-
PDF
- Split View
-
Views
-
Cite
Cite
Marta Araujo-Castro, Betina Biagetti, Edelmiro Menéndez Torre, Iría Novoa-Testa, Fernando Cordido, Eider Pascual Corrales, Víctor Rodríguez Berrocal, Fernando Guerrero-Pérez, Almudena Vicente, Juan Carlos Percovich, Rogelio García Centeno, Laura González, María Dolores Ollero García, Ana Irigaray Echarri, María Dolores Moure Rodríguez, Cristina Novo-Rodríguez, María Calatayud, Rocío Villar, Ignacio Bernabéu, Cristina Alvarez-Escola, Pamela Benítez Valderrama, Carmen Tenorio-Jimenéz, Pablo Abellán Galiana, Eva Venegas Moreno, Inmaculada González Molero, Pedro Iglesias, Concepción Blanco, Fernando Vidal-Ostos De Lara, Paz de Miguel, Elena López Mezquita, Felicia Hanzu, Iban Aldecoa, Cristina Lamas, Silvia Aznar, Anna Aulinas, Anna Calabrese, Paola Gracia, José María Recio-Córdova, Mariola Aviles, Diego Asensio-Wandosel, Miguel Sampedro, Ignacio Ruz-Caracuel, Rosa Camara, Miguel Paja, Carmen Fajardo-Montañana, Mónica Marazuela, Manel Puig-Domingo, Differences Between GH- and PRL-Cosecreting and GH-Secreting Pituitary Adenomas: a Series of 604 Cases, The Journal of Clinical Endocrinology & Metabolism, Volume 109, Issue 12, December 2024, Pages e2178–e2187, https://doi.org/10.1210/clinem/dgae126
Close - Share Icon Share
Abstract
Few data exist about the clinical course of acromegaly, surgical and medical outcomes in patients with GH- and prolactin cosecreting pituitary adenomas (GH&PRL-PAs). Nevertheless, some series described a more aggressive clinic-radiological behavior than in growth hormone–secreting pituitary adenomas (GH-PAs).
This work aims to evaluate differences in clinical presentation and in surgical outcomes between GH-PAs and GH&PRL-PAs.
A multicenter retrospective study was conducted of 604 patients with acromegaly who underwent pituitary surgery. Patients were classified into 2 groups according to serum PRL levels at diagnosis and immunohistochemistry (IHC) for PRL: a) GH&PRL-PAs when PRL levels were above the upper limit of normal (ULN) and IHC for GH and PRL was positive or PRL levels were greater than 100 ng/dL and PRL IHC was not available (n = 130) and b) GH-PA patients who did not meet the previously mentioned criteria (n = 474).
GH&PRL-PAs represented 21.5% (n = 130) of patients with acromegaly. The mean age at diagnosis was lower in GH&PRL-PAs than in GH-PAs (P < .001). GH&PRL-PAs were more frequently macroadenomas (90.6% vs 77.4%; P = .001) and tended to be more invasive (33.6% vs 24.7%; P = .057) than GH-PAs. Furthermore, they had presurgical hypopituitarism more frequently (odds ratio 2.8; 95% CI, 1.83-4.38). Insulin-like growth factor ULN levels at diagnosis were lower in patients with GH&PRL-PAs (median 2.4 [interquartile range (IQR) 1.73-3.29] vs 2.7 [IQR 1.91-3.67]; P = .023). There were no differences in the immediate (41.1% vs 43.3%; P = .659) or long-term postsurgical acromegaly biochemical cure rate (53.5% vs 53.1%; P = .936) between groups. However, there was a higher incidence of permanent arginine-vasopressin deficiency (AVP-D) (7.3% vs 2.4%; P = .011) in GH&PRL-PA patients.
GH&PRL-PAs are responsible for 20% of acromegaly cases. These tumors are more invasive, larger, and cause hypopituitarism more frequently than GH-PAs and are diagnosed at an earlier age. The biochemical cure rate is similar between both groups, but patients with GH&PRL-PAs tend to develop permanent postsurgical AVP-D more frequently.
Acromegaly is a rare disease characterized by excessive secretion of growth hormone (GH) and increased circulating levels of insulin-like growth factor-1 (IGF-1). Most cases are caused by isolated hypersecretion of GH by the pituitary. However, in around 16% to 27% of cases there is an increase both of GH and prolactin (PRL) levels (1). This prevalence is widely variable across studies since the diagnostic criteria used for cosecreting GH and PRL pituitary adenoma (GH&PRL-PA) are not homogeneous (2). In fact, most studies have based the diagnosis of GH&PRL-PA on PRL and GH staining alone (3-5) or on serum PRL levels alone (1, 6, 7), without the assessment of transcription factors that have been recently incorporated into the pathology report in clinical practice.
A great deal of data exist about the clinical course of acromegaly, surgical and medical outcomes, and potential predictors of surgical cure in GH-PAs. However, there are few data in relation to these aspects in patients presenting with GH&PRL-PAs (2). Although in general a worse long-term remission rate has been reported in mixed somatotroph–lactotroph PAs compared to mammosomatotrophs and GH-PAs (4) and in GH-PRL PAs than in GH-PAs (5), all these studies are retrospective, performed at a single center, and have included fewer than 100 acromegaly cases and less than 50 patients with GH&PRL-PAs. The differences in surgical outcomes may be related to the larger tumor size and a high prevalence of cavernous sinus invasion described in the GH&PRL-PA group (1, 4, 6). However, the prevalence of preoperative hypopituitarism was reported to be similar between both groups (1, 4, 5)
Considering this background, the aim of our study was to evaluate in a multicentric study including more than 600 patients with acromegaly if there are differences in the clinical presentation of acromegaly and in surgical outcomes, including biochemical cure and postoperative complications, between patients with GH&PRL-PAs and patients with GH-PAs. We hypothesized that patients with GH&PRL-PAs would have with more severe clinical presentation and worse surgical outcomes than GH-PAs.
Materials and Methods
A multicenter retrospective chart review was performed of patients with GH-PA treated between 2003 and 2023 at 33 tertiary Spanish hospitals (ACRO-SPAIN study, n = 679). Inclusion criteria in this study were the following: i) biochemical diagnosis of acromegaly established by clinical practice guidelines criteria; ii) available data of clinical, hormonal, and radiological tumor characteristics preoperatively and postoperatively and or premedical and postmedical treatment; patients who did not undergo pituitary surgery were excluded from this study (n = 75); and iii) patients with follow-up data for longer than 3 months after surgery (Fig. 1). Patients who did not meet the inclusion criteria were not considered for entrance in the ACRO-SPAIN study.
Study population. GH-PAs, growth hormone–secreting pituitary adenomas; GH&PRL-PAs, growth hormone–and prolactin-cosecreting pituitary adenomas.
A specific registry was set up to collect information about clinical characteristics including sex, age, history of diabetes mellitus, hypertension, cardiovascular disease, hypopituitarism, and other acromegaly comorbidities such as sleep apnea syndrome or colonic polyps, among others. Radiological evaluation was performed with magnetic resonance imaging (MRI) scan and data about tumor size (transversal, laterolateral, and craniocaudal diameter), cavernous sinus invasion (Knosp grade), and hypointensity or hyperintensity in T2 sequence were registered. Cavernous sinus invasion was evaluated using the Knosp-Steiner classification based on coronal T1-weighted contrasted imaging (8). Knosp scores 3 and 4 were considered as invasive PAs. Based on maximum tumor size, tumors were categorized as microadenoma (<1 cm), macroadenoma (1-4 cm) and giant pituitary adenoma (≥4 cm).
Patients were classified into 2 groups according to serum PRL levels at diagnosis and immunohistochemistry (IHC) for GH and PRL: a) GH&PRL-PAs when serum PRL levels were above the upper limit of normal (ULN) and IHC for GH and PRL was positive (n = 118) or if serum PRL levels were greater than 100 ng/dL regardless of PRL IHC (IHC was not available for 12 patients) (n = 130); and b) GH-PAs, the rest of the patients who did not meet the previously mentioned criteria (n = 474) (see Fig. 1). We have selected the threshold of 100 ng/dL because these levels are rarely associated with pituitary stalk compression or dopaminergic neuronal damage (9). In addition, situations that may lead to PRL elevations such as interfering medication use, renal failure, and untreated hypothyroidism were excluded. A total of 604 patients fulfilled the inclusion criteria.
The study was endorsed by the Spanish Society of Endocrinology and Nutrition (SEEN) and was distributed to all members of the Neuroendocrinology Task Force of the SEEN, which includes most of the endocrinologists who care for acromegaly patients in Spain. The local ethics committee of the Hospital Universitario Ramón y Cajal reviewed and approved the study (approval date: June 2, 2023; ACTA 454). The study was conducted according to the mandates of the Declaration of Helsinki and good clinical practices. Patient consent was waived due to the retrospective nature of the study. Informed consent was requested only from patients who continued follow-up or who were prospectively included.
Hormonal and Clinical Information and Definitions
The diagnosis of acromegaly was made following the recommendation of the recommended guidelines at the time of the diagnosis (10, 11). Serum IGF-1 and GH levels were determined locally in each center by immunochemiluminescence, immunoradiometric assay, or electrochemiluminescence using different assays (IMMULITE 2000, Liaison XL [Diasorin], ImmuliteXP). The precision of these measurements was evaluated in accordance with a protocol based on CLSI EP-5A2, “Evaluation of Precision Performance of Quantitative Measurement Methods.” Nevertheless, due to the variability in the assays used for the measurement of IGF-1, the percentage above the ULN was used. Hormones levels including GH and IGF-1 were measured in each patient at baseline, after surgery (immediate postoperative evaluation), and at the last follow-up visit (long-term postoperative evaluation).
Information about the status of biochemical and tumoral control was evaluated at short-term and long-term follow-up. Surgical remission was defined at least 3 months after surgery, using the Cortina definition (2000 criteria) (random GH < 2.5 ng/mL or GH nadir < 1 ng/mL on oral glucose tolerance test, along with normal age- and sex-matched IGF-1) (12). In addition, the 2010 criteria of the American Association of Clinical Endocrinologists and Endocrine Society guidelines (random GH < 1 ng/mL or GH nadir < 0.4 ng/mL on oral glucose tolerance test for GH, along with normal age- and sex-matched IGF-1) was also assessed in those patients treated from 2010 onward (11, 13).
We also registered information about presurgical treatment with first-generation somatostatin receptor ligands (fg-SRLs) (duration, doses, and biochemical and tumoral response before surgery).
Surgical Procedure and Histological Evaluation
A transsphenoidal approach was used in all surgeries. Surgeries were generally performed by 1 or 2 experienced pituitary neurosurgeons in each participant center. Most of the participant centers meet the criteria for being considering a Pituitary Tumor Center of Excellence (PTCOE) in terms of the numbers of pituitary surgeries performed per year (20-40 cases/years) (14); however, there were 7 centers with an average of fewer than 20 pituitary surgeries per year (that it is the threshold proposed by the Spanish Ministry of Health to take into consideration a center as a PTCOE). This information was extracted from the TESSPAIN database that include information about the pituitary surgeries/year in the different Spanish centers. Considering this point, we have described surgical outcomes in high-volume centers (≥20 surgeries/year) and low-volume centers (<20/year).
Major surgical complications were defined as the development of permanent neurological deficit (oculomotor or visual impairment), postsurgical meningitis, cerebrospinal fluid leak, postsurgical bleeding requiring reoperation, or intraoperative bleeding due to vascular injury. As we have previously defined, postsurgical arginine-vasopressin deficiency (AVP-D, formerly known as central diabetes insipidus) was classified as permanent when there was no recovery after 6 months, and transient if the duration was shorter than 6 months (15).
Surgically removed specimens were immediately fixed in 10% buffered formalin and subsequently embedded in paraffin. Standard hematoxylin and eosin–stained sections were used for diagnosis. The following features were included in the description of the pathological specimen: cellular atypia; immunostaining for human GH, PRL, thyrotropin (TSH), adrenocorticotropin (ACTH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and Ki-67. In addition, in 191 cases, according to the cytokeratin IHC pattern, PAs were separated in densely granulated and sparsely granulated categories (16). Information about the expression of the different transcription factors (PIT1, TPIT, SF1, GATA3, and ERα) was available only for 80 cases (17).
Statistical Analysis
Statistical analysis was performed with STATA.15. In the descriptive analysis, categorical variables were expressed as percentages and (absolute values of variable); quantitative variables were expressed as mean ±SD or as medians ± interquartile ranges (IQRs) depending on whether the normality assumption was fulfilled. For the comparison of the differences in continuous parameters between 2 subgroups, we used t tests and lineal regression tests. The chi-square (χ2) test was used to compare categorical data. Univariant logistic regression analysis was used to estimate odds ratio (OR), and multivariant logistic regression model for the adjustment of the OR by potential confounding factors. The Mantel-Haenszel (MH) test for linear trend test was used to calculate the linear correlation in the proportion of GH&PRL-PAs in the different Knosp grades. In all cases, a 2-tailed P value less than .05 was considered statistically significant.
Results
Baseline Differences in Clinical and Radiological Data Between Growth Hormone–Secreting Pituitary Adenomas and Growth Hormone– and Prolactin-Cosecreting Pituitary Adenomas
GH&PRL-PAs represented 21.5% (n = 130) of patients with acromegaly, while GH-PAs caused the others 78.5% (n = 474). All patients included in the GH&PRL-PA group with available immunostaining for PRL were positive (n = 118), but there were 12 cases without available PRL IHC classified as GH&PRL-PAs because serum PRL levels were greater than 100 ng/dL. The median serum PRL levels in these patients was 153 ng/dL (IQR 144-225 ng/dL) and 3 patient cases had serum PRL levels greater than 200 ng/dL. In the group of patients with available PRL IHC, there were 40 patients with slight elevation of serum PRL levels (between ULN and 50 ng/dL). Nevertheless, the median serum PRL level of this second group was 85.4 ng/dL (IQR 40.7-137.7). On the other hand, there were 50 patient cases in the GH-PA group with hyperprolactinemia, but with slightly elevated levels (mean levels of 19.8 ± 31.71 ng/dL). The mean age at diagnosis was lower in GH&PRL-PA than in GH-PA patients. A higher burden of cardiometabolic comorbidities was observed in the GH-PA group, but these differences disappeared after adjusting for age (adjusted OR for type 2 diabetes: 1.70; 95% CI, 0.96-2.99; adjusted OR for sleep apnea syndrome: 1.45; 95% CI, 0.89-2.35), except for hypertension, which continued being more frequent in GH-PAs after adjusting for age (adjusted OR for hypertension: 1.76; 95% CI, 1.07-2.89) (Table 1).
Differences in presurgical variables between patients with growth hormone–secreting pituitary adenomas and with growth hormone–and prolactin-cosecreting pituitary adenomas
| Variable . | GH-PAs (n = 474) . | GH&PRL-PAs (n = 130) . | P . |
|---|---|---|---|
| Age, years | 50.1 (IQR 39.1-58.6) | 43.5 (IQR 35.0-53.9) | <.001 |
| Female sex | 58.4% (n = 277) | 60.8% (n = 79) | .632 |
| Type 2 diabetes | 26.6% (n = 126) | 13.9% (n = 18) | .003 |
| Hypertension | 42.6% (n = 202) | 25.4% (n = 33) | <.001 |
| Sleep apnea syndrome | 35.2% (n = 163/463) | 22.3% (n = 29/130) | .005 |
| Cardiovascular disease | 3.9% (n = 18/464) | 3.1% (n = 4/130) | .669 |
| Headache | 42.9% (n = 186/464) | 53.2% (n = 67/126) | .040 |
| Visual involvement | 15.8% (n = 70/443) | 27.3% (n = 35/128) | .003 |
| Time of acromegaly symptoms before diagnosis, months | 24 (IQR 12-60) | 24 (8-48) | .046 |
| Macroadenoma | 77.4% (n = 342/442) | 90.6% (n = 115/127) | .001 |
| Maximum tumor size, mm | 15.9 ± 10.05 | 19.7 ± 11.83 | <.001 |
| Knosp grade >2 | 24.7% (n = 94/380) | 33.6% (n = 40/119) | .057 |
| T2-MRI hypointensity | 49.5% (n = 134/271) | 28.2% (n = 26/92) | <.001 |
| Hypopituitarism (≥1 deficits) | 16.7% (n = 75/450) | 36.2% (n = 47/130) | <.001 |
| TSH deficit | 5.5% (n = 25/453) | 14.6% (n = 19/130) | .001 |
| ACTH deficit | 3.6% (n = 16/450) | 9.23% (n = 12/130) | .008 |
| FSH/LH deficit | 14.6% (n = 66/453) | 33.1% (n = 43/130) | <.001 |
| IGF-1, %ULN | 2.7 (IQR 1.91-3.67) | 2.4 (IQR 1.73-3.29) | .023 |
| GH, ng/dL | 8.0 (IQR 3.69-20) | 9.3 (IQR 3.6-20.3) | .980 |
| Serum PRL, ng/dL | 11.8 (IQR 7.66-18.42) | 98.1 (IQR 41.69-146.2) | <.001 |
| Variable . | GH-PAs (n = 474) . | GH&PRL-PAs (n = 130) . | P . |
|---|---|---|---|
| Age, years | 50.1 (IQR 39.1-58.6) | 43.5 (IQR 35.0-53.9) | <.001 |
| Female sex | 58.4% (n = 277) | 60.8% (n = 79) | .632 |
| Type 2 diabetes | 26.6% (n = 126) | 13.9% (n = 18) | .003 |
| Hypertension | 42.6% (n = 202) | 25.4% (n = 33) | <.001 |
| Sleep apnea syndrome | 35.2% (n = 163/463) | 22.3% (n = 29/130) | .005 |
| Cardiovascular disease | 3.9% (n = 18/464) | 3.1% (n = 4/130) | .669 |
| Headache | 42.9% (n = 186/464) | 53.2% (n = 67/126) | .040 |
| Visual involvement | 15.8% (n = 70/443) | 27.3% (n = 35/128) | .003 |
| Time of acromegaly symptoms before diagnosis, months | 24 (IQR 12-60) | 24 (8-48) | .046 |
| Macroadenoma | 77.4% (n = 342/442) | 90.6% (n = 115/127) | .001 |
| Maximum tumor size, mm | 15.9 ± 10.05 | 19.7 ± 11.83 | <.001 |
| Knosp grade >2 | 24.7% (n = 94/380) | 33.6% (n = 40/119) | .057 |
| T2-MRI hypointensity | 49.5% (n = 134/271) | 28.2% (n = 26/92) | <.001 |
| Hypopituitarism (≥1 deficits) | 16.7% (n = 75/450) | 36.2% (n = 47/130) | <.001 |
| TSH deficit | 5.5% (n = 25/453) | 14.6% (n = 19/130) | .001 |
| ACTH deficit | 3.6% (n = 16/450) | 9.23% (n = 12/130) | .008 |
| FSH/LH deficit | 14.6% (n = 66/453) | 33.1% (n = 43/130) | <.001 |
| IGF-1, %ULN | 2.7 (IQR 1.91-3.67) | 2.4 (IQR 1.73-3.29) | .023 |
| GH, ng/dL | 8.0 (IQR 3.69-20) | 9.3 (IQR 3.6-20.3) | .980 |
| Serum PRL, ng/dL | 11.8 (IQR 7.66-18.42) | 98.1 (IQR 41.69-146.2) | <.001 |
Abbreviations: ACTH, adrenocorticotropin; FSH, follicle-stimulating hormone; GH, growth hormone; GH-PAs, growth hormone–secreting pituitary adenomas; GH&PRL-PAs, growth hormone– and prolactin-cosecreting pituitary adenomas; IGF-1, insulin-like growth factor-1; IQR, interquartile range; LH, luteinizing hormone; MRI, magnetic resonance imaging; PRL, prolactin; TSH, thyrotropin; ULN, upper limit of normal.
Differences in presurgical variables between patients with growth hormone–secreting pituitary adenomas and with growth hormone–and prolactin-cosecreting pituitary adenomas
| Variable . | GH-PAs (n = 474) . | GH&PRL-PAs (n = 130) . | P . |
|---|---|---|---|
| Age, years | 50.1 (IQR 39.1-58.6) | 43.5 (IQR 35.0-53.9) | <.001 |
| Female sex | 58.4% (n = 277) | 60.8% (n = 79) | .632 |
| Type 2 diabetes | 26.6% (n = 126) | 13.9% (n = 18) | .003 |
| Hypertension | 42.6% (n = 202) | 25.4% (n = 33) | <.001 |
| Sleep apnea syndrome | 35.2% (n = 163/463) | 22.3% (n = 29/130) | .005 |
| Cardiovascular disease | 3.9% (n = 18/464) | 3.1% (n = 4/130) | .669 |
| Headache | 42.9% (n = 186/464) | 53.2% (n = 67/126) | .040 |
| Visual involvement | 15.8% (n = 70/443) | 27.3% (n = 35/128) | .003 |
| Time of acromegaly symptoms before diagnosis, months | 24 (IQR 12-60) | 24 (8-48) | .046 |
| Macroadenoma | 77.4% (n = 342/442) | 90.6% (n = 115/127) | .001 |
| Maximum tumor size, mm | 15.9 ± 10.05 | 19.7 ± 11.83 | <.001 |
| Knosp grade >2 | 24.7% (n = 94/380) | 33.6% (n = 40/119) | .057 |
| T2-MRI hypointensity | 49.5% (n = 134/271) | 28.2% (n = 26/92) | <.001 |
| Hypopituitarism (≥1 deficits) | 16.7% (n = 75/450) | 36.2% (n = 47/130) | <.001 |
| TSH deficit | 5.5% (n = 25/453) | 14.6% (n = 19/130) | .001 |
| ACTH deficit | 3.6% (n = 16/450) | 9.23% (n = 12/130) | .008 |
| FSH/LH deficit | 14.6% (n = 66/453) | 33.1% (n = 43/130) | <.001 |
| IGF-1, %ULN | 2.7 (IQR 1.91-3.67) | 2.4 (IQR 1.73-3.29) | .023 |
| GH, ng/dL | 8.0 (IQR 3.69-20) | 9.3 (IQR 3.6-20.3) | .980 |
| Serum PRL, ng/dL | 11.8 (IQR 7.66-18.42) | 98.1 (IQR 41.69-146.2) | <.001 |
| Variable . | GH-PAs (n = 474) . | GH&PRL-PAs (n = 130) . | P . |
|---|---|---|---|
| Age, years | 50.1 (IQR 39.1-58.6) | 43.5 (IQR 35.0-53.9) | <.001 |
| Female sex | 58.4% (n = 277) | 60.8% (n = 79) | .632 |
| Type 2 diabetes | 26.6% (n = 126) | 13.9% (n = 18) | .003 |
| Hypertension | 42.6% (n = 202) | 25.4% (n = 33) | <.001 |
| Sleep apnea syndrome | 35.2% (n = 163/463) | 22.3% (n = 29/130) | .005 |
| Cardiovascular disease | 3.9% (n = 18/464) | 3.1% (n = 4/130) | .669 |
| Headache | 42.9% (n = 186/464) | 53.2% (n = 67/126) | .040 |
| Visual involvement | 15.8% (n = 70/443) | 27.3% (n = 35/128) | .003 |
| Time of acromegaly symptoms before diagnosis, months | 24 (IQR 12-60) | 24 (8-48) | .046 |
| Macroadenoma | 77.4% (n = 342/442) | 90.6% (n = 115/127) | .001 |
| Maximum tumor size, mm | 15.9 ± 10.05 | 19.7 ± 11.83 | <.001 |
| Knosp grade >2 | 24.7% (n = 94/380) | 33.6% (n = 40/119) | .057 |
| T2-MRI hypointensity | 49.5% (n = 134/271) | 28.2% (n = 26/92) | <.001 |
| Hypopituitarism (≥1 deficits) | 16.7% (n = 75/450) | 36.2% (n = 47/130) | <.001 |
| TSH deficit | 5.5% (n = 25/453) | 14.6% (n = 19/130) | .001 |
| ACTH deficit | 3.6% (n = 16/450) | 9.23% (n = 12/130) | .008 |
| FSH/LH deficit | 14.6% (n = 66/453) | 33.1% (n = 43/130) | <.001 |
| IGF-1, %ULN | 2.7 (IQR 1.91-3.67) | 2.4 (IQR 1.73-3.29) | .023 |
| GH, ng/dL | 8.0 (IQR 3.69-20) | 9.3 (IQR 3.6-20.3) | .980 |
| Serum PRL, ng/dL | 11.8 (IQR 7.66-18.42) | 98.1 (IQR 41.69-146.2) | <.001 |
Abbreviations: ACTH, adrenocorticotropin; FSH, follicle-stimulating hormone; GH, growth hormone; GH-PAs, growth hormone–secreting pituitary adenomas; GH&PRL-PAs, growth hormone– and prolactin-cosecreting pituitary adenomas; IGF-1, insulin-like growth factor-1; IQR, interquartile range; LH, luteinizing hormone; MRI, magnetic resonance imaging; PRL, prolactin; TSH, thyrotropin; ULN, upper limit of normal.
GH&PRL-PAs were more often macroadenomas and tended to be invasive tumors more commonly than GH-PAs. In fact, there was a positive significant linear tendency to detect a higher proportion of GH&PRL-PAs as the Knosp grade increased (MH test for linear trend: χ2(1) = 10.57; P = .001) (Table 2). Hypointensity in T2 sequence was 2 times more common in the group of GH-PA than in GH&PRL-PA patients (OR 2.5; 95% CI, 1.49-4.15). However, these differences disappeared after adjusting by the proportion of tumors with densely granulated patterns (adjusted OR 2.15; 95% CI, 0.96-4.83).
Proportion of growth hormone– and prolactin-cosecreting pituitary adenomas according to Knosp grade
| Knosp grade . | Cases of GH&PRL . | Total PAs . | Prevalence . | OR (95% CI) . |
|---|---|---|---|---|
| 0 | 26 | 170 | 15% | 1 (reference) |
| 1 | 27 | 104 | 26% | 1.94 (1.06-3.56) |
| 2 | 26 | 91 | 29% | 2.22 (1.94-4.11) |
| 3 | 20 | 82 | 24% | 1.79 (0.93-3.44) |
| 4 | 20 | 52 | 38% | 3.46 (1.72-6.95) |
| Total | 119 | 499 | 24% |
| Knosp grade . | Cases of GH&PRL . | Total PAs . | Prevalence . | OR (95% CI) . |
|---|---|---|---|---|
| 0 | 26 | 170 | 15% | 1 (reference) |
| 1 | 27 | 104 | 26% | 1.94 (1.06-3.56) |
| 2 | 26 | 91 | 29% | 2.22 (1.94-4.11) |
| 3 | 20 | 82 | 24% | 1.79 (0.93-3.44) |
| 4 | 20 | 52 | 38% | 3.46 (1.72-6.95) |
| Total | 119 | 499 | 24% |
Mantel-Haenszel test for linear trend: χ2(1) = 10.57 (P = .001); deviation from linearity: χ2(3) = 3.78 (P = .286).
Abbreviations: GH-PAs, growth hormone–secreting pituitary adenomas; GH&PRL-PAs, growth hormone– and prolactin-cosecreting pituitary adenomas; OR, odds ratio.
Proportion of growth hormone– and prolactin-cosecreting pituitary adenomas according to Knosp grade
| Knosp grade . | Cases of GH&PRL . | Total PAs . | Prevalence . | OR (95% CI) . |
|---|---|---|---|---|
| 0 | 26 | 170 | 15% | 1 (reference) |
| 1 | 27 | 104 | 26% | 1.94 (1.06-3.56) |
| 2 | 26 | 91 | 29% | 2.22 (1.94-4.11) |
| 3 | 20 | 82 | 24% | 1.79 (0.93-3.44) |
| 4 | 20 | 52 | 38% | 3.46 (1.72-6.95) |
| Total | 119 | 499 | 24% |
| Knosp grade . | Cases of GH&PRL . | Total PAs . | Prevalence . | OR (95% CI) . |
|---|---|---|---|---|
| 0 | 26 | 170 | 15% | 1 (reference) |
| 1 | 27 | 104 | 26% | 1.94 (1.06-3.56) |
| 2 | 26 | 91 | 29% | 2.22 (1.94-4.11) |
| 3 | 20 | 82 | 24% | 1.79 (0.93-3.44) |
| 4 | 20 | 52 | 38% | 3.46 (1.72-6.95) |
| Total | 119 | 499 | 24% |
Mantel-Haenszel test for linear trend: χ2(1) = 10.57 (P = .001); deviation from linearity: χ2(3) = 3.78 (P = .286).
Abbreviations: GH-PAs, growth hormone–secreting pituitary adenomas; GH&PRL-PAs, growth hormone– and prolactin-cosecreting pituitary adenomas; OR, odds ratio.
IGF-1 levels were lower in patients with GH&PRL-PAs than in GH-PAs, and they presented with presurgical hypopituitarism more frequently than GH-PA patients (OR 2.8; 95%, CI 1.83-4.38), including ACTH, FSH/LH, and TSH deficiencies (see Table 1). These differences remained statistically significant after adjusting for tumor size and Knosp grade (OR 2.2; 95% CI, 1.31-3.63).
A positive correlation was found between PRL levels at diagnosis with tumor size (r = 0.26; P < .001) and with Knosp grade (r = 0.10; P = .049), as well as between IGF-1 levels and tumor size (r = 0.10; P = .040), but not with Knosp grade (P > .05). However, when we evaluated the correlation between tumor size and IGF-1 levels in the group of GH&PRL-PA patients, this correlation disappeared (r = 0.01; P = .945), while it was maintained in the GH-PA group (r = 0.14; P = .01) (Fig. 2).
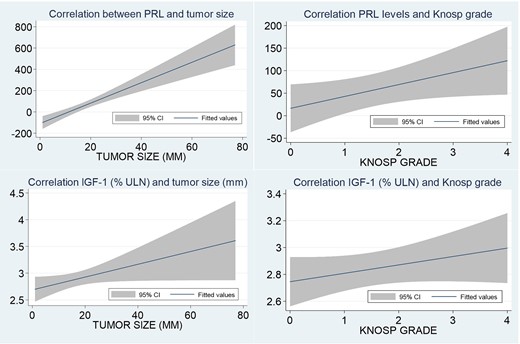
Correlation between prolactin (PRL) levels and insulin-like growth factor-1 (IGF-1) levels with tumor size and Knosp grade.
When we compared patients with serum PRL levels of 100 ng/dL or greater (n = 68) and those with values below this threshold (n = 536), we observed similar results as when comparing GH&PRL-PA and GH-PA based on our proposed definition except for headache prevalence, gonadotropic deficiency, and tumor size (Table 3).
Differences in presurgical variables between patients with serum prolactin levels of 100 ng/dL or greater and patients with levels less than 100 ng/dL
| Variable . | Serum PRL < 100 ng/dL (n = 536) . | Serum PRL ≥100 ng/dL (n = 68) . | P . |
|---|---|---|---|
| Age, years | 48.9 (IQR 37.70-58.02) | 44.9 (IQR 34.70-54.57) | .006 |
| Female sex | 59.7% (n = 320) | 52.9% (n = 36) | .286 |
| Type 2 diabetes | 24.4% (n = 131) | 19.1% (n = 13) | .332 |
| Hypertension | 39.7% (n = 213) | 32.4% (n = 22) | .239 |
| Sleep apnea syndrome | 33.5% (n = 176/525) | 23.5% (n = 16/68) | .097 |
| Cardiovascular disease | 4.2% (n = 22/526) | 0% | .086 |
| Headache | 43.2% (n = 214/496) | 60.9% (n = 39/64) | .007 |
| Visual involvement | 17.4% (n = 88/505) | 25.8% (n = 17/66) | .100 |
| Time of acromegaly symptoms before diagnosis, months | 24 (IQR 12-60) | 19 (IQR 9-52) | .374 |
| Macroadenoma | 78.7% (n = 396/503) | 92.4% (n = 61/66) | .009 |
| Maximum tumor size, mm | 16.1 ± 9.97 | 19.1 ± 9.97 | <.001 |
| Knosp grade >2 | 27.2% (n = 119/437) | 24.2% (n = 15/62) | .614 |
| T2-MRI hypointensity | 47.6% (n = 149/313) | 22.0% (n = 11/50) | .001 |
| Hypopituitarism (≥1 more deficits) | 18.6% (n = 95/512) | 39.7% (n = 27/68) | <.001 |
| TSH deficit | 6.4% (n = 33/515) | 16.2% (n = 11/68) | .004 |
| ACTH deficit | 4.1% (n = 21/512) | 10.3% (n = 7/68) | .025 |
| FSH/LH deficit | 16.3% (n = 84/515) | 36.8% (n = 25/68) | <.001 |
| IGF-1, %ULN | 2.6 (IQR 1.88-3.61) | 2.4 (IQR 1.82-3.38) | .372 |
| GH, ng/dL | 8.1 (IQR 3.66-19.7) | 9.3 (IQR 4-23.9) | .708 |
| Serum PRL, ng/dL | 13 (IQR 8-25.8) | 147.5 (IQR 121.0-224.5) | <.001 |
| Variable . | Serum PRL < 100 ng/dL (n = 536) . | Serum PRL ≥100 ng/dL (n = 68) . | P . |
|---|---|---|---|
| Age, years | 48.9 (IQR 37.70-58.02) | 44.9 (IQR 34.70-54.57) | .006 |
| Female sex | 59.7% (n = 320) | 52.9% (n = 36) | .286 |
| Type 2 diabetes | 24.4% (n = 131) | 19.1% (n = 13) | .332 |
| Hypertension | 39.7% (n = 213) | 32.4% (n = 22) | .239 |
| Sleep apnea syndrome | 33.5% (n = 176/525) | 23.5% (n = 16/68) | .097 |
| Cardiovascular disease | 4.2% (n = 22/526) | 0% | .086 |
| Headache | 43.2% (n = 214/496) | 60.9% (n = 39/64) | .007 |
| Visual involvement | 17.4% (n = 88/505) | 25.8% (n = 17/66) | .100 |
| Time of acromegaly symptoms before diagnosis, months | 24 (IQR 12-60) | 19 (IQR 9-52) | .374 |
| Macroadenoma | 78.7% (n = 396/503) | 92.4% (n = 61/66) | .009 |
| Maximum tumor size, mm | 16.1 ± 9.97 | 19.1 ± 9.97 | <.001 |
| Knosp grade >2 | 27.2% (n = 119/437) | 24.2% (n = 15/62) | .614 |
| T2-MRI hypointensity | 47.6% (n = 149/313) | 22.0% (n = 11/50) | .001 |
| Hypopituitarism (≥1 more deficits) | 18.6% (n = 95/512) | 39.7% (n = 27/68) | <.001 |
| TSH deficit | 6.4% (n = 33/515) | 16.2% (n = 11/68) | .004 |
| ACTH deficit | 4.1% (n = 21/512) | 10.3% (n = 7/68) | .025 |
| FSH/LH deficit | 16.3% (n = 84/515) | 36.8% (n = 25/68) | <.001 |
| IGF-1, %ULN | 2.6 (IQR 1.88-3.61) | 2.4 (IQR 1.82-3.38) | .372 |
| GH, ng/dL | 8.1 (IQR 3.66-19.7) | 9.3 (IQR 4-23.9) | .708 |
| Serum PRL, ng/dL | 13 (IQR 8-25.8) | 147.5 (IQR 121.0-224.5) | <.001 |
Abbreviations: ACTH, adrenocorticotropin; FSH, follicle-stimulating hormone; GH, growth hormone; GH-PAs, growth hormone–secreting pituitary adenomas; GH&PRL-PAs, growth hormone– and prolactin-cosecreting pituitary adenomas; IGF-1, insulin-like growth factor-1; IQR, interquartile range; LH, luteinizing hormone; MRI, magnetic resonance imaging; PRL, prolactin; TSH, thyrotropin; ULN, upper limit of normal.
Differences in presurgical variables between patients with serum prolactin levels of 100 ng/dL or greater and patients with levels less than 100 ng/dL
| Variable . | Serum PRL < 100 ng/dL (n = 536) . | Serum PRL ≥100 ng/dL (n = 68) . | P . |
|---|---|---|---|
| Age, years | 48.9 (IQR 37.70-58.02) | 44.9 (IQR 34.70-54.57) | .006 |
| Female sex | 59.7% (n = 320) | 52.9% (n = 36) | .286 |
| Type 2 diabetes | 24.4% (n = 131) | 19.1% (n = 13) | .332 |
| Hypertension | 39.7% (n = 213) | 32.4% (n = 22) | .239 |
| Sleep apnea syndrome | 33.5% (n = 176/525) | 23.5% (n = 16/68) | .097 |
| Cardiovascular disease | 4.2% (n = 22/526) | 0% | .086 |
| Headache | 43.2% (n = 214/496) | 60.9% (n = 39/64) | .007 |
| Visual involvement | 17.4% (n = 88/505) | 25.8% (n = 17/66) | .100 |
| Time of acromegaly symptoms before diagnosis, months | 24 (IQR 12-60) | 19 (IQR 9-52) | .374 |
| Macroadenoma | 78.7% (n = 396/503) | 92.4% (n = 61/66) | .009 |
| Maximum tumor size, mm | 16.1 ± 9.97 | 19.1 ± 9.97 | <.001 |
| Knosp grade >2 | 27.2% (n = 119/437) | 24.2% (n = 15/62) | .614 |
| T2-MRI hypointensity | 47.6% (n = 149/313) | 22.0% (n = 11/50) | .001 |
| Hypopituitarism (≥1 more deficits) | 18.6% (n = 95/512) | 39.7% (n = 27/68) | <.001 |
| TSH deficit | 6.4% (n = 33/515) | 16.2% (n = 11/68) | .004 |
| ACTH deficit | 4.1% (n = 21/512) | 10.3% (n = 7/68) | .025 |
| FSH/LH deficit | 16.3% (n = 84/515) | 36.8% (n = 25/68) | <.001 |
| IGF-1, %ULN | 2.6 (IQR 1.88-3.61) | 2.4 (IQR 1.82-3.38) | .372 |
| GH, ng/dL | 8.1 (IQR 3.66-19.7) | 9.3 (IQR 4-23.9) | .708 |
| Serum PRL, ng/dL | 13 (IQR 8-25.8) | 147.5 (IQR 121.0-224.5) | <.001 |
| Variable . | Serum PRL < 100 ng/dL (n = 536) . | Serum PRL ≥100 ng/dL (n = 68) . | P . |
|---|---|---|---|
| Age, years | 48.9 (IQR 37.70-58.02) | 44.9 (IQR 34.70-54.57) | .006 |
| Female sex | 59.7% (n = 320) | 52.9% (n = 36) | .286 |
| Type 2 diabetes | 24.4% (n = 131) | 19.1% (n = 13) | .332 |
| Hypertension | 39.7% (n = 213) | 32.4% (n = 22) | .239 |
| Sleep apnea syndrome | 33.5% (n = 176/525) | 23.5% (n = 16/68) | .097 |
| Cardiovascular disease | 4.2% (n = 22/526) | 0% | .086 |
| Headache | 43.2% (n = 214/496) | 60.9% (n = 39/64) | .007 |
| Visual involvement | 17.4% (n = 88/505) | 25.8% (n = 17/66) | .100 |
| Time of acromegaly symptoms before diagnosis, months | 24 (IQR 12-60) | 19 (IQR 9-52) | .374 |
| Macroadenoma | 78.7% (n = 396/503) | 92.4% (n = 61/66) | .009 |
| Maximum tumor size, mm | 16.1 ± 9.97 | 19.1 ± 9.97 | <.001 |
| Knosp grade >2 | 27.2% (n = 119/437) | 24.2% (n = 15/62) | .614 |
| T2-MRI hypointensity | 47.6% (n = 149/313) | 22.0% (n = 11/50) | .001 |
| Hypopituitarism (≥1 more deficits) | 18.6% (n = 95/512) | 39.7% (n = 27/68) | <.001 |
| TSH deficit | 6.4% (n = 33/515) | 16.2% (n = 11/68) | .004 |
| ACTH deficit | 4.1% (n = 21/512) | 10.3% (n = 7/68) | .025 |
| FSH/LH deficit | 16.3% (n = 84/515) | 36.8% (n = 25/68) | <.001 |
| IGF-1, %ULN | 2.6 (IQR 1.88-3.61) | 2.4 (IQR 1.82-3.38) | .372 |
| GH, ng/dL | 8.1 (IQR 3.66-19.7) | 9.3 (IQR 4-23.9) | .708 |
| Serum PRL, ng/dL | 13 (IQR 8-25.8) | 147.5 (IQR 121.0-224.5) | <.001 |
Abbreviations: ACTH, adrenocorticotropin; FSH, follicle-stimulating hormone; GH, growth hormone; GH-PAs, growth hormone–secreting pituitary adenomas; GH&PRL-PAs, growth hormone– and prolactin-cosecreting pituitary adenomas; IGF-1, insulin-like growth factor-1; IQR, interquartile range; LH, luteinizing hormone; MRI, magnetic resonance imaging; PRL, prolactin; TSH, thyrotropin; ULN, upper limit of normal.
Surgical Outcomes
The proportion of patients treated with presurgical fg-SRLs or with dopamine agonists did not differ between GH&PRL-PAs and GH-PAs (42.3% vs 38.7%; P = .453).
In the immediate postoperative evaluation, after a median follow-up of 5.6 ± 21.0 months, 42.8% of the patients had normalized IGF-1 levels, being 64% in microadenomas, 40% in macroadenomas in general, and 23% in invasive PAs. No differences in the rate of acromegaly biochemical control were observed between GH&PRL-PA and GH-PA patients (41.1% vs 43.3%; P = .659). However, the proportion of cases with postsurgical hyperprolactinemia was higher in the former (14.6% vs 2.9%; P < .001). The median level of PRL in those cases with persistent hyperprolactinemia (n = 30) was 70.1 ± 89.00 ng/dL, and most of these patients did not achieve postsurgical IGF-1 control (70%). Nevertheless, 85.4% of the group of GH&PRL-PAs had normalized PRL levels after surgery. Regarding surgical complications, no differences in the rate of major complications were detected between both groups. Nonetheless, patients with GH&PRL-PAs developed permanent AVP-D more frequently than the GH-PA group (OR 3.15; 95% CI, 1.25-7.94) (Table 4).
Differences in surgical outcomes between growth hormone–secreting pituitary adenomas and growth hormone– and prolactin-cosecreting pituitary adenomas
| Variable . | GH-PAs (n = 474) . | GH&PRL-PAs (n = 130) . | P . |
|---|---|---|---|
| Early follow-up assessment (after 6 mo of surgical treatment) | |||
| Rate of IGF-1 normalization | 43.3% (n = 199) | 41.1% (n = 53) | .659 |
| Hyperprolactinemia | 2.9% (n = 12) | 14.6% (n = 18) | <.001 |
| IGF-1, %ULN | 0.96 (IQR 0.71-1.57) | 1 (IQR 0.72-1.68) | .569 |
| Major complications | 13.5% (n = 64) | 16.9% (n = 22) | .323 |
| Cerebrospinal leakage | 5.8% (n = 25) | 8.0% (n = 10) | .362 |
| Meningitis | 1.0% (n = 4) | 2.4% (n = 3) | .190 |
| Intraoperative bleeding | 1.2% (n = 5) | 2.4% (n = 3) | .302 |
| Postsurgical bleeding | 2.5% (n = 11) | 1.6% (n = 2) | .541 |
| AVP-D | 16.9% (n = 80) | 23.1% (n = 30) | .105 |
| Headache resolution | 71.0% (n = 120/169) | 81.5% (n = 53/65) | .100 |
| Visual involvement recovery | 44.1% (n = 26/59) | 45.2% (n = 14/31) | .921 |
| Hospital stay, days | 7.6 ± 7.32 | 7.9 ± 7.96 | .719 |
| Last follow-up assessment (after median of 8 y of surgical treatment) | |||
| Rate of surgical cure (2000) | 53.1% (n = 249) | 53.5% (n = 69) | .936 |
| Rate of surgical cure (2010) | 41.5% (n = 189) | 41.4% (n = 53) | .993 |
| Not controlled acromegaly with medical treatmenta | 15.0% (n = 33/220) | 23.3% (n = 14/60) | .126 |
| Last IGF-1 ULN | 062 (IQR 0.44-0.80) | 0.67 (IQR 0.49-0.82) | .137 |
| Basal GH level, ng/mL | 0.6 (IQR 0.2-1.68) | 0.68 (IQR 0.25-1.69) | .108 |
| Permanent diabetes insipidus | 2.4% (n = 10) | 7.3% (n = 9) | .011 |
| Complete hypopituitarism recovery | 37.0% (n = 27/73) | 43.5% (n = 20/46) | .481 |
| ACTH deficit recovery | 46.7% (n = 7/15) | 36.4% (n = 4/11) | .599 |
| TSH deficit recovery | 17.4% (n = 4/23) | 31.6% (n = 6/19) | .283 |
| FSH/LH deficit recovery | 35.4% (n = 23/65) | 41.9% (n = 18/43) | .497 |
| Tumor recurrence | 17.2% (n = 69) | 9.8% (n = 11) | .057 |
| Variable . | GH-PAs (n = 474) . | GH&PRL-PAs (n = 130) . | P . |
|---|---|---|---|
| Early follow-up assessment (after 6 mo of surgical treatment) | |||
| Rate of IGF-1 normalization | 43.3% (n = 199) | 41.1% (n = 53) | .659 |
| Hyperprolactinemia | 2.9% (n = 12) | 14.6% (n = 18) | <.001 |
| IGF-1, %ULN | 0.96 (IQR 0.71-1.57) | 1 (IQR 0.72-1.68) | .569 |
| Major complications | 13.5% (n = 64) | 16.9% (n = 22) | .323 |
| Cerebrospinal leakage | 5.8% (n = 25) | 8.0% (n = 10) | .362 |
| Meningitis | 1.0% (n = 4) | 2.4% (n = 3) | .190 |
| Intraoperative bleeding | 1.2% (n = 5) | 2.4% (n = 3) | .302 |
| Postsurgical bleeding | 2.5% (n = 11) | 1.6% (n = 2) | .541 |
| AVP-D | 16.9% (n = 80) | 23.1% (n = 30) | .105 |
| Headache resolution | 71.0% (n = 120/169) | 81.5% (n = 53/65) | .100 |
| Visual involvement recovery | 44.1% (n = 26/59) | 45.2% (n = 14/31) | .921 |
| Hospital stay, days | 7.6 ± 7.32 | 7.9 ± 7.96 | .719 |
| Last follow-up assessment (after median of 8 y of surgical treatment) | |||
| Rate of surgical cure (2000) | 53.1% (n = 249) | 53.5% (n = 69) | .936 |
| Rate of surgical cure (2010) | 41.5% (n = 189) | 41.4% (n = 53) | .993 |
| Not controlled acromegaly with medical treatmenta | 15.0% (n = 33/220) | 23.3% (n = 14/60) | .126 |
| Last IGF-1 ULN | 062 (IQR 0.44-0.80) | 0.67 (IQR 0.49-0.82) | .137 |
| Basal GH level, ng/mL | 0.6 (IQR 0.2-1.68) | 0.68 (IQR 0.25-1.69) | .108 |
| Permanent diabetes insipidus | 2.4% (n = 10) | 7.3% (n = 9) | .011 |
| Complete hypopituitarism recovery | 37.0% (n = 27/73) | 43.5% (n = 20/46) | .481 |
| ACTH deficit recovery | 46.7% (n = 7/15) | 36.4% (n = 4/11) | .599 |
| TSH deficit recovery | 17.4% (n = 4/23) | 31.6% (n = 6/19) | .283 |
| FSH/LH deficit recovery | 35.4% (n = 23/65) | 41.9% (n = 18/43) | .497 |
| Tumor recurrence | 17.2% (n = 69) | 9.8% (n = 11) | .057 |
Abbreviations: ACTH, adrenocorticotropin; AVP-D, arginine-vasopressin deficiency; FSH, follicle-stimulating hormone; GH, growth hormone; GH-PAs, growth hormone–secreting pituitary adenomas; GH&PRL-PAs, growth hormone– and prolactin-cosecreting pituitary adenomas; IGF-1, insulin-like growth factor-1; IQR, interquartile range; LH, luteinizing hormone; MRI, magnetic resonance imaging; PRL, prolactin; TSH, thyrotropin; ULN, upper limit of normal.
aIGF-1 above ULN despite medical treatment.
Differences in surgical outcomes between growth hormone–secreting pituitary adenomas and growth hormone– and prolactin-cosecreting pituitary adenomas
| Variable . | GH-PAs (n = 474) . | GH&PRL-PAs (n = 130) . | P . |
|---|---|---|---|
| Early follow-up assessment (after 6 mo of surgical treatment) | |||
| Rate of IGF-1 normalization | 43.3% (n = 199) | 41.1% (n = 53) | .659 |
| Hyperprolactinemia | 2.9% (n = 12) | 14.6% (n = 18) | <.001 |
| IGF-1, %ULN | 0.96 (IQR 0.71-1.57) | 1 (IQR 0.72-1.68) | .569 |
| Major complications | 13.5% (n = 64) | 16.9% (n = 22) | .323 |
| Cerebrospinal leakage | 5.8% (n = 25) | 8.0% (n = 10) | .362 |
| Meningitis | 1.0% (n = 4) | 2.4% (n = 3) | .190 |
| Intraoperative bleeding | 1.2% (n = 5) | 2.4% (n = 3) | .302 |
| Postsurgical bleeding | 2.5% (n = 11) | 1.6% (n = 2) | .541 |
| AVP-D | 16.9% (n = 80) | 23.1% (n = 30) | .105 |
| Headache resolution | 71.0% (n = 120/169) | 81.5% (n = 53/65) | .100 |
| Visual involvement recovery | 44.1% (n = 26/59) | 45.2% (n = 14/31) | .921 |
| Hospital stay, days | 7.6 ± 7.32 | 7.9 ± 7.96 | .719 |
| Last follow-up assessment (after median of 8 y of surgical treatment) | |||
| Rate of surgical cure (2000) | 53.1% (n = 249) | 53.5% (n = 69) | .936 |
| Rate of surgical cure (2010) | 41.5% (n = 189) | 41.4% (n = 53) | .993 |
| Not controlled acromegaly with medical treatmenta | 15.0% (n = 33/220) | 23.3% (n = 14/60) | .126 |
| Last IGF-1 ULN | 062 (IQR 0.44-0.80) | 0.67 (IQR 0.49-0.82) | .137 |
| Basal GH level, ng/mL | 0.6 (IQR 0.2-1.68) | 0.68 (IQR 0.25-1.69) | .108 |
| Permanent diabetes insipidus | 2.4% (n = 10) | 7.3% (n = 9) | .011 |
| Complete hypopituitarism recovery | 37.0% (n = 27/73) | 43.5% (n = 20/46) | .481 |
| ACTH deficit recovery | 46.7% (n = 7/15) | 36.4% (n = 4/11) | .599 |
| TSH deficit recovery | 17.4% (n = 4/23) | 31.6% (n = 6/19) | .283 |
| FSH/LH deficit recovery | 35.4% (n = 23/65) | 41.9% (n = 18/43) | .497 |
| Tumor recurrence | 17.2% (n = 69) | 9.8% (n = 11) | .057 |
| Variable . | GH-PAs (n = 474) . | GH&PRL-PAs (n = 130) . | P . |
|---|---|---|---|
| Early follow-up assessment (after 6 mo of surgical treatment) | |||
| Rate of IGF-1 normalization | 43.3% (n = 199) | 41.1% (n = 53) | .659 |
| Hyperprolactinemia | 2.9% (n = 12) | 14.6% (n = 18) | <.001 |
| IGF-1, %ULN | 0.96 (IQR 0.71-1.57) | 1 (IQR 0.72-1.68) | .569 |
| Major complications | 13.5% (n = 64) | 16.9% (n = 22) | .323 |
| Cerebrospinal leakage | 5.8% (n = 25) | 8.0% (n = 10) | .362 |
| Meningitis | 1.0% (n = 4) | 2.4% (n = 3) | .190 |
| Intraoperative bleeding | 1.2% (n = 5) | 2.4% (n = 3) | .302 |
| Postsurgical bleeding | 2.5% (n = 11) | 1.6% (n = 2) | .541 |
| AVP-D | 16.9% (n = 80) | 23.1% (n = 30) | .105 |
| Headache resolution | 71.0% (n = 120/169) | 81.5% (n = 53/65) | .100 |
| Visual involvement recovery | 44.1% (n = 26/59) | 45.2% (n = 14/31) | .921 |
| Hospital stay, days | 7.6 ± 7.32 | 7.9 ± 7.96 | .719 |
| Last follow-up assessment (after median of 8 y of surgical treatment) | |||
| Rate of surgical cure (2000) | 53.1% (n = 249) | 53.5% (n = 69) | .936 |
| Rate of surgical cure (2010) | 41.5% (n = 189) | 41.4% (n = 53) | .993 |
| Not controlled acromegaly with medical treatmenta | 15.0% (n = 33/220) | 23.3% (n = 14/60) | .126 |
| Last IGF-1 ULN | 062 (IQR 0.44-0.80) | 0.67 (IQR 0.49-0.82) | .137 |
| Basal GH level, ng/mL | 0.6 (IQR 0.2-1.68) | 0.68 (IQR 0.25-1.69) | .108 |
| Permanent diabetes insipidus | 2.4% (n = 10) | 7.3% (n = 9) | .011 |
| Complete hypopituitarism recovery | 37.0% (n = 27/73) | 43.5% (n = 20/46) | .481 |
| ACTH deficit recovery | 46.7% (n = 7/15) | 36.4% (n = 4/11) | .599 |
| TSH deficit recovery | 17.4% (n = 4/23) | 31.6% (n = 6/19) | .283 |
| FSH/LH deficit recovery | 35.4% (n = 23/65) | 41.9% (n = 18/43) | .497 |
| Tumor recurrence | 17.2% (n = 69) | 9.8% (n = 11) | .057 |
Abbreviations: ACTH, adrenocorticotropin; AVP-D, arginine-vasopressin deficiency; FSH, follicle-stimulating hormone; GH, growth hormone; GH-PAs, growth hormone–secreting pituitary adenomas; GH&PRL-PAs, growth hormone– and prolactin-cosecreting pituitary adenomas; IGF-1, insulin-like growth factor-1; IQR, interquartile range; LH, luteinizing hormone; MRI, magnetic resonance imaging; PRL, prolactin; TSH, thyrotropin; ULN, upper limit of normal.
aIGF-1 above ULN despite medical treatment.
When we compared high- and low-volume centers, no differences were detected in the rate of biochemical control (43.2% [n = 220/509] vs 40.0% [n = 32/80]; P = .588) between surgeries performed in high-volume centers and those performed in low-volume centers, but the incidence of major complications was lower in the former (13.0% vs 22.0%; P = .027).
At the last follow-up visit, after a mean follow-up time of 91 months (IQR 45-163), 53.2% of the patients achieved surgical remission based on the Cortina criteria and 41.4% based on the 2010 classification. We did not observe differences in surgical remission between GH-PA and GH&PRL-PA patients based on the 2000 and 2010 definitions (see Table 4). Nevertheless, in the group of patients who did not achieve surgical cure, there was a nonsignificant tendency toward a higher rate of uncontrolled acromegaly patients in the group of GH&PRL-PA patients (23.3% vs 15.0%; P = .126). The rate of pituitary function recovery was similar between both groups as well when the axes were evaluated separately (see Table 4).
Pathological Results
Pathological findings were slightly different between the 2 groups, including a trend toward a higher proportion of tumors with a high Ki-67 index (>3%) in the GH&PRL-PA patients (14.6% vs 8.5%; P = .076). The proportion of sparsely granulated tumors did not differ between both groups (57.5% vs 52.1%; P = .522). However, we found that patients with densely granulated tumors were hypointense on T2-MRI more commonly than sparsely granulated tumors (61.9% vs 38.1%; P = .001). On the other hand, sparsely granulated tumors tended to be larger than densely granulated (18.4 ± 9.43 vs 13.8 ± 8.09 mm; P < .001) and also tended to be more invasive (proportion of Knosp grade >2 of 33.6% vs 24.7%; P = .057). In the GH-PA group, a positive IHC for PRL was detected in 40.6% (n = 112/276) of cases. Nevertheless, most of these cases had focal positive immunostaining for PRL (65.3% of cases).
Information of transcription factors was available only for 80 cases, and we observed that PIT-1 was positive in the same proportion of GH&PRL-PA and GH-PA patients (69.2% vs 85.7%; P = .215), while ERα tended to be more common in GH&PRL-PA patients (26.7% vs 6.7%; P = .142).
Discussion
In our study, GH&PRL-PAs represented 21.5% of the cases. They occurred in younger patients with larger tumors and tended to have more invasive PAs than patients with GH-PAs. However, the IGF-1 levels were lower, without differences in the rate of surgical remission between both groups, but with greater postoperative involvement of the posterior axis in the GH&PRL-PA group.
The prevalence of GH&PRL-PAs varies across studies and depends on factors like the diagnostic criteria used for their definition. Nevertheless, in general cosecretion of PRL in acromegaly is present in about 25% to 30% of cases (4, 5). In accordance with these results, we found a rate of cosecreting tumors of 21.5% in the whole Spanish cohort. To the best of our knowledge, our series is the largest study that has evaluated the prevalence and the effect of PRL cosecretion on surgical outcomes in patients with acromegaly ever reported. In addition, a combined definition has been used for the classification of GH&PRL-PAs (serum PRL levels and PRL IHC), which is actually the recommended approach (2, 17). In this regard, it is important to mention that some previous studies considered PRL and GH staining as the most important point for classification, considering cosecreting tumors when only both stainings were positive, regardless of serum PRL levels (3-5). The prevalence described by these authors ranges between 25% and 40%. However, as we have observed in our series, up to 41% of the GH-PAs, with normal serum PRL levels, had a positive PRL staining. In addition, some of the studies published so far have found normal serum PRL levels in approximately 60% of the cases classified as cosecreting tumors based on the pathological results, while 20% to 30% of the tumors considered pure GH-secreting PAs had high serum PRL levels (3). On the other hand, other authors differentiate between cosecretion and no cosecretion based only on the presence or lack of hyperprolactinemia (1, 6, 7), describing rates of cosecretion of up to 40% (6). In addition, they have not proposed a cutoff value of serum PRL to differentiate between hyperprolactinemia secondary to pituitary stalk compression and primary PRL hypersecretion by the pituitary tumor. The most accepted threshold to exclude hyperprolactinemia associated with pituitary stalk compression or dopaminergic neuronal damage has been arbitrarily proposed to be 100 ng/dL (9), which was the one employed in our study. Considering these aspects, our recommendation is to differentiate the group of GH&PRL-PAs and GH-PAs based on both serum PRL levels and PRL IHC. Furthermore, ideally, the information about the expression of the different transcription factors should be taken into account following the recommendations of the latest World Health Organization pituitary tumor classification (17). In fact, it has been described that the clinical behavior of mixed somatotroph–lactotroph PAs is more aggressive than of the mammosomatotroph PAs group (4).
Our study supports that GH&PRL-PAs and GH-PAs have different clinical and hormonal behaviors, as the former tended to present at an earlier age, had presurgical hypopituitarism more frequently, and lower IGF-1 levels at diagnosis than patients with GH-PAs. Regarding the age of the acromegaly diagnosis in cosecreting tumors, our results are in accordance with the reported by Guo et al (6), who described a younger age at presentation in patients with acromegaly and hyperprolactinemia compared to those without hyperprolactinemia. In fact, mammosomatotroph tumors have been described as the most common PA in young patients with acromegaly and in cases of childhood-onset gigantism (18). The incidence of presurgical hypopituitarism in our study could be explained by the higher proportion of macroadenomas and invasive tumors in the GH&PRL-PA group; however, the higher risk persisted after adjusting for tumor size and Knosp grade. Thus, other factors should have contributed to the higher proportion of hormonal deficits in the cosecreting group. Among these factors, it is known that hyperprolactinemia leads to the loss of stimulatory gonadotropin-releasing hormone effect with consequent secondary hypogonadism (19). In addition, it has been described as an interlink between PRL and TSH-releasing factor secretion, so a potential influence of PRL levels on the hypothalamic-pituitary-thyroid axis might also be expected (20). The lower IGF-1 level in GH&PRL-PA patients may be associated with an earlier diagnosed due to the presence of hyperprolactinemia-induced symptoms, such as decreased libido and menstrual cycle alterations in combination with larger tumor size leading to more tumor mass effect symptoms. However, GH-PRL-PAs were larger, and a larger tumor size might be expected to be associated with higher IGF1 levels, and this was not the case in our study. Tumor size may be larger in this group of patients because some tumor cells are secreting PRL whereas others secrete GH. Supporting this theory, we observed that there was a positive correlation between tumor size and IGF-1 levels in patients with GH-PAs, but not with GH&PRL-PAs. In addition, we observed that the time of the symptoms of GH excess until the acromegaly diagnosed was also shorter in the GH&PRL-PA compared to the GH-PA group. In fact, some authors have described that GH-PA patients had significantly more coarse facial features, large hands and feet, hypertension, and diabetes mellitus compared with GH&PRL-PA patients, despite a smaller mean maximum diameter than cosecreting tumors (1). However, we are aware that studies analyzing the proportion of GH- and PRL-secreting cells will be necessary to prove our hypothesis.
The radiological presentation of cosecreting PRL and GH and pure GH-secreting PAs is also quite different since GH&PRL-PAs were more frequently macroadenomas and tended to be invasive more commonly than GH-PAs. Accordingly, several previous studies described that GH tumors causing hyperprolactinemia were larger than those without PRL increase (1, 4, 6). For example, in the Wang series (1), the mean maximal diameter of the adenomas was higher in the GH&PRL-PA group than in the group of pure GH tumors (2.6 ± 1.1 vs 2.2 ± 0.9 cm; P = .004). As a higher tumor size was observed in the cosecreting group, a higher rate of invasive PAs was also expected in this group. Supporting the higher invasiveness of the GH&PRL group, we found that the proportion of these tumors increased as the Knosp grade increased, reaching a prevalence of 38% in Knosp-4 PAs. The proportion of invasive PAs was also higher in patients with GH&PRL-secreting tumors than in GH-PAs in the Lv series (4). All these data together, in combination with the fact that GH&PRL-secreting tumors tended to have an elevated proliferative index more commonly than GH-PAs, indicate that these tumors are generally more aggressive than pure GH tumors.
Another important characteristic of patients with GH&PRL-PAs when compared to GH-PAs was that they were hypointense on T2-MRI sequence less frequently. In addition, although we were not able to demonstrate differences in the proportion of densely granulated tumors between both groups, we found that the proportion of densely granulated tumors was significantly higher in hypointense tumors compared with nonhypointense (61.9% vs 35%). Data about T2 intensity are important in clinical practice since it has been observed that it may be a predictive factor of response to fg-SRL in patients who were not cured after surgery (21). In this regard, it has been described that patients with hypointense tumors were more likely to have a complete response than patients with hyperintense tumors (71% vs 20%; P = .004) (22). The higher rate of responders to fg-SRL in these hypointense tumors has been attributed to a higher proportion of densely granulated tumors, and thus also a higher expression of the somatostatin receptor 2 (23). Considering these findings, it may be expected that patients with GH&PRL-PAs had a worse response to SRL than those with GH-PAs. In fact, the Rick et al study (5) reported that patients with tumors with GH- and PRL-positive staining were more frequently on the maximum dosage of pegvisomant (30.0% vs 3.6%; P = .04) and lanreotide (20.0% vs 0.0%; P = .03) than patients with only GH-positive staining tumors. However, in this study only 91 patients who had acromegaly were included, 22 of whom had positive dual staining. Thus, further studies including a large number of cases and focused on the evaluation of response to the different available medical therapies for acromegaly will be necessary to confirm our hypothesis.
However, despite the fact that it is well known that cavernous sinus invasion is the most important limiting factor to achieve complete tumor resection in acromegaly (24), we did not find differences in the immediate postsurgical and long-term biochemical cure rate between groups in terms of IGF-1 control. Nonetheless, the rate of postsurgical hyperprolactinemia was 5 times greater in the group of cosecreting tumors, although in those cases in which persistence of hyperprolactinemia was present, a very important decrease of PRL was observed indicating a consistent debulking effect. Thus, this fact may be an indicator of tumor persistence in GH&PRL-secreting tumors. In this regard, the current consensus and guidelines do not propose specific biochemical remission criteria for GH&PRL-secreting tumors, considering that biochemical control is reached, even in these tumors, when normal IGF-1 values along with baseline GH levels less than 1.0 mcg/dL are achieved (10, 11). However, we should take into account that for GH&PRL-secreting tumors, both the normalization of GH and PRL levels are important goals of the treatment. The rate of IGF-1 control in the Wang et al study (1), which included 279 acromegalic patients undergoing transsphenoidal surgery, was similar in GH- and GH&PRL-PAs (68.4 vs 59.7%; P = .187). On the other hand, a worse long-term remission rate in GH&PRL-PA patients has been described by other research teams (4, 5). For example, the Rick study (5) described a lower rate of acromegaly surgical remission in tumors with positive GH and PRL staining compared to only GH-positive tumors (20% vs 68%; P = .01). Differences across these studies are probably explained by the different tumor characteristics at acromegaly diagnosis between GH&PRL tumors and GH tumors, since while some of them described GH&PRL tumors as aggressive tumors (4, 5), other series reported GH-PAs as worse tumors (7).
In our study, there was a higher incidence of permanent AVP-D in the GH&PRL-PA group. To the best of our knowledge, no previous studies have compared the incidence of postoperative AVP-D between patients with GH&PRL- and with GH-PAs. In relation to other complications, we did not find differences between groups. This is in line with that described in the Rick et al series (5), where there was no significant variation in cerebrospinal fluid leaks, need for lumbar drains, or achieved extent of resection between groups (5). The higher rate of AVP-D in our series is probably explained by a more aggressive pituitary resection since tumor size and invasiveness was higher in the cosecreting group, together with the younger age of the patients in the GH&PRL group. We have also previously described that a younger age may be a predictive factor of AVP-D development (15); however, on the other hand, younger patients seem to show better neurohypophyseal recovery, and most cases of AVP-D in young patients were usually transient.
A major limitation of our study is its retrospective nature. However, patients with missing values in the main variables of interest were not included in the study and a strict protocol was followed for the data inclusion and patient selection. We are also aware that although the multicentric design provides strength in terms of external validity, the fact that IHC and imaging analysis were performed by different techniques and evaluated by different observers is another limitation to consider. One of the most important strengths of our study in comparison with previous series is that the definitions employed for differentiating between GH&PRL-PAs and GH-PAs were based on serum PRL levels and PRL and GH staining. In addition, a robust threshold in serum PRL (100 ng/dL) was used for the diagnosis of cosecreting tumors when PRL staining was not available. Nevertheless, an even higher threshold is proposed by the last Pituitary Society International Consensus Statement, suggesting that the cutoff of 200 ng/mL is the most reliable for the diagnosis of endogenous hyperprolactinemia (25). However, at the time of the design of our study this consensus had not yet been published. Another strength of this study is the large number of patients included, being the largest multicenter study of acromegaly series reported to date focused on the role of PRL cosecretion in acromegaly.
Conclusion
GH&PRL-PAs are responsible for 20% of acromegaly cases. These tumors are more invasive, larger, and cause hypopituitarism more frequently than GH-PAs. The biochemical cure rate is similar between both groups, but patients with GH&PRL-PAs tend to present with postsurgical AVP-D more frequently.
Funding
This work was supported by the Foundation of the Spanish Society of Endocirnology and Nutrition (“Sociedad Española de Endocrinología y Nutrición”) (FSEEN): “Impacto de la co-secreción de prolactina en la expresión de marcadores moleculares y en la respuesta al tratamiento con análogos de somatostatina y agonistas dopaminérgicos en pacientes con acromegalia.”
Disclosures
The authors declare no conflict of interest.
Data Availability
The data that support the findings of this study are available on request from the corresponding authors.
References
Abbreviations
- ACTH
adrenocorticotropin
- AVP-D
arginine-vasopressin deficiency
- fg-SRL
first-generation somatostatin receptor ligand
- FSH
follicle-stimulating hormone
- GH
growth hormone
- GH-PAs
growth hormone–secreting pituitary adenomas
- GH&PRL-PAs
growth hormone– and prolactin-cosecreting pituitary adenomas
- IGF-1
insulin-like growth factor-1
- IHC
immunohistochemistry
- IQR
interquartile range
- LH
luteinizing hormone
- MRI
magnetic resonance imaging
- PRL
prolactin
- PTCOE
Pituitary Tumor Center of Excellence
- SEEN
Spanish Society of Endocrinology and Nutrition
- TSH
thyrotropin
- ULN
upper limit of normal



