-
PDF
- Split View
-
Views
-
Cite
Cite
Mark E. Wilson, Jeffrey Fisher, Kathy Chikazawa, Ruth Yoda, Ariadne Legendre, Deborah Mook, Kenneth G. Gould, Leptin Administration Increases Nocturnal Concentrations of Luteinizing Hormone and Growth Hormone in Juvenile Female Rhesus Monkeys, The Journal of Clinical Endocrinology & Metabolism, Volume 88, Issue 10, 1 October 2003, Pages 4874–4883, https://doi.org/10.1210/jc.2003-030782
Close - Share Icon Share
Abstract
The importance of leptin in regulating sexual maturation is supported by data showing that deletions of the leptin gene or alterations in the leptin receptor result in infertility. However, attempts to define a role for leptin in normal puberty have produced equivocal results, leading to the conclusion that, if leptin is involved in puberty, its role is permissive and not obligatory. To better define the importance of leptin in primate puberty, the present study tested the hypothesis that a premature elevation in nocturnal leptin concentrations would accelerate indices of puberty, including nocturnal LH secretion in female rhesus monkeys (Macaca mulatta). Juvenile, gonadally intact females were treated daily with leptin (n = 6; 30 μg/kg, sc at 1700 h) from 12–30 months of age and were compared with age-matched control females (n = 13). Chronic elevation in peripheral concentrations of leptin increased serum levels of both daytime and nighttime bioactive LH at a significantly younger age compared with control females. The earlier rise in LH in leptin-treated females was associated with an earlier increase in serum estradiol and occurrence of menarche. Despite this effect of leptin, nocturnal serum LH was significantly higher at each age assessed in non-leptin-treated ovariectomized controls (n = 6). In addition, leptin increased skeletal lengths and maturity that were associated with significantly higher serum levels of nocturnal GH and daytime IGF-I. Although body weights were not consistently affected by treatment, body mass index, as an index of body fat, was consistently lower in leptin-treated females. Taken together, these data indicate that the chronic elevation in serum leptin concentrations advances the nocturnal increase in serum LH as well as other parameters of female puberty. Furthermore, the observation that nocturnal LH was higher in age-matched, agonadal females compared with the leptin-treated females suggests that the nongonadal drive to LH secretion is operative in female macaques as early as 14 months of age, suggesting that the effect of leptin on puberty in female primates may involve a diminution in gonadal negative feedback suppression of LH secretion. Such a role would suggest that leptin is permissive yet critical for advancing female puberty.
THE NOTION THAT a critical amount of somatic mass, reflecting metabolic fuel reserves, is essential to initiate and sustain puberty in mammals has historically been an attractive hypothesis (1, 2). With the identification of the fat-derived protein leptin in 1995, a number of studies using a variety of model systems have investigated the importance of this metabolic cue for sexual maturation. Support for the hypothesis that leptin is important derives from the observation that the gonadotropin deficits and infertility exhibited by mice lacking the leptin gene are corrected by leptin administration (3–5). Furthermore, the hypogonadotropic hypogonadism observed in a cohort of children with mutations in the leptin gene that prevent leptin secretion is also corrected by leptin replacement therapy (6, 7). These observations are consistent with those in humans that mutations in the leptin receptor result in an absence of puberty and infertility (8).
Despite the rather compelling evidence from these genetic models linking leptin to sexual maturation, the precise role for leptin in the onset and maintenance of puberty is quite controversial. The genetic models indicate that a presence of leptin is necessary for puberty to proceed. The remaining question is whether leptin provides a critical signal initiating puberty or whether leptin plays a permissive role enabling puberty to proceed once the essential neuroendocrine mechanisms have matured (9). In answering this question, two issues must be addressed (10): 1) does leptin change in a physiologically meaningful way during prepuberty such that it could act on neuroendocrine mechanisms regulating puberty, and 2) does leptin administration advance sexual maturation in otherwise normal individuals. The experiments designed to answer these questions using a range of animal models have provided equivocal results and, thus, no accepted role for leptin in puberty regulation has emerged.
Circulating concentrations of leptin, reflecting acquisition of sc fat (11, 12), progressively increase from prepuberty through puberty in girls, whereas the developmental increase in leptin in boys peaks during early puberty before declining (13–19). The sexual dimorphism in this developmental pattern of leptin is related to the pubertal rise of testosterone in boys (11) as well as gender differences in fat distribution (20). Furthermore, the developmental increase in circulating leptin predicts the timing of menarche in girls (21). Although daytime leptin levels were observed to increase in female rats during the transition to puberty (22), other data from male and female rodents suggest that puberty is not characterized by a developmental increase in daytime leptin (23–25). However, nocturnal levels of leptin from serial blood samples do increase before vaginal opening in rats (26, 27). Studies of male monkeys report no change in daytime serum leptin levels before puberty, defined by a rise in either daytime testosterone or LH (28–30). On the other hand, an increase in nocturnal leptin occurs before the increase in pulsatile nocturnal LH release in adolescent males (31), although the precise timing of this relationship has been questioned (32). Thus, although the data from children indicate that daytime leptin may change developmentally in a meaningful fashion in relationship to puberty, data from animal models are mixed but do suggest a developmental increase in nighttime values.
If leptin is an important metabolic cue regulating puberty, it must act to regulate the hypothalamic release of GnRH. A number of in vitro studies indicate that leptin stimulates hypothalamic GnRH and pituitary gonadotropin release. Leptin increases the frequency of GnRH release from hypothalamic explants (33, 34) and dispersed hypothalamic neurons (34). Although leptin receptors have not been found to be expressed in GnRH neurons in vivo but rather in other neuronal populations in the arcuate and ventomedial nuclei (9, 35, 36), leptin receptors are expressed in immortalized GnRH neurons, and leptin stimulates GnRH release in this cell line (37). Pituitary gonadotropes also express the leptin receptor (38), and leptin stimulates LH and FSH release from incubated pituitaries (39).
The in vitro data notwithstanding, studies assessing the effects of exogenous leptin on parameters of sexual maturation in vivo have produced mixed results. Peripheral leptin administration advances parameters of puberty in normal female mice (40, 41) and prematurely increases serum LH in female rats (42). Leptin administered intracerebroventricularly (icv) also produces an increase in LH during the late juvenile phase, mediated by an increase in GnRH (22). Infusions of leptin to the hypothalamus increase the release of GnRH in fasted but not fed female rats (43). Finally, genetically altered mice that overexpress leptin also have accelerated puberty compared with wild-type littermates (44). However, other studies show that leptin administered icv (45) or peripherally (46) to female rats or mice (47) reduces food intake and pubertal growth and does not accelerate parameters of puberty relative to control animals. In this experimental context, leptin administration did normalize the timing of puberty compared with animals fed a comparable restricted diet. Finally, acute treatment with human leptin given for up to 22 d iv (48) or icv (49) stimulated GH but not LH in agonadal male monkeys.
It is clear from these data that there is no consensus for a role of leptin in mammalian puberty. The involvement of leptin in reproductive maturation may not only vary between species, but may also differ between males and females (49, 50). In an attempt to further understand the importance of leptin for sexual maturation in primates, the present study used prepubertal female rhesus monkeys (Macaca mulatta) to test the hypothesis that a premature elevation in nocturnal leptin concentrations in peripheral circulation would accelerate indices of puberty, including nocturnal LH, serum estradiol, secondary sexual characteristics, menarche, and first ovulation. The developmental increase in LH secretion in female primates is regulated by a nongonadal drive to GnRH followed by a change in estradiol negative feedback inhibition, appearance of secondary sexual characteristics, and menarche (51–53). Furthermore, puberty onset is most clearly defined as a significant elevation in nocturnal LH concentrations in both female monkeys (51, 54) and girls (52). Consequently, this study tested the hypothesis that leptin administration to gonadally intact females would significantly advance the developmental increase in nocturnal LH relative to age-matched, gonadally intact controls, but not to females in which estradiol negative feedback was not operative (age-matched ovariectomized controls). In addition, the study tested the hypothesis that leptin administration would accelerate skeletal growth through corresponding increases in GH and, consequently, IGF-I. Knowing the role of leptin in primate puberty may not only provide a better understanding of the cues that induce the neurobiological mechanisms responsible for puberty (55) but also provide insight into mechanisms responsible for the early onset of puberty in obese girls (56).
Materials and Methods
Female rhesus monkeys, born and raised at the Yerkes National Primate Research Center (Atlanta, GA), were subjects. Animals were born outdoors and, between 9 and 10 months of age, were moved indoors where they were housed under a fixed photoperiod (12-h light, 12-h dark) and temperature range (21–25 C). Animals were housed socially in small groups (n = 4) until 22 months of age and in pairs thereafter. Animals were fed commercial monkey chow (Ralston Purina Company, St. Louis, MO) ad libitum twice daily and received a daily supplement of fresh fruit and vegetables. The protocol was approved by the Emory University Institutional Animal Care and Use Committee in accordance with the Animal Welfare Act and the U.S. Department of Health and Human Services Guide for Care and Use of Laboratory Animals.
All females were born in Spring 2000. At 11 months of age, they were randomly assigned to a control group (Con, n = 13) or a leptin-treated group (Ob, n = 6). The average birth date for the Con (April 27) and Ob females (April 21) did not differ significantly. A second cohort of females was ovariectomized at 11 months of age (Ovx-Con, n = 6; birth date, May 13) and housed in similar rooms as the gonadally intact females. The ovariectomized, untreated control females were included to compare developmental increases in LH in the absence of gonadal negative feedback to that observed in untreated and leptin-treated gonadally intact females. Recombinant human leptin, purchased from U.S. Biological (Swampscott, MA), was administered at a dose of 30 μg/kg sc every day at 1700 h. This dose was chosen because it represents the dose administered to leptin-deficient children that produces a reduction in body weight and initiates puberty (6). Because nocturnal levels of leptin show a developmental increase in children (57), leptin was administered in the late afternoon to stimulate a nocturnal rise 4–8 h later (58). This also follows a protocol used with female mice to assess effects of leptin on puberty (40). Finally, a sc route of administration was chosen because the majority of circulating leptin is produced peripherally in adipocytes. Treatments were initiated when the females were 12 months of age (April 24, 2001), approximately 1 yr before the expected age of menarche and onset of perineal swelling (59, 60) resulting from a change in sensitivity to estradiol negative feedback inhibition on LH (53). We reasoned that if leptin were important for puberty, initiating treatment at this age would advance these and other parameters of puberty. Treatment continued daily for 18 months (October 31, 2002) to the approximate age of first ovulation (i.e. 31 months) for our colony (59, 60).
The primary dependent variable was the developmental increase in LH assessed in morning (1000 and 1030 h) and evening (2200 and 2230 h) serum samples collected at 14, 18, 21, and 24 months of age. To obtain a better estimate of LH secretion, the LH values in the 1000 and 1030 h were averaged for each female at each assessment to yield a morning value, as were the 2200 and 2230 h samples to yield an evening value. A significant elevation in bioactive LH (bioLH) for an individual animal was defined as an increase 2 sd values above the mean bioLH concentration at the youngest age for Con females (14 months). For the gonadally intact females, daytime serum samples were collected once weekly until menarche and twice weekly thereafter. First ovulation for the Con and Ob females was inferred from sustained elevation in serum progesterone (7–10 d, >1.00 ng/ml) from samples collected twice weekly (60). All subjects were habituated to conscious venipuncture as described previously (61). Juvenile females readily adapt to these procedures, showing no adverse effects on growth or fertility (60).
All assays were performed in the Yerkes Endocrine Core Laboratory. Serum estradiol was determined by a modification of a commercially available RIA (Diagnostics Products Corp., Los Angeles, CA). Before assay, samples (250 μl) were extracted twice with 5 ml of anesthesia grade ether. After evaporation of the solvent, samples were reconstituted with 250 μl of the zero calibrator, and 100-μl aliquots were assayed in duplicate. The assay has a sensitivity of 5 pg/ml using 100 μl of extracted serum, with inter- and intraassay coefficients of variation (CV) of 6.1 and 11.2%, respectively. Sample values of estradiol were corrected for extraction efficiencies, which exceeded 95%. Serum IGF-I was determined by a commercially available ELISA (Diagnostic Systems Laboratory, Webster, TX). The assay has a sensitivity of 50 ng/ml using 5 μl of serum with inter- and intraassay CV of 5.89 and 4.5%, respectively. Serum progesterone was determined by a commercially available RIA (Diagnostics Products Corp.). The assay has a sensitivity of 0.15 ng/ml using 100 μl of serum with inter- and intraassay CV of 10.26 and 9.08%, respectively. Serum GH was determined by a commercially available ELISA (Diagnostic Systems Laboratory). The assay has a sensitivity of 0.10 ng/ml using 20 μl of serum with inter- and intraassay CV of 9.13 and 6.33%, respectively. LH concentrations were determined using the mouse interstitial cell bioassay (62). The standard for the assay was the recombinant monkey LH (purchased from the National Hormone and Pituitary Program, National Institute of Diabetes & Digestive Kidney Diseases). Using 5 μl of serum, the LH bioassay has a sensitivity of 0.20 ng/ml with inter- and intraassay CV of 12.00 and 10.31%, respectively. The LH potency in the standards, control, and unknown samples was based on the production of testosterone in the incubates. Testosterone was determined using a commercially available RIA (Diagnostic Systems Laboratory). To ensure that leptin in the serum of treated monkeys did not bias the results of the LH bioassay, three concentrations of leptin in incubation media, equivalent to 25, 75, and 100 ng/ml, were assayed. Resulting values of testosterone, and thus bioLH, were below the sensitivity of the assay (0.20 ng LH/ml). Thus, the use of the bioassay provided an unbiased means to quantify LH in serum from Con and Ob monkeys. Leptin binding activity in serum, reflecting either leptin binding protein or antibodies to administered human leptin, was measured as previously described (63) to determine whether activity decreases in Con females as it does in children (63) and to determine whether binding activity increased in Ob females. Serum concentrations of leptin were measured by RIA using a commercially available kit validated for nonhuman primates (Linco, St. Louis, MO). Assaying 100 μl, the assay has a range of 0.5–100 ng/ml. Intraassay CV was 5.5%, and interassay CV was 8.8%.
For the gonadally intact females, the occurrence of perineal coloration and swelling as well as menstrual bleeding was assessed by daily visual inspection. Perineal changes in color and swelling are characteristic of perimenarchal rhesus monkeys (62) and are induced by increases in estradiol and activation of estrogen receptors (64). Nonfasted body weights were obtained bimonthly. Morphometric measures in gonadally intact females were obtained at 13, 15, 18, 21, 25, and 29 months of age, while the animals were anesthetized with Telazol (5 mg/kg, im; Henry Schein, Melville, NY). Measurements of crown-rump (distance from the crown of the head to the ischial callosities) and height (distance from the crown of the head to the bottom of the heel) were obtained using previously described procedures (59). Body mass index (BMI, or weight in kilograms divided by height in meters squared) was calculated for each age. Bone mineral content was also determined at these times using a dual energy x-ray absorptiometer (Norland XR-26 System, Fort Atkinson, WI) as described previously (28). Bone ages were estimated from radiographs of hands and wrists according to the Tanner-Whitehouse system for humans as described previously (59).
Data were summarized as mean ± sem. Differences between Con and Ob females throughout development were evaluated with ANOVA models for repeated measures. Post hoc comparisons were made using Fisher’s exact test. Differences between groups on a single variable were evaluated with t tests. Log transformation was applied to data that were not normally distributed. Statistical analyses were performed using SPSS software (SPSS, Inc., Chicago, IL), and tests having a P ≤ 0.05 were considered significant.
Results
The daily, late afternoon injection of leptin to Ob females significantly elevated serum concentrations of the protein compared with untreated Con females (Table 1). Within 1 month of the initiation of treatment, serum leptin rose significantly within 2 h after the injection (Fig. 1A). However, after 12 months of treatment, serum leptin levels were further elevated and did not show a significant change after the injection. Importantly, serum leptin was increased above that observed in Con females throughout the treatment period (Fig. 1A and Table 1). The sustained elevation in serum leptin throughout a 24-h period after 12 months of treatment corresponded to a sustained elevation in leptin binding activity in treated females (Table 1). In contrast, leptin binding activity was significantly less in Con compared with Ob females (F1,17 = 20.66; P = 0.001) and showed a significant decline with advancing age (P < 0.05; Table 1). With respect to changes in serum leptin during maturation in Con females (Fig. 1B), both daytime (1000 h) and nighttime (2200 h) concentrations were slightly, yet significantly, elevated at the age of the nocturnal increase in bioLH concentrations (see below) compared with a younger age (14 months) before any increase in bioLH (F1,17 = 6.79; P = 0.02). At both developmental time points, nocturnal values were significantly higher than daytime values (F1,17 = 21.96; P = 0.007).
Serum concentrations of leptin and leptin binding activity for Con and Ob females at 14, 18, 21, and 24 months of age
| . | Nocturnal leptin (ng/ml) . | Leptin binding activity (%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Age (months) . | Age (months) . | |||||||
| 14 . | 18 . | 21 . | 24 . | 14 . | 18 . | 21 . | 24 . | |
| Con | 1.22 ± 0.09 | 1.44 ± 0.13 | 1.11 ± 0.09 | 1.75 ± 0.31 | 4.6 ± 1.62 | 2.07 ± 0.68 | 0.6 ± 0.24 | 1.18 ± 0.40 |
| Ob | 26.42 ± 2.18 | 63.72 ± 6.64 | 74.79 ± 6.76 | 73.57 ± 6.27 | 13.91 ± 4.33 | 15.69 ± 3.62 | 14.69 ± 3.64 | 19.7 ± 3.42 |
| . | Nocturnal leptin (ng/ml) . | Leptin binding activity (%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Age (months) . | Age (months) . | |||||||
| 14 . | 18 . | 21 . | 24 . | 14 . | 18 . | 21 . | 24 . | |
| Con | 1.22 ± 0.09 | 1.44 ± 0.13 | 1.11 ± 0.09 | 1.75 ± 0.31 | 4.6 ± 1.62 | 2.07 ± 0.68 | 0.6 ± 0.24 | 1.18 ± 0.40 |
| Ob | 26.42 ± 2.18 | 63.72 ± 6.64 | 74.79 ± 6.76 | 73.57 ± 6.27 | 13.91 ± 4.33 | 15.69 ± 3.62 | 14.69 ± 3.64 | 19.7 ± 3.42 |
Data represent mean ± sem.
Serum concentrations of leptin and leptin binding activity for Con and Ob females at 14, 18, 21, and 24 months of age
| . | Nocturnal leptin (ng/ml) . | Leptin binding activity (%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Age (months) . | Age (months) . | |||||||
| 14 . | 18 . | 21 . | 24 . | 14 . | 18 . | 21 . | 24 . | |
| Con | 1.22 ± 0.09 | 1.44 ± 0.13 | 1.11 ± 0.09 | 1.75 ± 0.31 | 4.6 ± 1.62 | 2.07 ± 0.68 | 0.6 ± 0.24 | 1.18 ± 0.40 |
| Ob | 26.42 ± 2.18 | 63.72 ± 6.64 | 74.79 ± 6.76 | 73.57 ± 6.27 | 13.91 ± 4.33 | 15.69 ± 3.62 | 14.69 ± 3.64 | 19.7 ± 3.42 |
| . | Nocturnal leptin (ng/ml) . | Leptin binding activity (%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Age (months) . | Age (months) . | |||||||
| 14 . | 18 . | 21 . | 24 . | 14 . | 18 . | 21 . | 24 . | |
| Con | 1.22 ± 0.09 | 1.44 ± 0.13 | 1.11 ± 0.09 | 1.75 ± 0.31 | 4.6 ± 1.62 | 2.07 ± 0.68 | 0.6 ± 0.24 | 1.18 ± 0.40 |
| Ob | 26.42 ± 2.18 | 63.72 ± 6.64 | 74.79 ± 6.76 | 73.57 ± 6.27 | 13.91 ± 4.33 | 15.69 ± 3.62 | 14.69 ± 3.64 | 19.7 ± 3.42 |
Data represent mean ± sem.
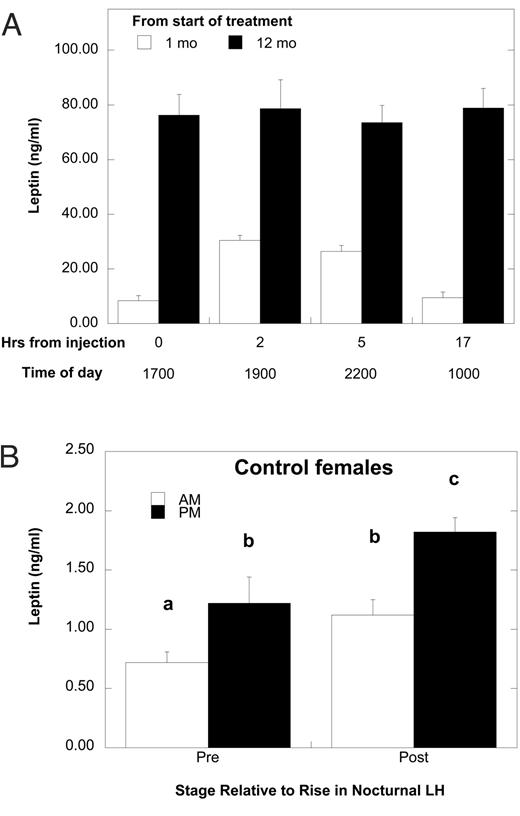
A, Mean ± sem serum concentrations of leptin after the daily injection of leptin at month 1 (white bars) and month 12 (black bars) of the study. Hour 17 also corresponds to 7 h before the next daily injection. B, Mean ± sem serum concentrations of daytime and nighttime leptin in Con females before the developmental increase in bioLH (14 months of age) and at age of the first detectable increase in bioLH. Different letters above the bars indicate that values are significantly different from each other (P < 0.05).
Leptin administration significantly increased serum concentration of bioLH compared with the untreated gonadally intact controls but not to the Ovx-Con (Fig. 2). Nocturnal levels of bioLH were significantly elevated in Ob compared with Con females at 14, 18, and 21 months of age, corresponding to d 84, 198, and 265 of treatment, respectively (F3,36 = 11.68; P = 0.005). Nighttime levels at 24 months of age were not different between Con and Ob females. Daytime levels of bioLH were significantly higher in Ob compared with Con females from 14–21 months of age and were lower at 24 months. For Con females, nocturnal concentrations of serum bioLH were similar between 14 and 21 months but increased significantly at 24 months. For Ob females, nocturnal concentrations of serum bioLH increased significantly from 14–18 months with no significant increment thereafter. Assessing the first significant rise in nocturnal concentrations of bioLH for each individual within each treatment group revealed that this occurred significantly younger for Ob females (14.9 ± 0.1 months) compared with Con (21.1 ± 0.8 months; t17 = 5.58; P < 0.0001). Despite this significant increase in nocturnal bioLH by leptin, serum concentrations were nevertheless significantly lower at all ages studied compared with those observed for the Ovx-Con females (Fig. 2; F2,22 = 54.29; P < 0.0001). A developmental increase in nocturnal LH was observed in these animals (F3,18 = 6.05; P = 0.007), with concentrations similar between 14 and 21 months of age and significantly lower than those observed at 24 months.

Mean ± sem concentrations of bioLH for Con (white bars), Ob females (black bars), and Ovx-con females (gray bars) in daytime (AM) and nighttime (PM) samples collected at 14, 18, 21, and 24 months of age. Different letters at each time point indicate groups are significantly different (P < 0.05).
In addition to this significantly earlier rise in nocturnal levels of bioLH, leptin administration also advanced the appearance of secondary sexual characteristics and menarche (Fig. 3A). The age at the first signs of perineal color and swelling was significantly younger for Ob compared with Con females (t17 = 2.31; P = 0.04). Furthermore, age at menarche was also younger for Ob compared with Con females (t17 = 2.11; P = 0.05). The acceleration in the timing of these external signs of puberty was associated with significantly elevated serum estradiol concentrations in Ob compared with Con females (Fig. 3B; F1,17 = 11.08; P = 0.004). For individual females, serum estradiol rose above the sensitivity of the assay (5.0 pg/ml) by 18.2 ± 1.3 months in Ob females, whereas this was delayed until 23.0 ± 0.5 months in Con females. Finally, analyses of twice weekly blood samples collected after menarche indicated that leptin did not advance the occurrence of first ovulation. One of the Ob females and three of the 13 Con females had ovulated by the time data collection was stopped (November 2002, or 30 months of age).
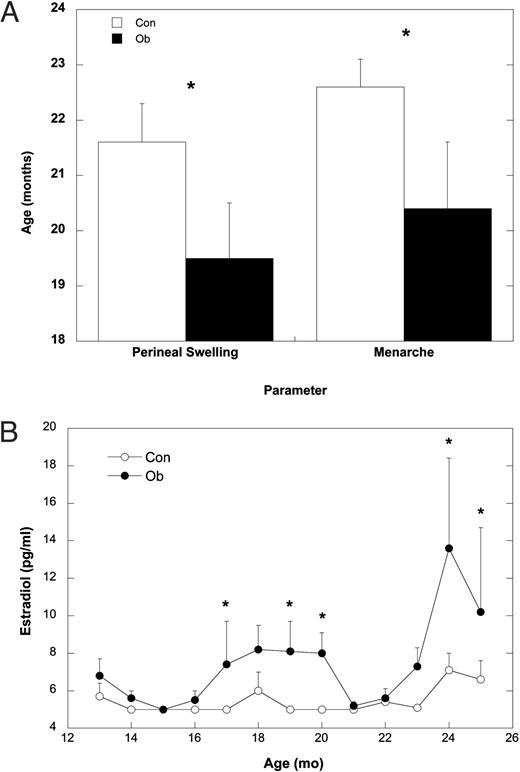
A, Mean ± sem age at the initial signs of perineal swelling and age at menarche for Con (white bars) and Ob females (black bars). B, Mean ± sem of serum estradiol concentrations from 12–25 months of age for Con (○) and Ob females (•). Asterisks indicate that the groups are significantly different (P < 0.05).
Leptin administration also differentially affected body growth. Leptin-treated animals had significantly lower body weights (P < 0.05) compared with Con females at 21 and 22 months and again from 25–27 months of age, whereas weights at other ages were similar (Fig. 4A; F18,306 = 1.93; P = 0.01). Overall, the increase in body weight from the start of treatment to 30 months of age was not different in Ob (2.33 ± 0.12 kg) compared with Con females (2.47 ± 0.11 kg; t17 = 0.70; P = 0.44). Height increased significantly during development in both groups (Fig. 4B; F5,85 = 392.78; P < 0.0001) and was significantly greater at specific ages in Ob compared with Con females. As an estimate of body fat, BMI was significantly different at specific ages (F5,85 = 3.45; P = 0.006), with Ob females having significantly lower BMI than Con from 18–29 months of age (Fig. 4C). Furthermore, the significant increase in crown rump lengths during development (F5,85 = 337.82; P < 0.0001) differed between Con and Ob females (Fig. 5A) with Ob females significantly longer at 15, 21, and 28 months but not at 13, 18, or 24 months. Skeletal maturity, assessed from hand and wrist radiographs, increased in both groups (Fig. 5B; F5,85 = 136.10; P < 0.0001) but was significantly advanced in Ob compared with Con females at 24 months of age (F5,85 = 2.76; P = 0.03). In contrast, the increase in bone mineral content did not differ between Con and Ob females throughout the study (Fig. 5C; F1,17 = 0.15; P = 0.71).
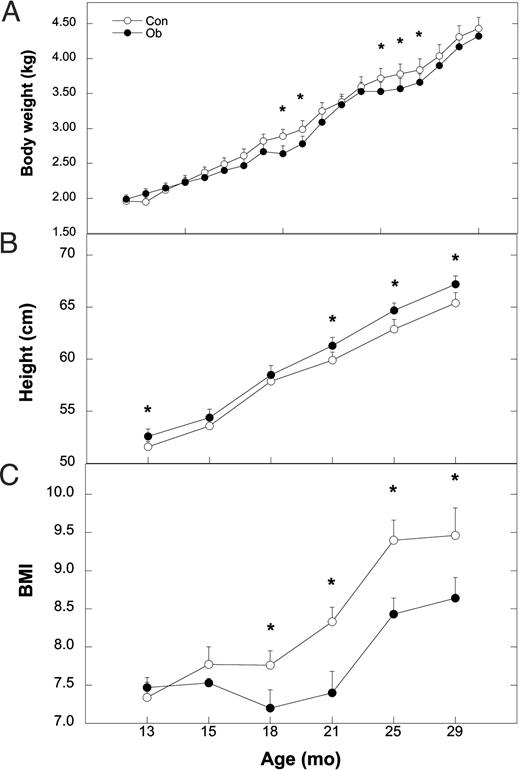
Mean ± sem values of body weight (A), height (B), and BMI (C) for Con (○) and Ob females (•) throughout the course of the study. Asterisks indicate that the difference between Con and Ob females at that time point is significant (P < 0.05).
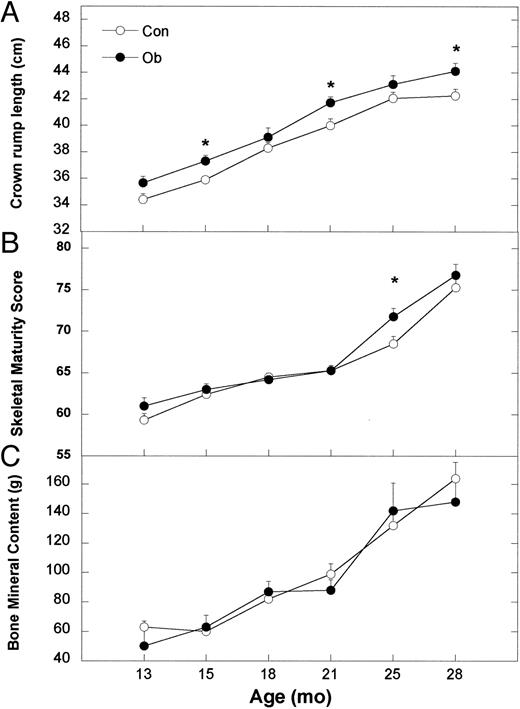
Mean ± sem values of crown rump length (A), skeletal maturity score (B), and bone mineral content (C) for Con (○) and Ob females (•) throughout the course of the study. Asterisks indicate that the difference between Con and Ob females at that time point is significant (P < 0.05).
Leptin administration also had a significant effect on GH and IGF-I concentrations in circulation (Fig. 6). Daytime GH concentrations varied significantly (F1,17 = 4.43; P = 0.05), with values higher in Ob compared with Con females at 14 and 18 months. Furthermore, nighttime GH concentrations were also significantly different (F1,17 = 4.63; P = 0.04), with higher levels in Ob compared with Con females at 14, 18, and 21 months of age, corresponding to 84, 198, and 265 d on treatment, respectively (Fig. 6A). For Con females, nocturnal levels of GH were similar at 14 and 18 months before rising significantly at 21 and 24 months. For Ob females, nocturnal GH also increased significantly at 21 months compared with values observed at 14 and 18 months. Furthermore, daytime concentrations of serum IGF-I (Fig. 6B) were significantly higher at 14 and 18 months of age in Ob compared with Con females but were not different at 21 and 24 months of age (F1,17 = 6.61; P = 0.02). Serum IGF-I increased significantly at each successive age for Con females, whereas the increase was significant through 21 months in Ob females.
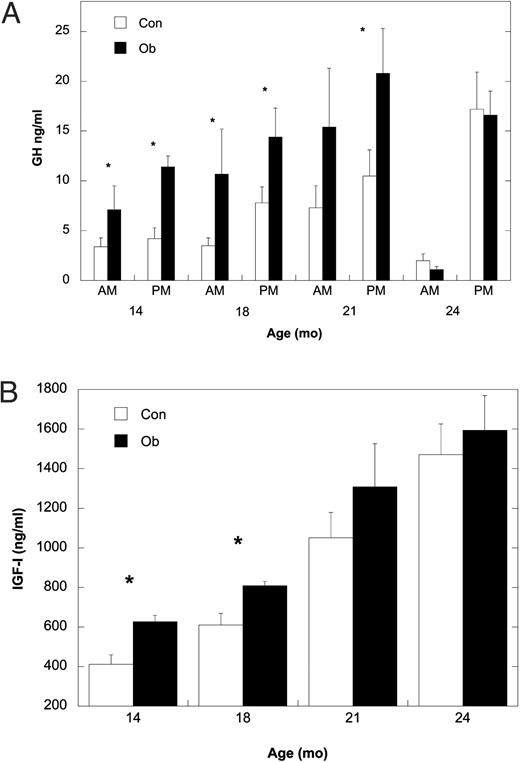
A, Mean ± sem serum concentrations of GH for Con (white bars) and Ob females (black bars) in daytime and nighttime samples collected from 14–24 months of age. B, Mean ± sem serum concentrations of IGF-I at 14, 18, 21, and 24 months of age for Con (white bars) and Ob females (black bars). Asterisk indicates that the difference between Con and Ob females at that time point is significant (P < 0.05).
Discussion
The results from the present study show that the chronic elevation in peripheral concentrations of leptin increases serum levels of both day and nighttime bioLH at a significantly younger age compared with the gonadally intact Con females. The earlier rise in LH in Ob females was associated with an earlier increase in serum estradiol, onset of secondary sexual characteristics, and occurrence of menarche. However, the timing of first ovulation was not affected by leptin treatment, at least within the duration of the treatment period. Furthermore, leptin increased crown rump lengths and height and advanced skeletal maturity during the course of the study, and these were associated with significantly higher serum concentrations of nocturnal GH and daytime IGF-I concentrations. Although body weights were not consistently affected by treatment and were even similar between groups at the completion of the study, BMI, as an index of body fat, was lower in Ob females.
These results could lead one to conclude that, in the female primate, leptin is a key signal for the onset of puberty. However, using an increase in nocturnal LH secretion as a definition for puberty onset must take into account whether the increase is due to a change in steroid-independent drive or the result of a decrease in steroid inhibition. Thus, drawing inferences from the significantly earlier rise in nocturnal LH in the Ob compared with Con gonadally intact females must be balanced against the robust secretion of LH at the same age in the ovariectomized, non-leptin-treated controls. At every age assessed, LH concentrations in these ovariectomized, age-matched females exceeded those in the Ob, intact females and even more so in the intact Con animals. Thus, the steroid-independent drive to GnRH, inferred from LH, was well under way in females by 14 months of age, and the change in LH secretion in nonovariectomized females would be due to alterations in gonadal restraint of the pulse generator (51).
The comparison of gonadally intact females with ovariectomized females emphasizes two points. The first is that the onset of puberty for female primates may not be easily defined as simply an increase in nocturnal LH without reference to the gonadal status of the individual. Secondly, understanding factors that influence the progression of puberty in gonadally intact females is important. The elevated levels of bioLH in the ovariectomized females at 14 months of age supports other data that, unlike the male primate, developmental increases in LH in females are influenced by both nongonadal factors and estradiol negative feedback inhibition (51, 65). We cannot say how nocturnal LH changed in these agonadal females from the time of ovariectomy to the first assessment at 14 months or whether leptin would have affected this pattern. The use of the sensitive bioassay for LH in this study clearly showed robust nocturnal concentrations of LH at 14 months that did not increase further in these animals until 24 months. Data using other approaches certainly indicate that LH, as well as GnRH, increase developmentally in female monkeys in the absence of gonadal negative feedback (66–68). However, understanding how gonadal negative feedback changes during maturation is still relevant. The significant elevation in nocturnal LH in the ovariectomized females by 14 months occurred some 9 months before menarche in the intact controls and approximately 18 months before first ovulation for animals in our indoor colony (60). This delay underscores the critical role that estradiol negative feedback inhibition imposes on the progression of puberty. Elegant data from the Terasawa laboratory (69) support the importance of this notion. Although the administration of the γ-aminobutyric acidA antagonist, bicuculline, to gonadally intact female monkeys significantly advances age at menarche and first ovulation, the effect is not immediate. Rather, menarche occurs approximately 2.8 months after the initiation of treatment and ovulation 14 months later, an interval similar to the interval between menarche and first ovulation in the saline controls. The point is that, despite unleashing the nongonadal break on the GnRH pulse generator by antagonism of γ-aminobutyric acid receptors, the progression of puberty, timed by gonadal negative feedback, was similar to that of normal monkeys. The bicuculline-treated animals simply started earlier. Thus, focusing on the process rather than the onset of puberty for the female may provide a more comprehensive understanding of factors responsible for the attainment of fertility (50).
Given this perspective, the results of the present study suggest that leptin administration accelerates the progression of puberty in gonadally intact animals. Leptin advanced not only the age of the developmental elevation in nocturnal LH, but also a significantly earlier rise in serum estradiol, an earlier age at menarche, and the appearance of secondary sexual characteristics. It is not surprising that a detectable increase in serum estradiol lagged the significant elevation in nocturnal LH because the specific pulsatile pattern of LH and FSH must be attained to drive ovarian steroid production (70, 71). Furthermore, the availability of an ultrasensitive assay for estradiol, typically used for girls (72), may have detected the increase in estradiol at an earlier age. We were not able to adequately determine whether leptin administration advanced first ovulation in our study because data collection was stopped before the typical age of first ovulation of 31–32 months in our indoor-housed colony (59, 60). Nevertheless, a possible explanation for the acceleration of the other parameters of puberty is that leptin may diminish the efficacy of estradiol negative feedback inhibition of LH secretion. A pubertal change in estradiol negative feedback in the monkey has been previously attributed to developmental increases in IGF-I (53, 60). Because leptin administration also elevated serum IGF-I in the present study, one cannot rule out the possibility that the increase in LH at younger ages in treated females was due to leptin stimulation of IGF-I and not a direct effect of leptin. Whether direct or indirect, gonadal restraint is not completely unleashed by leptin, because LH concentrations did not increase to levels seen in ovariectomized females. Nevertheless, prospective studies will need to determine whether leptin impacts LH secretion in agonadal females and how leptin and IGF-I may differentially affect estradiol negative feedback during puberty in female primates.
An important question is how to reconcile these data in the female with previous studies of male monkeys which show that leptin does not increase pulsatile LH secretion in castrated, GnRH-sensitized animals (48). Although the route of administration and dose of leptin differed, it is unlikely that these accounted for the opposite results. However, males were treated for approximately 3 wk, whereas the females in the present study were treated for 78 wk. Two issues influenced the treatment parameters for the males. In the early stages of the study, the investigators serendipitously discovered that leptin was stimulating the production of antibodies or leptin binding proteins. Thus, they chose to limit the treatment duration and analysis to a period before the binding proteins rose. However, as pointed out by the authors, it was unlikely that these antibodies were neutralizing because GH levels were nonetheless elevated in Ob animals despite the presence of the binding proteins. Using a different technique (63), we too detected the presence of leptin binding activity in serum as early as 1 month into treatment. As found in the male study, it was unlikely that these were neutralizing because treatment differences in bioLH and GH were still evident some 265 d into treatment, and height, crown rump, and skeletal maturity were greater in Ob females at the completion of the study.
In the previous study of male monkeys, the a priori rationale for limiting the treatment to a 3-wk period was the observation that the administration of the glutamate receptor agonist, N-methyl-d-aspartate, immediately initiates an adult-like pattern of LH and testosterone secretion in prepubertal male monkeys (73). The investigators argued (48) that if leptin is important, it too should elicit an immediate response. Although the activation of glutamate receptors has been implicated as one of several neurobiological mechanisms responsible for the developmental increase in GnRH and LH (55), the actual developmental time course of these neurobiological changes in vivo is not known and may involve a remodeling of inputs into hypothalamic GnRH neurons (74). It is not known whether a longer treatment period of leptin for the males would have produced a different outcome. In the present study, the first assessment of nocturnal LH was made 84 d into treatment when bioLH was already elevated in Ob compared with Con females. It is not known whether a significant elevation in serum LH would have been observed before this time.
This methodological issue aside, the difference in outcome between the two studies may reflect sex differences in the control of puberty. Given the differential importance of the gonad in regulating the postnatal secretion of gonadotropins between male and female primates (51, 75), it is plausible that the neurobiological control of this process is distinctive as well. This is certainly the case with other mammals. Male and female rats show a differential sensitivity to estradiol negative feedback during development that appears to be due to androgenization during the perinatal period (76). In sheep, leptin- and growth-related cues are important in males, whereas photoperiodic information and changing sensitivity to estradiol negative feedback is critical for females (50). Obesity results in precocious puberty in girls, whereas obese boys tend to mature later (56). It is possible that this sex difference is due to a differential sensitivity to obesity-induced hyperleptinemia associated with obesity in girls (77). This is not to say that energetics and cues associated with positive energy balance are not important for male primates. However, previous attempts to evaluate leptin as a metabolic signal for the maintenance of LH secretion in postpubertal or young adult males have produced mixed results (35, 78). A systematic comparison of sex differences in factors, including leptin, affecting puberty in primates has not been done.
In addition to its effects on the reproductive axis, leptin administration also significantly advanced the developmental increase in daytime and nighttime GH and, consequently, IGF-I secretion. Although this could be due to an increase in gonadal estradiol secretion coincident with an increase in nocturnal LH, as occurs during puberty (79, 80), it is more likely the result of a direct action of leptin on the neural mechanisms controlling GH secretion because serum estradiol in the Ob females did not show appreciable increases until approximately 18 months of age. Furthermore, leptin increases GH release in castrated, juvenile male monkeys (48), and icv administration of leptin increases GH in sheep (81). The observation that both GH and IGF-I were similar between groups at 24 months of age reflects the activation of the GH axis by rising estradiol levels, because menarche had occurred by this age in the untreated control females.
Associated with this acceleration in GH and IGF-I release were significant developmental differences in crown rump lengths and height. This was evident at the first measurement taken at 13 months, or 1 month after treatment had started, as well as at 30 months of age when treatment was stopped. Because skeletal measurements were not taken at the initiation of leptin treatment at 12 months of age, it is possible that Ob females were larger to begin with. Although this possibility cannot be ruled out, it seems unlikely, because all females were randomly assigned to treatment groups, body weights were not different at 12 months of age, and group differences in crown rump lengths and heights increased with time on treatment. The effect of leptin on skeletal growth in monkeys supports similar data in ob/ob mice (82) and leptin-deficient children (83). Although leptin may act in a paracrine fashion to stimulate bone growth via IGF-I (84), data from the present study suggest that, at least in nonobese females, leptin-induced elevations in GH augment adolescent growth. In contrast, leptin had no effect on the developmental increase in bone mineral content. This supports observations in children that leptin is unrelated to bone mineral content or density (85). The possible leptin-induced decreases in bone mass (86) could have been offset by the significant activation of the GH axis in these developing females. Furthermore, leptin administration advanced skeletal maturity, but the effect was not seen until 25 months of age. Because skeletal maturity, reflecting calcification of the epiphyses, is driven by developmental increases in estradiol (87), the emergence of differences at 25 months likely reflects the continued exposure to higher levels of estradiol in the Ob females. Finally, body weight was not consistently affected by the 18-month leptin treatment. It was not possible to quantify food intake in the present study, given the social housing of the subjects. Nevertheless, calculations of BMI, as an index of body fat, were consistently lower in Ob monkeys.
Our observations that leptin accelerates indices of puberty are not merely academic but may provide insight into the relationship between early puberty and obesity in girls (56) and the consequences of this accelerated maturation on adult health. Although some studies suggest no relationship between fat mass and puberty (88), the majority of evidence indicates that age at puberty, defined in a number of ways, has been decreasing for decades and is associated with degree of fat mass (56, 89, 90). The results of the present study suggest that an elevation in serum leptin concentrations, resulting from accumulating fat mass, could increase LH secretion and advance the progression of puberty. We observed small but significant increases in diurnal leptin in non-leptin-treated monkeys associated with elevations in nocturnal LH. Furthermore, leptin binding activity decreased as sexual maturation advanced in non-leptin-treated females, a situation similar to that of children (63, 91). It is not known whether this change in serum binding activity increases leptin bioactivity. However, the relationship between changes in unbound leptin and tissue-specific effects is likely complex, because unbound leptin is higher in obese individuals compared with lean individuals (92, 93). What is clear from the present study is that producing excessively high levels of leptin through exogenous treatment, as would be observed in obese girls (94), advanced signs of reproductive maturation. This signal may be one of several used by the brain to assess positive energy balance to time the occurrence of puberty (50).
Acknowledgments
The invaluable technical assistance of Susie Lackey, Ilana Brown, and Caryn Rowland of the Yerkes Endocrine Core Laboratory is greatly appreciated. We also appreciate the help of Jill Johnson-Ward in collection of bone scan data.
This work was supported by National Institutes of Health Grants HD37583, HD39153, and RR00165. The Yerkes National Primate Research Center is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Abbreviations:
- bioLH,
Bioactive LH;
- BMI,
body mass index;
- Con,
control group;
- CV,
coefficient(s) of variation;
- icv,
intracerebroventricular(ly);
- Ob,
leptin-treated group;
- Ovx-Con,
ovariectomized controls.



