-
PDF
- Split View
-
Views
-
Cite
Cite
Leili Rahimi, Annop Kittithaworn, Raul Gregg Garcia, Jasmine Saini, Prerna Dogra, Elizabeth J Atkinson, Sara J Achenbach, Andrea Kattah, Irina Bancos, Kidney Function in Patients With Adrenal Adenomas: A Single-Center Retrospective Cohort Study, The Journal of Clinical Endocrinology & Metabolism, Volume 109, Issue 9, September 2024, Pages e1750–e1758, https://doi.org/10.1210/clinem/dgad765
Close - Share Icon Share
Abstract
Patients with nonfunctioning adrenal adenomas (NFA) and mild autonomous cortisol secretion (MACS) demonstrate an increased risk of chronic kidney disease (CKD); however, factors associated with CKD are unknown.
We aimed to identify the factors associated with CKD and assess the effect of adrenalectomy on kidney function in patients with NFA or MACS.
A single-center cohort study of patients with NFA and MACS, 1999 to 2020, was conducted. MACS was diagnosed based on post dexamethasone suppression test (DST) cortisol greater than or equal to 1.8 mcg/dL. Age, sex, dysglycemia, hypertension, therapy with statin, angiotensin-converting enzyme inhibitor, or angiotensin II receptor blocker were included in the multivariable analysis. Outcomes included estimated glomerular filtration rate (eGFR) at the time of diagnosis with MACS or NFA and postadrenalectomy delta eGFR.
Of 972 patients, 429 (44%) had MACS and 543 (56%) had NFA. At the time of diagnosis, patients with MACS had lower eGFR (median 79.6 vs 83.8 mL/min/1.73 m2; P < .001) than patients with NFA. In a multivariable analysis, factors associated with lower eGFR were older age, hypertension, and higher DST. In 204 patients (MACS: 155, 76% and NFA: 49, 24%) treated with adrenalectomy, postadrenalectomy eGFR improved in both groups starting at 18 months up to 3.5 years of follow-up. Factors associated with increased eGFR were younger age, lower preadrenalectomy eGFR, and longer follow-up period.
DST cortisol is an independent risk factor for lower eGFR in patients with adrenal adenomas. Patients with both MACS and NFA demonstrate an increase in eGFR post adrenalectomy, especially younger patients with lower eGFR pre adrenalectomy.
Incidentally discovered adrenal tumors are reported in about 3% to 7% of adults undergoing cross-sectional imaging, and most are benign adrenal cortical adenomas (1, 2). Adrenal adenomas may present with overt or mild adrenal hormone excess or be nonfunctioning (NFA). Mild autonomous cortisol secretion (MACS) is the most common hormonal abnormality noted in up to 30% to 50% of cases (1, 3, 4). MACS is defined as abnormal cortisol following the overnight dexamethasone suppression of 1.8 mcg/dL or greater in a patient without overt features of cortisol excess (5, 6).
MACS, and to a lesser extent NFA, were reported to be associated with increased prevalence and incidence of cardiovascular risk factors, such as hypertension, dyslipidemia, diabetes mellitus type 2, and obesity (7-13). Data on adrenal adenomas and chronic kidney disease (CKD) are scarce with discrepant results. Some studies demonstrate increased incidence of CKD in a mixed cohort of patients with NFA and MACS (19% vs 12%) (3) and in patients with NFA when compared to referent individuals (7.3% vs 2.3%) (14). However, when examining kidney function in MACS vs NFA, one study reported a higher prevalence of CKD in patients with MACS (25.2% vs 16.9% in NFA) (9), while another study showed no subgroup differences in kidney function (15).
Even less information is available on the effect of adrenalectomy on kidney function in patients with MACS, and none in patients with NFA. Current evidence consists of 3 small studies in mixed cohorts of patients with both MACS and overt Cushing syndrome (CS) that demonstrated discordant results, with one study demonstrating improvement in kidney function (16), another study—no change (17), and the third study—worsening of kidney function (15). Available evidence is limited by small sample size, inclusion of mixed subgroups of patients, lack of consideration of concomitant cardiovascular comorbidities that may have an effect on kidney function, and insufficient duration of follow-up to assess for kidney function change post adrenalectomy.
In this study, we aimed to (1) determine the prevalence of CKD in patients with MACS vs NFA, (2) distinguish the effect of associated cardiovascular comorbidities and medications vs abnormal postdexamethasone cortisol on kidney function as determined by estimated glomerular filtration rate (eGFR), and (3) determine the effect of adrenalectomy on kidney function and factors associated with change in eGFR in patients with MACS and NFA.
Materials and Methods
Study Design
We conducted a single-center, retrospective cohort study of patients evaluated for adrenal adenoma between April 3, 1999 and March 17, 2020. The study protocol was approved by the institutional review board (No. 14-008336), and only patients who provided a research consent waiver were included.
Participants
We identified all consecutive adults with adrenal adenoma evaluated either for NFA or MACS at our institution during the study period. Adenomas were defined based on the following reference standard: 1) imaging with unenhanced computed tomography Hounsfield units less than 10, 2) follow-up imaging of at least 6 months demonstrating no tumor growth, 3) histology positive for adenoma in those undergoing adrenalectomy, and 4) clinical follow-up of at least 5 years without development of adrenal malignancy. MACS was diagnosed based on post dexamethasone suppression test (DST) cortisol greater than or equal to 1.8 mcg/dL, and NFA was based on DST less than 1.8 mcg/dL (5). We excluded patients with overt hormone excess, primary aldosteronism, presence of urological disorders (such as kidney or bladder cancer, nephrolithiasis), and unavailable data on serum creatinine and eGFR measurement within 6 months of diagnosis with NFA or MACS.
Variables of Interest
Clinical, biochemical, radiological, and surgical information was obtained from the medical record for each patient. We collected data on age, sex, body mass index (BMI), comorbidities, including dysglycemia (prediabetes/diabetes), hypertension, and dyslipidemia, therapy with statin, therapy with angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, adrenalectomy (if performed), and date of adrenalectomy. We calculated the eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation with serum creatinine, if available within 6 months prior or after the date of first hormonal workup. In patients treated with adrenalectomy, only the closest eGFR available within 6 months prior to adrenalectomy was considered. For postadrenalectomy outcomes, all eGFR measurements up to 3.5 years post adrenalectomy were considered. The diagnosis of CKD was made based on eGFR. As data on albuminuria were not available, CKD stage 1 (eGFR >90 mL/min/1.73 m2 and albuminuria) and CKD stage 2 (eGFR 60-89 mL/min/1.73 m2 and albuminuria) were not included. CKD stages 3a, 3b, 4, and 5 were defined by eGFR 45 to 59, 30 to 44, 15 to 29, and less than 15 mL/min/1.73 m2, respectively.
Outcomes
Primary outcomes included eGFR in patients with MACS and NFA at the time of diagnosis, and delta eGFR in those who underwent adrenalectomy. Secondary outcomes were effect of individual variables on eGFR at the time of diagnosis and post adrenalectomy.
Statistical Analysis
Continuous variables were reported as median and interquartile range (IQR); comparisons were performed using a Kruskal-Wallis test. Categorical variables were reported as counts and percentages and compared using the chi-square test. As it was not possible to determine whether the indication for statin therapy was dyslipidemia, we have included only statin therapy as a variable of interest in the multivariable analysis. Linear regression models were used to assess the associations between eGFR levels at the time of adenoma diagnosis and clinical covariates including age, sex, BMI, hypertension, dysglycemia, statin therapy, smoking, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, and postdexamethasone cortisol levels. Linear mixed-effects models were used to predict the change in kidney function over the course of the first 3 years post adrenalectomy including assessment of an interaction between time and group. All analyses were conducted using R version 4.1.2.
Results
Of the 972 patients meeting inclusion criteria (Fig. 1), 429 (44%) had MACS, diagnosed at a median age of 62.8 years (IQR 54.6-71.1 years) and 543 (56%) had NFA, diagnosed at a median age of 59.4 years (IQR 50.5-67.3 years) (P < .001). The proportion of women was higher both in the MACS and NFA groups (66.4% vs 63.4%; P = .318) (Table 1).
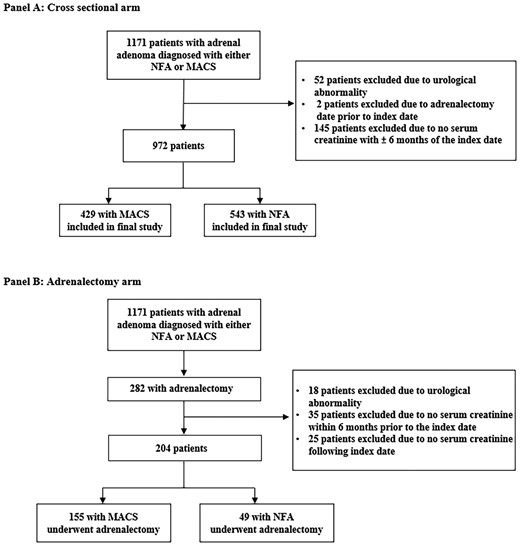
Flow diagram depicting the selection process of patients entering the study. A, Cross sectional arm. B, Adrenalectomy arm. Index date is the date of adrenalectomy. MACS, mild autonomous cortisol secretion; NFA, nonfunctioning adrenal adenoma.
Demographic and baseline characteristics of patients with mild autonomous cortisol secretion or nonfunctioning adrenal adenomas
| . | n available . | All patients (n = 972) . | Nonfunctioning adrenal adenomas (n = 543) . | Mild autonomous cortisol secretion (n = 429) . | P . |
|---|---|---|---|---|---|
| Age, y median (IQR) | 972 | 60.9 (52.6-68.7) | 59.4 (50.5-67.3) | 62.8 (54.6-71.1) | <.001 |
| Women, n (%) | 972 | 629 (64.7%) | 344 (63.4%) | 285 (66.4%) | .318 |
| Race, n (%) | 972 | .151 | |||
| Black | 10 (1.1%) | 5 (0.9%) | 5 (1.2%) | ||
| Asian | 14 (1.5%) | 9 (1.7%) | 5 (1.2%) | ||
| White | 894 (95.0%) | 495 (93.9%) | 399 (96.4%) | ||
| Other | 23 (2.4%) | 18 (3.4%) | 5 (1.2%) | ||
| Unknown | 31 | 16 | 15 | ||
| Smoking history, n (%) | 918 | 609 (66.3%) | 329 (63.0%) | 280 (70.7%) | .015 |
| Alcohol abuse history, n (%) | 972 | 160 (16.5%) | 85 (15.7%) | 75 (17.5%) | .445 |
| BMI, median (IQR) | 965 | 31.0 (26.8-36.3) | 31.4 (27.3-36.6) | 30.7 (26.1-36.0) | .035 |
| BMI, n (%) | 958 | .044 | |||
| ≤18.5 | 7 (0.7%) | 4 (0.7%) | 3 (0.7%) | ||
| 18.5-24.9 | 146 (15.1%) | 66 (12.2%) | 80 (18.8%) | ||
| 25.0-29.9 | 277 (28.7%) | 157 (29.1%) | 120 (28.2%) | ||
| ≥30.0 | 535 (55.4%) | 312 (57.9%) | 223 (52.3%) | ||
| Hypertension, n (%) | 972 | 515 (53.0%) | 266 (49.0%) | 249 (58.0%) | .005 |
| Outpatient angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, n (%) | 972 | 331 (34.1%) | 178 (32.8%) | 153 (35.7%) | .346 |
| Dyslipidemia, n (%) | 972 | 429 (44.1%) | 241 (44.4%) | 188 (43.8%) | .861 |
| Statin therapy, n (%) | 341 (35.1%) | 185 (34.1%) | 156 (36.4%) | .457 | |
| Dyslipidemia and statin prescription in prior 5 y | 266 (27.4%) | 147 (27.1%) | 119 (27.7%) | .817 | |
| Dysglycemia, n (%) | 972 | .133 | |||
| Prediabetes | 91 (9.4%) | 56 (10.3%) | 35 (8.2%) | ||
| Diabetes mellitus | 218 (22.4%) | 110 (20.3%) | 108 (25.2%) | ||
| Tumor size, mm median (IQR) | 964 | 20.0 (14-30) | 17.0 (12-24) | 27.0 (17-37) | <.001 |
| Laterality, n (%) | 964 | <.001 | |||
| Left | 516 (53.3%) | 310 (57.2%) | 206 (48.4%) | ||
| Right | 273 (28.2%) | 166 (30.6%) | 107 (25.1%) | ||
| Bilateral | 179 (18.5%) | 66 (12.2%) | 113 (26.5%) | ||
| Postdexamethasone cortisol, mcg/dL median (IQR) | 972 | 1.7 (1.1-2.7) | 1.2 (1.0-1.5) | 3.0 (2.3-4.5) | — |
| Creatinine, mg/dL median (IQR) | 972 | 0.83 (0.72-1.00) | 0.83 (0.71-1.00) | 0.85 (0.72-1.01) | .003 |
| eGFR mL/min/1.73 m2 median (IQR) | 972 | 82.3 (67.7-94.9) | 83.8 (69.2-96.5) | 79.6 (66.4-92.0) | <.001 |
| Categories based on eGFR, n (%) | 972 | <.001 | |||
| eGFR >90 mL/min/1.73 m2 | 350 (36.0%) | 222 (40.9%) | 128 (29.8%) | ||
| eGFR 60-89 mL/min/1.73 m2 | 483 (49.7%) | 257 (47.3%) | 226 (52.7%) | ||
| EPI-CKDa stage 3a | 87 (9.0%) | 48 (8.8%) | 39 (9.1%) | ||
| EPI-CKD stage 3b | 35 (3.6%) | 12 (2.2%) | 23 (5.4%) | ||
| EPI-CKD stage 4 | 12 (1.2%) | 4 (0.7%) | 8 (1.9%) | ||
| EPI-CKD stage 5 | 5 (0.5%) | 0 (0.0%) | 5 (1.2%) |
| . | n available . | All patients (n = 972) . | Nonfunctioning adrenal adenomas (n = 543) . | Mild autonomous cortisol secretion (n = 429) . | P . |
|---|---|---|---|---|---|
| Age, y median (IQR) | 972 | 60.9 (52.6-68.7) | 59.4 (50.5-67.3) | 62.8 (54.6-71.1) | <.001 |
| Women, n (%) | 972 | 629 (64.7%) | 344 (63.4%) | 285 (66.4%) | .318 |
| Race, n (%) | 972 | .151 | |||
| Black | 10 (1.1%) | 5 (0.9%) | 5 (1.2%) | ||
| Asian | 14 (1.5%) | 9 (1.7%) | 5 (1.2%) | ||
| White | 894 (95.0%) | 495 (93.9%) | 399 (96.4%) | ||
| Other | 23 (2.4%) | 18 (3.4%) | 5 (1.2%) | ||
| Unknown | 31 | 16 | 15 | ||
| Smoking history, n (%) | 918 | 609 (66.3%) | 329 (63.0%) | 280 (70.7%) | .015 |
| Alcohol abuse history, n (%) | 972 | 160 (16.5%) | 85 (15.7%) | 75 (17.5%) | .445 |
| BMI, median (IQR) | 965 | 31.0 (26.8-36.3) | 31.4 (27.3-36.6) | 30.7 (26.1-36.0) | .035 |
| BMI, n (%) | 958 | .044 | |||
| ≤18.5 | 7 (0.7%) | 4 (0.7%) | 3 (0.7%) | ||
| 18.5-24.9 | 146 (15.1%) | 66 (12.2%) | 80 (18.8%) | ||
| 25.0-29.9 | 277 (28.7%) | 157 (29.1%) | 120 (28.2%) | ||
| ≥30.0 | 535 (55.4%) | 312 (57.9%) | 223 (52.3%) | ||
| Hypertension, n (%) | 972 | 515 (53.0%) | 266 (49.0%) | 249 (58.0%) | .005 |
| Outpatient angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, n (%) | 972 | 331 (34.1%) | 178 (32.8%) | 153 (35.7%) | .346 |
| Dyslipidemia, n (%) | 972 | 429 (44.1%) | 241 (44.4%) | 188 (43.8%) | .861 |
| Statin therapy, n (%) | 341 (35.1%) | 185 (34.1%) | 156 (36.4%) | .457 | |
| Dyslipidemia and statin prescription in prior 5 y | 266 (27.4%) | 147 (27.1%) | 119 (27.7%) | .817 | |
| Dysglycemia, n (%) | 972 | .133 | |||
| Prediabetes | 91 (9.4%) | 56 (10.3%) | 35 (8.2%) | ||
| Diabetes mellitus | 218 (22.4%) | 110 (20.3%) | 108 (25.2%) | ||
| Tumor size, mm median (IQR) | 964 | 20.0 (14-30) | 17.0 (12-24) | 27.0 (17-37) | <.001 |
| Laterality, n (%) | 964 | <.001 | |||
| Left | 516 (53.3%) | 310 (57.2%) | 206 (48.4%) | ||
| Right | 273 (28.2%) | 166 (30.6%) | 107 (25.1%) | ||
| Bilateral | 179 (18.5%) | 66 (12.2%) | 113 (26.5%) | ||
| Postdexamethasone cortisol, mcg/dL median (IQR) | 972 | 1.7 (1.1-2.7) | 1.2 (1.0-1.5) | 3.0 (2.3-4.5) | — |
| Creatinine, mg/dL median (IQR) | 972 | 0.83 (0.72-1.00) | 0.83 (0.71-1.00) | 0.85 (0.72-1.01) | .003 |
| eGFR mL/min/1.73 m2 median (IQR) | 972 | 82.3 (67.7-94.9) | 83.8 (69.2-96.5) | 79.6 (66.4-92.0) | <.001 |
| Categories based on eGFR, n (%) | 972 | <.001 | |||
| eGFR >90 mL/min/1.73 m2 | 350 (36.0%) | 222 (40.9%) | 128 (29.8%) | ||
| eGFR 60-89 mL/min/1.73 m2 | 483 (49.7%) | 257 (47.3%) | 226 (52.7%) | ||
| EPI-CKDa stage 3a | 87 (9.0%) | 48 (8.8%) | 39 (9.1%) | ||
| EPI-CKD stage 3b | 35 (3.6%) | 12 (2.2%) | 23 (5.4%) | ||
| EPI-CKD stage 4 | 12 (1.2%) | 4 (0.7%) | 8 (1.9%) | ||
| EPI-CKD stage 5 | 5 (0.5%) | 0 (0.0%) | 5 (1.2%) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
aEPI-CKD: Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation: CKD stage 3a: eGFR 45-59 mL/min/1.73 m2; CKD stage 3b: eGFR 30-44 mL/min/1.73 m2; CKD stage 4: eGFR 15-29 mL/min/1.73 m2, CKD stage 5: < 15 mL/min/1.73 m2.
Demographic and baseline characteristics of patients with mild autonomous cortisol secretion or nonfunctioning adrenal adenomas
| . | n available . | All patients (n = 972) . | Nonfunctioning adrenal adenomas (n = 543) . | Mild autonomous cortisol secretion (n = 429) . | P . |
|---|---|---|---|---|---|
| Age, y median (IQR) | 972 | 60.9 (52.6-68.7) | 59.4 (50.5-67.3) | 62.8 (54.6-71.1) | <.001 |
| Women, n (%) | 972 | 629 (64.7%) | 344 (63.4%) | 285 (66.4%) | .318 |
| Race, n (%) | 972 | .151 | |||
| Black | 10 (1.1%) | 5 (0.9%) | 5 (1.2%) | ||
| Asian | 14 (1.5%) | 9 (1.7%) | 5 (1.2%) | ||
| White | 894 (95.0%) | 495 (93.9%) | 399 (96.4%) | ||
| Other | 23 (2.4%) | 18 (3.4%) | 5 (1.2%) | ||
| Unknown | 31 | 16 | 15 | ||
| Smoking history, n (%) | 918 | 609 (66.3%) | 329 (63.0%) | 280 (70.7%) | .015 |
| Alcohol abuse history, n (%) | 972 | 160 (16.5%) | 85 (15.7%) | 75 (17.5%) | .445 |
| BMI, median (IQR) | 965 | 31.0 (26.8-36.3) | 31.4 (27.3-36.6) | 30.7 (26.1-36.0) | .035 |
| BMI, n (%) | 958 | .044 | |||
| ≤18.5 | 7 (0.7%) | 4 (0.7%) | 3 (0.7%) | ||
| 18.5-24.9 | 146 (15.1%) | 66 (12.2%) | 80 (18.8%) | ||
| 25.0-29.9 | 277 (28.7%) | 157 (29.1%) | 120 (28.2%) | ||
| ≥30.0 | 535 (55.4%) | 312 (57.9%) | 223 (52.3%) | ||
| Hypertension, n (%) | 972 | 515 (53.0%) | 266 (49.0%) | 249 (58.0%) | .005 |
| Outpatient angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, n (%) | 972 | 331 (34.1%) | 178 (32.8%) | 153 (35.7%) | .346 |
| Dyslipidemia, n (%) | 972 | 429 (44.1%) | 241 (44.4%) | 188 (43.8%) | .861 |
| Statin therapy, n (%) | 341 (35.1%) | 185 (34.1%) | 156 (36.4%) | .457 | |
| Dyslipidemia and statin prescription in prior 5 y | 266 (27.4%) | 147 (27.1%) | 119 (27.7%) | .817 | |
| Dysglycemia, n (%) | 972 | .133 | |||
| Prediabetes | 91 (9.4%) | 56 (10.3%) | 35 (8.2%) | ||
| Diabetes mellitus | 218 (22.4%) | 110 (20.3%) | 108 (25.2%) | ||
| Tumor size, mm median (IQR) | 964 | 20.0 (14-30) | 17.0 (12-24) | 27.0 (17-37) | <.001 |
| Laterality, n (%) | 964 | <.001 | |||
| Left | 516 (53.3%) | 310 (57.2%) | 206 (48.4%) | ||
| Right | 273 (28.2%) | 166 (30.6%) | 107 (25.1%) | ||
| Bilateral | 179 (18.5%) | 66 (12.2%) | 113 (26.5%) | ||
| Postdexamethasone cortisol, mcg/dL median (IQR) | 972 | 1.7 (1.1-2.7) | 1.2 (1.0-1.5) | 3.0 (2.3-4.5) | — |
| Creatinine, mg/dL median (IQR) | 972 | 0.83 (0.72-1.00) | 0.83 (0.71-1.00) | 0.85 (0.72-1.01) | .003 |
| eGFR mL/min/1.73 m2 median (IQR) | 972 | 82.3 (67.7-94.9) | 83.8 (69.2-96.5) | 79.6 (66.4-92.0) | <.001 |
| Categories based on eGFR, n (%) | 972 | <.001 | |||
| eGFR >90 mL/min/1.73 m2 | 350 (36.0%) | 222 (40.9%) | 128 (29.8%) | ||
| eGFR 60-89 mL/min/1.73 m2 | 483 (49.7%) | 257 (47.3%) | 226 (52.7%) | ||
| EPI-CKDa stage 3a | 87 (9.0%) | 48 (8.8%) | 39 (9.1%) | ||
| EPI-CKD stage 3b | 35 (3.6%) | 12 (2.2%) | 23 (5.4%) | ||
| EPI-CKD stage 4 | 12 (1.2%) | 4 (0.7%) | 8 (1.9%) | ||
| EPI-CKD stage 5 | 5 (0.5%) | 0 (0.0%) | 5 (1.2%) |
| . | n available . | All patients (n = 972) . | Nonfunctioning adrenal adenomas (n = 543) . | Mild autonomous cortisol secretion (n = 429) . | P . |
|---|---|---|---|---|---|
| Age, y median (IQR) | 972 | 60.9 (52.6-68.7) | 59.4 (50.5-67.3) | 62.8 (54.6-71.1) | <.001 |
| Women, n (%) | 972 | 629 (64.7%) | 344 (63.4%) | 285 (66.4%) | .318 |
| Race, n (%) | 972 | .151 | |||
| Black | 10 (1.1%) | 5 (0.9%) | 5 (1.2%) | ||
| Asian | 14 (1.5%) | 9 (1.7%) | 5 (1.2%) | ||
| White | 894 (95.0%) | 495 (93.9%) | 399 (96.4%) | ||
| Other | 23 (2.4%) | 18 (3.4%) | 5 (1.2%) | ||
| Unknown | 31 | 16 | 15 | ||
| Smoking history, n (%) | 918 | 609 (66.3%) | 329 (63.0%) | 280 (70.7%) | .015 |
| Alcohol abuse history, n (%) | 972 | 160 (16.5%) | 85 (15.7%) | 75 (17.5%) | .445 |
| BMI, median (IQR) | 965 | 31.0 (26.8-36.3) | 31.4 (27.3-36.6) | 30.7 (26.1-36.0) | .035 |
| BMI, n (%) | 958 | .044 | |||
| ≤18.5 | 7 (0.7%) | 4 (0.7%) | 3 (0.7%) | ||
| 18.5-24.9 | 146 (15.1%) | 66 (12.2%) | 80 (18.8%) | ||
| 25.0-29.9 | 277 (28.7%) | 157 (29.1%) | 120 (28.2%) | ||
| ≥30.0 | 535 (55.4%) | 312 (57.9%) | 223 (52.3%) | ||
| Hypertension, n (%) | 972 | 515 (53.0%) | 266 (49.0%) | 249 (58.0%) | .005 |
| Outpatient angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, n (%) | 972 | 331 (34.1%) | 178 (32.8%) | 153 (35.7%) | .346 |
| Dyslipidemia, n (%) | 972 | 429 (44.1%) | 241 (44.4%) | 188 (43.8%) | .861 |
| Statin therapy, n (%) | 341 (35.1%) | 185 (34.1%) | 156 (36.4%) | .457 | |
| Dyslipidemia and statin prescription in prior 5 y | 266 (27.4%) | 147 (27.1%) | 119 (27.7%) | .817 | |
| Dysglycemia, n (%) | 972 | .133 | |||
| Prediabetes | 91 (9.4%) | 56 (10.3%) | 35 (8.2%) | ||
| Diabetes mellitus | 218 (22.4%) | 110 (20.3%) | 108 (25.2%) | ||
| Tumor size, mm median (IQR) | 964 | 20.0 (14-30) | 17.0 (12-24) | 27.0 (17-37) | <.001 |
| Laterality, n (%) | 964 | <.001 | |||
| Left | 516 (53.3%) | 310 (57.2%) | 206 (48.4%) | ||
| Right | 273 (28.2%) | 166 (30.6%) | 107 (25.1%) | ||
| Bilateral | 179 (18.5%) | 66 (12.2%) | 113 (26.5%) | ||
| Postdexamethasone cortisol, mcg/dL median (IQR) | 972 | 1.7 (1.1-2.7) | 1.2 (1.0-1.5) | 3.0 (2.3-4.5) | — |
| Creatinine, mg/dL median (IQR) | 972 | 0.83 (0.72-1.00) | 0.83 (0.71-1.00) | 0.85 (0.72-1.01) | .003 |
| eGFR mL/min/1.73 m2 median (IQR) | 972 | 82.3 (67.7-94.9) | 83.8 (69.2-96.5) | 79.6 (66.4-92.0) | <.001 |
| Categories based on eGFR, n (%) | 972 | <.001 | |||
| eGFR >90 mL/min/1.73 m2 | 350 (36.0%) | 222 (40.9%) | 128 (29.8%) | ||
| eGFR 60-89 mL/min/1.73 m2 | 483 (49.7%) | 257 (47.3%) | 226 (52.7%) | ||
| EPI-CKDa stage 3a | 87 (9.0%) | 48 (8.8%) | 39 (9.1%) | ||
| EPI-CKD stage 3b | 35 (3.6%) | 12 (2.2%) | 23 (5.4%) | ||
| EPI-CKD stage 4 | 12 (1.2%) | 4 (0.7%) | 8 (1.9%) | ||
| EPI-CKD stage 5 | 5 (0.5%) | 0 (0.0%) | 5 (1.2%) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
aEPI-CKD: Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation: CKD stage 3a: eGFR 45-59 mL/min/1.73 m2; CKD stage 3b: eGFR 30-44 mL/min/1.73 m2; CKD stage 4: eGFR 15-29 mL/min/1.73 m2, CKD stage 5: < 15 mL/min/1.73 m2.
Patients with MACS had a higher prevalence of hypertension (58% vs 49%; P = .005) and smoking (70.7% vs 63%; P = .02) when compared to patients with NFA, while the prevalence of dysglycemia (33.4% vs 30.6%; P = .133) and dyslipidemia (43.8% vs 44.4%; P = .861) was similar in both groups. BMI was slightly lower in patients with MACS (median 30.7 vs 31.4 in NFA; P = .035). The use of angiotensin-converting enzyme inhibitor or angiotensin II receptor blockers, and statin therapy at the time of initial presentation was similar in both groups (see Table 1). Patients with MACS had lower eGFR (median 79.6 vs 83.8 mL/min/1.73 m2; P < .001) and a higher prevalence of CKD (17.6% vs 11.7%; P < .001).
Factors Associated With Estimated Glomerular Filtration Rate
In a multivariable analysis of age, sex, BMI, hypertension, dysglycemia, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers use, statin therapy, smoking, and (log) postdexamethasone cortisol, the factors associated with lower eGFR were older age (est −7.94; P = .001), hypertension (est −2.72; P = .038), and higher (log2) postdexamethasone cortisol (est −1.01; P = .017), Table 2.
Multivariable analysis of factors associated with estimated glomerular filtration rate
| Model 1 . | Model 2 . | ||||||
|---|---|---|---|---|---|---|---|
| Variables . | Estimate . | 95% CI . | P . | Variables . | Estimate . | 95% CI . | P . |
| Age (per 10-y increase) | −7.94 | −8.93 to −6.93 | <.001 | Age (per 10-y increase) | −7.92 | −8.91 to −6.93 | <.001 |
| Sex (male) | 0.62 | −1.735 to 2.979 | .605 | Sex (male) | 0.61 | −1.76 to 2.97 | .615 |
| Body mass index (per 5-point increase) | −0.01 | −0.77 to 0.76 | .983 | Body mass index (per 5-point increase) | 0.06 | −0.70 to 0.83 | .874 |
| Hypertension | −2.72 | −5.29 to −0.15 | .038 | Hypertension | −2.77 | −5.35 to −0.19 | .035 |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use | −0.49 | −3.22 to 2.25 | .728 | Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use | −0.56 | −3.29 to 2.18 | .690 |
| Dysglycemia | 0.98 | −1.66 to 3.62 | .465 | Dysglycemia | 0.99 | −1.65 to 3.63 | .462 |
| Statin therapy | −2.20 | −4.94 to 0.54 | .116 | Statin therapy | −2.22 | −4.96 to 0.53 | .114 |
| Smoking | 1.70 | −0.68 to 4.07 | .161 | Smoking | 1.66 | −0.72 to 4.04 | .172 |
| Log2 (postdexamethasone cortisol) | −1.50 | −2.74 to −0.27 | .017 | Postdexamethasone cortisol category (>1.8 mcg/dL vs ≤ 1.8 mcg/dL) | −2.05 | −4.34 to 0.25 | .080 |
| Model 1 . | Model 2 . | ||||||
|---|---|---|---|---|---|---|---|
| Variables . | Estimate . | 95% CI . | P . | Variables . | Estimate . | 95% CI . | P . |
| Age (per 10-y increase) | −7.94 | −8.93 to −6.93 | <.001 | Age (per 10-y increase) | −7.92 | −8.91 to −6.93 | <.001 |
| Sex (male) | 0.62 | −1.735 to 2.979 | .605 | Sex (male) | 0.61 | −1.76 to 2.97 | .615 |
| Body mass index (per 5-point increase) | −0.01 | −0.77 to 0.76 | .983 | Body mass index (per 5-point increase) | 0.06 | −0.70 to 0.83 | .874 |
| Hypertension | −2.72 | −5.29 to −0.15 | .038 | Hypertension | −2.77 | −5.35 to −0.19 | .035 |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use | −0.49 | −3.22 to 2.25 | .728 | Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use | −0.56 | −3.29 to 2.18 | .690 |
| Dysglycemia | 0.98 | −1.66 to 3.62 | .465 | Dysglycemia | 0.99 | −1.65 to 3.63 | .462 |
| Statin therapy | −2.20 | −4.94 to 0.54 | .116 | Statin therapy | −2.22 | −4.96 to 0.53 | .114 |
| Smoking | 1.70 | −0.68 to 4.07 | .161 | Smoking | 1.66 | −0.72 to 4.04 | .172 |
| Log2 (postdexamethasone cortisol) | −1.50 | −2.74 to −0.27 | .017 | Postdexamethasone cortisol category (>1.8 mcg/dL vs ≤ 1.8 mcg/dL) | −2.05 | −4.34 to 0.25 | .080 |
Multivariable analysis of factors associated with estimated glomerular filtration rate
| Model 1 . | Model 2 . | ||||||
|---|---|---|---|---|---|---|---|
| Variables . | Estimate . | 95% CI . | P . | Variables . | Estimate . | 95% CI . | P . |
| Age (per 10-y increase) | −7.94 | −8.93 to −6.93 | <.001 | Age (per 10-y increase) | −7.92 | −8.91 to −6.93 | <.001 |
| Sex (male) | 0.62 | −1.735 to 2.979 | .605 | Sex (male) | 0.61 | −1.76 to 2.97 | .615 |
| Body mass index (per 5-point increase) | −0.01 | −0.77 to 0.76 | .983 | Body mass index (per 5-point increase) | 0.06 | −0.70 to 0.83 | .874 |
| Hypertension | −2.72 | −5.29 to −0.15 | .038 | Hypertension | −2.77 | −5.35 to −0.19 | .035 |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use | −0.49 | −3.22 to 2.25 | .728 | Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use | −0.56 | −3.29 to 2.18 | .690 |
| Dysglycemia | 0.98 | −1.66 to 3.62 | .465 | Dysglycemia | 0.99 | −1.65 to 3.63 | .462 |
| Statin therapy | −2.20 | −4.94 to 0.54 | .116 | Statin therapy | −2.22 | −4.96 to 0.53 | .114 |
| Smoking | 1.70 | −0.68 to 4.07 | .161 | Smoking | 1.66 | −0.72 to 4.04 | .172 |
| Log2 (postdexamethasone cortisol) | −1.50 | −2.74 to −0.27 | .017 | Postdexamethasone cortisol category (>1.8 mcg/dL vs ≤ 1.8 mcg/dL) | −2.05 | −4.34 to 0.25 | .080 |
| Model 1 . | Model 2 . | ||||||
|---|---|---|---|---|---|---|---|
| Variables . | Estimate . | 95% CI . | P . | Variables . | Estimate . | 95% CI . | P . |
| Age (per 10-y increase) | −7.94 | −8.93 to −6.93 | <.001 | Age (per 10-y increase) | −7.92 | −8.91 to −6.93 | <.001 |
| Sex (male) | 0.62 | −1.735 to 2.979 | .605 | Sex (male) | 0.61 | −1.76 to 2.97 | .615 |
| Body mass index (per 5-point increase) | −0.01 | −0.77 to 0.76 | .983 | Body mass index (per 5-point increase) | 0.06 | −0.70 to 0.83 | .874 |
| Hypertension | −2.72 | −5.29 to −0.15 | .038 | Hypertension | −2.77 | −5.35 to −0.19 | .035 |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use | −0.49 | −3.22 to 2.25 | .728 | Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use | −0.56 | −3.29 to 2.18 | .690 |
| Dysglycemia | 0.98 | −1.66 to 3.62 | .465 | Dysglycemia | 0.99 | −1.65 to 3.63 | .462 |
| Statin therapy | −2.20 | −4.94 to 0.54 | .116 | Statin therapy | −2.22 | −4.96 to 0.53 | .114 |
| Smoking | 1.70 | −0.68 to 4.07 | .161 | Smoking | 1.66 | −0.72 to 4.04 | .172 |
| Log2 (postdexamethasone cortisol) | −1.50 | −2.74 to −0.27 | .017 | Postdexamethasone cortisol category (>1.8 mcg/dL vs ≤ 1.8 mcg/dL) | −2.05 | −4.34 to 0.25 | .080 |
When examining MACS vs NFA as a categorical variable (based on post DST cortisol cutoff of 1.8 mcg/dL), older age and hypertension were associated with lower eGFR; however, MACS was not significantly associated with eGFR (estimate of −2.05; P = .08) (see Table 2).
Adrenalectomy Cohort
Of 204 patients treated with adrenalectomy, 155 (76.0%) were diagnosed with MACS and 49 (24.0%) had NFA (see Fig. 1). In patients with NFA, the primary reasons for undergoing adrenalectomy were indeterminate imaging characteristics and the patients’ preference to opt for adrenalectomy rather than monitoring. No significant differences in age, sex, and BMI were found between the groups (Table 3). The prevalence of hypertension (71.0% vs 53.1%; P = .02) and therapy with angiotensin-converting enzyme inhibitor or angiotensin II receptor blockers (37.4% vs 20.4%; P = .028) was higher in patients with MACS vs NFA, while the prevalence of dysglycemia (31.7% vs 34.7%; P = .893), dyslipidemia (47.7% vs 46.9%; P = .922), smoking (74.2% vs 68.9%; P = .489), and alcohol use (13.5% vs 16.3%; P = .627) was similar between the 2 groups. Prior to adrenalectomy, patients with MACS and NFA had similar eGFR (median 84.4 vs 89.2 mL/min/1.73 m2; P = .088) and no differences in the prevalence of CKD (see Table 3).
| Variables . | n available . | All patients (n = 204) . | Nonfunctioning adrenal adenomas (n = 49) . | Mild autonomous cortisol secretion (n = 155) . | P . |
|---|---|---|---|---|---|
| Age, y median (IQR) | 204 | 58.9 (48.5-67.0) | 54.6 (41.6-66.9) | 59.2 (50.9-67.0) | .052 |
| Women, n (%) | 204 | 145 (71.1%) | 34 (69.4%) | 111 (71.6%) | .765 |
| Race, n (%) | 204 | .103 | |||
| Black | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Asian | 3 (1.5%) | 0 (0.0%) | 3 (2.0%) | ||
| White | 188 (95.9%) | 44 (93.6%) | 144 (96.6%) | ||
| Other | 5 (2.6%) | 3 (6.4%) | 2 (1.3%) | ||
| Unknown | 8 | 2 | 6 | ||
| Smoking history, n (%) | 173 | 126 (72.8%) | 31 (68.9%) | 95 (74.2%) | .489 |
| Alcohol abuse history, n (%) | 204 | 29 (14.2%) | 8 (16.3%) | 21 (13.5%) | .627 |
| BMI, median (IQR) | 163 | 30.8 (26.5-37.7) | 31.8 (28.0-37.9) | 30.4 (25.4-37.4) | .351 |
| BMI, n (%) | 173 | .145 | |||
| ≤ 18.5 | 3 (1.8%) | 1 (2.4%) | 2 (1.7%) | ||
| 18.5-24.9 | 26 (16.0%) | 2 (4.8%) | 24 (19.8%) | ||
| 25.0-29.9 | 49 (30.1%) | 15 (35.7%) | 34 (28.1%) | ||
| ≥ 30.0 | 85 (52.1%) | 24 (57.1%) | 61 (50.4%) | ||
| Hypertension, n (%) | 204 | 136 (66.7%) | 26 (53.1%) | 110 (71.0%) | .020 |
| Outpatient angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, n (%) | 204 | 68 (33.3%) | 10 (20.4%) | 58 (37.4%) | .028 |
| Dyslipidemia, n (%) | 204 | 97 (47.5%) | 23 (46.9%) | 74 (47.7%) | .922 |
| Statin therapy, n (%) | 101 (49.5%) | 21 (42.9%) | 80 (51.6%) | .285 | |
| Dyslipidemia and statin prescription in prior 5 y | 77 (37.8%) | 21 (42.9%) | 56 (36.1%) | .397 | |
| Dysglycemia, n (%) | 204 | .893 | |||
| Prediabetes | 13 (6.4%) | 3 (6.1%) | 10 (6.5%) | ||
| Diabetes mellitus | 53 (26.0%) | 14 (28.6%) | 39 (25.2%) | ||
| Tumor size, mm median (IQR) | 204 | 35.0 (24.0-42.0) | 30.0 (21.0-45.0) | 35.0 (24.0-41.5) | .941 |
| Laterality, n (%) | 204 | <.001 | |||
| Left | 96 (47.1%) | 25 (51.0%) | 71 (45.8%) | ||
| Right | 59 (28.9%) | 23 (46.9%) | 36 (23.2%) | ||
| Bilateral | 49 (24.0%) | 1 (2.0%) | 48 (31.0%) | ||
| Postdexamethasone cortisol, mcg/dL median (IQR) | 204 | 3.0 (1.9-4.7) | 1.3 (1.0-1.5) | 3.7 (2.6-5.8) | — |
| Creatinine, mg/dL median (IQR) | 204 | 0.83 (0.70-1.00) | 0.83 (0.70-0.94) | 0.83 (0.70-1.00) | .273 |
| eGFR mL/min/1.73 m2 median (IQR) | 204 | 85.5 (71.0-97.3) | 89.2 (76.8-99.1) | 84.4 (69.3-96.9) | .088 |
| Categories based on eGFR, n (%) | 204 | .714 | |||
| eGFR >90 mL/min/1.73 m2 | 77 (37.7%) | 22 (44.9%) | 55 (35.5%) | ||
| eGFR 60-89 mL/min/1.73 m2 | 98 (48.0%) | 23 (46.9%) | 75 (48.4%) | ||
| EPI-CKDa stage 3a | 20 (9.8%) | 3 (6.1%) | 17 (11.0%) | ||
| EPI-CKD stage 3b | 6 (2.9%) | 1 (2.0%) | 5 (3.2%) | ||
| EPI-CKD stage 4 | 1 (0.5%) | 0 (0.0%) | 1 (0.6%) | ||
| EPI-CKD stage 5 | 2 (1.0%) | 0 (0.0%) | 2 (1.3%) |
| Variables . | n available . | All patients (n = 204) . | Nonfunctioning adrenal adenomas (n = 49) . | Mild autonomous cortisol secretion (n = 155) . | P . |
|---|---|---|---|---|---|
| Age, y median (IQR) | 204 | 58.9 (48.5-67.0) | 54.6 (41.6-66.9) | 59.2 (50.9-67.0) | .052 |
| Women, n (%) | 204 | 145 (71.1%) | 34 (69.4%) | 111 (71.6%) | .765 |
| Race, n (%) | 204 | .103 | |||
| Black | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Asian | 3 (1.5%) | 0 (0.0%) | 3 (2.0%) | ||
| White | 188 (95.9%) | 44 (93.6%) | 144 (96.6%) | ||
| Other | 5 (2.6%) | 3 (6.4%) | 2 (1.3%) | ||
| Unknown | 8 | 2 | 6 | ||
| Smoking history, n (%) | 173 | 126 (72.8%) | 31 (68.9%) | 95 (74.2%) | .489 |
| Alcohol abuse history, n (%) | 204 | 29 (14.2%) | 8 (16.3%) | 21 (13.5%) | .627 |
| BMI, median (IQR) | 163 | 30.8 (26.5-37.7) | 31.8 (28.0-37.9) | 30.4 (25.4-37.4) | .351 |
| BMI, n (%) | 173 | .145 | |||
| ≤ 18.5 | 3 (1.8%) | 1 (2.4%) | 2 (1.7%) | ||
| 18.5-24.9 | 26 (16.0%) | 2 (4.8%) | 24 (19.8%) | ||
| 25.0-29.9 | 49 (30.1%) | 15 (35.7%) | 34 (28.1%) | ||
| ≥ 30.0 | 85 (52.1%) | 24 (57.1%) | 61 (50.4%) | ||
| Hypertension, n (%) | 204 | 136 (66.7%) | 26 (53.1%) | 110 (71.0%) | .020 |
| Outpatient angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, n (%) | 204 | 68 (33.3%) | 10 (20.4%) | 58 (37.4%) | .028 |
| Dyslipidemia, n (%) | 204 | 97 (47.5%) | 23 (46.9%) | 74 (47.7%) | .922 |
| Statin therapy, n (%) | 101 (49.5%) | 21 (42.9%) | 80 (51.6%) | .285 | |
| Dyslipidemia and statin prescription in prior 5 y | 77 (37.8%) | 21 (42.9%) | 56 (36.1%) | .397 | |
| Dysglycemia, n (%) | 204 | .893 | |||
| Prediabetes | 13 (6.4%) | 3 (6.1%) | 10 (6.5%) | ||
| Diabetes mellitus | 53 (26.0%) | 14 (28.6%) | 39 (25.2%) | ||
| Tumor size, mm median (IQR) | 204 | 35.0 (24.0-42.0) | 30.0 (21.0-45.0) | 35.0 (24.0-41.5) | .941 |
| Laterality, n (%) | 204 | <.001 | |||
| Left | 96 (47.1%) | 25 (51.0%) | 71 (45.8%) | ||
| Right | 59 (28.9%) | 23 (46.9%) | 36 (23.2%) | ||
| Bilateral | 49 (24.0%) | 1 (2.0%) | 48 (31.0%) | ||
| Postdexamethasone cortisol, mcg/dL median (IQR) | 204 | 3.0 (1.9-4.7) | 1.3 (1.0-1.5) | 3.7 (2.6-5.8) | — |
| Creatinine, mg/dL median (IQR) | 204 | 0.83 (0.70-1.00) | 0.83 (0.70-0.94) | 0.83 (0.70-1.00) | .273 |
| eGFR mL/min/1.73 m2 median (IQR) | 204 | 85.5 (71.0-97.3) | 89.2 (76.8-99.1) | 84.4 (69.3-96.9) | .088 |
| Categories based on eGFR, n (%) | 204 | .714 | |||
| eGFR >90 mL/min/1.73 m2 | 77 (37.7%) | 22 (44.9%) | 55 (35.5%) | ||
| eGFR 60-89 mL/min/1.73 m2 | 98 (48.0%) | 23 (46.9%) | 75 (48.4%) | ||
| EPI-CKDa stage 3a | 20 (9.8%) | 3 (6.1%) | 17 (11.0%) | ||
| EPI-CKD stage 3b | 6 (2.9%) | 1 (2.0%) | 5 (3.2%) | ||
| EPI-CKD stage 4 | 1 (0.5%) | 0 (0.0%) | 1 (0.6%) | ||
| EPI-CKD stage 5 | 2 (1.0%) | 0 (0.0%) | 2 (1.3%) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
aEPI-CKD: Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation: CKD stage 3a: eGFR 45-59. CKD stage 3b: eGFR 30-44. CKD stage 4: eGFR 15-29, CKD stage 5: < 15 mL/min/1.73 m2.
| Variables . | n available . | All patients (n = 204) . | Nonfunctioning adrenal adenomas (n = 49) . | Mild autonomous cortisol secretion (n = 155) . | P . |
|---|---|---|---|---|---|
| Age, y median (IQR) | 204 | 58.9 (48.5-67.0) | 54.6 (41.6-66.9) | 59.2 (50.9-67.0) | .052 |
| Women, n (%) | 204 | 145 (71.1%) | 34 (69.4%) | 111 (71.6%) | .765 |
| Race, n (%) | 204 | .103 | |||
| Black | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Asian | 3 (1.5%) | 0 (0.0%) | 3 (2.0%) | ||
| White | 188 (95.9%) | 44 (93.6%) | 144 (96.6%) | ||
| Other | 5 (2.6%) | 3 (6.4%) | 2 (1.3%) | ||
| Unknown | 8 | 2 | 6 | ||
| Smoking history, n (%) | 173 | 126 (72.8%) | 31 (68.9%) | 95 (74.2%) | .489 |
| Alcohol abuse history, n (%) | 204 | 29 (14.2%) | 8 (16.3%) | 21 (13.5%) | .627 |
| BMI, median (IQR) | 163 | 30.8 (26.5-37.7) | 31.8 (28.0-37.9) | 30.4 (25.4-37.4) | .351 |
| BMI, n (%) | 173 | .145 | |||
| ≤ 18.5 | 3 (1.8%) | 1 (2.4%) | 2 (1.7%) | ||
| 18.5-24.9 | 26 (16.0%) | 2 (4.8%) | 24 (19.8%) | ||
| 25.0-29.9 | 49 (30.1%) | 15 (35.7%) | 34 (28.1%) | ||
| ≥ 30.0 | 85 (52.1%) | 24 (57.1%) | 61 (50.4%) | ||
| Hypertension, n (%) | 204 | 136 (66.7%) | 26 (53.1%) | 110 (71.0%) | .020 |
| Outpatient angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, n (%) | 204 | 68 (33.3%) | 10 (20.4%) | 58 (37.4%) | .028 |
| Dyslipidemia, n (%) | 204 | 97 (47.5%) | 23 (46.9%) | 74 (47.7%) | .922 |
| Statin therapy, n (%) | 101 (49.5%) | 21 (42.9%) | 80 (51.6%) | .285 | |
| Dyslipidemia and statin prescription in prior 5 y | 77 (37.8%) | 21 (42.9%) | 56 (36.1%) | .397 | |
| Dysglycemia, n (%) | 204 | .893 | |||
| Prediabetes | 13 (6.4%) | 3 (6.1%) | 10 (6.5%) | ||
| Diabetes mellitus | 53 (26.0%) | 14 (28.6%) | 39 (25.2%) | ||
| Tumor size, mm median (IQR) | 204 | 35.0 (24.0-42.0) | 30.0 (21.0-45.0) | 35.0 (24.0-41.5) | .941 |
| Laterality, n (%) | 204 | <.001 | |||
| Left | 96 (47.1%) | 25 (51.0%) | 71 (45.8%) | ||
| Right | 59 (28.9%) | 23 (46.9%) | 36 (23.2%) | ||
| Bilateral | 49 (24.0%) | 1 (2.0%) | 48 (31.0%) | ||
| Postdexamethasone cortisol, mcg/dL median (IQR) | 204 | 3.0 (1.9-4.7) | 1.3 (1.0-1.5) | 3.7 (2.6-5.8) | — |
| Creatinine, mg/dL median (IQR) | 204 | 0.83 (0.70-1.00) | 0.83 (0.70-0.94) | 0.83 (0.70-1.00) | .273 |
| eGFR mL/min/1.73 m2 median (IQR) | 204 | 85.5 (71.0-97.3) | 89.2 (76.8-99.1) | 84.4 (69.3-96.9) | .088 |
| Categories based on eGFR, n (%) | 204 | .714 | |||
| eGFR >90 mL/min/1.73 m2 | 77 (37.7%) | 22 (44.9%) | 55 (35.5%) | ||
| eGFR 60-89 mL/min/1.73 m2 | 98 (48.0%) | 23 (46.9%) | 75 (48.4%) | ||
| EPI-CKDa stage 3a | 20 (9.8%) | 3 (6.1%) | 17 (11.0%) | ||
| EPI-CKD stage 3b | 6 (2.9%) | 1 (2.0%) | 5 (3.2%) | ||
| EPI-CKD stage 4 | 1 (0.5%) | 0 (0.0%) | 1 (0.6%) | ||
| EPI-CKD stage 5 | 2 (1.0%) | 0 (0.0%) | 2 (1.3%) |
| Variables . | n available . | All patients (n = 204) . | Nonfunctioning adrenal adenomas (n = 49) . | Mild autonomous cortisol secretion (n = 155) . | P . |
|---|---|---|---|---|---|
| Age, y median (IQR) | 204 | 58.9 (48.5-67.0) | 54.6 (41.6-66.9) | 59.2 (50.9-67.0) | .052 |
| Women, n (%) | 204 | 145 (71.1%) | 34 (69.4%) | 111 (71.6%) | .765 |
| Race, n (%) | 204 | .103 | |||
| Black | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Asian | 3 (1.5%) | 0 (0.0%) | 3 (2.0%) | ||
| White | 188 (95.9%) | 44 (93.6%) | 144 (96.6%) | ||
| Other | 5 (2.6%) | 3 (6.4%) | 2 (1.3%) | ||
| Unknown | 8 | 2 | 6 | ||
| Smoking history, n (%) | 173 | 126 (72.8%) | 31 (68.9%) | 95 (74.2%) | .489 |
| Alcohol abuse history, n (%) | 204 | 29 (14.2%) | 8 (16.3%) | 21 (13.5%) | .627 |
| BMI, median (IQR) | 163 | 30.8 (26.5-37.7) | 31.8 (28.0-37.9) | 30.4 (25.4-37.4) | .351 |
| BMI, n (%) | 173 | .145 | |||
| ≤ 18.5 | 3 (1.8%) | 1 (2.4%) | 2 (1.7%) | ||
| 18.5-24.9 | 26 (16.0%) | 2 (4.8%) | 24 (19.8%) | ||
| 25.0-29.9 | 49 (30.1%) | 15 (35.7%) | 34 (28.1%) | ||
| ≥ 30.0 | 85 (52.1%) | 24 (57.1%) | 61 (50.4%) | ||
| Hypertension, n (%) | 204 | 136 (66.7%) | 26 (53.1%) | 110 (71.0%) | .020 |
| Outpatient angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, n (%) | 204 | 68 (33.3%) | 10 (20.4%) | 58 (37.4%) | .028 |
| Dyslipidemia, n (%) | 204 | 97 (47.5%) | 23 (46.9%) | 74 (47.7%) | .922 |
| Statin therapy, n (%) | 101 (49.5%) | 21 (42.9%) | 80 (51.6%) | .285 | |
| Dyslipidemia and statin prescription in prior 5 y | 77 (37.8%) | 21 (42.9%) | 56 (36.1%) | .397 | |
| Dysglycemia, n (%) | 204 | .893 | |||
| Prediabetes | 13 (6.4%) | 3 (6.1%) | 10 (6.5%) | ||
| Diabetes mellitus | 53 (26.0%) | 14 (28.6%) | 39 (25.2%) | ||
| Tumor size, mm median (IQR) | 204 | 35.0 (24.0-42.0) | 30.0 (21.0-45.0) | 35.0 (24.0-41.5) | .941 |
| Laterality, n (%) | 204 | <.001 | |||
| Left | 96 (47.1%) | 25 (51.0%) | 71 (45.8%) | ||
| Right | 59 (28.9%) | 23 (46.9%) | 36 (23.2%) | ||
| Bilateral | 49 (24.0%) | 1 (2.0%) | 48 (31.0%) | ||
| Postdexamethasone cortisol, mcg/dL median (IQR) | 204 | 3.0 (1.9-4.7) | 1.3 (1.0-1.5) | 3.7 (2.6-5.8) | — |
| Creatinine, mg/dL median (IQR) | 204 | 0.83 (0.70-1.00) | 0.83 (0.70-0.94) | 0.83 (0.70-1.00) | .273 |
| eGFR mL/min/1.73 m2 median (IQR) | 204 | 85.5 (71.0-97.3) | 89.2 (76.8-99.1) | 84.4 (69.3-96.9) | .088 |
| Categories based on eGFR, n (%) | 204 | .714 | |||
| eGFR >90 mL/min/1.73 m2 | 77 (37.7%) | 22 (44.9%) | 55 (35.5%) | ||
| eGFR 60-89 mL/min/1.73 m2 | 98 (48.0%) | 23 (46.9%) | 75 (48.4%) | ||
| EPI-CKDa stage 3a | 20 (9.8%) | 3 (6.1%) | 17 (11.0%) | ||
| EPI-CKD stage 3b | 6 (2.9%) | 1 (2.0%) | 5 (3.2%) | ||
| EPI-CKD stage 4 | 1 (0.5%) | 0 (0.0%) | 1 (0.6%) | ||
| EPI-CKD stage 5 | 2 (1.0%) | 0 (0.0%) | 2 (1.3%) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
aEPI-CKD: Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation: CKD stage 3a: eGFR 45-59. CKD stage 3b: eGFR 30-44. CKD stage 4: eGFR 15-29, CKD stage 5: < 15 mL/min/1.73 m2.
Postadrenalectomy Outcomes:
In patients with MACS, after an initial decline of eGFR within 18 months post adrenalectomy (mean decrease of 5.3 mL/min/1.73 m2), eGFR increased from baseline by a mean of 4.9 points at 18 to 30 months, and by a mean of 8.1 points at 30 to 42 months post adrenalectomy (P < .001) (Fig. 2). Patients with NFA followed a similar delta eGFR trajectory as patients with MACS, which was not statistically significant (P = .378) (see Fig. 2).
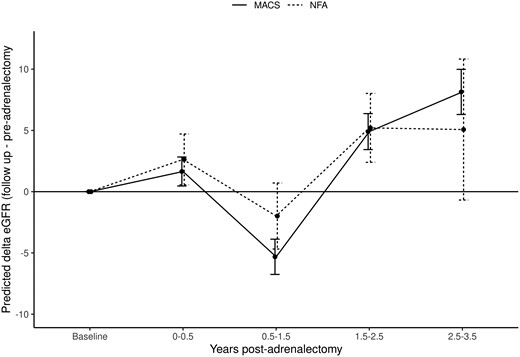
Effect of adrenalectomy on delta eGFR in patients with adrenal adenomas. In the adrenalectomy cohort, only the closest eGFR available within 6 months prior to adrenalectomy was considered. For postadrenalectomy outcomes, all eGFR measurements up to 3.5 years post adrenalectomy were considered. Patients with MACS show initial decline of eGFR within 1.5 years post adrenalectomy (mean decrease of 5.3 mL/min/1.73 m2), followed by an increase in eGFR by a mean of 4.9 points at 1.5 to 2.5 years, and by a mean of 8.1 points at 2.5 to 3.5 years post adrenalectomy (P < .001). Patients with NFA follow a similar delta eGFR trend as patients with MACS (NFA vs MACS; P = .378). eGFR, estimated glomerular filtration rate; MACS, mild autonomous cortisol secretion; NFA, nonfunctioning adrenal adenoma.
In the univariable analysis, longer duration of follow-up and lower eGFR pre adrenalectomy was associated with postadrenalectomy increase in eGFR (Table 4). Multivariable analysis also showed that lower preadrenalectomy eGFR (estimate of −0.27; P < .001) and longer duration of follow-up (estimate of 1.9; P = .001) was associated with eGFR increase. In addition, younger age (estimate of −2.70; P = .004) was associated with improvement in kidney function. Post-DST cortisol category (MACS vs NFA) was not associated with eGFR increase (see Table 4).
Factors associated with improvement of kidney function post adrenalectomy during 3 years of follow-up
| Variables . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| Estimate . | 95% CI . | P . | Estimate . | 95% CI . | P . | |
| Age (per 10-y increase) | −1.05 | −2.48 to 0.37 | .146 | −2.79 | −4.69 to −0.88 | .004 |
| Sex (male) | 1.90 | −2.16 to 5.95 | .359 | 3.15 | −1.40 to 7.70 | .175 |
| Body mass index (per 5-point increase) | 0.07 | −0.22 to 0.35 | .647 | 0.33 | −1.08 to 1.75 | .643 |
| Hypertension | 0.03 | −4.11 to 4.16 | .990 | 0.44 | −4.73 to 5.61 | .868 |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use | −1.93 | −5.87 to 2.01 | .337 | −1.88 | −7.07 to 3.32 | .479 |
| Dysglycemia | 0.35 | −3.60 to 4.30 | .862 | −0.67 | −5.30 to 3.97 | .777 |
| Statin therapy | −2.25 | −6.03 to 1.53 | .242 | 2.95 | −7.80 to 1.91 | .234 |
| Smoking | 2.90 | −1.85 to 7.65 | .231 | 3.08 | −1.73 to 7.88 | .210 |
| eGFR pre adrenalectomy | −0.15 | −0.23 to −0.07 | <.001 | −0.27 | −0.38 to −0.17 | <.001 |
| Follow-up time post adrenalectomy (per 1-mo increase) | 1.19 | 0.21 to 2.17 | .017 | 1.90 | 0.81 to 2.98 | .001 |
| Postdexamethasone cortisol category (>1.8 mcg/dL vs ≤ 1.8 mcg/dL) | −0.57 | −4.98 to 3.84 | .800 | −1.15 | −5.86 to 3.55 | .631 |
| Variables . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| Estimate . | 95% CI . | P . | Estimate . | 95% CI . | P . | |
| Age (per 10-y increase) | −1.05 | −2.48 to 0.37 | .146 | −2.79 | −4.69 to −0.88 | .004 |
| Sex (male) | 1.90 | −2.16 to 5.95 | .359 | 3.15 | −1.40 to 7.70 | .175 |
| Body mass index (per 5-point increase) | 0.07 | −0.22 to 0.35 | .647 | 0.33 | −1.08 to 1.75 | .643 |
| Hypertension | 0.03 | −4.11 to 4.16 | .990 | 0.44 | −4.73 to 5.61 | .868 |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use | −1.93 | −5.87 to 2.01 | .337 | −1.88 | −7.07 to 3.32 | .479 |
| Dysglycemia | 0.35 | −3.60 to 4.30 | .862 | −0.67 | −5.30 to 3.97 | .777 |
| Statin therapy | −2.25 | −6.03 to 1.53 | .242 | 2.95 | −7.80 to 1.91 | .234 |
| Smoking | 2.90 | −1.85 to 7.65 | .231 | 3.08 | −1.73 to 7.88 | .210 |
| eGFR pre adrenalectomy | −0.15 | −0.23 to −0.07 | <.001 | −0.27 | −0.38 to −0.17 | <.001 |
| Follow-up time post adrenalectomy (per 1-mo increase) | 1.19 | 0.21 to 2.17 | .017 | 1.90 | 0.81 to 2.98 | .001 |
| Postdexamethasone cortisol category (>1.8 mcg/dL vs ≤ 1.8 mcg/dL) | −0.57 | −4.98 to 3.84 | .800 | −1.15 | −5.86 to 3.55 | .631 |
Abbreviation: eGFR, estimated glomerular filtration rate.
Factors associated with improvement of kidney function post adrenalectomy during 3 years of follow-up
| Variables . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| Estimate . | 95% CI . | P . | Estimate . | 95% CI . | P . | |
| Age (per 10-y increase) | −1.05 | −2.48 to 0.37 | .146 | −2.79 | −4.69 to −0.88 | .004 |
| Sex (male) | 1.90 | −2.16 to 5.95 | .359 | 3.15 | −1.40 to 7.70 | .175 |
| Body mass index (per 5-point increase) | 0.07 | −0.22 to 0.35 | .647 | 0.33 | −1.08 to 1.75 | .643 |
| Hypertension | 0.03 | −4.11 to 4.16 | .990 | 0.44 | −4.73 to 5.61 | .868 |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use | −1.93 | −5.87 to 2.01 | .337 | −1.88 | −7.07 to 3.32 | .479 |
| Dysglycemia | 0.35 | −3.60 to 4.30 | .862 | −0.67 | −5.30 to 3.97 | .777 |
| Statin therapy | −2.25 | −6.03 to 1.53 | .242 | 2.95 | −7.80 to 1.91 | .234 |
| Smoking | 2.90 | −1.85 to 7.65 | .231 | 3.08 | −1.73 to 7.88 | .210 |
| eGFR pre adrenalectomy | −0.15 | −0.23 to −0.07 | <.001 | −0.27 | −0.38 to −0.17 | <.001 |
| Follow-up time post adrenalectomy (per 1-mo increase) | 1.19 | 0.21 to 2.17 | .017 | 1.90 | 0.81 to 2.98 | .001 |
| Postdexamethasone cortisol category (>1.8 mcg/dL vs ≤ 1.8 mcg/dL) | −0.57 | −4.98 to 3.84 | .800 | −1.15 | −5.86 to 3.55 | .631 |
| Variables . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| Estimate . | 95% CI . | P . | Estimate . | 95% CI . | P . | |
| Age (per 10-y increase) | −1.05 | −2.48 to 0.37 | .146 | −2.79 | −4.69 to −0.88 | .004 |
| Sex (male) | 1.90 | −2.16 to 5.95 | .359 | 3.15 | −1.40 to 7.70 | .175 |
| Body mass index (per 5-point increase) | 0.07 | −0.22 to 0.35 | .647 | 0.33 | −1.08 to 1.75 | .643 |
| Hypertension | 0.03 | −4.11 to 4.16 | .990 | 0.44 | −4.73 to 5.61 | .868 |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use | −1.93 | −5.87 to 2.01 | .337 | −1.88 | −7.07 to 3.32 | .479 |
| Dysglycemia | 0.35 | −3.60 to 4.30 | .862 | −0.67 | −5.30 to 3.97 | .777 |
| Statin therapy | −2.25 | −6.03 to 1.53 | .242 | 2.95 | −7.80 to 1.91 | .234 |
| Smoking | 2.90 | −1.85 to 7.65 | .231 | 3.08 | −1.73 to 7.88 | .210 |
| eGFR pre adrenalectomy | −0.15 | −0.23 to −0.07 | <.001 | −0.27 | −0.38 to −0.17 | <.001 |
| Follow-up time post adrenalectomy (per 1-mo increase) | 1.19 | 0.21 to 2.17 | .017 | 1.90 | 0.81 to 2.98 | .001 |
| Postdexamethasone cortisol category (>1.8 mcg/dL vs ≤ 1.8 mcg/dL) | −0.57 | −4.98 to 3.84 | .800 | −1.15 | −5.86 to 3.55 | .631 |
Abbreviation: eGFR, estimated glomerular filtration rate.
Discussion
In this large study, we evaluated kidney function in patients with MACS and NFA at the time of diagnosis, and in a subgroup of patients, post adrenalectomy. We found that patients with MACS have lower eGFR and higher prevalence of CKD at diagnosis when compared to patients with NFA. In addition, we demonstrated that higher post-DST cortisol is associated with lower eGFR, independent of traditional cardiovascular risk factors. We also showed that in patients with MACS and NFA treated with adrenalectomy, eGFR improved in both the MACS and NFA group starting at 18 months post adrenalectomy. Factors associated with kidney function improvement were younger age, lower eGFR pre adrenalectomy, and a longer duration of follow-up.
MACS may affect kidney function in several ways. First, kidney function may be affected by MACS-associated comorbidities, such as hypertension, diabetes, and dyslipidemia leading to glomerular dysfunction, albuminuria, and proteinuria (18-20). Second, chronic hypercortisolism may have a direct effect on renal vascular tone, leading to increased renal vascular resistance and hypertension (18). Third, cortisol excess may lead to kidney damage through activation of the mineralocorticoid receptor resulting in increased sodium and water reabsorption in renal tubular cells, volume expansion, and hypertension (19). The mineralocorticoid receptor is present not only in renal epithelial tubular cells, but also in endothelial cells, podocytes, and fibroblasts, and excess activation of mineralocorticoid receptor is associated with progression of CKD (21).
In our study, we demonstrated a lower eGFR and higher prevalence of CKD in patients with MACS vs NFA. We were able to show that postdexamethasone cortisol was independently associated with lower eGFR, as well as CKD, after adjustment for demographic factors, cardiovascular comorbidities, smoking, and certain medications. These findings suggest that MACS affects kidney function both due to higher prevalence of cardiovascular comorbidities, as well as through a direct effect of cortisol on kidney function. Two previous studies compared kidney function in patients with NFA vs those with MACS (Table 5). In a smaller study from our institution that relied on the International Classification of Diseases codes and did not distinguish based on CKD stage, patients with MACS were more likely to have CKD when compared to patients with NFA (25% vs 17%) (10). In contrast, in another smaller study looking at baseline eGFR, the authors reported similar eGFR in patients with MACS and NFA (18). Neither of the 2 previous studies considered associated cardiovascular risk factors or medication therapy (10, 18).
Studies investigating kidney function in patients with nonfunctioning adrenal adenomas and/or mild autonomous cortisol secretion
| Author . | Study Design . | Country . | Participants . | Measurement timepoints . | Follow-up . | Outcome in relation to kidney function . |
|---|---|---|---|---|---|---|
| Lopez et al, 2016 (11) | Retrospective (1991-2014) | USA | NFA (n = 242) Referent individuals (n = 1237) | Baseline + conservative follow-up (available only for 110 patients with NFA and 615 referent individuals) | Mean 7.7 y (≥3 y) | Baseline: CKD prevalence: 6.2% vs 5.7% (referent individuals, n = 1237); P = .76 Follow-up: CKD incidence: 7.3% (NFA) vs 2.4% (referent individuals); P = .015 |
| Numakura et al, 2019 (13) | Retrospective (1997-2017) | Japan | MACS (n = 12) CS (n = 18) | Post adrenalectomy | ≥1 y | Baseline: Median eGFR 78.4 mL/min/1.73 m2 Follow-up post adrenalectomy: eGFR increased when compared to baseline (median of eGFR 84.1 vs 78.4 mL/min/1.73 m2); P < .05 |
| Singh et al, 2020 (8) | Retrospective (2003-2018) | USA | NFA (n = 275) MACS (n = 168) | Baseline | NA | Baseline: CKD prevalence: 16.9% (NFA) vs 25.3% (MACS); P = .044 |
| Naka et al, 2020 (12) | Retrospective (2010-2018) | Japan | NFA (n = 232) MACS (n = 84) CS (n = 23) | Baseline + post adrenalectomy (available only for 19 patients) | Not reported | Baseline: eGFR: 82.2 ± 2.2 (MACS) vs 81.6 ± 1.2 (NFA); P = NS Follow-up post adrenalectomy in patients with MACS and CS: eGFR pre surgery: 94.0 ± 4.7 vs post surgery: 81.7 ± 3.7; P < .01 |
| Zhang et al, 2021 (3) | Retrospective (1995-2017) | USA | Adrenal adenoma (NFA and MACS) (n = 1004) Referent individuals (n = 1004) | Baseline + conservative follow-up | Median 6.8 y | Baseline: CKD prevalence: 6.5% (adrenal adenoma) vs 5.2% (referent individuals), P = .216 Follow-up: CKD incidence: 19% (adrenal adenoma) vs 12% (referent individuals), adjusted hazard ratio: 1.46 (95% CI, 1.14-1.86) |
| Araujo-Castro, 2021 (20) | Case control (2018-2020) | Spain | MACS (n = 57) | Baseline + conservative follow-up (n = 55) and postadrenalectomy follow-up (n = 2) | Median 2.25 y | Baseline: CKD prevalence: 7.4% Follow-up: CKD incidence 0.0%, mean eGFR decline: −2.3 mL/min/1.73 m2 (P value not reported) |
| Kufukihara et al, 2022 (14) | Retrospective (2001-2018) | Japan | CS (n = 59) MACS (n = 17) with postdexamethasone cortisol >5 mcg/dL | Baseline and postadrenalectomy follow-up | 1 mo and 1 y post adrenalectomy | Baseline: eGFR 82.2 ± 19.5 mL/min/1.73 m2 1 mo follow-up from baseline: 71.7 ± 17.3 mL/min/1.73 m2, in comparison to baseline: P < .001 1 y follow-up from baseline: 79.5 ± 20.2 mL/min/1.73 m2, in comparison to baseline: P = .108 |
| Author . | Study Design . | Country . | Participants . | Measurement timepoints . | Follow-up . | Outcome in relation to kidney function . |
|---|---|---|---|---|---|---|
| Lopez et al, 2016 (11) | Retrospective (1991-2014) | USA | NFA (n = 242) Referent individuals (n = 1237) | Baseline + conservative follow-up (available only for 110 patients with NFA and 615 referent individuals) | Mean 7.7 y (≥3 y) | Baseline: CKD prevalence: 6.2% vs 5.7% (referent individuals, n = 1237); P = .76 Follow-up: CKD incidence: 7.3% (NFA) vs 2.4% (referent individuals); P = .015 |
| Numakura et al, 2019 (13) | Retrospective (1997-2017) | Japan | MACS (n = 12) CS (n = 18) | Post adrenalectomy | ≥1 y | Baseline: Median eGFR 78.4 mL/min/1.73 m2 Follow-up post adrenalectomy: eGFR increased when compared to baseline (median of eGFR 84.1 vs 78.4 mL/min/1.73 m2); P < .05 |
| Singh et al, 2020 (8) | Retrospective (2003-2018) | USA | NFA (n = 275) MACS (n = 168) | Baseline | NA | Baseline: CKD prevalence: 16.9% (NFA) vs 25.3% (MACS); P = .044 |
| Naka et al, 2020 (12) | Retrospective (2010-2018) | Japan | NFA (n = 232) MACS (n = 84) CS (n = 23) | Baseline + post adrenalectomy (available only for 19 patients) | Not reported | Baseline: eGFR: 82.2 ± 2.2 (MACS) vs 81.6 ± 1.2 (NFA); P = NS Follow-up post adrenalectomy in patients with MACS and CS: eGFR pre surgery: 94.0 ± 4.7 vs post surgery: 81.7 ± 3.7; P < .01 |
| Zhang et al, 2021 (3) | Retrospective (1995-2017) | USA | Adrenal adenoma (NFA and MACS) (n = 1004) Referent individuals (n = 1004) | Baseline + conservative follow-up | Median 6.8 y | Baseline: CKD prevalence: 6.5% (adrenal adenoma) vs 5.2% (referent individuals), P = .216 Follow-up: CKD incidence: 19% (adrenal adenoma) vs 12% (referent individuals), adjusted hazard ratio: 1.46 (95% CI, 1.14-1.86) |
| Araujo-Castro, 2021 (20) | Case control (2018-2020) | Spain | MACS (n = 57) | Baseline + conservative follow-up (n = 55) and postadrenalectomy follow-up (n = 2) | Median 2.25 y | Baseline: CKD prevalence: 7.4% Follow-up: CKD incidence 0.0%, mean eGFR decline: −2.3 mL/min/1.73 m2 (P value not reported) |
| Kufukihara et al, 2022 (14) | Retrospective (2001-2018) | Japan | CS (n = 59) MACS (n = 17) with postdexamethasone cortisol >5 mcg/dL | Baseline and postadrenalectomy follow-up | 1 mo and 1 y post adrenalectomy | Baseline: eGFR 82.2 ± 19.5 mL/min/1.73 m2 1 mo follow-up from baseline: 71.7 ± 17.3 mL/min/1.73 m2, in comparison to baseline: P < .001 1 y follow-up from baseline: 79.5 ± 20.2 mL/min/1.73 m2, in comparison to baseline: P = .108 |
Abbreviations: CKD, chronic kidney disease; CS, Cushing syndrome; eGFR, estimated glomerular filtration rate; MACS, mild autonomous cortisol secretion; NA, not applicable; NFA, nonfunctioning adrenal adenoma; USA, United States of America.
Studies investigating kidney function in patients with nonfunctioning adrenal adenomas and/or mild autonomous cortisol secretion
| Author . | Study Design . | Country . | Participants . | Measurement timepoints . | Follow-up . | Outcome in relation to kidney function . |
|---|---|---|---|---|---|---|
| Lopez et al, 2016 (11) | Retrospective (1991-2014) | USA | NFA (n = 242) Referent individuals (n = 1237) | Baseline + conservative follow-up (available only for 110 patients with NFA and 615 referent individuals) | Mean 7.7 y (≥3 y) | Baseline: CKD prevalence: 6.2% vs 5.7% (referent individuals, n = 1237); P = .76 Follow-up: CKD incidence: 7.3% (NFA) vs 2.4% (referent individuals); P = .015 |
| Numakura et al, 2019 (13) | Retrospective (1997-2017) | Japan | MACS (n = 12) CS (n = 18) | Post adrenalectomy | ≥1 y | Baseline: Median eGFR 78.4 mL/min/1.73 m2 Follow-up post adrenalectomy: eGFR increased when compared to baseline (median of eGFR 84.1 vs 78.4 mL/min/1.73 m2); P < .05 |
| Singh et al, 2020 (8) | Retrospective (2003-2018) | USA | NFA (n = 275) MACS (n = 168) | Baseline | NA | Baseline: CKD prevalence: 16.9% (NFA) vs 25.3% (MACS); P = .044 |
| Naka et al, 2020 (12) | Retrospective (2010-2018) | Japan | NFA (n = 232) MACS (n = 84) CS (n = 23) | Baseline + post adrenalectomy (available only for 19 patients) | Not reported | Baseline: eGFR: 82.2 ± 2.2 (MACS) vs 81.6 ± 1.2 (NFA); P = NS Follow-up post adrenalectomy in patients with MACS and CS: eGFR pre surgery: 94.0 ± 4.7 vs post surgery: 81.7 ± 3.7; P < .01 |
| Zhang et al, 2021 (3) | Retrospective (1995-2017) | USA | Adrenal adenoma (NFA and MACS) (n = 1004) Referent individuals (n = 1004) | Baseline + conservative follow-up | Median 6.8 y | Baseline: CKD prevalence: 6.5% (adrenal adenoma) vs 5.2% (referent individuals), P = .216 Follow-up: CKD incidence: 19% (adrenal adenoma) vs 12% (referent individuals), adjusted hazard ratio: 1.46 (95% CI, 1.14-1.86) |
| Araujo-Castro, 2021 (20) | Case control (2018-2020) | Spain | MACS (n = 57) | Baseline + conservative follow-up (n = 55) and postadrenalectomy follow-up (n = 2) | Median 2.25 y | Baseline: CKD prevalence: 7.4% Follow-up: CKD incidence 0.0%, mean eGFR decline: −2.3 mL/min/1.73 m2 (P value not reported) |
| Kufukihara et al, 2022 (14) | Retrospective (2001-2018) | Japan | CS (n = 59) MACS (n = 17) with postdexamethasone cortisol >5 mcg/dL | Baseline and postadrenalectomy follow-up | 1 mo and 1 y post adrenalectomy | Baseline: eGFR 82.2 ± 19.5 mL/min/1.73 m2 1 mo follow-up from baseline: 71.7 ± 17.3 mL/min/1.73 m2, in comparison to baseline: P < .001 1 y follow-up from baseline: 79.5 ± 20.2 mL/min/1.73 m2, in comparison to baseline: P = .108 |
| Author . | Study Design . | Country . | Participants . | Measurement timepoints . | Follow-up . | Outcome in relation to kidney function . |
|---|---|---|---|---|---|---|
| Lopez et al, 2016 (11) | Retrospective (1991-2014) | USA | NFA (n = 242) Referent individuals (n = 1237) | Baseline + conservative follow-up (available only for 110 patients with NFA and 615 referent individuals) | Mean 7.7 y (≥3 y) | Baseline: CKD prevalence: 6.2% vs 5.7% (referent individuals, n = 1237); P = .76 Follow-up: CKD incidence: 7.3% (NFA) vs 2.4% (referent individuals); P = .015 |
| Numakura et al, 2019 (13) | Retrospective (1997-2017) | Japan | MACS (n = 12) CS (n = 18) | Post adrenalectomy | ≥1 y | Baseline: Median eGFR 78.4 mL/min/1.73 m2 Follow-up post adrenalectomy: eGFR increased when compared to baseline (median of eGFR 84.1 vs 78.4 mL/min/1.73 m2); P < .05 |
| Singh et al, 2020 (8) | Retrospective (2003-2018) | USA | NFA (n = 275) MACS (n = 168) | Baseline | NA | Baseline: CKD prevalence: 16.9% (NFA) vs 25.3% (MACS); P = .044 |
| Naka et al, 2020 (12) | Retrospective (2010-2018) | Japan | NFA (n = 232) MACS (n = 84) CS (n = 23) | Baseline + post adrenalectomy (available only for 19 patients) | Not reported | Baseline: eGFR: 82.2 ± 2.2 (MACS) vs 81.6 ± 1.2 (NFA); P = NS Follow-up post adrenalectomy in patients with MACS and CS: eGFR pre surgery: 94.0 ± 4.7 vs post surgery: 81.7 ± 3.7; P < .01 |
| Zhang et al, 2021 (3) | Retrospective (1995-2017) | USA | Adrenal adenoma (NFA and MACS) (n = 1004) Referent individuals (n = 1004) | Baseline + conservative follow-up | Median 6.8 y | Baseline: CKD prevalence: 6.5% (adrenal adenoma) vs 5.2% (referent individuals), P = .216 Follow-up: CKD incidence: 19% (adrenal adenoma) vs 12% (referent individuals), adjusted hazard ratio: 1.46 (95% CI, 1.14-1.86) |
| Araujo-Castro, 2021 (20) | Case control (2018-2020) | Spain | MACS (n = 57) | Baseline + conservative follow-up (n = 55) and postadrenalectomy follow-up (n = 2) | Median 2.25 y | Baseline: CKD prevalence: 7.4% Follow-up: CKD incidence 0.0%, mean eGFR decline: −2.3 mL/min/1.73 m2 (P value not reported) |
| Kufukihara et al, 2022 (14) | Retrospective (2001-2018) | Japan | CS (n = 59) MACS (n = 17) with postdexamethasone cortisol >5 mcg/dL | Baseline and postadrenalectomy follow-up | 1 mo and 1 y post adrenalectomy | Baseline: eGFR 82.2 ± 19.5 mL/min/1.73 m2 1 mo follow-up from baseline: 71.7 ± 17.3 mL/min/1.73 m2, in comparison to baseline: P < .001 1 y follow-up from baseline: 79.5 ± 20.2 mL/min/1.73 m2, in comparison to baseline: P = .108 |
Abbreviations: CKD, chronic kidney disease; CS, Cushing syndrome; eGFR, estimated glomerular filtration rate; MACS, mild autonomous cortisol secretion; NA, not applicable; NFA, nonfunctioning adrenal adenoma; USA, United States of America.
We showed that patients with NFA and MACS demonstrate an improvement in eGFR within 3.5 years of postadrenalectomy follow-up. A potential mechanism involves the improvement of subtle hypercortisolism as a direct contributing factor to kidney function impairment, along with the amelioration of MACS-associated cardiovascular comorbidities following adrenalectomy. We further showed that younger patients with lower eGFR were more likely to have improved kidney function following adrenalectomy. Previous small studies examining change in eGFR post adrenalectomy were limited by small sample size and included mixed adenoma subtypes (see Table 5). In a study of 30 patients with either MACS or CS, the authors reported a 5-point increase in eGFR when reassessed at least 1 year after adrenalectomy (16). In contrast, in another study of 19 patients with either MACS or CS, the authors showed that eGFR worsens after adrenalectomy, without providing the timeline for eGFR assessment (15). In a larger study of 59 patients with CS and 17 patients with MACS, eGFR declined 1 month after adrenalectomy and increased back to the preadrenalectomy level 1 year later (17). Comparison of the results from our study to the literature is difficult as none of the studies reported on patients with MACS separately from patients with CS, or in patients with NFA (see Table 5).
Our study has several strengths, including a large sample size of consecutive patients with MACS and NFA, definition of CKD based on eGFR rather than diagnostic codes, and consideration of several confounding factors, such as cardiovascular risk factors, smoking, and certain medications. We were able to also evaluate the effect of adrenalectomy on eGFR and identify factors associated with improvement. We defined MACS according to the most recent guidelines in adrenal incidentaloma (5). Several limitations apply to this study. First, we acknowledge a referral, selection, and information bias. It is possible that patients with MACS and NFA evaluated at our institution have a higher prevalence of CKD; however, we do not expect that the differences found between patients with MACS and NFA were affected by the selection bias. Second, we classified our groups (MACS vs NFA) based on the 1-mg DST, as recommended by the guidelines (5). However, 1-mg DST presents with a proportion of false-positive results, and as such, some of the patients with NFA may have been misclassified as MACS. We previously showed that the proportion of false-positive results due to dexamethasone malabsorption is low when performed at our institution (22). Third, we acknowledge the potential influence of low eGFR on cortisol level. Nevertheless, it is noteworthy that within our cohort of 972 patients, only 17 patients exhibited severe renal impairment (eGFR < 30 mL/min/1.73 m²). Given the limited number of cases, it is unlikely that this subgroup significantly affects our findings. Fourth, we relied on the eGFR measurement closest to the diagnosis of MACS or NFA and were not able to account for eGFR intraindividual variability. Fifth, 92% of participants were White; as a result, findings may not be generalizable to more diverse populations. Sixth, we acknowledge heterogeneous follow-up and timing for assessment of eGFR in our postadrenalectomy cohort. Finally, while our adrenalectomy cohort was 7 times larger than in previous reports, the sample size was still small, and future studies are needed to identify the differential effect of MACS vs NFA on eGFR improvement, as well as whether treatment with mineralocorticoid receptor antagonists or other agents are renoprotective in conservatively followed patients.
In conclusion, we showed that patients with MACS demonstrate lower eGFR and higher prevalence of advanced CKD at the time of adenoma diagnosis compared to patients with NFA. We further demonstrate that higher post-DST cortisol is associated with lower eGFR independent of cardiovascular risk factors known to affect kidney function, such as BMI, hypertension, dysglycemia, and dyslipidemia. After adrenalectomy, patients with both MACS and NFA demonstrate an increase in eGFR. We identified young age and lower eGFR as factors associated with kidney function improvement.
Funding
This work was partly supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health USA (under award Nos. K23DK121888 and R03DK132121 to I.B). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
I.B. reports advisory board participation/consulting (fees to institution) with HRA Pharma, Corcept, Recordati, Sparrow Pharmaceutics, Neurocrine, Spruce, and Diurnal outside the submitted work, and data monitoring and safety board participation for Adrenas. The remainder of the authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Abbreviations
- BMI
body mass index
- CKD
chronic kidney disease
- CS
Cushing syndrome
- DST
dexamethasone suppression test
- eGFR
estimated glomerular filtration rate
- IQR
interquartile range
- MACS
mild autonomous cortisol secretion
- NFA
nonfunctioning adrenal adenoma



