloma (32–37), defined as an adrenal mass detected incidentally during imaging performed for extraadrenal reasons. | Median, 2% (range, 1.1–10%) One retrospective study that excluded patients with hypokalemia and severe hypertension found APA in 16 of 1004 subjects (37). |
DBP, Diastolic blood pressure; SBP, systolic blood pressure.
| Patient group . | Prevalence . |
|---|---|
| Moderate/severe hypertension. The prevalence rates cited here are from Mosso et al. (16). Others have reported similar estimates (18, 23–25). The classification of blood pressure for adults (aged 18 yr and older) was based on the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, which establishes three different stages: stage 1, SBP 140–159, DBP 90–99; stage 2, SBP 160–179, DBP 100–109; stage 3, SBP > 180, DBP > 110 (10). When SBP and DBP were in different categories, the higher category was selected to classify the individual’s blood pressure status. | Overall, 6.1%; stage 1 (mild), 2%; stage 2 (moderate), 8%; stage 3 (severe), 13% |
| Resistant hypertension, defined as SBP > 140 and DBP > 90 despite treatment with three hypertensive medications. The prevalence rates cited here are from Refs. 26–31. | 17–23% |
| Hypertensive patients with spontaneous or diuretic-induced hypokalemia. | Specific prevalence figures are not available, but PA is more frequently found in this group. |
| Hypertension with adrenal incidentaloma (32–37), defined as an adrenal mass detected incidentally during imaging performed for extraadrenal reasons. | Median, 2% (range, 1.1–10%) One retrospective study that excluded patients with hypokalemia and severe hypertension found APA in 16 of 1004 subjects (37). |
| Patient group . | Prevalence . |
|---|---|
| Moderate/severe hypertension. The prevalence rates cited here are from Mosso et al. (16). Others have reported similar estimates (18, 23–25). The classification of blood pressure for adults (aged 18 yr and older) was based on the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, which establishes three different stages: stage 1, SBP 140–159, DBP 90–99; stage 2, SBP 160–179, DBP 100–109; stage 3, SBP > 180, DBP > 110 (10). When SBP and DBP were in different categories, the higher category was selected to classify the individual’s blood pressure status. | Overall, 6.1%; stage 1 (mild), 2%; stage 2 (moderate), 8%; stage 3 (severe), 13% |
| Resistant hypertension, defined as SBP > 140 and DBP > 90 despite treatment with three hypertensive medications. The prevalence rates cited here are from Refs. 26–31. | 17–23% |
| Hypertensive patients with spontaneous or diuretic-induced hypokalemia. | Specific prevalence figures are not available, but PA is more frequently found in this group. |
| Hypertension with adrenal incidentaloma (32–37), defined as an adrenal mass detected incidentally during imaging performed for extraadrenal reasons. | Median, 2% (range, 1.1–10%) One retrospective study that excluded patients with hypokalemia and severe hypertension found APA in 16 of 1004 subjects (37). |
DBP, Diastolic blood pressure; SBP, systolic blood pressure.
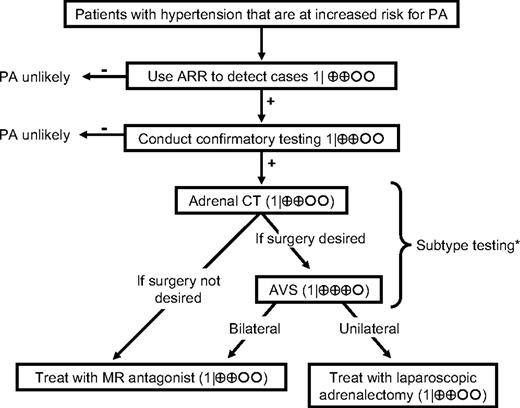
Algorithm for the detection, confirmation, subtype testing, and treatment of PA. We recommend the case detection of PA in patient groups with relatively high prevalence of PA (1|⊕⊕OO); these include patients with moderate, severe, or resistant hypertension, spontaneous or diuretic-induced hypokalemia, hypertension with adrenal incidentaloma, or a family history of early-onset hypertension or cerebrovascular accident at a young age (<40 yr). We recommend use of the plasma ARR to detect cases of PA in these patient groups (1|⊕⊕OO). We recommend that patients with a positive ARR undergo testing, using any of four confirmatory tests, to definitively confirm or exclude the diagnosis (1|⊕⊕OO). We recommend that all patients with PA undergo an adrenal CT scan as the initial study in subtype testing and to exclude adrenocortical carcinoma (1|⊕⊕OO). When surgical treatment is practicable and desired by the patient, the distinction between unilateral and bilateral adrenal disease should be made by AVS (1|⊕⊕⊕O). We recommend that treatment by unilateral laparoscopic adrenalectomy be offered to patients with AVS-documented unilateral APA (1|⊕⊕OO). If a patient is unable or unwilling to undergo surgery, we recommend medical treatment with an MR antagonist (1|⊕⊕OO). In patients with PA due to bilateral adrenal disease, we recommend medical treatment with an MR antagonist (1|⊕OOO). *, In patients with confirmed PA who have a family history of PA or of strokes at young age (<40 yr), or with onset of hypertension earlier than at 20 yr of age, we suggest genetic testing for GRA (2|⊕OOO). In patients with GRA, we recommend the use of the lowest dose of glucocorticoid receptor agonist that can normalize blood pressure and serum potassium levels (1|⊕OOO).
1.1 Evidence
Indirect evidence links the detection of PA with improved patient outcomes. There are no clinical trials of screening that measure the impact of this practice on morbidity, mortality, or quality-of-life outcomes. Patients could potentially be harmed by the work-up and treatment (i.e. by withdrawal of antihypertensive medication, invasive vascular examination, or adrenalectomy) aimed at vascular protection along with easier and better blood pressure control. There is strong evidence linking improved blood pressure control and reduction in aldosterone levels to improved cardiac and cerebrovascular outcomes (38). Until prospective studies inform us differently, we recommend that all hypertensive first-degree relatives of patients with PA undergo ARR testing.
1.1 Values
Our recommendation to detect cases of PA places a high value on avoiding the risks associated with missing the diagnosis (and thus forgoing the opportunity of a surgical cure or improved control of hypertension through specific medical treatment) and a lower value on avoiding the risk of falsely classifying a hypertensive patient as having PA and exposing him or her to additional diagnostic testing.
1.2 We recommend use of the plasma ARR to detect cases of PA in these patient groups (Fig. 1). (1|⊕⊕OO)
1.2 Evidence
The ARR is currently the most reliable available means of screening for PA. Although valid estimates of test characteristics of the ARR are lacking (mainly due to limitations in the design of studies that have addressed this issue) (39), numerous studies have demonstrated the ARR to be superior to measurement of potassium or aldosterone (both of which have lower sensitivity) or of renin (which is less specific) in isolation (40–42).
Like all biochemical case detection tests, the ARR is not without false positives and negatives (17, 18, 39, 43–45). Table 2 documents the effect of medications and conditions on the ARR. The ARR should therefore be regarded as a detection test only and should be repeated if the initial results are inconclusive or difficult to interpret because of suboptimal sampling conditions (e.g. maintenance of some medications listed in Table 2).
Medications that have minimal effects on plasma aldosterone levels and can be used to control hypertension during case finding and confirmatory testing for PA
| Drug . | Class . | Usual dose . | Comments . |
|---|---|---|---|
| Verapamil slow-release | Non-dihydropyridine calcium channel antagonist | 90–120 mg twice daily | Use singly or in combination with the other agents listed in this table. |
| Hydralazine | Vasodilator | 10–12.5 mg twice daily, increasing as required | Commence verapamil slow release first to prevent reflex tachycardia. Commencement at low doses reduces risk of side effects (including headaches, flushing, and palpitations). |
| Prazosin hydrochloride | α-Adrenergic blocker | 0.5–1 mg two to three times daily, increasing as required | Monitor for postural hypotension |
| Doxazosin mesylate | α-Adrenergic blocker | 1–2 mg once daily, increasing as required | Monitor for postural hypotension |
| Terazosin hydrochloride | α-Adrenergic blocker | 1–2 mg once daily, increasing as required | Monitor for postural hypotension |
| Drug . | Class . | Usual dose . | Comments . |
|---|---|---|---|
| Verapamil slow-release | Non-dihydropyridine calcium channel antagonist | 90–120 mg twice daily | Use singly or in combination with the other agents listed in this table. |
| Hydralazine | Vasodilator | 10–12.5 mg twice daily, increasing as required | Commence verapamil slow release first to prevent reflex tachycardia. Commencement at low doses reduces risk of side effects (including headaches, flushing, and palpitations). |
| Prazosin hydrochloride | α-Adrenergic blocker | 0.5–1 mg two to three times daily, increasing as required | Monitor for postural hypotension |
| Doxazosin mesylate | α-Adrenergic blocker | 1–2 mg once daily, increasing as required | Monitor for postural hypotension |
| Terazosin hydrochloride | α-Adrenergic blocker | 1–2 mg once daily, increasing as required | Monitor for postural hypotension |
Medications that have minimal effects on plasma aldosterone levels and can be used to control hypertension during case finding and confirmatory testing for PA
| Drug . | Class . | Usual dose . | Comments . |
|---|---|---|---|
| Verapamil slow-release | Non-dihydropyridine calcium channel antagonist | 90–120 mg twice daily | Use singly or in combination with the other agents listed in this table. |
| Hydralazine | Vasodilator | 10–12.5 mg twice daily, increasing as required | Commence verapamil slow release first to prevent reflex tachycardia. Commencement at low doses reduces risk of side effects (including headaches, flushing, and palpitations). |
| Prazosin hydrochloride | α-Adrenergic blocker | 0.5–1 mg two to three times daily, increasing as required | Monitor for postural hypotension |
| Doxazosin mesylate | α-Adrenergic blocker | 1–2 mg once daily, increasing as required | Monitor for postural hypotension |
| Terazosin hydrochloride | α-Adrenergic blocker | 1–2 mg once daily, increasing as required | Monitor for postural hypotension |
| Drug . | Class . | Usual dose . | Comments . |
|---|---|---|---|
| Verapamil slow-release | Non-dihydropyridine calcium channel antagonist | 90–120 mg twice daily | Use singly or in combination with the other agents listed in this table. |
| Hydralazine | Vasodilator | 10–12.5 mg twice daily, increasing as required | Commence verapamil slow release first to prevent reflex tachycardia. Commencement at low doses reduces risk of side effects (including headaches, flushing, and palpitations). |
| Prazosin hydrochloride | α-Adrenergic blocker | 0.5–1 mg two to three times daily, increasing as required | Monitor for postural hypotension |
| Doxazosin mesylate | α-Adrenergic blocker | 1–2 mg once daily, increasing as required | Monitor for postural hypotension |
| Terazosin hydrochloride | α-Adrenergic blocker | 1–2 mg once daily, increasing as required | Monitor for postural hypotension |
1.2 Values
Similar values underpin our recommendation to target subjects in groups with documented high prevalence of PA and to test them by ARR. In particular, this recommendation acknowledges the costs currently associated with ARR testing of all patients with essential hypertension. Against this recommendation for selective testing, however, must be weighed the risk of missing or at least delaying the diagnosis of PA in some hypertensive individuals. The consequences of this may include the later development of more severe and resistant hypertension resulting from failure to lower levels of aldosterone or to block its actions. Furthermore, duration of hypertension has been reported by several investigators to be a negative predictor of outcome after unilateral adrenalectomy for APA (46, 47), suggesting that delays in diagnosis may result in a poorer response to specific treatment once PA is finally diagnosed.
1.2 Remarks: technical aspects required for the correct implementation of recommendation 1.2
Testing conditions (Tables 3 and 4)
| ARR measurement . |
|---|
| A. Preparation for ARR measurement: agenda |
| 1. Attempt to correct hypokalemia, after measuring plasma potassium in blood collected slowly with a syringe and needle (preferably not a Vacutainer to minimize the risk of spuriously raising potassium); avoid fist clenching during collection; wait at least 5 sec after tourniquet release (if used to achieve insertion of needle) and ensure separation of plasma from cells within 30 min of collection. |
| 2. Encourage patient to liberalize (rather than restrict) sodium intake. |
| 3. Withdraw agents that markedly affect the ARR (48) for at least 4 wk: |
| a. Spironolactone, eplerenone, amiloride, and triamterene |
| b. Potassium-wasting diuretics |
| c. Products derived from licorice root (e.g. confectionary licorice, chewing tobacco) |
| 4. If the results of ARR off the above agents are not diagnostic, and if hypertension can be controlled with relatively noninterfering medications (see Table 2), withdraw other medications that may affect the ARR (48) for at least 2 wk: |
| a. β-Adrenergic blockers, central α-2 agonists (e.g. clonidine and α-methyldopa), nonsteroidal antiinflammatory drugs |
| b. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, renin inhibitors, dihydropyridine calcium channel antagonists |
| 5. If necessary to maintain hypertension control, commence other antihypertensive medications that have lesser effects on the ARR [e.g. verapamil slow-release, hydralazine (with verapamil slow-release, to avoid reflex tachycardia), prazosin, doxazosin, terazosin; see Table 2]. |
| 6. Establish OC and HRT status, because estrogen-containing medications may lower DRC and cause false-positive ARR when DRC (rather than PRA) is measured. Do not withdraw OC unless confident of alternative effective contraception. |
| B. Conditions for collection of blood |
| 1. Collect blood mid-morning, after the patient has been up (sitting, standing, or walking) for at least 2 h and seated for 5–15 min. |
| 2. Collect blood carefully, avoiding stasis and hemolysis (see A.1 above). |
| 3. Maintain sample at room temperature (and not on ice, because this will promote conversion of inactive to active renin) during delivery to laboratory and before centrifugation and rapid freezing of plasma component pending assay. |
| C. Factors to take into account when interpreting results (see Table 4) |
| 1. Age: in patients aged >65 yr, renin can be lowered more than aldosterone by age alone, leading to a raised ARR |
| 2. Time of day, recent diet, posture, and length of time in that posture |
| 3. Medications |
| 4. Method of blood collection, including any difficulty doing so |
| 5. Level of potassium |
| 6. Level of creatinine (renal failure can lead to false-positive ARR) |
| ARR measurement . |
|---|
| A. Preparation for ARR measurement: agenda |
| 1. Attempt to correct hypokalemia, after measuring plasma potassium in blood collected slowly with a syringe and needle (preferably not a Vacutainer to minimize the risk of spuriously raising potassium); avoid fist clenching during collection; wait at least 5 sec after tourniquet release (if used to achieve insertion of needle) and ensure separation of plasma from cells within 30 min of collection. |
| 2. Encourage patient to liberalize (rather than restrict) sodium intake. |
| 3. Withdraw agents that markedly affect the ARR (48) for at least 4 wk: |
| a. Spironolactone, eplerenone, amiloride, and triamterene |
| b. Potassium-wasting diuretics |
| c. Products derived from licorice root (e.g. confectionary licorice, chewing tobacco) |
| 4. If the results of ARR off the above agents are not diagnostic, and if hypertension can be controlled with relatively noninterfering medications (see Table 2), withdraw other medications that may affect the ARR (48) for at least 2 wk: |
| a. β-Adrenergic blockers, central α-2 agonists (e.g. clonidine and α-methyldopa), nonsteroidal antiinflammatory drugs |
| b. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, renin inhibitors, dihydropyridine calcium channel antagonists |
| 5. If necessary to maintain hypertension control, commence other antihypertensive medications that have lesser effects on the ARR [e.g. verapamil slow-release, hydralazine (with verapamil slow-release, to avoid reflex tachycardia), prazosin, doxazosin, terazosin; see Table 2]. |
| 6. Establish OC and HRT status, because estrogen-containing medications may lower DRC and cause false-positive ARR when DRC (rather than PRA) is measured. Do not withdraw OC unless confident of alternative effective contraception. |
| B. Conditions for collection of blood |
| 1. Collect blood mid-morning, after the patient has been up (sitting, standing, or walking) for at least 2 h and seated for 5–15 min. |
| 2. Collect blood carefully, avoiding stasis and hemolysis (see A.1 above). |
| 3. Maintain sample at room temperature (and not on ice, because this will promote conversion of inactive to active renin) during delivery to laboratory and before centrifugation and rapid freezing of plasma component pending assay. |
| C. Factors to take into account when interpreting results (see Table 4) |
| 1. Age: in patients aged >65 yr, renin can be lowered more than aldosterone by age alone, leading to a raised ARR |
| 2. Time of day, recent diet, posture, and length of time in that posture |
| 3. Medications |
| 4. Method of blood collection, including any difficulty doing so |
| 5. Level of potassium |
| 6. Level of creatinine (renal failure can lead to false-positive ARR) |
HRT, Hormone replacement therapy; OC, oral contraceptive.
| ARR measurement . |
|---|
| A. Preparation for ARR measurement: agenda |
| 1. Attempt to correct hypokalemia, after measuring plasma potassium in blood collected slowly with a syringe and needle (preferably not a Vacutainer to minimize the risk of spuriously raising potassium); avoid fist clenching during collection; wait at least 5 sec after tourniquet release (if used to achieve insertion of needle) and ensure separation of plasma from cells within 30 min of collection. |
| 2. Encourage patient to liberalize (rather than restrict) sodium intake. |
| 3. Withdraw agents that markedly affect the ARR (48) for at least 4 wk: |
| a. Spironolactone, eplerenone, amiloride, and triamterene |
| b. Potassium-wasting diuretics |
| c. Products derived from licorice root (e.g. confectionary licorice, chewing tobacco) |
| 4. If the results of ARR off the above agents are not diagnostic, and if hypertension can be controlled with relatively noninterfering medications (see Table 2), withdraw other medications that may affect the ARR (48) for at least 2 wk: |
| a. β-Adrenergic blockers, central α-2 agonists (e.g. clonidine and α-methyldopa), nonsteroidal antiinflammatory drugs |
| b. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, renin inhibitors, dihydropyridine calcium channel antagonists |
| 5. If necessary to maintain hypertension control, commence other antihypertensive medications that have lesser effects on the ARR [e.g. verapamil slow-release, hydralazine (with verapamil slow-release, to avoid reflex tachycardia), prazosin, doxazosin, terazosin; see Table 2]. |
| 6. Establish OC and HRT status, because estrogen-containing medications may lower DRC and cause false-positive ARR when DRC (rather than PRA) is measured. Do not withdraw OC unless confident of alternative effective contraception. |
| B. Conditions for collection of blood |
| 1. Collect blood mid-morning, after the patient has been up (sitting, standing, or walking) for at least 2 h and seated for 5–15 min. |
| 2. Collect blood carefully, avoiding stasis and hemolysis (see A.1 above). |
| 3. Maintain sample at room temperature (and not on ice, because this will promote conversion of inactive to active renin) during delivery to laboratory and before centrifugation and rapid freezing of plasma component pending assay. |
| C. Factors to take into account when interpreting results (see Table 4) |
| 1. Age: in patients aged >65 yr, renin can be lowered more than aldosterone by age alone, leading to a raised ARR |
| 2. Time of day, recent diet, posture, and length of time in that posture |
| 3. Medications |
| 4. Method of blood collection, including any difficulty doing so |
| 5. Level of potassium |
| 6. Level of creatinine (renal failure can lead to false-positive ARR) |
| ARR measurement . |
|---|
| A. Preparation for ARR measurement: agenda |
| 1. Attempt to correct hypokalemia, after measuring plasma potassium in blood collected slowly with a syringe and needle (preferably not a Vacutainer to minimize the risk of spuriously raising potassium); avoid fist clenching during collection; wait at least 5 sec after tourniquet release (if used to achieve insertion of needle) and ensure separation of plasma from cells within 30 min of collection. |
| 2. Encourage patient to liberalize (rather than restrict) sodium intake. |
| 3. Withdraw agents that markedly affect the ARR (48) for at least 4 wk: |
| a. Spironolactone, eplerenone, amiloride, and triamterene |
| b. Potassium-wasting diuretics |
| c. Products derived from licorice root (e.g. confectionary licorice, chewing tobacco) |
| 4. If the results of ARR off the above agents are not diagnostic, and if hypertension can be controlled with relatively noninterfering medications (see Table 2), withdraw other medications that may affect the ARR (48) for at least 2 wk: |
| a. β-Adrenergic blockers, central α-2 agonists (e.g. clonidine and α-methyldopa), nonsteroidal antiinflammatory drugs |
| b. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, renin inhibitors, dihydropyridine calcium channel antagonists |
| 5. If necessary to maintain hypertension control, commence other antihypertensive medications that have lesser effects on the ARR [e.g. verapamil slow-release, hydralazine (with verapamil slow-release, to avoid reflex tachycardia), prazosin, doxazosin, terazosin; see Table 2]. |
| 6. Establish OC and HRT status, because estrogen-containing medications may lower DRC and cause false-positive ARR when DRC (rather than PRA) is measured. Do not withdraw OC unless confident of alternative effective contraception. |
| B. Conditions for collection of blood |
| 1. Collect blood mid-morning, after the patient has been up (sitting, standing, or walking) for at least 2 h and seated for 5–15 min. |
| 2. Collect blood carefully, avoiding stasis and hemolysis (see A.1 above). |
| 3. Maintain sample at room temperature (and not on ice, because this will promote conversion of inactive to active renin) during delivery to laboratory and before centrifugation and rapid freezing of plasma component pending assay. |
| C. Factors to take into account when interpreting results (see Table 4) |
| 1. Age: in patients aged >65 yr, renin can be lowered more than aldosterone by age alone, leading to a raised ARR |
| 2. Time of day, recent diet, posture, and length of time in that posture |
| 3. Medications |
| 4. Method of blood collection, including any difficulty doing so |
| 5. Level of potassium |
| 6. Level of creatinine (renal failure can lead to false-positive ARR) |
HRT, Hormone replacement therapy; OC, oral contraceptive.
Factors that may affect the ARR and thus lead to false-positive or false-negative results
| Factor . | Effect on aldosterone levels . | Effect on renin levels . | Effect on ARR . |
|---|---|---|---|
| Medications | |||
| β-Adrenergic blockers | ↓ | ↓↓ | ↑ (FP) |
| Central α-2 agonists (e.g. clonidine and α-methyldopa) | ↓ | ↓↓ | ↑ (FP) |
| NSAIDs | ↓ | ↓↓ | ↑ (FP) |
| K+-wasting diuretics | →↑ | ↑↑ | ↓ (FN) |
| K+-sparing diuretics | ↑ | ↑↑ | ↓ (FN) |
| ACE inhibitors | ↓ | ↑↑ | ↓ (FN) |
| ARBs | ↓ | ↑↑ | ↓ (FN) |
| Ca2+ blockers (DHPs) | →↓ | ↑ | ↓ (FN) |
| Renin inhibitors | ↓ | ↓↑a | ↑ (FP)a |
| ↓ (FN)a | |||
| Potassium status | |||
| Hypokalemia | ↓ | →↑ | ↓ (FN) |
| Potassium loading | ↑ | →↓ | ↑ (FP) |
| Dietary sodium | |||
| Sodium restricted | ↑ | ↑↑ | ↓ (FN) |
| Sodium loaded | ↓ | ↓↓ | ↑ (FP) |
| Advancing age | ↓ | ↓↓ | ↑ (FP) |
| Other conditions | |||
| Renal impairment | → | ↓ | ↑ (FP) |
| PHA-2 | → | ↓ | ↑ (FP) |
| Pregnancy | ↑ | ↑↑ | ↓ (FN) |
| Renovascular HT | ↑ | ↑↑ | ↓ (FN) |
| Malignant HT | ↑ | ↑↑ | ↓ (FN) |
| Factor . | Effect on aldosterone levels . | Effect on renin levels . | Effect on ARR . |
|---|---|---|---|
| Medications | |||
| β-Adrenergic blockers | ↓ | ↓↓ | ↑ (FP) |
| Central α-2 agonists (e.g. clonidine and α-methyldopa) | ↓ | ↓↓ | ↑ (FP) |
| NSAIDs | ↓ | ↓↓ | ↑ (FP) |
| K+-wasting diuretics | →↑ | ↑↑ | ↓ (FN) |
| K+-sparing diuretics | ↑ | ↑↑ | ↓ (FN) |
| ACE inhibitors | ↓ | ↑↑ | ↓ (FN) |
| ARBs | ↓ | ↑↑ | ↓ (FN) |
| Ca2+ blockers (DHPs) | →↓ | ↑ | ↓ (FN) |
| Renin inhibitors | ↓ | ↓↑a | ↑ (FP)a |
| ↓ (FN)a | |||
| Potassium status | |||
| Hypokalemia | ↓ | →↑ | ↓ (FN) |
| Potassium loading | ↑ | →↓ | ↑ (FP) |
| Dietary sodium | |||
| Sodium restricted | ↑ | ↑↑ | ↓ (FN) |
| Sodium loaded | ↓ | ↓↓ | ↑ (FP) |
| Advancing age | ↓ | ↓↓ | ↑ (FP) |
| Other conditions | |||
| Renal impairment | → | ↓ | ↑ (FP) |
| PHA-2 | → | ↓ | ↑ (FP) |
| Pregnancy | ↑ | ↑↑ | ↓ (FN) |
| Renovascular HT | ↑ | ↑↑ | ↓ (FN) |
| Malignant HT | ↑ | ↑↑ | ↓ (FN) |
ACE, Angiotensin-converting enzyme; ARB, angiotensin II type 1 receptor blocker; DHP, dihydropyridine; FP, false positive; FN, false negative; HT, hypertension; NSAID, nonsteroidal antiinflammatory drug; PHA-2, pseudohypoaldosteronism type 2 (familial hypertension and hyperkalemia with normal glomerular filtration rate).
Renin inhibitors lower PRA but raise DRC. This would be expected to result in false-positive ARR levels for renin measured as PRA and false negatives for renin measured as DRC.
Factors that may affect the ARR and thus lead to false-positive or false-negative results
| Factor . | Effect on aldosterone levels . | Effect on renin levels . | Effect on ARR . |
|---|---|---|---|
| Medications | |||
| β-Adrenergic blockers | ↓ | ↓↓ | ↑ (FP) |
| Central α-2 agonists (e.g. clonidine and α-methyldopa) | ↓ | ↓↓ | ↑ (FP) |
| NSAIDs | ↓ | ↓↓ | ↑ (FP) |
| K+-wasting diuretics | →↑ | ↑↑ | ↓ (FN) |
| K+-sparing diuretics | ↑ | ↑↑ | ↓ (FN) |
| ACE inhibitors | ↓ | ↑↑ | ↓ (FN) |
| ARBs | ↓ | ↑↑ | ↓ (FN) |
| Ca2+ blockers (DHPs) | →↓ | ↑ | ↓ (FN) |
| Renin inhibitors | ↓ | ↓↑a | ↑ (FP)a |
| ↓ (FN)a | |||
| Potassium status | |||
| Hypokalemia | ↓ | →↑ | ↓ (FN) |
| Potassium loading | ↑ | →↓ | ↑ (FP) |
| Dietary sodium | |||
| Sodium restricted | ↑ | ↑↑ | ↓ (FN) |
| Sodium loaded | ↓ | ↓↓ | ↑ (FP) |
| Advancing age | ↓ | ↓↓ | ↑ (FP) |
| Other conditions | |||
| Renal impairment | → | ↓ | ↑ (FP) |
| PHA-2 | → | ↓ | ↑ (FP) |
| Pregnancy | ↑ | ↑↑ | ↓ (FN) |
| Renovascular HT | ↑ | ↑↑ | ↓ (FN) |
| Malignant HT | ↑ | ↑↑ | ↓ (FN) |
| Factor . | Effect on aldosterone levels . | Effect on renin levels . | Effect on ARR . |
|---|---|---|---|
| Medications | |||
| β-Adrenergic blockers | ↓ | ↓↓ | ↑ (FP) |
| Central α-2 agonists (e.g. clonidine and α-methyldopa) | ↓ | ↓↓ | ↑ (FP) |
| NSAIDs | ↓ | ↓↓ | ↑ (FP) |
| K+-wasting diuretics | →↑ | ↑↑ | ↓ (FN) |
| K+-sparing diuretics | ↑ | ↑↑ | ↓ (FN) |
| ACE inhibitors | ↓ | ↑↑ | ↓ (FN) |
| ARBs | ↓ | ↑↑ | ↓ (FN) |
| Ca2+ blockers (DHPs) | →↓ | ↑ | ↓ (FN) |
| Renin inhibitors | ↓ | ↓↑a | ↑ (FP)a |
| ↓ (FN)a | |||
| Potassium status | |||
| Hypokalemia | ↓ | →↑ | ↓ (FN) |
| Potassium loading | ↑ | →↓ | ↑ (FP) |
| Dietary sodium | |||
| Sodium restricted | ↑ | ↑↑ | ↓ (FN) |
| Sodium loaded | ↓ | ↓↓ | ↑ (FP) |
| Advancing age | ↓ | ↓↓ | ↑ (FP) |
| Other conditions | |||
| Renal impairment | → | ↓ | ↑ (FP) |
| PHA-2 | → | ↓ | ↑ (FP) |
| Pregnancy | ↑ | ↑↑ | ↓ (FN) |
| Renovascular HT | ↑ | ↑↑ | ↓ (FN) |
| Malignant HT | ↑ | ↑↑ | ↓ (FN) |
ACE, Angiotensin-converting enzyme; ARB, angiotensin II type 1 receptor blocker; DHP, dihydropyridine; FP, false positive; FN, false negative; HT, hypertension; NSAID, nonsteroidal antiinflammatory drug; PHA-2, pseudohypoaldosteronism type 2 (familial hypertension and hyperkalemia with normal glomerular filtration rate).
Renin inhibitors lower PRA but raise DRC. This would be expected to result in false-positive ARR levels for renin measured as PRA and false negatives for renin measured as DRC.
The ARR is most sensitive when used in patients from whom samples are collected in the morning after patients have been out of bed for at least 2 h, usually after they have been seated for 5–15 min. Ideally, patients should have unrestricted dietary salt intake before testing. In many cases, the ARR can be confidently interpreted with knowledge of the effect on the ARR of continued medications or suboptimal conditions of testing, avoiding delay and allowing the patient to proceed directly to confirmatory/exclusion testing. Washout of all interfering antihypertensive medications is feasible in patients with mild hypertension but is potentially problematic in others, and perhaps unnecessary in that medications with minimal effect on the ARR can be used in their place (Table 2).
Assay reliability
Although newer techniques are evolving, we prefer to use validated immunometric assays for plasma renin activity (PRA) or direct renin concentration (DRC); PRA takes into account factors (such as estrogen-containing preparations) that affect endogenous substrate levels. Laboratories should use aliquots from human plasma pools, carefully selected to cover the critical range of measurements, rather than the lyophilized controls provided by the manufacturer to monitor intra- and interassay reproducibility and long-term stability. Because the ARR is mathematically highly dependent on renin (49), renin assays should be sufficiently sensitive to measure levels as low as 0.2–0.3 ng/ml·h (DRC 2 mU/liter) (10, 16). For PRA, but not DRC, sensitivity for levels less than 1 ng/ml·h can be improved by prolonging the duration of the assay incubation phase as suggested by Sealey and Laragh (50). Although most laboratories use RIA for plasma and urinary aldosterone, measured levels of standards have been shown to be unacceptably different in some instances (51). Tandem mass spectrometry is increasingly used and has proved to be much more consistent in performance (52).
Interpretation
There are important and confusing differences between laboratories in the methods and units used to report values of renin and aldosterone. For aldosterone, 1 ng/dl converts to 27.7 pmol/liter in Système International (SI) units. For immunometric methods of directly measuring renin concentration, a PRA level of 1 ng/ml·h (12.8 pmol/liter·min in SI units) converts to a DRC of approximately 8.2 mU/liter (5.2 ng/liter in traditional units) when measured by either the Nichols Institute Diagnostics automated chemiluminescence immunoassay (previously widely used but recently withdrawn) or the Bio-Rad Renin II RIA. Because DRC assays are still in evolution, these conversion factors may change. For example, 1 ng/ml·h PRA converts to a DRC of approximately 12 mU/liter (7.6 ng/liter) when measured by the recently introduced and already widely used Diasorin automated chemiluminescence immunoassay. Here, we express aldosterone and PRA levels in conventional units (aldosterone in nanograms per deciliter; PRA in nanograms per milliliter per hour) with SI units for aldosterone and DRC (using the 8.2 conversion factor) given in parentheses. Lack of uniformity in diagnostic protocols and assay methods for ARR measurement has been associated with substantial variability in cutoff values used by different groups ranging from 20–100 (68–338) (11, 14, 15, 19, 29, 53, 54). Most groups, however, use cutoffs of 20–40 (68–135) when testing is performed in the morning on a seated ambulatory patient. Table 5 lists ARR cutoff values using some commonly expressed assay units for plasma aldosterone concentration (PAC), PRA, and direct measurement of plasma renin concentration.
ARR cutoff values, depending on assay and based on whether PAC, PRA, and DRC are measured in conventional or SI units
| . | PRA (ng/ml·h) . | PRA (pmol/liter·min) . | DRCa (mU/liter) . | DRCa (ng/liter) . |
|---|---|---|---|---|
| PAC (ng/dl) | 20 | 1.6 | 2.4 | 3.8 |
| 30b | 2.5 | 3.7 | 5.7 | |
| 40 | 3.1 | 4.9 | 7.7 | |
| PAC (pmol/liter) | 750b | 60 | 91 | 144 |
| 1000 | 80 | 122 | 192 |
| . | PRA (ng/ml·h) . | PRA (pmol/liter·min) . | DRCa (mU/liter) . | DRCa (ng/liter) . |
|---|---|---|---|---|
| PAC (ng/dl) | 20 | 1.6 | 2.4 | 3.8 |
| 30b | 2.5 | 3.7 | 5.7 | |
| 40 | 3.1 | 4.9 | 7.7 | |
| PAC (pmol/liter) | 750b | 60 | 91 | 144 |
| 1000 | 80 | 122 | 192 |
Values shown are on the basis of a conversion factor of PRA (ng/ml·h) to DRC (mU/liter) of 8.2. DRC assays are still in evolution, and in a recently introduced and already commonly used automated DRC assay, the conversion factor is 12 (see text).
The most commonly adopted cutoff values are shown in bold: 30 for PAC and PRA in conventional units (equivalent to 830 when PAC is in SI units) and 750 when PAC is expressed in SI units (equivalent to 27 in conventional units).
ARR cutoff values, depending on assay and based on whether PAC, PRA, and DRC are measured in conventional or SI units
| . | PRA (ng/ml·h) . | PRA (pmol/liter·min) . | DRCa (mU/liter) . | DRCa (ng/liter) . |
|---|---|---|---|---|
| PAC (ng/dl) | 20 | 1.6 | 2.4 | 3.8 |
| 30b | 2.5 | 3.7 | 5.7 | |
| 40 | 3.1 | 4.9 | 7.7 | |
| PAC (pmol/liter) | 750b | 60 | 91 | 144 |
| 1000 | 80 | 122 | 192 |
| . | PRA (ng/ml·h) . | PRA (pmol/liter·min) . | DRCa (mU/liter) . | DRCa (ng/liter) . |
|---|---|---|---|---|
| PAC (ng/dl) | 20 | 1.6 | 2.4 | 3.8 |
| 30b | 2.5 | 3.7 | 5.7 | |
| 40 | 3.1 | 4.9 | 7.7 | |
| PAC (pmol/liter) | 750b | 60 | 91 | 144 |
| 1000 | 80 | 122 | 192 |
Values shown are on the basis of a conversion factor of PRA (ng/ml·h) to DRC (mU/liter) of 8.2. DRC assays are still in evolution, and in a recently introduced and already commonly used automated DRC assay, the conversion factor is 12 (see text).
The most commonly adopted cutoff values are shown in bold: 30 for PAC and PRA in conventional units (equivalent to 830 when PAC is in SI units) and 750 when PAC is expressed in SI units (equivalent to 27 in conventional units).
Some investigators require elevated aldosterone levels in addition to elevated ARR for a positive screening test for PA [usually aldosterone >15 ng/dl (416 pmol/liter)] (55). An alternative approach is to avoid a formal cutoff level for plasma aldosterone but to recognize that the likelihood of a false-positive ARR becomes greater when renin levels are very low (11). Against a formal cutoff level for aldosterone are the findings of several studies. In one study, seated plasma aldosterone levels were less than 15 ng/dl (<416 pmol/liter) in 36% of 74 patients diagnosed with PA after screening positive by ARR defined as more than 30 (>100) and showing failure of aldosterone to suppress during fludrocortisone suppression testing (FST), and in four of 21 patients found by AVS to have unilateral, surgically correctable PA (56). Another study reported plasma aldosterone levels of 9–16 ng/dl (250–440 pmol/liter) in 16 of 37 patients diagnosed with PA by FST (16). Although it would clearly be desirable to provide firm recommendations for ARR and plasma aldosterone cutoffs, the variability of assays between laboratories and the divided literature to date make it more prudent to point out relative advantages and disadvantages, leaving clinicians the flexibility to judge for themselves.
2.0 Case Confirmation
2.1 Instead of proceeding directly to subtype classification, we recommend that patients with a positive aldosterone-renin ratio (ARR) measurement undergo testing, by any of four confirmatory tests, to definitively confirm or exclude the diagnosis (Fig. 1). (1|⊕⊕OO)
2.1 Evidence
The current literature does not identify a gold standard confirmatory test for PA. Test performance has been evaluated only retrospectively, in relatively small series of patients selected with high prior (pretest) probability of PA, commonly in comparison with other tests rather than toward a conclusive diagnosis of PA.
Some of these limitations are illustrated in the following example. There is empirical evidence that case-control designs for establishing the accuracy of diagnostic tests overestimate their accuracy. Giacchetti et al. (57) used such a design including 61 PA patients (26 with confirmed APA) and 157 patients with essential hypertension. In this context, they found that a post-sodium infusion test (SIT) with a cutoff value for plasma aldosterone of at least 7 ng/dl showed a sensitivity of 88% and a specificity of 100% when evaluated by receiver-operating characteristic curve in the 76 cases with ARR more than 40 ng/dl per ng/ml·h. In the prospective PAPY study, analysis of sensitivity/specificity in the 317 patients undergoing a SIT gave a best aldosterone cutoff value of 6.8 ng/dl. The sensitivity and specificity, however, were moderate (respectively, 83 and 75%), reflecting values overlapping between patients with and without disease; use of the aldosterone-cortisol ratio did not improve the accuracy of the test (17, 58).
Four testing procedures (oral sodium loading, saline infusion, fludrocortisone suppression, and captopril challenge) are in common use, and there is currently insufficient direct evidence to recommend one over the others. Although it is acknowledged that these tests may differ in terms of sensitivity, specificity, and reliability, the choice of confirmatory test is commonly determined by considerations of cost, patient compliance, laboratory routine, and local expertise (Table 6). It should be noted that confirmatory tests requiring oral or iv sodium loading should be administered with caution in patients with uncontrolled hypertension or congestive heart failure. We recommend that the pharmacological agents with minimal or no effects on the renin-angiotensin-aldosterone system shown in Table 2 be used to control blood pressure during confirmatory testing.
| Confirmatory test . | Procedure . | Interpretation . | Concerns . |
|---|---|---|---|
| Oral sodium loading test | Patients should increase their sodium intake to >200 mmol/d (∼6 g/d) for 3 d, verified by 24-h urine sodium content. Patients should receive adequate slow-release potassium chloride supplementation to maintain plasma potassium in the normal range. Urinary aldosterone is measured in the 24-h urine collection from the morning of d 3 to the morning of d 4. | PA is unlikely if urinary aldosterone is lower than 10 μg/24 h (27.7 nmol/d) in the absence of renal disease where PA may coexist with lower measured urinary aldosterone levels. Elevated urinary aldosterone excretion [>12 μg/24 h (>33.3 nmol/d) at the Mayo Clinic, >14 μg/24 h (38.8 nmol/d) at the Cleveland Clinic] makes PA highly likely. | This test should not be performed in patients with severe uncontrolled hypertension, renal insufficiency, cardiac insufficiency, cardiac arrhythmia, or severe hypokalemia. The 24-h urine collection may be inconvenient. Laboratory-specific poor performance of the RIA for urinary aldosterone (aldosterone 18-oxo-glucuronide or acid-labile metabolite) may blunt diagnostic accuracy, a problem obviated by the currently available HPLC-tandem mass spectrometry methodology (52). Aldosterone 18-oxo-glucuronide is a renal metabolite, and its excretion may not rise in patients with renal disease. |
| SIT | Patients stay in the recumbent position for at least 1 h before and during the infusion of 2 liters of 0.9% saline iv over 4 h, starting at 0800–0930 h. Blood samples for renin, aldosterone, cortisol, and plasma potassium are drawn at time zero and after 4 h, with blood pressure and heart rate monitored throughout the test. | Postinfusion plasma aldosterone levels <5 ng/dl make the diagnosis of PA unlikely, and levels >10 ng/dl are a very probable sign of PA. Values between 5 and 10 ng/dl are indeterminate (57–60). | This test should not be performed in patients with severe uncontrolled hypertension, renal insufficiency, cardiac insufficiency, cardiac arrhythmia, or severe hypokalemia. |
| FST | Patients receive 0.1 mg oral fludrocortisone every 6 h for 4 d, together with slow-release KCl supplements (every 6 h at doses sufficient to keep plasma K+, measured four times a day, close to 4.0 mmol/liter), slow-release NaCl supplements (30 mmol three times daily with meals) and sufficient dietary salt to maintain a urinary sodium excretion rate of at least 3 mmol/kg body weight. On d 4, plasma aldosterone and PRA are measured at 1000 h with the patient in the seated posture, and plasma cortisol is measured at 0700 and 1000 h. | Upright plasma aldosterone >6 ng/dl on d 4 at 1000 h confirms PA, provided PRA is < 1 ng/ml·h and plasma cortisol concentration is lower than the value obtained at 0700 h (to exclude a confounding ACTH effect) (42, 43, 56, 61–63). | Although some centers (10, 16) conduct this test in the outpatient setting (provided that patients are able to attend frequently to monitor their potassium), in other centers, several days of hospitalization are customary. |
| Most of the data available come from the Brisbane group (42, 43, 56, 61–63) who have established, on the basis of a very large series of patients, a cutoff of a plasma aldosterone concentration of 6 ng/dl at 1000 h in an ambulatory patient on d 4. | |||
| Proponents of the FST argue that 1) it is the most sensitive for confirming PA, 2) it is a less intrusive method of sodium loading than SIT and therefore less likely to provoke non-renin-dependent alterations of aldosterone levels, 3) it allows for the potentially confounding effects of potassium to be controlled and for ACTH (via cortisol) to be monitored and detected, and 4) it is safe when performed by experienced hands. | |||
| Captopril challenge test | Patients receive 25–50 mg captopril orally after sitting or standing for at least 1 h. Blood samples are drawn for measurement of PRA, plasma aldosterone, and cortisol at time zero and at 1 or 2 h after challenge, with the patient remaining seated during this period. | Plasma aldosterone is normally suppressed by captopril (>30%). In patients with PA, it remains elevated and PRA remains suppressed. Differences may be seen between patients with APA and those with IHA, in that some decrease of aldosterone levels is occasionally seen in IHA (23, 64–66). | There are reports of a substantial number of false-negative or equivocal results (67, 68). |
| Confirmatory test . | Procedure . | Interpretation . | Concerns . |
|---|---|---|---|
| Oral sodium loading test | Patients should increase their sodium intake to >200 mmol/d (∼6 g/d) for 3 d, verified by 24-h urine sodium content. Patients should receive adequate slow-release potassium chloride supplementation to maintain plasma potassium in the normal range. Urinary aldosterone is measured in the 24-h urine collection from the morning of d 3 to the morning of d 4. | PA is unlikely if urinary aldosterone is lower than 10 μg/24 h (27.7 nmol/d) in the absence of renal disease where PA may coexist with lower measured urinary aldosterone levels. Elevated urinary aldosterone excretion [>12 μg/24 h (>33.3 nmol/d) at the Mayo Clinic, >14 μg/24 h (38.8 nmol/d) at the Cleveland Clinic] makes PA highly likely. | This test should not be performed in patients with severe uncontrolled hypertension, renal insufficiency, cardiac insufficiency, cardiac arrhythmia, or severe hypokalemia. The 24-h urine collection may be inconvenient. Laboratory-specific poor performance of the RIA for urinary aldosterone (aldosterone 18-oxo-glucuronide or acid-labile metabolite) may blunt diagnostic accuracy, a problem obviated by the currently available HPLC-tandem mass spectrometry methodology (52). Aldosterone 18-oxo-glucuronide is a renal metabolite, and its excretion may not rise in patients with renal disease. |
| SIT | Patients stay in the recumbent position for at least 1 h before and during the infusion of 2 liters of 0.9% saline iv over 4 h, starting at 0800–0930 h. Blood samples for renin, aldosterone, cortisol, and plasma potassium are drawn at time zero and after 4 h, with blood pressure and heart rate monitored throughout the test. | Postinfusion plasma aldosterone levels <5 ng/dl make the diagnosis of PA unlikely, and levels >10 ng/dl are a very probable sign of PA. Values between 5 and 10 ng/dl are indeterminate (57–60). | This test should not be performed in patients with severe uncontrolled hypertension, renal insufficiency, cardiac insufficiency, cardiac arrhythmia, or severe hypokalemia. |
| FST | Patients receive 0.1 mg oral fludrocortisone every 6 h for 4 d, together with slow-release KCl supplements (every 6 h at doses sufficient to keep plasma K+, measured four times a day, close to 4.0 mmol/liter), slow-release NaCl supplements (30 mmol three times daily with meals) and sufficient dietary salt to maintain a urinary sodium excretion rate of at least 3 mmol/kg body weight. On d 4, plasma aldosterone and PRA are measured at 1000 h with the patient in the seated posture, and plasma cortisol is measured at 0700 and 1000 h. | Upright plasma aldosterone >6 ng/dl on d 4 at 1000 h confirms PA, provided PRA is < 1 ng/ml·h and plasma cortisol concentration is lower than the value obtained at 0700 h (to exclude a confounding ACTH effect) (42, 43, 56, 61–63). | Although some centers (10, 16) conduct this test in the outpatient setting (provided that patients are able to attend frequently to monitor their potassium), in other centers, several days of hospitalization are customary. |
| Most of the data available come from the Brisbane group (42, 43, 56, 61–63) who have established, on the basis of a very large series of patients, a cutoff of a plasma aldosterone concentration of 6 ng/dl at 1000 h in an ambulatory patient on d 4. | |||
| Proponents of the FST argue that 1) it is the most sensitive for confirming PA, 2) it is a less intrusive method of sodium loading than SIT and therefore less likely to provoke non-renin-dependent alterations of aldosterone levels, 3) it allows for the potentially confounding effects of potassium to be controlled and for ACTH (via cortisol) to be monitored and detected, and 4) it is safe when performed by experienced hands. | |||
| Captopril challenge test | Patients receive 25–50 mg captopril orally after sitting or standing for at least 1 h. Blood samples are drawn for measurement of PRA, plasma aldosterone, and cortisol at time zero and at 1 or 2 h after challenge, with the patient remaining seated during this period. | Plasma aldosterone is normally suppressed by captopril (>30%). In patients with PA, it remains elevated and PRA remains suppressed. Differences may be seen between patients with APA and those with IHA, in that some decrease of aldosterone levels is occasionally seen in IHA (23, 64–66). | There are reports of a substantial number of false-negative or equivocal results (67, 68). |
| Confirmatory test . | Procedure . | Interpretation . | Concerns . |
|---|---|---|---|
| Oral sodium loading test | Patients should increase their sodium intake to >200 mmol/d (∼6 g/d) for 3 d, verified by 24-h urine sodium content. Patients should receive adequate slow-release potassium chloride supplementation to maintain plasma potassium in the normal range. Urinary aldosterone is measured in the 24-h urine collection from the morning of d 3 to the morning of d 4. | PA is unlikely if urinary aldosterone is lower than 10 μg/24 h (27.7 nmol/d) in the absence of renal disease where PA may coexist with lower measured urinary aldosterone levels. Elevated urinary aldosterone excretion [>12 μg/24 h (>33.3 nmol/d) at the Mayo Clinic, >14 μg/24 h (38.8 nmol/d) at the Cleveland Clinic] makes PA highly likely. | This test should not be performed in patients with severe uncontrolled hypertension, renal insufficiency, cardiac insufficiency, cardiac arrhythmia, or severe hypokalemia. The 24-h urine collection may be inconvenient. Laboratory-specific poor performance of the RIA for urinary aldosterone (aldosterone 18-oxo-glucuronide or acid-labile metabolite) may blunt diagnostic accuracy, a problem obviated by the currently available HPLC-tandem mass spectrometry methodology (52). Aldosterone 18-oxo-glucuronide is a renal metabolite, and its excretion may not rise in patients with renal disease. |
| SIT | Patients stay in the recumbent position for at least 1 h before and during the infusion of 2 liters of 0.9% saline iv over 4 h, starting at 0800–0930 h. Blood samples for renin, aldosterone, cortisol, and plasma potassium are drawn at time zero and after 4 h, with blood pressure and heart rate monitored throughout the test. | Postinfusion plasma aldosterone levels <5 ng/dl make the diagnosis of PA unlikely, and levels >10 ng/dl are a very probable sign of PA. Values between 5 and 10 ng/dl are indeterminate (57–60). | This test should not be performed in patients with severe uncontrolled hypertension, renal insufficiency, cardiac insufficiency, cardiac arrhythmia, or severe hypokalemia. |
| FST | Patients receive 0.1 mg oral fludrocortisone every 6 h for 4 d, together with slow-release KCl supplements (every 6 h at doses sufficient to keep plasma K+, measured four times a day, close to 4.0 mmol/liter), slow-release NaCl supplements (30 mmol three times daily with meals) and sufficient dietary salt to maintain a urinary sodium excretion rate of at least 3 mmol/kg body weight. On d 4, plasma aldosterone and PRA are measured at 1000 h with the patient in the seated posture, and plasma cortisol is measured at 0700 and 1000 h. | Upright plasma aldosterone >6 ng/dl on d 4 at 1000 h confirms PA, provided PRA is < 1 ng/ml·h and plasma cortisol concentration is lower than the value obtained at 0700 h (to exclude a confounding ACTH effect) (42, 43, 56, 61–63). | Although some centers (10, 16) conduct this test in the outpatient setting (provided that patients are able to attend frequently to monitor their potassium), in other centers, several days of hospitalization are customary. |
| Most of the data available come from the Brisbane group (42, 43, 56, 61–63) who have established, on the basis of a very large series of patients, a cutoff of a plasma aldosterone concentration of 6 ng/dl at 1000 h in an ambulatory patient on d 4. | |||
| Proponents of the FST argue that 1) it is the most sensitive for confirming PA, 2) it is a less intrusive method of sodium loading than SIT and therefore less likely to provoke non-renin-dependent alterations of aldosterone levels, 3) it allows for the potentially confounding effects of potassium to be controlled and for ACTH (via cortisol) to be monitored and detected, and 4) it is safe when performed by experienced hands. | |||
| Captopril challenge test | Patients receive 25–50 mg captopril orally after sitting or standing for at least 1 h. Blood samples are drawn for measurement of PRA, plasma aldosterone, and cortisol at time zero and at 1 or 2 h after challenge, with the patient remaining seated during this period. | Plasma aldosterone is normally suppressed by captopril (>30%). In patients with PA, it remains elevated and PRA remains suppressed. Differences may be seen between patients with APA and those with IHA, in that some decrease of aldosterone levels is occasionally seen in IHA (23, 64–66). | There are reports of a substantial number of false-negative or equivocal results (67, 68). |
| Confirmatory test . | Procedure . | Interpretation . | Concerns . |
|---|---|---|---|
| Oral sodium loading test | Patients should increase their sodium intake to >200 mmol/d (∼6 g/d) for 3 d, verified by 24-h urine sodium content. Patients should receive adequate slow-release potassium chloride supplementation to maintain plasma potassium in the normal range. Urinary aldosterone is measured in the 24-h urine collection from the morning of d 3 to the morning of d 4. | PA is unlikely if urinary aldosterone is lower than 10 μg/24 h (27.7 nmol/d) in the absence of renal disease where PA may coexist with lower measured urinary aldosterone levels. Elevated urinary aldosterone excretion [>12 μg/24 h (>33.3 nmol/d) at the Mayo Clinic, >14 μg/24 h (38.8 nmol/d) at the Cleveland Clinic] makes PA highly likely. | This test should not be performed in patients with severe uncontrolled hypertension, renal insufficiency, cardiac insufficiency, cardiac arrhythmia, or severe hypokalemia. The 24-h urine collection may be inconvenient. Laboratory-specific poor performance of the RIA for urinary aldosterone (aldosterone 18-oxo-glucuronide or acid-labile metabolite) may blunt diagnostic accuracy, a problem obviated by the currently available HPLC-tandem mass spectrometry methodology (52). Aldosterone 18-oxo-glucuronide is a renal metabolite, and its excretion may not rise in patients with renal disease. |
| SIT | Patients stay in the recumbent position for at least 1 h before and during the infusion of 2 liters of 0.9% saline iv over 4 h, starting at 0800–0930 h. Blood samples for renin, aldosterone, cortisol, and plasma potassium are drawn at time zero and after 4 h, with blood pressure and heart rate monitored throughout the test. | Postinfusion plasma aldosterone levels <5 ng/dl make the diagnosis of PA unlikely, and levels >10 ng/dl are a very probable sign of PA. Values between 5 and 10 ng/dl are indeterminate (57–60). | This test should not be performed in patients with severe uncontrolled hypertension, renal insufficiency, cardiac insufficiency, cardiac arrhythmia, or severe hypokalemia. |
| FST | Patients receive 0.1 mg oral fludrocortisone every 6 h for 4 d, together with slow-release KCl supplements (every 6 h at doses sufficient to keep plasma K+, measured four times a day, close to 4.0 mmol/liter), slow-release NaCl supplements (30 mmol three times daily with meals) and sufficient dietary salt to maintain a urinary sodium excretion rate of at least 3 mmol/kg body weight. On d 4, plasma aldosterone and PRA are measured at 1000 h with the patient in the seated posture, and plasma cortisol is measured at 0700 and 1000 h. | Upright plasma aldosterone >6 ng/dl on d 4 at 1000 h confirms PA, provided PRA is < 1 ng/ml·h and plasma cortisol concentration is lower than the value obtained at 0700 h (to exclude a confounding ACTH effect) (42, 43, 56, 61–63). | Although some centers (10, 16) conduct this test in the outpatient setting (provided that patients are able to attend frequently to monitor their potassium), in other centers, several days of hospitalization are customary. |
| Most of the data available come from the Brisbane group (42, 43, 56, 61–63) who have established, on the basis of a very large series of patients, a cutoff of a plasma aldosterone concentration of 6 ng/dl at 1000 h in an ambulatory patient on d 4. | |||
| Proponents of the FST argue that 1) it is the most sensitive for confirming PA, 2) it is a less intrusive method of sodium loading than SIT and therefore less likely to provoke non-renin-dependent alterations of aldosterone levels, 3) it allows for the potentially confounding effects of potassium to be controlled and for ACTH (via cortisol) to be monitored and detected, and 4) it is safe when performed by experienced hands. | |||
| Captopril challenge test | Patients receive 25–50 mg captopril orally after sitting or standing for at least 1 h. Blood samples are drawn for measurement of PRA, plasma aldosterone, and cortisol at time zero and at 1 or 2 h after challenge, with the patient remaining seated during this period. | Plasma aldosterone is normally suppressed by captopril (>30%). In patients with PA, it remains elevated and PRA remains suppressed. Differences may be seen between patients with APA and those with IHA, in that some decrease of aldosterone levels is occasionally seen in IHA (23, 64–66). | There are reports of a substantial number of false-negative or equivocal results (67, 68). |
2.1 Values
Confirmatory testing places a high value on sparing individuals with false-positive ARR tests costly and intrusive lateralization procedures.
2.1 Remarks
For each of the four confirmatory tests, procedures, interpretations, and concerns are described in Table 6.
3.0 Subtype Classification
3.1 We recommend that all patients with PA undergo an adrenal CT scan as the initial study in subtype testing and to exclude large masses that may represent adrenocortical carcinoma (Fig. 1). (1|⊕⊕OO)
3.1 Evidence
The findings on adrenal CT—normal-appearing adrenals, unilateral macroadenoma (>1 cm), minimal unilateral adrenal limb thickening, unilateral microadenomas (≤1 cm), or bilateral macro- or microadenomas (or a combination of the two)—are used in conjunction with AVS and, if needed, ancillary tests to guide treatment decisions in patients with PA. APA may be visualized as small hypodense nodules (usually <2 cm in diameter) on CT. IHA adrenal glands may be normal on CT or show nodular changes. Aldosterone-producing adrenal carcinomas are almost always more than 4 cm in diameter, but occasionally smaller, and like most adrenocortical carcinomas have a suspicious imaging phenotype on CT (69).
Adrenal CT has several limitations. Small APAs may be interpreted incorrectly by the radiologist as IHA on the basis of CT findings of bilateral nodularity or normal-appearing adrenals. Moreover, apparent adrenal microadenomas may actually represent areas of hyperplasia, and unilateral adrenalectomy would be inappropriate. In addition, nonfunctioning unilateral adrenal macroadenomas are not uncommon, especially in older patients (>40 yr) (70) and are indistinguishable from APAs on CT. Unilateral UAH may be visible on CT, or the UAH adrenal may appear normal on CT.
In one study, CT contributed to lateralization in only 59 of 111 patients with surgically proven APA; CT detected fewer than 25% of the APAs that were smaller than 1 cm in diameter (62). In another study of 203 patients with PA who were evaluated with both CT and AVS, CT was accurate in only 53% of patients (71). On the basis of CT findings, 42 patients (22%) would have been incorrectly excluded as candidates for adrenalectomy, and 48 (25%) might have had unnecessary or inappropriate surgery (71). In a recent study, AVS was performed in 41 patients with PA, and concordance between CT and AVS was only 54% (72). Therefore, AVS is essential to direct appropriate therapy in patients with PA who seek a potential surgical cure. CT is particularly useful, however, for detecting larger lesions (e.g. >2.5 cm) that may warrant consideration for removal based on malignant potential and also for localizing the right adrenal vein as it enters into the inferior vena cava (IVC), thus aiding cannulation of the vein during AVS (73, 74).
3.1 Remarks
Magnetic resonance imaging has no advantage over CT in subtype evaluation of PA, being more expensive and having less spatial resolution than CT.
3.2 We recommend that, when surgical treatment is practicable and desired by the patient, the distinction between unilateral and bilateral adrenal disease be made by AVS by an experienced radiologist (Fig. 1). (1|⊕⊕⊕O)
Evidence
Lateralization of the source of the excessive aldosterone secretion is critical to guide the management of PA. Distinguishing between unilateral and bilateral disease is important because unilateral adrenalectomy in patients with APA or UAH results in normalization of hypokalemia in all; hypertension is improved in all and cured in 30–60% (46, 75, 76). In bilateral IHA and GRA, unilateral or bilateral adrenalectomy seldom corrects the hypertension (77–81), and medical therapy is the treatment of choice (82). Unilateral disease may be treated medically if the patient declines or is not a candidate for surgery.
Imaging cannot reliably visualize microadenomas or distinguish incidentalomas from APAs with confidence (71), making AVS the most accurate means of differentiating unilateral from bilateral forms of PA. AVS is expensive and invasive, and so it is highly desirable to avoid this test in patients who do not have PA (83). Because ARR testing can be associated with false positives, confirmatory testing should eliminate the potential for patients with false-positive ARR to undergo AVS.
The sensitivity and specificity of AVS (95 and 100%, respectively) for detecting unilateral aldosterone excess are superior to that of adrenal CT (78 and 75%, respectively) (62, 71, 72). Importantly, CT has the potential to be frankly misleading by demonstrating unilateral nodules in patients with bilateral disease and thereby to lead to inappropriate surgery.
AVS is the reference standard test to differentiate unilateral (APA or UAH) from bilateral (IHA) disease in patients with PA (62, 71). Although AVS can be a difficult procedure, especially in terms of successfully cannulating the right adrenal vein (which is smaller than the left and usually empties directly into the IVC rather than the renal vein), the success rate usually improves quickly as the angiographer becomes more experienced. According to a review of 47 reports, the success rate for cannulating the right adrenal vein in 384 patients was 74% (82). With experience, the success rate increased to 90–96% (71, 73, 74, 84). The addition of rapid intraprocedural measurement of adrenal vein cortisol concentrations has facilitated improved accuracy of catheter placement in AVS (85). Some centers perform AVS in all patients who have the diagnosis of PA (62), and others advocate its selective use (e.g. AVS may not be needed in patients younger than age 40 with solitary unilateral apparent adenoma on CT scan) (71, 86).
At centers with experienced AVS radiologists, the complication rate is 2.5% or lower (71, 73). The risk of adrenal hemorrhage can be minimized by employing a radiologist skilled in the technique and by avoiding adrenal venography and limiting use of contrast to the smallest amounts necessary to assess the catheter tip position (74). Where there is a clinical suspicion of a procoagulant disorder, the risk of thromboembolism may be reduced by performing tests for such conditions before the procedure and administering heparin after the procedure in patients at risk.
3.2 Values
Our recommendation to pursue AVS in the subtype evaluation of the patient with PA who is a candidate for surgery places a high value on avoiding the risk of an unnecessary unilateral adrenalectomy based on adrenal CT and a relatively low value on avoiding the potential complications of AVS.
3.2 Remarks
A radiologist experienced with and dedicated to AVS is needed to implement this recommendation.
There are three protocols for AVS: 1) unstimulated sequential or simultaneous bilateral AVS, 2) unstimulated sequential or simultaneous bilateral AVS followed by bolus cosyntropin-stimulated sequential or simultaneous bilateral AVS, and 3) continuous cosyntropin infusion with sequential bilateral AVS. Simultaneous bilateral AVS is difficult to perform and is not used at most centers. Many groups advocate the use of continuous cosyntropin infusion during AVS 1) to minimize stress-induced fluctuations in aldosterone secretion during nonsimultaneous (sequential) AVS, 2) to maximize the gradient in cortisol from adrenal vein to IVC and thus confirm successful sampling of the adrenal vein, and 3) to maximize the secretion of aldosterone from an APA (71, 81, 84, 87) and thus avoid the risk of sampling during a relatively quiescent phase of aldosterone secretion.
The criteria used to determine lateralization of aldosterone hypersecretion depend on whether the sampling is done under cosyntropin administration. Dividing the right and left adrenal vein PACs by their respective cortisol concentrations corrects for dilutional effects of the inferior phrenic vein flowing into the left adrenal vein and, if suboptimally sampled, of IVC flow into the right adrenal vein. These are termed cortisol-corrected aldosterone ratios. With continuous cosyntropin administration, a cutoff of the cortisol-corrected aldosterone ratio from high side to low side more than 4:1 is used to indicate unilateral aldosterone excess (71); a ratio less than 3:1 is suggestive of bilateral aldosterone hypersecretion (71). With these cutoffs, AVS for detecting unilateral aldosterone hypersecretion (APA or UAH) has a sensitivity of 95% and specificity of 100% (71). Patients with lateralization ratios between 3:1 and 4:1 may have either unilateral or bilateral disease, and the AVS results must be interpreted in conjunction with the clinical setting, CT scan, and ancillary tests.
Some investigators consider a cortisol-corrected aldosterone lateralization ratio (high to low side) of more than 2:1 in the absence of cosyntropin as consistent with unilateral disease (83). Other groups rely primarily on comparing the adrenal vein aldosterone-cortisol ratios to those in a simultaneously collected peripheral venous sample (62). When the aldosterone-cortisol ratio from an adrenal vein is significantly (usually at least 2.5 times) greater than that of the peripheral vein (cubital fossa or IVC), and the aldosterone-cortisol ratio in the contralateral adrenal vein is no higher than peripheral (indicating contralateral suppression), the ratio is considered to show lateralization, an indication that unilateral adrenalectomy should cure or improve the hypertension.
Cosyntropin use
If cosyntropin infusion is not used, AVS should be performed in the morning hours after overnight recumbency. This approach avoids the confounding effects of changes in posture on aldosterone levels in patients with angiotensin II-responsive varieties of PA and takes advantage of the effect of high early morning endogenous ACTH levels on aldosterone production in all subtypes of PA (74).
If cosyntropin infusion is used, it may be continuous or bolus. For continuous cosyntropin, an infusion of 50 μg cosyntropin per hour is begun 30 min before adrenal vein catheterization and continued throughout the procedure (71, 81, 84). The bolus cosyntropin technique involves AVS before and after the iv administration of 250 μg cosyntropin. However, some groups have suggested that when given as a bolus injection and when the adrenal veins are sampled simultaneously, cosyntropin administration does not improve the diagnostic accuracy of AVS and that cosyntropin may in fact increase secretion from the nonadenomatous gland to a greater degree than from the APA (88).
Catheterization
The adrenal veins are catheterized through the percutaneous femoral vein approach, and the position of the catheter tip is verified by gentle injection of a small amount of nonionic contrast medium and radiographic documentation (73). Blood is obtained from both adrenal veins and a peripheral vein, e.g. cubital fossa or iliac vein, and labeled peripheral and assayed for aldosterone and cortisol concentrations. To be sure there is no cross-contamination, the peripheral sample should be obtained from a cubital or iliac vein. The venous sample from the left side typically is obtained with the catheter tip at the junction of the inferior phrenic and left adrenal vein. The right adrenal vein may be especially difficult to catheterize because it is short and enters the IVC at an acute angle (84). The cortisol concentrations from the adrenal veins and peripheral vein are used to confirm successful catheterization. The adrenal/peripheral vein cortisol ratio is typically more than 10:1 with the continuous cosyntropin infusion protocol (71) and more than 3:1 without the use of cosyntropin (43).
Unsuccessful AVS
When both adrenal veins are not successfully catheterized, the clinician may 1) repeat AVS, 2) treat the patient with MR antagonist, or 3) consider surgery based on the findings of other studies (e.g. adrenal CT). Additional studies that may guide the clinician in this setting include posture stimulation test and iodocholesterol scintigraphy.
Posture stimulation test
In patients with unsuccessful AVS and with a CT scan showing a unilateral adrenal mass, some experts use the posture stimulation test. This test, developed in the 1970s, was based on the finding that the PAC in patients with APA showed diurnal variation and was relatively unaffected by changes in angiotensin II levels, whereas IHA was characterized by enhanced sensitivity to a small change in angiotensin II that occurred with standing (89). In a review of 16 published reports, the accuracy of the posture stimulation test was 85% in 246 patients with surgically verified APA (82). The lack of accuracy is explained by the fact that some APAs are sensitive to angiotensin II and some patients with IHA have diurnal variation in aldosterone secretion (90). Thus, the posture stimulation test, particularly if it shows lack of responsiveness [consistent with angiotensin II-unresponsive APA or familial hyperaldosteronism type I (FH-I), with the latter readily confirmed or excluded by genetic testing] may serve an ancillary role, for example, in those patients for whom AVS was unsuccessful and CT shows a unilateral adrenal mass (91, 92).
Iodocholesterol scintigraphy
[131I]19-Iodocholesterol scintigraphy was first used in the early 1970s (93), and an improved agent, [6β-131I]iodomethyl-19-norcholesterol (NP-59), was introduced in 1977 (94). The NP-59 scan, performed with dexamethasone suppression, had the putative advantage of correlating function with anatomical abnormalities. However, the sensitivity of this test depends heavily on the size of the adenoma (95, 96). Because tracer uptake was poor in adenomas smaller than 1.5 cm in diameter, this method often is not helpful in interpreting micronodular findings obtained with high-resolution CT (97) and rarely plays a role in subtype evaluation. Currently, it is no longer used in most centers.
18-Hydroxycorticosterone levels
18-Hydroxycorticosterone is formed by 18-hydroxylation of corticosterone. Patients with APA generally have recumbent plasma 18-hydroxycorticosterone levels greater than 100 ng/dl at 0800 h, whereas patients with IHA have levels that are usually less than 100 ng/dl (98). However, this test lacks the accuracy needed to guide the clinician in the subtype evaluation of PA (82).
3.3 In patients with onset of confirmed PA earlier than at 20 yr of age and in those who have a family history of PA or of strokes at young age, we suggest genetic testing for GRA (Fig. 1). (2|⊕OOO)
3.3 Evidence
Testing for familial forms of PA: FH-I (GRA)
The FH-I syndrome is inherited in an autosomal dominant fashion and is responsible for fewer than 1% of cases of PA (99). GRA presentation is highly variable, with some patients presenting with normal blood pressure and some characterized by aldosterone excess, suppressed PRA, and hypertension of early onset that is usually severe and refractory to conventional antihypertensive therapies.
Some studies suggest a high pretest probability for GRA in children or young adults with severe or resistant hypertension and a positive family history of early-onset hypertension and/or premature hemorrhagic stroke (100, 101). In the study by Dluhy and colleagues (100), 50% of children under 18 yr of age with GRA had moderate or severe hypertension (blood pressure >99th percentile for age and sex) at diagnosis. Moreover, Litchfield et al. (101) reported in 376 patients from 27 genetically proven GRA pedigrees that 48% of all GRA pedigrees and 18% of all GRA patients had cerebrovascular complications, with the mean age at the time of the initial event being 32 ± 11.3 yr. Seventy percent of events were hemorrhagic strokes with an overall case fatality rate of 61% (101). The study design used in these reports does not allow estimation of the yield of new GRA patients that case detection could have in such populations.
Genetic testing by either Southern blot (102) or long PCR (103) techniques is sensitive and specific for GRA and obviates the need to measure the urinary levels of 18-oxocortisol and 18-hydroxycortisol or to perform dexamethasone suppression testing, both of which may be misleading (104). Genetic testing for GRA should be considered for PA patients with a family history of PA or of strokes at a young age (101, 105), or with an onset at a young age (e.g. <20 yr).
Testing for familial forms of PA: FH-II
FH-II is an autosomal dominant disorder and possibly genetically heterogeneous (106). Unlike FH-I, the hyperaldosteronism in FH-II does not suppress with dexamethasone, and GRA mutation testing is negative (107). FH-II families may have APA, IHA, or both and are clinically indistinguishable from patients with apparent nonfamilial PA (108). Although FH-II is more common than FH-I, accounting for at least 7% of patients with PA in one series, its prevalence is unknown (108). The molecular basis for FH-II is unclear, although several linkage analyses have shown an association with chromosomal region 7p22 (106, 109).
Finally, APA may rarely but on occasion be seen in multiple endocrine neoplasia type 1.
4.0 Treatment
4.1 We recommend that unilateral laparoscopic adrenalectomy be offered to patients with documented unilateral PA (i.e. APA or UAH) (Fig. 1). (1|⊕⊕OO) If a patient is unable or unwilling to undergo surgery, we recommend medical treatment with an MR antagonist (Fig. 1). (1|⊕⊕OO)
4.1 Evidence
Unilateral laparoscopic adrenalectomy is used in patients with unilateral PA because blood pressure and serum potassium concentrations improve in nearly 100% of patients postoperatively (76, 110–114). Hypertension is cured (defined as blood pressure <140/90 mm Hg without the aid of antihypertensive drugs) in about 50% (range, 35–60%) of patients with APA after unilateral adrenalectomy (75, 110), with a cure rate as high as 56–77% when the cure threshold was blood pressure less than 160/95 mm Hg (46, 115, 116). There is no high-quality evidence linking adrenalectomy with improved quality of life, morbidity, or mortality because studies of this nature have not, to our knowledge, been published.
Factors associated with resolution of hypertension in the postoperative period include having one or no first-degree relative with hypertension and preoperative use of two or fewer antihypertensive drugs (76). Other factors have been reported to predict cure but have been evaluated by only univariate analysis or when the cutoff for blood pressure resolution was less than 160/95 mm Hg (46, 110, 113), duration of hypertension less than 5 yr (46, 47, 75, 76), higher PAC to PRA ratio preoperatively (75, 76), higher urinary aldosterone secretion (75, 76), or positive preoperative response to spironolactone (75, 111). The most common reasons for persistently increased blood pressure after adrenalectomy are coexistent hypertension of unknown cause (46, 76) and older age and/or longer duration of hypertension.
As compared with open adrenalectomy, laparoscopic adrenalectomy is associated with shorter hospital stays and fewer complications (112, 117, 118). Because AVS is able to identify only which gland (and not which part of the gland) is overproducing aldosterone, partial adrenalectomy (removal of an adenoma leaving the remaining adrenal intact) may result in persistent hypertension; continued elevation of PAC is found in up to 10% of patients with unilateral APA, and 27% of extirpated adrenal glands are found to contain multiple nodules (119).
Medical management is recommended for patients who do not undergo surgery. In a retrospective study of 24 patients with APA who were treated for 5 yr with spironolactone or amiloride, systolic and diastolic blood pressure decreased from an average of 175/106 to 129/79 mm Hg (120) with 83% of these patients requiring additional antihypertensive medication to achieve this result. Furthermore, several of the patients experienced side effects from the spironolactone therapy including breast tenderness (54%), breast engorgement (33%), muscle cramps (29%), and decreased libido (13%). In the long term, adrenalectomy is more cost effective than lifelong medical therapy for patients with unilateral PA (121).
Therefore, because unilateral laparoscopic adrenalectomy can either eliminate the need for medication or reduce medication-related side effects, it is the preferred procedure for the treatment of unilateral disease in patients with PA.
4.1 Values
Our recommendation to subject patients with unilateral adrenal disease to laparoscopic adrenalectomy in preference to other methods of treatment places a high value on reduction of blood pressure and/or the number of medications necessary to control blood pressure, on normalization of endogenous aldosterone secretion, and on the resolution of hypokalemia. This benefit is far greater than the risks of surgery and postoperative management, which are extremely low.
4.1 Remarks
This recommendation requires the availability of a surgeon experienced in laparoscopic adrenalectomy.
Preoperative management
In the patient scheduled for surgery, both hypertension and hypokalemia should be well controlled preoperatively. Obtaining such control may require a delay in surgery and the addition of an MR antagonist.
Postoperative management
Plasma aldosterone and renin activity levels should be measured shortly after surgery as an early indication of biochemical response (114), and on postoperative d 1, potassium supplementation should be withdrawn, spironolactone discontinued, and antihypertensive therapy reduced, if appropriate (122).
Postoperative iv fluids should be normal saline without potassium chloride unless serum potassium levels remain very low (i.e. <3.0 mmol/liter), and during the first few weeks after surgery, a generous sodium diet should be recommended to avoid the hyperkalemia that can develop from hypoaldosteronism due to chronic contralateral adrenal gland suppression (122). In rare instances, temporary fludrocortisone therapy may be required.
Blood pressure typically normalizes or shows maximal improvement in 1–6 months after unilateral adrenalectomy for unilateral APA but can continue to fall for up to 1 yr in some patients. Some investigators have employed postoperative FST (performed at least 3 months after surgery to permit recovery of the contralateral gland) to assess whether the PA has been cured from a biochemical perspective (123).
4.2 In patients with PA due to bilateral adrenal disease, we recommend medical treatment with an MR antagonist (1|⊕⊕OO); we suggest spironolactone as the primary agent with eplerenone as an alternative (Fig. 1). (2|⊕OOO)
4.2 Evidence
Bilateral adrenal disease includes idiopathic adrenal hyperplasia, bilateral APA, and GRA. In 99 surgically treated patients with IHA reported in the literature, the hypertension cure rate was only 19% after unilateral or bilateral adrenalectomy (77–81). No randomized placebo-controlled trials have evaluated the relative efficacy of drugs in the treatment of PA. However, the pathophysiology of PA due to bilateral adrenal hyperplasia and longstanding clinical experience suggest several pharmacological targets.
MR antagonists
MR antagonists appear to be effective at controlling blood pressure and to provide blood pressure-independent target organ protection.
Spironolactone
For more than four decades, the MR antagonist spironolactone has been the agent of choice in the medical treatment of PA. Several observational studies in patients with IHA (combined n = 122) have reported a mean reduction in systolic blood pressure of 25% and diastolic blood pressure of 22% in response to spironolactone 50–400 mg/d for 1–96 months (124–130). In a study of 28 hypertensive subjects with an ARR more than 750 pmol/liter (27 ng/dl) per ng/ml·h who failed to suppress their PAC after salt loading and without evidence of adenoma on adrenal CT scan, spironolactone therapy (25–50 mg/d) reduced the need for antihypertensive drugs by −0.5 (from a mean of 2.3 to 1.8 drugs) drugs, as well as reducing systolic blood pressure by −15 mm Hg (from a mean of 161 to 146 mm Hg) and diastolic blood pressure by −8 mm Hg (from a mean of 91 to 83 mm Hg); 48% of subjects achieved a blood pressure less than 140/90 mm Hg, and about half were able to be managed with spironolactone monotherapy (131). The dose of spironolactone employed in that study was much lower than previously considered necessary for the treatment of PA.
The incidence of gynecomastia with spironolactone therapy is dose related, with one study reporting an incidence after 6 months of 6.9% at a dose of less than 50 mg/d and 52% at a dose of more than 150 mg/d (132). The exact incidence of menstrual disturbances in premenopausal women with spironolactone therapy is unknown. Where available, canrenone (an active metabolite of spironolactone) or potassium canrenoate, its open E-ring water-soluble congener, might be considered, in that they possibly have fewer sex steroid-related side effects. In addition, a small dose of a thiazide diuretic, triamterine, or amiloride can be added to attempt to avoid a higher dose of spironolactone, which may cause side effects.
Eplerenone
Eplerenone is a newer, selective MR antagonist without antiandrogen and progesterone agonist effects (133), thus reducing the rate of adverse endocrine side effects. It has been approved for the treatment of primary (essential) hypertension (134, 135) in the United States and Japan and for heart failure after myocardial infarction (136) in the United States and a number of other countries. Eplerenone has 60% of the MR antagonist potency of spironolactone; its better tolerability profile needs to be balanced against its higher cost and the lack of current clinical trial evidence for its use in PA. Reflecting its shorter half-life, eplerenone should be given twice daily for optimal effect.
Other agents
Up-regulation of distal tubular sodium epithelial channel activity is a major mechanism whereby aldosterone exerts its actions on sodium and potassium handling. Of the available epithelial sodium channel antagonists (amiloride and triamterene), amiloride has been the most studied as a mode of treatment for PA. Although less efficacious than spironolactone, amiloride may be useful (28, 137). Being a potassium-sparing diuretic, amiloride can ameliorate both hypertension and hypokalemia in patients with PA and is generally well tolerated, lacking the sex steroid-related side effects of spironolactone, but without the beneficial effects on endothelial function (138, 139).
Calcium channel blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers have been evaluated in very few patients with PA, and in general, they are antihypertensive without a major effect on aldosterone excess. Supportive studies are small and methodologically weak and have not measured patient-important outcomes. Aldosterone synthase inhibitors may play a role in the future.
4.2 Values
This recommendation places a relatively higher value on reduction of blood pressure normalization of serum potassium concentrations and abrogation of the vascular, cardiac, and renal effects of aldosterone with the minimum number of pharmacological agents and a relatively lower value on side effects such as gynecomastia and erectile dysfunction in men and menstrual irregularities in women. Eplerenone, given its selectivity and despite its cost, is an alternative if the side effects of spironolactone prove difficult to tolerate.
4.2 Remarks
The starting dose for spironolactone should be 12.5–25 mg daily in a single dose. The lowest effective dose should be found by very gradually titrating upward if necessary to a maximum dose of 100 mg/d. The starting dose for eplerenone is 25 mg once or twice daily. In patients with stage III chronic kidney disease (i.e. glomerular filtration rate <60 ml/min·1.73m2), spironolactone and eplerenone may be used with caution because of the risk of hyperkalemia, but MR antagonists should be avoided in those with stage IV disease.
4.3 In patients with GRA, we recommend the use of the lowest dose of glucocorticoid that can normalize blood pressure and potassium levels rather than first-line treatment with an MR antagonist (Fig. 1). (1|⊕OOO)
4.3 Evidence
GRA should be treated medically with a glucocorticoid to partially suppress pituitary ACTH secretion. We recommend use of a synthetic glucocorticoid that is longer acting than hydrocortisone, such as dexamethasone or prednisone, to suppress ACTH secretion. Ideally, the glucocorticoid should be taken at bedtime to suppress the early morning ACTH surge. PRA and aldosterone concentrations may be helpful in assessing the effectiveness of treatment and the prevention of overtreatment.
Overtreatment with exogenous steroids must be avoided; iatrogenic Cushing’s syndrome and impaired linear growth in children have resulted from such overdosing (100). In general, the lowest possible dose of glucocorticoid that normalizes blood pressure and/or serum potassium concentration should be used (74). Treatment with a glucocorticoid may not always normalize blood pressure, and addition of an MR antagonist should be considered in these cases.
The use of eplerenone may be preferred in the case of affected children, in whom there may be concerns with respect to growth retardation and antiandrogenic effects of glucocorticoids and spironolactone, respectively.
4.3 Values
The treatment of GRA places a high value on preventing the potential consequences of hyperaldosteronism and a lower value on the possible side effects of chronic glucocorticoid administration.
4.3 Remarks
The starting dose of dexamethasone in adults is 0.125–0.25 mg daily. The starting dose of prednisone is 2.5–5 mg daily. For each, treatment is usually administered at bedtime.
In addition to the members of the Task Force, there have been a number of people whose contribution to these guidelines has been invaluable. First, we thank Dr. Robert Vigersky, the members of the Clinical Guidelines Subcommittee, the Clinical Affairs Core Committee, and the Council of The Endocrine Society for their careful reading of and very useful suggestions for improving the guidelines. Second, we thank the members of The Endocrine Society at large for their input when the draft guidelines were posted on the Society’s website; all the responses received were considered by the authors, and many incorporated. Third, we thank the members of our sister societies around the world for their enthusiastic involvement in reading the draft guidelines, and in offering their support for this publication. Fourth, we thank Lisa Marlow of The Endocrine Society, who has provided first-class administrative and logistic back-up, without which such a geographically disperse task force would have faced considerable difficulty in operating. Last, but very much not least, we are indebted to Dr. Patricia A. Stephens, medical writer, for her unfailing patience, her meticulous checking of both text and references, and most importantly her ability to meld half a dozen disparate writing styles into a seamless whole.
Disclaimer
Clinical Practice Guidelines are developed to be of assistance to endocrinologists by providing guidance and recommendations for particular areas of practice. The Guidelines should not be considered inclusive of all proper approaches or methods, or exclusive of others. The Guidelines cannot guarantee any specific outcome, nor do they establish a standard of care. The Guidelines are not intended to dictate the treatment of a particular patient. Treatment decisions must be made based on the independent judgment of health care providers and each patient’s individual circumstances.
The Endocrine Society makes no warranty, express or implied, regarding the Guidelines and specifically excludes any warranties of merchantability and fitness for a particular use or purpose. The Society shall not be liable for direct, indirect, special, incidental, or consequential damages related to the use of the information contained herein.
Financial Disclosure of Task Force
John W. Funder, M.D., Ph.D. (chair) – Financial or Business/Organizational Interests: Schering-Plough, Daiichi-Sankyo, Pfizer, Cancer Institute of New South Wales, Speedel, Garnett Passe and Rodney Williams Memorial Foundation, Merck, Eli Lilly, P3 Panel (Commonwealth of Australia); Significant Financial Interest or Leadership Position: Schering-Plough, Pfizer, Daiichi-Sankyo, and Cancer Institute of New South Wales. Robert M. Carey, M.D. – Financial or Business/Organizational Interests: Novartis, Pfizer, and Daiichi-Sankyo; Significant Financial Interest or Leadership Position: none declared. Carlos Fardella, M.D. – Financial or Business/Organizational Interests: National Fund for Scientific and Technological Development (Fondo Nacional de Desarrollo Científico y Tecnológico [FONDECYT]); Significant Financial Interest or Leadership Position: none declared. Celso E. Gomez-Sanchez, M.D. – Financial or Business/Organizational Interests: American Heart Association; Significant Financial Interest or Leadership Position: Associate Editor for the journal Hypertension. Franco Mantero, M.D., Ph.D. – Financial or Business/Organizational Interests: none declared; Significant Financial Interest or Leadership Position: Executive Committee of the International Society of Endocrinology. Michael Stowasser, MBBS, FRACP, Ph.D. – Financial or Business/Organizational Interests: none declared; Significant Financial Interest or Leadership Position: none declared. William F. Young Jr., M.Sc., M.D. – Financial or Business/Organizational Interests: none declared; Significant Financial Interest or Leadership Position: Mayo Clinic, Clinical Endocrinology. *Victor M. Montori, M.D. – Financial or Business/Organizational Interests: KER Unit (Mayo Clinic); Significant Financial Interest or Leadership Position: none declared.
Cosponsoring organizations include the European Society of Endocrinology, European Society of Hypertension, International Society of Endocrinology, International Society of Hypertension, and The Japanese Society of Hypertension.
First Published Online June 13, 2008
Evidence-based reviews for this guideline were prepared under contract with The Endocrine Society.
Abbreviations
- ARR
Aldosterone to renin ratio
- APA
aldosterone-producing adenoma
- AVS
adrenal venous sampling
- CT
computed tomography
- DRC
direct renin concentration
- FH-I
familial hyperaldosteronism type I
- FST
fludrocortisone suppression testing
- GRA
glucocorticoid-remediable aldosteronism
- IHA
idiopathic hyperaldosteronism
- IVC
inferior vena cava
- MR
mineralocorticoid receptor
- PA
primary aldosteronism
- PAC
plasma aldosterone concentration
- PRA
plasma renin activity
- SIT
sodium infusion test
- UAH
unilateral adrenal hyperplasia



