-
PDF
- Split View
-
Views
-
Cite
Cite
Manon Rosimont, Dulanjalee Kariyawasam, Dinane Samara-Boustani, Elisa Giani, Jacques Beltrand, Stephanie Bolle, Brice Fresneau, Stephanie Puget, Christian Sainte-Rose, Claire Alapetite, Graziella Pinto, Philippe Touraine, Marie-Liesse Piketty, Séverine Brabant, Samuel Abbou, Isabelle Aerts, Kevin Beccaria, Marie Bourgeois, Thomas Roujeau, Thomas Blauwblomme, Federico Di Rocco, Caroline Thalassinos, Charlotte Rigaud, Syril James, Kanetee Busiah, Albane Simon, Franck Bourdeaut, Lauriane Lemelle, Léa Guerrini-Rousseau, Daniel Orbach, François Doz, Christelle Dufour, Jacques Grill, Michel Polak, Laura González Briceño, Assessment of Puberty and Hypothalamic–Pituitary–Gonadal Axis Function After Childhood Brain Tumor Treatment, The Journal of Clinical Endocrinology & Metabolism, Volume 108, Issue 9, September 2023, Pages e823–e831, https://doi.org/10.1210/clinem/dgad097
Close - Share Icon Share
Abstract
Endocrine complications are common in pediatric brain tumor patients.
To describe hypothalamic–pituitary–gonadal axis (HPGA) function in patients treated in childhood for a primary brain tumor more than 5 years earlier, in order to identify risk factors for HPGA impairment.
We retrospectively included 204 patients diagnosed with a primary brain tumor before 18 years of age and monitored at the pediatric endocrinology unit of the Necker Enfants-Malades University Hospital (Paris, France) between January 2010 and December 2015. Patients with pituitary adenoma or untreated glioma were excluded.
Among patients with suprasellar glioma not treated by radiotherapy, the prevalence of advanced puberty was 65% overall and 70% when the diagnosis occurred before 5 years of age. Medulloblastoma chemotherapy caused gonadal toxicity in 70% of all patients and in 87.5% of those younger than 5 years at diagnosis. In the group with craniopharyngioma, 70% of patients had hypogonadotropic hypogonadism, which was consistently accompanied by growth hormone deficiency.
Tumor type, location, and treatment were the risk main factors for HPGA impairment. Awareness that onset can be delayed is essential to guide information of parents and patients, patient monitoring, and timely hormone replacement therapy.
Primary malignant central nervous system tumors are the most common solid-organ tumors in pediatric patients (1–3). Their histology governs treatment decisions and predicts the 5-year survival rate. Impairments in hypothalamic–pituitary–gonadal axis (HPGA) function with alterations in puberty and fertility are major threats to quality of life in survivors (4–6). Optimal long-term follow-up with corrective hormonal treatment as appropriate is therefore essential.
Many studies have sought to identify risk factors for HPGA dysfunction after central nervous system tumor treatment in children. Risk factors for precocious puberty include tumor location within or near the hypothalamic–pituitary region and treatment with cranial radiotherapy in a dose of 18 to 50 Gy (7). Gonadal toxicity is a well-established side effect of several chemotherapy agents including busulfan, melphalan, thiotepa, platinum-based drugs, and cyclophosphamide. The possible roles for chemotherapy dose, tumor type, and tumor location remain unclear. The links between adverse effects and a given drug are often difficult to confirm, since several drugs are generally given over time.
Available studies focused chiefly on the long-term endocrine side effects of specific treatments used for various pediatric malignancies or for brain tumors considered as a whole (3, 8–11). None separated brain tumors by histological type. Moreover, the timing of puberty according to tumor type has received little attention. Knowledge of the specific side effects seen with each tumor type and location would help pediatric and adult endocrinologists optimize follow-up care (12).
The aims of this study were to assess HPGA function and puberty timing in patients treated for primary brain tumors at least 5 years earlier and to identify risk factors for specific HPGA impairments. Knowledge of these factors should allow risk stratification of patients in order to optimize the timing and nature of fertility preservation strategies.
Materials and Methods
Study Design and Participants
We conducted a single-center, retrospective, observational cohort study after obtaining approval from our institutional review board. Inclusion criteria were a diagnosis of primary brain tumor before 18 years of age and follow-up provided by the pediatric endocrinology department of the Necker-Enfants Malades university hospital (Paris, France) between 1 January 2010 and 31 December 2015. Follow-up involved visits at intervals of 6 to 12 months for clinical evaluations, combined with basal hormone assays performed once a year. Exclusion criteria were insufficient data, pituitary adenoma, untreated glioma, and refusal to participate in the study.
Data Collection
Standardized forms were used to abstract data from the medical records of each patient. The features of the tumor and treatment, together with data on pubertal chronology, were collected from the first visit until June 15, 2020. At the first visit, the following were collected for each patient: height, weight, and adult height recorded using French growth charts; Tanner pubertal stage according to pubic and axillary hair growth and to breast development or testis volume and penis length. A basal fasting blood sample was drawn at 8:00 Am for hormone assays. If indicated by the clinical findings and basal hormone levels, dynamic tests were performed. Insulin-like growth factor 1 (IGF-1) levels were measured using the Cis Bio International IRMA assay before August 2013 and the IDS-iSYS IGF-1 assay thereafter (Cat# IS3900, RRID:AB_2861357). The results were compared with reference IGF-1 values for age and pubertal status. Growth hormone (GH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), free thyroxine, thyrotropin, and cortisol were measured using Beckman Coulter Access assays and testosterone levels using the Cis Bio International Testo-RIA-CT assay (respectively, Cat#33580, RRID:AB_2756876/cat#33520, RRID:AB_2750983/cat#33510, RRID:AB_2750984/cat#33880 and B63284/cat#33600, RRID:AB_2802133/cat# MG12191). Antimullerian hormone (AMH) was assayed using the AMH GEN II kit assay from Beckman Coulter (Cat#A79765, RRID:AB_2800500) until 2015, and Beckman Coulter Access 2 thereafter (Cat#B13127, RRID:AB_2892998). Finally, inhibin B was measured using Inhibin B GEN II kit assay from Beckman Coulter (Cat#A81303, RRID:AB_2827405).
Definitions
Growth hormone deficiency (GHD) was defined as a growth hormone peak of less than 20 mIU/L during 2 stimulation tests, or during a single test done after a radiotherapy dose to the pituitary area of at least 18 Gy (7). Stimulation was with glucagon or arginine–insulin. The optimal frequency of GH testing in children at high risk for GH deficiency, due for instance to a pituitary tumor or to pituitary radiotherapy >18 Gy, is unclear (13). In our patients, the diagnosis of GHD was guided by clinical findings, the treatments used, and a single GH stimulation test in case of pituitary radiotherapy ≥18 Gy (since normal IGF-1 levels do not rule out GHD in patients with brain tumors). The clinical criteria for GH testing were a growth velocity decrease of at least 0.5 SD, absence of growth acceleration despite rapid weight gain, or diagnosis of other pituitary deficiencies.
Precocious puberty was diagnosed when pubertal signs (breast development in girls, testicular enlargement, or detectable levels of testosterone >0.07 ng/mL in boys) developed before 8 years of age in girls and 9 in boys. Early puberty was defined as the same signs developing between 8 and 9 years of age in girls and between 9 and 11 years in boys. We classified patients with precocious and early puberty into the same group, designated “advanced puberty,” for 2 reasons: early puberty can progress rapidly in patients with brain tumors, and the diagnosis of precocious puberty can be delayed due to the frequent blunting of the pubertal growth spurt in the event of GHD or spinal radiotherapy and to the challenges in assessing testis volume as the first pubertal sign in boys with chemotherapy-induced gonadal toxicity. Moreover, the management is the same in precocious puberty and early puberty, with gonadotropin-releasing hormone analog therapy improving statural growth when the predicted adult height is markedly decreased (14).
Absence of puberty was defined as absence of breast development or testis enlargement by 13 years of age in girls and 14 in boys, or as lack of appropriate pubertal progression with more than 4 years elapsing between the first pubertal signs and menarche in girls or testis volume >15 mL in boys. Patients with no pubertal development at all and those with stalled puberty were classified in the same group, designated “absence of puberty”.
Hypogonadotropic hypogonadism (HypoH) was defined as absence of puberty requiring steroid therapy in patients without the gonadal toxicity criteria described below. All patients were followed by pediatric endocrinologists, and none of the boys diagnosed with HypoH exhibited sufficient testicular growth to allow testosterone therapy withdrawal. Given the high likelihood of HypoH (as opposed to pubertal delay) in boys without testicular growth progression, testosterone replacement therapy was continued until statural growth and pubertal development were complete. At adulthood, all patients were transferred to an adult department, where the tests for HPGA were repeated at a distance from testosterone treatment.
Gonadal toxicity was defined as basal LH or FSH levels above the upper limit of the reference range and/or as low AMH levels in girls or low inhibin B levels in boys.
Assessing Positive Predictive Values
The positive predictive value (PPV) reflects the probability of the condition being present when the test is positive. PPV determination is relevant only for tests classified as either positive or negative. In our study, tumor type was likened to a test for detecting HPGA dysfunction, to allow an assessment of potential associations between the 2.
To assess the risk of advanced puberty, we considered only girls younger than 9 years and boys younger than 11 years at tumor diagnosis. Patients who had not yet reached the cut-off age for diagnosing delayed puberty (13 years for girls and 14 for boys) were excluded from the HypoH and normal puberty subgroups but were kept in the study for the hypergonadotropic hypogonadism subgroup.
Patient Classification According to Treatment
Radiotherapy, when used, was delivered to the cranium only or to the cranium and spine and consisted in either conventional radiotherapy or proton therapy. No patient received pelvic radiotherapy. To assess risks associated with radiotherapy, we compared the groups with vs without radiotherapy. The group with radiotherapy was divided into 7 subgroups based on the dose delivered (<10 Gy, 11-20 Gy, 21-30 Gy, 31-40 Gy, 41-50 Gy, 51-60 Gy, and >60 Gy).
Chemotherapy was classified as generating a high, moderate, or low risk of gonadal toxicity (15). The risk was defined as low in patients given only low-risk drugs (VP16, antimetabolites, bleomycin, vincristine, and vinblastine). Moderate risk was defined as having received a single moderate-risk chemotherapy drug (a low-dose alkylating agent such as cyclophosphamide, ifosfamide, or procarbazine; an anthracycline such as daunorubicin, doxorubicin, or mitoxantrone; lomustine; or carmustine). The risk was classified as high in patients given a high-risk drug (busulfan, melphalan, thiotepa, cisplatin, carboplatin) or more than 1 moderate-risk drug.
Patient Classification According to Tumor Type
We classified patients into 5 groups based on whether they had craniopharyngioma, glioma, medulloblastoma, dysgerminoma, or other tumor types (ependymoma, pineal tumor, or chordoma). To compare patients according to pituitary involvement, we divided the patients into 2 groups, 1 with suprasellar tumors (SSTs) involving the sellar and/or suprasellar region, hypothalamus, or optic pathways; and the other with nonsuprasellar tumors (NSSTs) involving only other sites. The SSTs included craniopharyngiomas, hypothalamic and optic-tract gliomas, germ cell tumors, and chordomas and the NSSTs were medulloblastomas, other gliomas, ependymomas, rhabdoid tumors, pineal tumors including germ cell tumors, and other tumor types. We hypothesized that pituitary dysfunction would be seen in the SST group, possibly as early as at diagnosis, and that NSSTs would be associated with delayed treatment-related endocrine disorders.
Statistical Analysis
All statistical analyses were performed using STATA software version 13.0 (StataCorp, College Station, TX). Median or mean ± SD were computed for each variable. The data were assessed using normality and equal variance tests, and parametric tests were used for comparisons when the underlying assumptions were met. Multiple logistic regression analysis was performed to identify factors associated with normal puberty. Values of P no greater than .05 were taken to indicate significant differences.
Results
Population and Endocrine Disorders
Of 239 initially identified patients, 35 were excluded due to incomplete data, death, refusal to participate, or untreated glioma. Table 1 reports the main features in the 204 included patients. Mean age was 6.5 years (range 0-15.1 years) in the 102 girls and 7.7 years (range 0.1-15.9) in the 102 boys. Mean follow-up after treatment completion was 8.4 years in girls and 10 years in boys. Of the 204 patients, 149 were younger than 9 or 11 years (girls or boys, respectively) at tumor diagnosis and were included in the assessment of the risk of advanced puberty; 187 patients reached the cut-off age for diagnosing delayed puberty.
Main features in the 186 patients with glioma, medulloblastoma, craniopharyngioma, or dysgerminoma
| . | Glioma n = 44 . | Medulloblastoma n = 71 . | Craniopharyngioma n = 63 . | Dysgerminoma n = 8 . | |
|---|---|---|---|---|---|
| Location, n (%) | SST: 34 (77%) | NSST: 10 (23%) | NSST: 71 (100%) | SST: 63 (100%) | SST: 7 (87.5%) |
| Females/Males | 25/9 | 5/5 | 31/40 | 28/35 | 4/4 |
| Surgery | 24 | 10 | 69 | 62 | 1 |
| Chemotherapy | 29 | 9 | 53 | 1 | 8 |
| Radiotherapy | 8 | 7 | 70 | 45 | 8 |
| Median age at diagnosis (5th-95th) | 3.7 (0.3-11.4) | 7.5 (1.5-14) | 6.8 (3.2-14.6) | 11 (8.1-14.6) | |
| Normal puberty (NP) | |||||
| NP, n/N (%) | 9/40 (22.5) | 28/68 (41.2) | 10/56 (17.8) | 1/7 (14.2) | |
| Females/Males | 2/2 | 3/2 | 10/18 | 5/5 | 1 |
| Median age at B2 (5th-95th) | 10.0 (9.8-10.0) | 9.6 (8.2-12.5) | 11.5 (9.7-12.3) | 11.4 (9.8-12.4) | 10.6 |
| Median age at G2 (5th-95th) | 12.6 (12.0-13.0) | 13.4 (13.0-13.8) | 12.3 (11.1-15.4) | 11.7 (10.9-14.4) | |
| Advanced puberty | |||||
| Advanced puberty, n/N (%) | 19/41 (46.3) | 1/52 (1.9) | 4/42 (9.5) | 1/3 (33.3) | |
| Females/Males | 14/4 | 1/0 | 1/0 | 3/1 | 0/1 |
| Median age at B2 (5th-95th) | 7.0 (4.7-8.7) | 8.0 | 7.7 | 7.5 (6.5-8.6) | 11.1 |
| Median age at G2 (5th-95th) | 8.5 (6.4-9.8) | 11.0 | |||
| Gonadal toxicity | |||||
| Gonadal toxicity, n/N (%) | 7/44 (15.9) | 37/71 (52.1) | 4/63 (6.3) | 2/8 (25.0) | |
| Females/Males | 5/0 | 0/2 | 18/19 | 0/4 | 1/1 |
| Median age at B2 (5th-95th) | 11.5 (4.7-11.9) | 12.0 | 12.5 (8.2-16.7) | 15.1 | 13.1 |
| Median age at G2 (5th-95th) | 12.6 (10.7-14.6) | (15-15.1) | 12.5 | ||
| Hypogonadotropic hypogonadism | |||||
| Hypogonadotropic hypogonadism, n/N (%) | 8/40 (20.0) | 2/62 (2.9) | 38/56 (69.6) | 2/7 (28.4%) | |
| Females/Males | 4/3 | 0/1 | 0/2 | 14/24 | 1/1 |
| Median age at B2 (5th-95th) | 9.8 (9.7-11.9) | 13.3 (9.2-17.0) | 17.0 | ||
| Median age at G2 (5th-95th) | 12.9 (7.5-13.9) | 14.5 | 12.3 (10.0-14.5) | 14.3 (11.7-17.0) | 12.0 |
| . | Glioma n = 44 . | Medulloblastoma n = 71 . | Craniopharyngioma n = 63 . | Dysgerminoma n = 8 . | |
|---|---|---|---|---|---|
| Location, n (%) | SST: 34 (77%) | NSST: 10 (23%) | NSST: 71 (100%) | SST: 63 (100%) | SST: 7 (87.5%) |
| Females/Males | 25/9 | 5/5 | 31/40 | 28/35 | 4/4 |
| Surgery | 24 | 10 | 69 | 62 | 1 |
| Chemotherapy | 29 | 9 | 53 | 1 | 8 |
| Radiotherapy | 8 | 7 | 70 | 45 | 8 |
| Median age at diagnosis (5th-95th) | 3.7 (0.3-11.4) | 7.5 (1.5-14) | 6.8 (3.2-14.6) | 11 (8.1-14.6) | |
| Normal puberty (NP) | |||||
| NP, n/N (%) | 9/40 (22.5) | 28/68 (41.2) | 10/56 (17.8) | 1/7 (14.2) | |
| Females/Males | 2/2 | 3/2 | 10/18 | 5/5 | 1 |
| Median age at B2 (5th-95th) | 10.0 (9.8-10.0) | 9.6 (8.2-12.5) | 11.5 (9.7-12.3) | 11.4 (9.8-12.4) | 10.6 |
| Median age at G2 (5th-95th) | 12.6 (12.0-13.0) | 13.4 (13.0-13.8) | 12.3 (11.1-15.4) | 11.7 (10.9-14.4) | |
| Advanced puberty | |||||
| Advanced puberty, n/N (%) | 19/41 (46.3) | 1/52 (1.9) | 4/42 (9.5) | 1/3 (33.3) | |
| Females/Males | 14/4 | 1/0 | 1/0 | 3/1 | 0/1 |
| Median age at B2 (5th-95th) | 7.0 (4.7-8.7) | 8.0 | 7.7 | 7.5 (6.5-8.6) | 11.1 |
| Median age at G2 (5th-95th) | 8.5 (6.4-9.8) | 11.0 | |||
| Gonadal toxicity | |||||
| Gonadal toxicity, n/N (%) | 7/44 (15.9) | 37/71 (52.1) | 4/63 (6.3) | 2/8 (25.0) | |
| Females/Males | 5/0 | 0/2 | 18/19 | 0/4 | 1/1 |
| Median age at B2 (5th-95th) | 11.5 (4.7-11.9) | 12.0 | 12.5 (8.2-16.7) | 15.1 | 13.1 |
| Median age at G2 (5th-95th) | 12.6 (10.7-14.6) | (15-15.1) | 12.5 | ||
| Hypogonadotropic hypogonadism | |||||
| Hypogonadotropic hypogonadism, n/N (%) | 8/40 (20.0) | 2/62 (2.9) | 38/56 (69.6) | 2/7 (28.4%) | |
| Females/Males | 4/3 | 0/1 | 0/2 | 14/24 | 1/1 |
| Median age at B2 (5th-95th) | 9.8 (9.7-11.9) | 13.3 (9.2-17.0) | 17.0 | ||
| Median age at G2 (5th-95th) | 12.9 (7.5-13.9) | 14.5 | 12.3 (10.0-14.5) | 14.3 (11.7-17.0) | 12.0 |
Eighteen patients had other tumor types.
Main features in the 186 patients with glioma, medulloblastoma, craniopharyngioma, or dysgerminoma
| . | Glioma n = 44 . | Medulloblastoma n = 71 . | Craniopharyngioma n = 63 . | Dysgerminoma n = 8 . | |
|---|---|---|---|---|---|
| Location, n (%) | SST: 34 (77%) | NSST: 10 (23%) | NSST: 71 (100%) | SST: 63 (100%) | SST: 7 (87.5%) |
| Females/Males | 25/9 | 5/5 | 31/40 | 28/35 | 4/4 |
| Surgery | 24 | 10 | 69 | 62 | 1 |
| Chemotherapy | 29 | 9 | 53 | 1 | 8 |
| Radiotherapy | 8 | 7 | 70 | 45 | 8 |
| Median age at diagnosis (5th-95th) | 3.7 (0.3-11.4) | 7.5 (1.5-14) | 6.8 (3.2-14.6) | 11 (8.1-14.6) | |
| Normal puberty (NP) | |||||
| NP, n/N (%) | 9/40 (22.5) | 28/68 (41.2) | 10/56 (17.8) | 1/7 (14.2) | |
| Females/Males | 2/2 | 3/2 | 10/18 | 5/5 | 1 |
| Median age at B2 (5th-95th) | 10.0 (9.8-10.0) | 9.6 (8.2-12.5) | 11.5 (9.7-12.3) | 11.4 (9.8-12.4) | 10.6 |
| Median age at G2 (5th-95th) | 12.6 (12.0-13.0) | 13.4 (13.0-13.8) | 12.3 (11.1-15.4) | 11.7 (10.9-14.4) | |
| Advanced puberty | |||||
| Advanced puberty, n/N (%) | 19/41 (46.3) | 1/52 (1.9) | 4/42 (9.5) | 1/3 (33.3) | |
| Females/Males | 14/4 | 1/0 | 1/0 | 3/1 | 0/1 |
| Median age at B2 (5th-95th) | 7.0 (4.7-8.7) | 8.0 | 7.7 | 7.5 (6.5-8.6) | 11.1 |
| Median age at G2 (5th-95th) | 8.5 (6.4-9.8) | 11.0 | |||
| Gonadal toxicity | |||||
| Gonadal toxicity, n/N (%) | 7/44 (15.9) | 37/71 (52.1) | 4/63 (6.3) | 2/8 (25.0) | |
| Females/Males | 5/0 | 0/2 | 18/19 | 0/4 | 1/1 |
| Median age at B2 (5th-95th) | 11.5 (4.7-11.9) | 12.0 | 12.5 (8.2-16.7) | 15.1 | 13.1 |
| Median age at G2 (5th-95th) | 12.6 (10.7-14.6) | (15-15.1) | 12.5 | ||
| Hypogonadotropic hypogonadism | |||||
| Hypogonadotropic hypogonadism, n/N (%) | 8/40 (20.0) | 2/62 (2.9) | 38/56 (69.6) | 2/7 (28.4%) | |
| Females/Males | 4/3 | 0/1 | 0/2 | 14/24 | 1/1 |
| Median age at B2 (5th-95th) | 9.8 (9.7-11.9) | 13.3 (9.2-17.0) | 17.0 | ||
| Median age at G2 (5th-95th) | 12.9 (7.5-13.9) | 14.5 | 12.3 (10.0-14.5) | 14.3 (11.7-17.0) | 12.0 |
| . | Glioma n = 44 . | Medulloblastoma n = 71 . | Craniopharyngioma n = 63 . | Dysgerminoma n = 8 . | |
|---|---|---|---|---|---|
| Location, n (%) | SST: 34 (77%) | NSST: 10 (23%) | NSST: 71 (100%) | SST: 63 (100%) | SST: 7 (87.5%) |
| Females/Males | 25/9 | 5/5 | 31/40 | 28/35 | 4/4 |
| Surgery | 24 | 10 | 69 | 62 | 1 |
| Chemotherapy | 29 | 9 | 53 | 1 | 8 |
| Radiotherapy | 8 | 7 | 70 | 45 | 8 |
| Median age at diagnosis (5th-95th) | 3.7 (0.3-11.4) | 7.5 (1.5-14) | 6.8 (3.2-14.6) | 11 (8.1-14.6) | |
| Normal puberty (NP) | |||||
| NP, n/N (%) | 9/40 (22.5) | 28/68 (41.2) | 10/56 (17.8) | 1/7 (14.2) | |
| Females/Males | 2/2 | 3/2 | 10/18 | 5/5 | 1 |
| Median age at B2 (5th-95th) | 10.0 (9.8-10.0) | 9.6 (8.2-12.5) | 11.5 (9.7-12.3) | 11.4 (9.8-12.4) | 10.6 |
| Median age at G2 (5th-95th) | 12.6 (12.0-13.0) | 13.4 (13.0-13.8) | 12.3 (11.1-15.4) | 11.7 (10.9-14.4) | |
| Advanced puberty | |||||
| Advanced puberty, n/N (%) | 19/41 (46.3) | 1/52 (1.9) | 4/42 (9.5) | 1/3 (33.3) | |
| Females/Males | 14/4 | 1/0 | 1/0 | 3/1 | 0/1 |
| Median age at B2 (5th-95th) | 7.0 (4.7-8.7) | 8.0 | 7.7 | 7.5 (6.5-8.6) | 11.1 |
| Median age at G2 (5th-95th) | 8.5 (6.4-9.8) | 11.0 | |||
| Gonadal toxicity | |||||
| Gonadal toxicity, n/N (%) | 7/44 (15.9) | 37/71 (52.1) | 4/63 (6.3) | 2/8 (25.0) | |
| Females/Males | 5/0 | 0/2 | 18/19 | 0/4 | 1/1 |
| Median age at B2 (5th-95th) | 11.5 (4.7-11.9) | 12.0 | 12.5 (8.2-16.7) | 15.1 | 13.1 |
| Median age at G2 (5th-95th) | 12.6 (10.7-14.6) | (15-15.1) | 12.5 | ||
| Hypogonadotropic hypogonadism | |||||
| Hypogonadotropic hypogonadism, n/N (%) | 8/40 (20.0) | 2/62 (2.9) | 38/56 (69.6) | 2/7 (28.4%) | |
| Females/Males | 4/3 | 0/1 | 0/2 | 14/24 | 1/1 |
| Median age at B2 (5th-95th) | 9.8 (9.7-11.9) | 13.3 (9.2-17.0) | 17.0 | ||
| Median age at G2 (5th-95th) | 12.9 (7.5-13.9) | 14.5 | 12.3 (10.0-14.5) | 14.3 (11.7-17.0) | 12.0 |
Eighteen patients had other tumor types.
The proportion of patients with normal puberty varied considerably across tumor types, from 41.2% in medulloblastoma to 22.5% in glioma and 17.8% in craniopharyngioma. Advanced puberty was most common in glioma (46.3%). HypoH developed in 69.6% of patients with craniopharyngioma but only 20.0% of those with glioma. Gonadal toxicity was found in 52.1% of patients with medulloblastoma compared with only 15.9% of those with glioma and 6.3% of those with craniopharyngioma (Fig. 1). HPGA dysfunction was demonstrated in similar proportions of girls and boys.
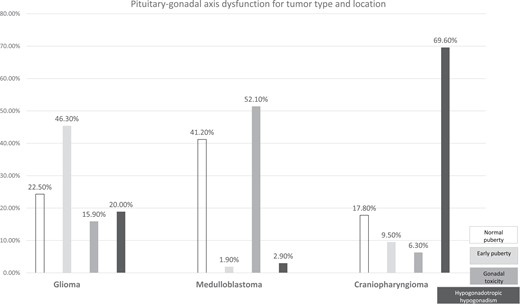
Pituitary–gonadal axis dysfunction for tumor type and location. Incidence rate of each type of pubertal progression according to tumor type.
Predictors of HPGA Dysfunction
Association between HPGA dysfunction and tumor type
Tumor type was significantly associated with the development of HPGA dysfunction (P = .0001). Advanced puberty was significantly more common in the group with glioma (P = .030), HypoH in the group with craniopharyngioma (P = .001), and gonadal toxicity in the group with medulloblastoma (P = .03). The small number of patients with dysgerminoma (n = 8) did not allow a statistical analysis (Table 1).
We then sought to determine whether the associations of HPGA dysfunction with tumor type were mediated by differences across tumor types regarding tumor location and treatments used.
Association between HPGA dysfunction and tumor location
Figure 1 reports the frequency and patterns of HPGA seen with the different tumor types and locations. The prevalence of HPGA dysfunction differed significantly between the SST and NSST groups, with HypoH being more common in the SST group (PPV, 63.1% vs 1.3% in the NSST group, P = .001) and gonadal toxicity in the NSST group (29.6% vs 2.8% in the SST group, P = .001). Advanced puberty was slightly more common in the SST group (PPV, 16.0% vs 12.2% in the NSST group) but the difference was not statistically significant (P = .250). Advanced puberty was also more common in suprasellar gliomas than in nonsuprasellar gliomas, indicating that location rather than type was the relevant factor. In the subgroup of 26 patients who had suprasellar gliomas not treated with radiotherapy, the proportions of patients with advanced puberty were similar for optic-tract tumors and hypothalamic-tract tumors (9/14 and 8/12, respectively, P = .800); however, the small sample size limited the ability to detect a significant difference. Thus, the main risk factor for advanced puberty was suprasellar glioma.
Association between HPGA dysfunction and tumor treatment
Chemotherapy
Chemotherapy was associated with a significantly higher risk of gonadal toxicity (P = .002). All 46 patients with gonadal toxicity received chemotherapy. However, this association was entirely ascribable to high-risk chemotherapy (P = .002): of the 83 patients given high-risk chemotherapy (medulloblastoma, n = 43; glioma, n = 27; dysgerminoma, n = 4; other tumors, n = 9), 39 had gonadal toxicity. It is worth noting that we diagnosed gonadal toxicity based on FSH elevation (>10 IU/L in boys and >20 IU/L in girls). Boys also had a routine inhibin B assay and girls a routine AMH assay. Of the 24 girls with FSH elevation, 2 had no available AMH data and 22 had low AMH levels. Of the 26 boys with FSH elevation, 5 had no available inhibin B data, including 3 for whom spermograms were available and showed azoospermia; 19 had inhibin B levels <80 ng/L and 2 levels of 88 and 126 ng/L, respectively.
In summary, HypoH and advanced puberty were associated with tumor type and gonadal toxicity with high-risk chemotherapy.
Radiotherapy
Advanced puberty
The risk of advanced puberty was nonsignificantly higher in patients given a cranial radiation dose of 21 to 30 Gy (P = .09).
HypoH
Doses of 51 to 60 Gy were associated with a higher risk of HypoH (P = .001). By multivariable regression, only doses between 51 and 60 Gy were associated with a significant risk increase (P < .001).
Of the 64 patients with craniopharyngioma, 61 had GHD. The remaining 3 patients had sellar craniopharyngioma (2/3) or hypothalamo–pituitary craniopharyngioma requiring no surgery or radiotherapy (1/3). The location of craniopharyngioma, rather than tumor type, was probably the main determinant of endocrine disorders, although the large proportion of patients with surgery or radiotherapy in our cohort precluded confirmation of this possibility. Neither could associations of endocrine disorders with surgery be assessed. Of the 50 patients with HypoH, 38 had craniopharyngioma and panhypopituitarism.
In the NSST group, the large proportion of patients with gonadal toxicity complicated the assessment of HypoH. We therefore focused on diagnosing gonadal toxicity based on the above-described criteria. Nonetheless, some patients with gonadal toxicity may have had HPGA dysfunction.
Surgery
Surgery was performed in 182 (89.2%) of the 204 patients. This high proportion precluded an assessment of potential links between surgery and endocrine disorders.
PPVs for HPGA Dysfunction According to Tumor Type
We computed the PPVs to determine how often each tumor type was associated with each endocrine disorder (Fig. 2).
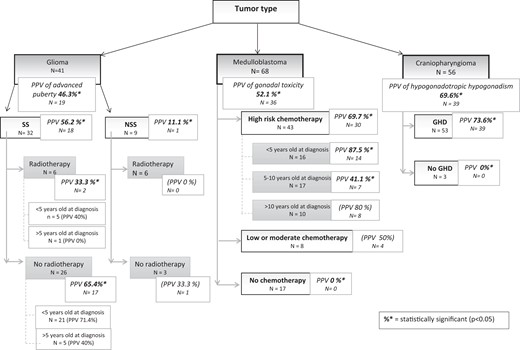
Positive predictive value (PPV) for tumor type and risk factors. The PPV of each hit is made compared with data from the same category. For example, radiotherapy vs no radiotherapy or <5 years vs >5 years. Values in bold and italics are those that are statistically significant while those in brackets are statistically insignificant.
Glioma
The PPV for advanced puberty in the 41 patients with glioma was 46.3% (n = 19). The PPVs were 11.1% (n = 1) in the 9 patients with NSSTs and 56.2% (n = 18) in the 32 patients with SSTs (P = .02). This highest PPV was 65.4% in patients who received either no radiotherapy or a pituitary dose <40 Gy (n = 26). The PPV was 33.3% in patients with radiotherapy >40 Gy (P = .02) and was nonsignificantly higher (71.4%) in patients who were younger than 5 years at diagnosis and received no radiotherapy or doses <20 Gy.
The proportion of patients with suprasellar glioma who developed advanced puberty was not significantly different between girls and boys. In the NSST glioma subgroup, advanced puberty was nonsignificantly more common in girls (PPV, 20% vs 0% in boys, P = .95.
Medulloblastoma
Medulloblastoma had PPVs for gonadal toxicity of 69.7% and 0% in patients with vs without high-risk chemotherapy (P = .001). With moderate-risk chemotherapy, the PPV was nonsignificantly lower (50%) than with high-risk drugs. The PPV was 87.5% (P = .001) in patients diagnosed before 5 years of age and given high-risk chemotherapy compared with 41.1% (P = .001) when the diagnosis occurred between 5 and 10 years of age and 80% when the patient was older than 10 years at diagnosis (P > .05).
Craniopharyngioma
Craniopharyngioma had a PPV of 69.6% for HypoH. The PPVs were similar in the subgroups with vs without radiotherapy. PPV increased nonsignificantly with age at diagnosis, from 68.7% before 5 years to 73.7% at 5 to 10 years and 80.9% after 10 years. Finally, the main risk factor for HypoH was GHD, which had a PPV of 73.6% (P = .001) compared with 0% in the absence of GHD (Fig. 2).
Timing of Puberty
Mean age at puberty onset in patients who achieved normal puberty was 11 ± 2.8 years in girls and 12.7 ± 2 years in boys.
In the group with advanced puberty, mean age at puberty onset was 7.83 and 9.88 years in girls and boys, respectively; mean time from diagnosis of the tumor to diagnosis of advanced puberty was 4.2 years and 5.9 years, respectively. In the subgroup with suprasellar glioma (the tumor type and location associated with the highest risk of advanced puberty), mean age at diagnosis of advanced puberty was 7.0 years in girls and 8.5 years for boys, with a time since the tumor diagnosis of 3.5 and 4.3 years, respectively.
Mean age at the diagnosis of absent puberty leading to pubertal induction was 12.8 years in girls and 13.3 years in boys.
Mean age at the diagnosis of gonadal toxicity was 16.6 years in girls, in other words 7.2 years after treatment completion, and 16.0 years in boys, in other words 10.1 years after treatment completion. In the group with medulloblastoma, the median time from the tumor diagnosis and the diagnosis of gonadal toxicity was 7.0 years in girls and 10.0 years in boys.
Discussion
The incidence of the different forms of HPGA dysfunction has varied across studies according to the features of the included patients.
Advanced Puberty
Advanced puberty has been reported in 5% to 15% of patients with a childhood diagnosis of brain tumor (3, 8, 9, 16). The main factors associated with advanced puberty in earlier studies were glioma, hydrocephalus, diagnosis before 5 years of age, pituitary radiation doses of 18 to 30 Gy, female sex, and a high body mass index (3, 8, 9, 16–18). In keeping with these findings, the strongest risk factors in our cohort were glioma diagnosed before 5 years of age and radiation therapy in a dose lower than 30 Gy. Compared with previous work, our study adds a quantification of the risk.
Pitfalls of Diagnosis and Management
In girls, the physical examination readily identifies puberty onset in girls by showing development of the breasts. In boys, the increase in testicular volume that is the main criterion for puberty onset may be absent after chemotherapy: alkylating agents can destroy the Sertoli cells but often have no effect on testosterone secretion (19–21). Slight LH elevation can reflect gonadal damage and should therefore not be interpreted as a sign of puberty onset in girls or boys (9). Patients who have GHD or have received radiotherapy to the spine may fail to experience the pubertal growth spurt despite ongoing puberty. These factors explain why the diagnosis of advanced puberty is sometimes delayed. Consequently, advanced puberty may exist in patients in whom pubertal development is documented only after 8 years (girls) or 9 years (boys).
Hypogonadotropic Hypogonadism
In populations with childhood brain tumors, the proportion of affected patients has ranged from 4.0% to 10.8% for HypoH and has reached 30.4% for gonadal toxicity (8, 22–24). Risk factors for HypoH were pituitary tumor location and pituitary radiotherapy, in doses above 50 Gy (23) or 40 Gy (5, 24) or even in lower doses of at least 30 Gy (9, 24). In our study, SSTs were associated with the highest frequency of HypoH, and additional risk factors were craniopharyngioma and GHD. Radiotherapy was associated with HypoH only when used in doses greater than 50 Gy. In the group with NSSTs, the high prevalence of gonadal toxicity precluded an assessment of risk factors for HypoH.
In addition to quantification of the risk, new information from our study is that HypoH was associated with GHD. Knowledge of this association may facilitate the diagnosis and allow earlier treatment. We did not observe the associations of HypoH with male sex and younger age at treatment initiation reported in another study (22).
Pitfalls of Diagnosis and Management
Low LH/FSH levels suggest HypoH, which may be difficult to differentiate from delayed puberty. Information provided by older patients on libido, erections, and sexual activity is important.
Gonadal Toxicity
Gonadal toxicity is the main side effect of gonadal radiotherapy and alkylating chemotherapy agents in both sexes. However, the risks associated with each agent differs between girls and boys. Agents associated per se with Sertoli cell failure in boys include busulfan, lomustine, and thiotepa, while those associated with dose-dependent toxicity are ifosfamide (>2500 mg/m²), procarbazine (>3000 mg/m²), and cisplatin (>475 mg/m²) (25). In girls, single-agent chemotherapy does not impair fertility except with busulfan; older age and combination chemotherapy increases the risks of gonadal toxicity and early menopause (26).
None of our patients received radiotherapy to the gonads. Of patients given high-risk chemotherapy, over two-thirds had gonadal toxicity, as well as about one-third of patients given moderate-risk chemotherapy.
An FSH level above 10.4 IU/L in boys has 83% sensitivity and 81% specificity for azoospermia (18). FSH elevation with an inhibin B level below 80 pg/mL is 100% specific of oligospermia (25). Inhibin B levels provide information on gonadal function of boys when FSH levels are low or normal due to radiotherapy to the hypothalamus or pituitary (25). In females, FSH elevation remains the most specific sign of gonadal toxicity until 25 years of age but occurs late (18) and may be absent after cranial radiotherapy. A 2021 study suggested AMH levels as a possibly useful marker for ovarian failure after irradiation for stem cell transplantation (27). Routine AMH monitoring is not yet recommended due to the absence of data on AMH kinetics after treatment. In our cohort, high FSH and low AMH indicated gonadal toxicity.
Pitfalls of Diagnosis and Management
The retrospective design is the main limitation of our study. The single-center recruitment resulted in small numbers of patients in each subgroup. Links between surgery and abnormalities of puberty could not be assessed, because most patients were treated surgically. Larger cohorts are needed to further investigate the risk factors for endocrine disorders after childhood brain tumor treatment.
Conclusion
Our study demonstrated links between various forms of HPGA dysfunction and specific tumor types, tumor locations, treatments, and age groups at diagnosis. Knowledge of these links should help pediatric and adult endocrinologists to optimize the follow-up they provide to survivors of childhood brain tumors. More specifically, glioma in a suprasellar location warrants a high level of alertness to advanced puberty, notably if the patient was young at diagnosis and/or received no or less than 30 Gy of radiation. After radiotherapy, the development of GHD may mask the pubertal growth spurt. Medulloblastoma treated with high-risk chemotherapy warrants careful attention to FSH levels and to AMH levels in girls and inhibin B levels in boys. Puberty is usually normal in patients given no high-risk or moderate-risk chemotherapy agents. Cranial radiotherapy in a dose above 50 Gy may produce HypoH, and if gonadal toxicity is associated LH and FSH levels can be lower than expected. Finally, craniopharyngioma treatment may be followed by multiple pituitary deficiencies including GHD and HypoH; in the absence of GHD, puberty is usually normal.
An endocrine evaluation and long-term follow-up are necessary in all patients with a brain tumor diagnosis in childhood, to identify potential threats to normal puberty and fertility. Awareness of the risk factors identified in our study will help to make proactive choices during follow-up. In patients at high risk for absence of puberty, for instance due to HPGA dysfunction combined with other pituitary dysfunctions such as GHD, pubertal induction may deserve to be considered early, before the age cut-off for defining delayed puberty.
Acknowledgments
We are indebted to David Lopez Rodriguez, a statistician at the GIGA Neurosciences-Neuroendocrinology laboratory in Liège, who donated his time and expertise to perform the statistical analyses and to Laurence Seidel, a statistician at the Liège University Hospital, who carried out the last statistical analyses under considerable time pressure. We thank Magali Viaud for assistance with understanding the ethical regulations applicable to this study. Finally, we are grateful to the physicians and nurses at the Necker-Enfants Malades University Hospital for their excellent care to the study patients.
Funding
This study was partially supported by Novo-Nordisk France.
Author Contributions
M.R., L.G.G.B., D.K., and M.P. wrote the manuscript. All other authors provided follow-up to the study patients, reviewed the manuscript for important intellectual content, and approved the final version of the manuscript.
Disclosures
D.K., D.S.B., E.G., J.B., S.B., B.F., S.P., C.S.R., C.A., G.P., M.L.P., S.B., S.A., I.A., K.B., M.B., T.R., T.B., F.D.R., C.T., C.P., C.R., S.J., K.B., A.S., F.B., L.L., L.G.R., D.O., P.T., C.D., J.G., and M.P. have nothing to declare. F.D. has received fees for advisory board roles from Bayer, BMS, Roche, Servier; travel expenses from Bayer, BMS, Roche; and consultancy roles from Servier.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.
References
Abbreviations
- AMH
antimullerian hormone
- FSH
follicle-stimulating hormone
- GH
growth hormone
- GHD
growth hormone deficiency
- HypoH
hypogonadotropic hypogonadism
- HPGA
hypothalamic–pituitary–gonadal axis
- IGF
insulin-like growth factor
- LH
luteinizing hormone
- NSST
nonsuprasellar tumor
- PPV
positive predictive value
- SST
suprasellar tumor



