-
PDF
- Split View
-
Views
-
Cite
Cite
Fabrizio Buffolo, Jacopo Pieroni, Federico Ponzetto, Vittorio Forestiero, Denis Rossato, Paolo Fonio, Antonello Nonnato, Fabio Settanni, Paolo Mulatero, Giulio Mengozzi, Silvia Monticone, Prevalence of Cortisol Cosecretion in Patients With Primary Aldosteronism: Role of Metanephrine in Adrenal Vein Sampling, The Journal of Clinical Endocrinology & Metabolism, Volume 108, Issue 9, September 2023, Pages e720–e725, https://doi.org/10.1210/clinem/dgad179
Close - Share Icon Share
Abstract
Adrenal venous sampling (AVS) is the gold standard procedure for subtype diagnosis in patients with primary aldosteronism (PA). Cortisol is usually adopted for the normalization of aldosterone levels in peripheral and adrenal samples. However, asymmetrical cortisol secretion can potentially affect the lateralization index, leading to subtype misdiagnosis.
We aimed to assess the prevalence of asymmetrical cortisol secretion in patients undergoing AVS and whether variations in adrenal vein cortisol might influence AVS interpretations. We then evaluated the use of metanephrines for the normalization of aldosterone levels for lateralization index.
We retrospectively included 101 patients with PA who underwent AVS: 49 patients underwent unstimulated AVS, while 52 patients underwent both unstimulated and cosyntropin-stimulated AVS. Eighty-eight patients had bilateral successful AVS according to metanephrine ratio. We assessed the prevalence of asymmetrical cortisol secretion through the cortisol to metanephrine (C/M) lateralization index (LI). We then evaluated whether the use of aldosterone to metanephrine (A/M) LI can improve the diagnostic accuracy of AVS compared with aldosterone to cortisol (A/C) LI.
Asymmetrical cortisol secretion is present in 18% of patients with PA. Diagnosis with A/M LI and A/C LI is discordant in 14% of patients: 9% had a diagnosis of unilateral PA with A/M LI instead of bilateral PA with A/C LI and 5% had a diagnosis of bilateral PA with A/M LI instead of unilateral PA.
The assessment of metanephrine levels in AVS is useful for the determination of selectivity and lateralization, allowing an accurate diagnosis, especially in patients with asymmetrical cortisol secretion.
Primary aldosteronism is the cause of hypertension in about 5% to 6% of patients in the primary care setting (1–3) and in 10% of patients referred to tertiary centers (4). One-third of patients are affected by unilateral and thus surgically curable forms, while two-thirds of patients display a bilateral disease, usually treated with mineralocorticoid receptor antagonists (MRAs) (5). Subtype diagnosis is pivotal for the identification of patients that are candidates for unilateral adrenalectomy (6, 7). Given the limited accuracy of a computed tomography (CT) scan for subtype diagnosis (8), current international guidelines recommend adrenal venous sampling (AVS) for subtype definition in the majority of patients with PA (5, 7, 9). Despite being considered the gold standard test, AVS interpretation is not unequivocal, since in some cases the final diagnosis can change from unstimulated to stimulated procedure (10). Serum cortisol levels, collected from the adrenal veins and inferior vena cava, are conventionally adopted for the definition of adrenal selectivity and for the normalization of aldosterone levels in peripheral and adrenal samples (5, 7, 9). However, the use of cortisol measurements has some limitations: (i) the cortisol secretion is characterized by circadian fluctuations, which makes mandatory the execution of unstimulated AVS in the early morning, when cortisol production is higher (5, 7, 9, 11); (ii) cortisol circulating half-life is relatively high with consequent small step-ups between adrenal and peripheral concentrations, especially for unstimulated procedures (12); (iii) cortisol secretion is affected by stress, which can alter the lateralization index when the sampling is performed sequentially with long time-gaps (5); (iv) the presence of autonomous cortisol secretion may reduce the cortisol secretion from the contralateral gland below the standard cutoffs that define selectivity (12); and (v) autonomous cortisol secretion can modify the lateralization indexes and subtype definition (13).
Some authors have proposed the use of metanephrines (12, 14, 15) as a more accurate alternative to define selectivity for AVS procedures. However, the role of aldosterone-to-metanephrine ratio for AVS lateralization has been poorly characterized by previous studies. Moreover, no study has investigated the impact of asymmetrical cortisol secretion on subtype diagnosis of PA.
The aim of this study is to evaluate: (i) the prevalence of asymmetrical cortisol secretion in PA; (ii) whether the presence of asymmetrical cortisol secretion might influence AVS interpretation; and (iii) the role of the aldosterone-to-metanephrine ratio in AVS for the determination of subtype diagnosis of PA.
Methods
Study Design and PA Diagnosis
We retrospectively included in our study 101 patients referred to the Division of Internal Medicine—Hypertension Unit of the University of Torino, who underwent AVS between 2008 and 2020. We excluded patients who had AVS exclusively with cosyntropin stimulation or with insufficient stocked material for metanephrine assay.
PA diagnosis was performed according to the recommendations of the European Society of Hypertension and the Endocrine Society (5–7). Screening tests were considered positive with aldosterone-to-renin ratio (ARR) ≥ 30 ng/dL/ng mL−1 h−1 or aldosterone-to-active renin ratio (AARR) ≥ 2.0 ng/dL/mU/L and aldosterone ≥ 10 ng/dL. Diagnosis of PA was confirmed by intravenous saline load and/or captopril challenge test in agreement with the guidelines (6, 7). Subtype diagnosis was obtained by adrenal computed tomography and AVS, according to Endocrine Society and European Society of Hypertension recommendations (5, 7). AVS were performed with sequential unstimulated catheterization (with < 5 minutes interval between cannulation of the right and the left adrenal vein) in 49 (48.5%) patients and with both, unstimulated and cosyntropin-stimulated procedure, in 52 (51.5%) patients (Table 1). Stimulated procedures were performed under continuous cosyntropin infusion (50 μg/h, Mayo clinic protocol) (16). Selectivity indices (SI) with C-ratio and M-ratio were defined as Cortisoladrenal vein/Cortisolperipheral vein and Metanephrineadrenal vein/Metanephrineperipheral vein, respectively. Lateralization indices (LI) were defined as (Aldosterone/Cortisol)dominant adrenal vein/(Aldosterone/Cortisol)nondominant adrenal vein (A/C LI), (Aldosterone/Metanephrine)dominant adrenal vein/(Aldosterone/Metanephrine)nondominant adrenal vein (A/M LI) or (Cortisol/Metanephrine)dominant adrenal vein/(Cortisol/Metanephrine)nondominant adrenal vein (C/M LI). Contralateral ratio as (Aldosterone/Cortisol)nondominant adrenal vein/(Aldosterone/Cortisol)peripheral vein. Contralateral suppression was defined as contralateral ratio <1. The analysis for the definition of AVS selectivity were performed using either C-ratio and M-ratio ≥ 2 or ≥3 for unstimulated procedures and C-ratio ≥ 5 and M-ratio ≥ 3 for cosyntropin-stimulated procedures, in agreement with international recommendations (5) and previous reports for the use of metanephrine for selectivity index (14). Patients with bilateral M-ratio ≥ 3 were considered for the analysis on lateralization indexes. Aldosterone production was considered unilateral with A/C LI ≥ 4 and cortisol production with C/M LI ≥ 4. The cutoff for A/M LI was derived from A/C LI through univariate linear regression, after exclusion of patients with asymmetrical cortisol secretion.
| Variables . | Patients (n = 101) . |
|---|---|
| Age at screening (years) | 50 ± 9 |
| Female sex, n (%) | 37 (36.6%) |
| Systolic BP (mmHg) | 157 ± 19 |
| Diastolic BP (mmHg) | 96 ± 11 |
| BMI (kg/sqm) | 26.3 ± 3.8 |
| Lowest Potassium (mEq/L) | 3.4 ± 0.6 |
| Creatinine (mg/dL) | 0.88 ± 0.20 |
| Diabetes, n (%) | 6 (5.9) |
| PRA (ng/mL/h) (n = 88) | 0.20 (0.10-0.40) |
| Renin (mU/L) (n = 13) | 1.80 (0.55-3.75) |
| Aldosterone (ng/dL) | 33.4 (24.0-45.4) |
| Adrenal vein sampling (%) | |
| Unstimulated | 49 (48.5) |
| Unstimulated and cosyntropin-stimulated | 52 (51.5) |
| Subtype diagnosis (%) | |
| Unilateral PA | 50 (49.5) |
| Bilateral PA | 35 (34.7) |
| Undetermined | 16 (15.8) |
| Variables . | Patients (n = 101) . |
|---|---|
| Age at screening (years) | 50 ± 9 |
| Female sex, n (%) | 37 (36.6%) |
| Systolic BP (mmHg) | 157 ± 19 |
| Diastolic BP (mmHg) | 96 ± 11 |
| BMI (kg/sqm) | 26.3 ± 3.8 |
| Lowest Potassium (mEq/L) | 3.4 ± 0.6 |
| Creatinine (mg/dL) | 0.88 ± 0.20 |
| Diabetes, n (%) | 6 (5.9) |
| PRA (ng/mL/h) (n = 88) | 0.20 (0.10-0.40) |
| Renin (mU/L) (n = 13) | 1.80 (0.55-3.75) |
| Aldosterone (ng/dL) | 33.4 (24.0-45.4) |
| Adrenal vein sampling (%) | |
| Unstimulated | 49 (48.5) |
| Unstimulated and cosyntropin-stimulated | 52 (51.5) |
| Subtype diagnosis (%) | |
| Unilateral PA | 50 (49.5) |
| Bilateral PA | 35 (34.7) |
| Undetermined | 16 (15.8) |
Values are mean ± SD, median (IQR), or absolute number (%).
Abbreviations: BP, blood pressure; BMI, body mass index; PA, primary aldosteronism; PRA, plasma renin activity.
| Variables . | Patients (n = 101) . |
|---|---|
| Age at screening (years) | 50 ± 9 |
| Female sex, n (%) | 37 (36.6%) |
| Systolic BP (mmHg) | 157 ± 19 |
| Diastolic BP (mmHg) | 96 ± 11 |
| BMI (kg/sqm) | 26.3 ± 3.8 |
| Lowest Potassium (mEq/L) | 3.4 ± 0.6 |
| Creatinine (mg/dL) | 0.88 ± 0.20 |
| Diabetes, n (%) | 6 (5.9) |
| PRA (ng/mL/h) (n = 88) | 0.20 (0.10-0.40) |
| Renin (mU/L) (n = 13) | 1.80 (0.55-3.75) |
| Aldosterone (ng/dL) | 33.4 (24.0-45.4) |
| Adrenal vein sampling (%) | |
| Unstimulated | 49 (48.5) |
| Unstimulated and cosyntropin-stimulated | 52 (51.5) |
| Subtype diagnosis (%) | |
| Unilateral PA | 50 (49.5) |
| Bilateral PA | 35 (34.7) |
| Undetermined | 16 (15.8) |
| Variables . | Patients (n = 101) . |
|---|---|
| Age at screening (years) | 50 ± 9 |
| Female sex, n (%) | 37 (36.6%) |
| Systolic BP (mmHg) | 157 ± 19 |
| Diastolic BP (mmHg) | 96 ± 11 |
| BMI (kg/sqm) | 26.3 ± 3.8 |
| Lowest Potassium (mEq/L) | 3.4 ± 0.6 |
| Creatinine (mg/dL) | 0.88 ± 0.20 |
| Diabetes, n (%) | 6 (5.9) |
| PRA (ng/mL/h) (n = 88) | 0.20 (0.10-0.40) |
| Renin (mU/L) (n = 13) | 1.80 (0.55-3.75) |
| Aldosterone (ng/dL) | 33.4 (24.0-45.4) |
| Adrenal vein sampling (%) | |
| Unstimulated | 49 (48.5) |
| Unstimulated and cosyntropin-stimulated | 52 (51.5) |
| Subtype diagnosis (%) | |
| Unilateral PA | 50 (49.5) |
| Bilateral PA | 35 (34.7) |
| Undetermined | 16 (15.8) |
Values are mean ± SD, median (IQR), or absolute number (%).
Abbreviations: BP, blood pressure; BMI, body mass index; PA, primary aldosteronism; PRA, plasma renin activity.
Biochemical Measurements
Aldosterone concentrations were measured by radioimmunoassay ACTIVE Aldosterone RIA kit (Beckman Coulter, Brea, CA, USA) and cortisol concentrations were determined by electrochemiluminescence immunoassay with the Elecsys Cortisol II kit (Roche, Basel, Switzerland). Metanephrine concentrations were assessed through liquid chromatography coupled with tandem mass spectrometry detection employing the MassChrom Free Metanephrines in Plasmakit (Chromsystems Instruments & Chemicals GmbH, Gräfelfing, Germany).
Hypercortisolism was investigated by 1-mg dexamethasone suppression test or 24-hour urinary free cortisol in patients with an adrenal nodule ≥ 1 cm at CT scan or with clinical features or comorbidities considered indicative of possible hypercortisolism. Autonomous cortisol secretion was defined by cortisol ≥ 18 µg/L after 1-mg dexamethasone suppression test or 24-hour urinary free cortisol ≥ 150 µg/24 hours.
Statistical Analysis
Variables were treated as parametric or nonparametric according to their distribution. Categorical variables were expressed as absolute number and percentage. Continuous variables with a normal distribution were expressed as mean ± SD. Nonnormally distributed variables were expressed as median [interquartile range]. We defined the statistical significance by Student t test for independent samples of parametric variables and Mann–Whitney U test for nonparametric variables. The χ2 test was adopted for comparison of unpaired categorical variables and McNemar's test for paired categorical variables. We used the Bland–Altman plot to show the relationship between lateralization index defined with A/C LI and lateralization index defined with A/M LI. Univariate linear regression was adopted to define the association between A/C LI and A/M LI.
Results
Characteristics of Patients
Clinical characteristics of patients with PA are summarized in Table 1. Eleven patients had autonomous cortisol secretion, but only one of them had overt hypercortisolism (with 1-mg dexamethasone suppression test >50 µg/L). Subtype diagnosis, defined with A/C LI, was the same using unstimulated or cosyntropin-stimulated procedure in 47 out of 52 AVS (91%) (Supplementary Figure S1) (17).
Selectivity Index
Using C-ratio ≥ 3, 68 of 101 unstimulated AVS (67%) were bilaterally successful (Supplementary Figure S2A-S2C) (17). Using M-ratio ≥ 3, 88 AVS procedures (87%) were bilaterally successful (Supplementary Figure S2A, S2B, S2D) (17), with a consequent 31% increase in bilaterally successful procedures (P < .001). Using C-ratio ≥ 2 to define selectivity, the number of bilaterally successful AVS procedures increased up to 76 (75%) (P = .005) (Supplementary Figure S3A-S3C) (17) and up to 89 (88%) with M-ratio ≥ 2 (Supplementary Figure S3A, S3B, S3D) (17). After cosyntropin stimulation, 50 of 52 (96%) AVS procedures were considered bilaterally successful with C-ratio ≥ 5 (Supplementary Figure S4A-S4C) (17), and 52 of 52 (100%) with M-ratio ≥ 3 (P = .157) (Supplementary Figure S4A, S4B, S4D) (17).
Asymmetrical Cortisol Secretion
We used C/M LI to define cortisol lateralization for 88 unstimulated AVS procedures that were bilaterally successful according to metanephrine selectivity index. Sixteen of 88 AVS procedures (18%) showed asymmetrical cortisol secretion (C/M LI ≥ 4) (Fig. 1A-1C). Nine of them (56%) showed cortisol lateralization on the same side of unilateral PA (UPA), 5 on the opposite side of UPA, and 2 showed asymmetrical cortisol secretion in patients with bilateral PA (BiPA) (Fig. 1D).
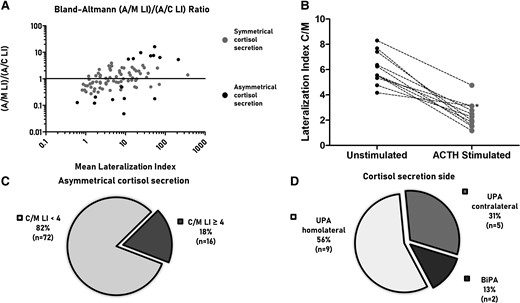
The Bland–Altmann plot (A) shows the correlation between A/M LI and A/C LI for unstimulated AVS (n = 88). The y-axis reports the ratio between A/M LI and A/C LI, while the x-axis reports the mean value of A/M LI and A/C LI. The plot highlights that patients with asymmetrical cortisol secretion (black dots) show greater dissimilarity between A/M LI or A/C LI than patients with symmetrical cortisol secretion (gray dots). The panel (B) shows C/M LI with and without cosyntropin stimulation in patients who had both procedures and showed asymmetrical cortisol secretion with unstimulated procedure (n = 11); Asterisk indicates an AVS where the dominant side of C/M LI changed after cosyntropin stimulation. The pie charts show the prevalence of asymmetrical cortisol secretion defined as C/M LI ≥4 (C) and the distribution of C/M LI compared to the subtype diagnosis (D). Abbreviations: A/C LI, aldosterone to cortisol lateralization index; A/M LI, aldosterone to metanephrine lateralization index; AVS, adrenal venous sampling; BiPA, bilateral primary aldosteronism; C/M LI: cortisol to metanephrine lateralization index; UPA; unilateral primary aldosteronism.
Among the 5 patients with cortisol secretion contralateral to UPA, 2 had a CT scan showing bilateral nodules, and 1 patient had overt hypercortisolism with 3 nodules on the side of cortisol lateralization and a normal adrenal gland on the contralateral adrenal where the A/C LI diagnosed lateralized aldosterone production; the remaining 2 patients had a normal contralateral adrenal gland.
Patients with asymmetrical cortisol secretion displayed the greatest differences between A/M LI and A/C LI (Fig. 1A). After cosyntropin stimulation, C/M LI decreased in all the patients with asymmetrical cortisol secretion (Fig. 1B), suggesting a direct stimulation of the contralateral gland, where cortisol secretion is suppressed in unstimulated conditions. No clinical or biochemical differences were observed between patients with symmetrical and asymmetrical cortisol secretion (Supplementary Table S1) (17). Four of the 16 patients with asymmetrical cortisol secretion had autonomous cortisol secretion diagnosed with biochemical tests (1-mg overnight dexamethasone suppression test and 24-hour urinary free cortisol); the other 12 patients were negative at these tests. Seven of the 11 patients with autonomous cortisol secretion displayed symmetrical cortisol secretion (C/M LI < 4).
Lateralization Index
After exclusion of patients with asymmetrical cortisol secretion, we performed a linear regression to predict A/M LI on the basis of A/C LI (Supplementary Table S2) (17). An A/C LI of 4 corresponded to 4.3 of A/M LI, that was rounded up to 4 for the following analysis.
Twelve of 88 patients (14%) had a different diagnosis with A/M LI vs A/C LI (Fig. 2A, 2B). Eight patients (9%) had a diagnosis of UPA with A/M LI instead of BiPA with A/C LI (Fig. 2B): 5 have been treated with medical therapy, while 3 underwent adrenalectomy on the basis of the A/C LI after cosyntropin stimulation. All patients who underwent adrenalectomy showed a complete biochemical success after surgery, with a histological examination showing a solitary aldosterone-producing adenoma (APA) (18).
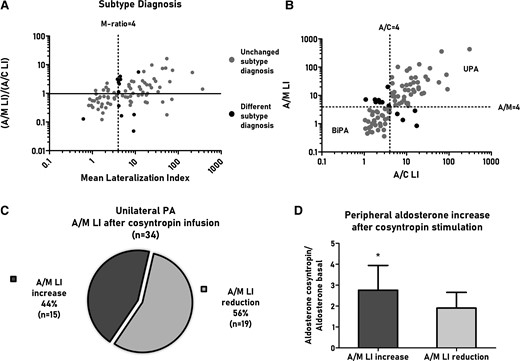
The Bland–Altmann plot (A) shows the correlation between A/M LI and A/C LI for unstimulated AVS (n = 88). The y-axis reports the ratio between A/M LI and A/C LI, while the x-axis reports the mean value of A/M LI and A/C LI. The scatterplot (B) shows A/M LI and A/C LI for unstimulated AVS (n = 88), highlighting the final diagnosis according to A/M LI or A/C LI. In both plots, we highlighted patients with a different subtype diagnosis (black dots) and those with a similar diagnosis (gray dots). The pie chart (C) reports the proportion of patients with unilateral PA that show an increase or reduction of A/M LI after cosyntropin stimulation. The panel (D) show peripheral aldosterone changes after cosyntropin stimulation in the subgroup of patients with A/M LI increase after cosyntropin stimulation vs patients with A/M LI reduction. *P value is considered significant for P < 0.05. Abbreviations: A/C LI, aldosterone to cortisol lateralization index; A/M LI, aldosterone to metanephrine lateralization index; AVS, adrenal venous sampling; BiPA, bilateral primary aldosteronism; C/M LI, cortisol to metanephrine lateralization index; LI, lateralization index; PA, primary aldosteronism.
Four patients (5%) had a diagnosis of BiPA with A/M LI instead of UPA with A/C LI (Fig. 2B). Two of them underwent surgical adrenalectomy with complete biochemical success: at the histological examination 1 patient had multiple aldosterone-producing nodules and 1 had a solitary APA (18). One patient refused adrenalectomy. The fourth patient (described in “Asymmetrical Cortisol Secretion”) had overt hypercortisolism. The patient has been treated with adrenalectomy on the side of cortisol lateralization, with complete resolution of hypercortisolism. The biochemical features of PA persisted after surgical treatment consistent with diagnosis of BiPA in agreement with the A/M LI.
Cosyntropin Effect on A/M LI
In 56% of patients with UPA A/M LI decreased after cosyntropin stimulation, suggesting a stimulation of the contralateral gland (Fig. 2C). However, in 44% of patients, A/M LI increased after cosyntropin stimulation, suggesting a stimulation of aldosterone secretion from the APA. Compared with patients with reduced A/M LI, patients with increased A/M LI showed a greater increase of peripheral aldosterone levels after cosyntropin stimulation, corroborating the hypothesis of cosyntropin-sensitive APAs (Fig. 2D). The effects of cosyntropin infusion on A/M LI changed the subtype diagnosis in 5 patients with UPA (defined with unstimulated A/M LI) to BiPA. On the other side, in 7 patients with BiPA (defined with unstimulated A/M LI), the diagnosis changed to UPA.
Discussion
In this study, we evaluated for the first time the presence of asymmetrical cortisol secretion, by the use of C/M LI, in patients with PA and its influence in subtype diagnosis. We then reported how the use of metanephrine normalization, through the use of A/M LI, avoids the biases of asymmetrical cortisol secretion, allowing a more accurate definition of PA subtype.
Previous studies investigated the role of metanephrine measurements in AVS as a more sensitive approach for selectivity index, improving the successful rate between 17% and 39% compared with unstimulated AVS (12, 14, 15). Our study confirmed previous reports, with an increase of 20% for unstimulated AVS and 4% for stimulated procedures.
Autonomous cortisol secretion is defined as an alteration of the hypothalamic–pituitary–adrenal axis with increased peripheral cortisol concentration and without the clinical phenotype of overt hypercortisolism (19). Autonomous cortisol secretion is assessed by various tests, including 1-mg dexamethasone suppression test, 24-hour urinary free cortisol, or late-night salivary cortisol, and it is present in 5% to 78% of patients with PA (19). The large heterogeneity is justified by the differences in the number and type of tests and the different cutoffs adopted in various studies (20). Autonomous and asymmetrical cortisol secretion can cause misinterpretation of the LI, when assessed by A/C LI (13). However, in our study, asymmetrical cortisol production was evident even in patients without autonomous cortisol secretion, suggesting that a confounding effect using cortisol normalization cannot be excluded even in patients with apparently normal cortisol production. On the other side, it should be noted that not all the patients with autonomous cortisol secretion have asymmetrical cortisol secretion (only 36% in our cohort).
Theoretically, an asymmetrical production of both hormones (metanephrine and cortisol) can cause the observation of C/M LI ≥ 4. However, the fact that cosyntropin stimulation is able to reduce C/M LI in all the patients with C/M LI ≥ 4 strongly suggest that these findings are caused by asymmetrical cortisol production.
The majority of the patients displayed asymmetrical cortisol secretion homolateral to the side of UPA, suggesting cosecretion of aldosterone and cortisol from the same adenoma/nodule. In 2 cases, the asymmetrical cortisol secretion was contralateral to the UPA with CT scan showing bilateral nodules, suggesting the presence of a cortisol-secreting nodule on the opposite side of the UPA. This is not surprising, since autonomous cortisol secretion is the most frequent functional abnormality in patients with an adrenal incidentaloma, being observed in up to 20% of cases (20). However, 4 patients with asymmetrical cortisol secretion had bilaterally normal glands at CT scanning, suggesting that the asymmetrical cortisol production can be the consequence of CT-undetectable micronodules. This observation has an important clinical implication, suggesting that asymmetrical cortisol secretion cannot be ruled out by the presence of bilaterally normal adrenal glands at CT scan.
As expected, patients with asymmetrical cortisol secretion displayed the greatest differences in LI defined with A/M LI vs A/C LI, with potential effect on the final diagnosis. However, when LI is close to 4, even a mild asymmetrical cortisol secretion can alter the subtype definition. In order to avoid the bias of asymmetrical cortisol secretion, we proposed the use of A/M LI when AVS is performed under unstimulated conditions. Three patients with UPA defined with A/M LI (but BiPA with A/C LI), showed complete biochemical success after adrenalectomy, while in 5 cases the patients have been treated with medical therapy.
Four patients displayed an UPA with A/C LI, but BiPA with A/M LI. One had overt unilateral hypercortisolism and BiPA correctly identified by A/M LI. Two showed complete biochemical success after adrenalectomy, indicating potential missing of UPAs by the use of metanephrine normalization. Both of these patients had a C/M LI ≥ 3 on the opposite side of the UPA and increase of A/M LI after cosyntropin stimulation, suggestive of cosyntropin-sensitive APAs. A third patient, who refused adrenalectomy, showed a similar pattern with C/M LI ≥ 3 and increase of A/M LI after cosyntropin stimulation. Intriguingly, in all these 3 patients, the cosyntropin stimulation increased the A/M LI above 4, allowing the correct identification of the UPAs with the stimulated procedure; A/C LI remained > 4 after cosyntropin stimulation.
These cases highlight that, in specific scenarios, cosyntropin stimulation can add important information, both with A/M LI and A/C LI, allowing the identification of cosyntropin-sensitive APA that could go otherwise unrecognized. Moreover, cosyntropin stimulation reduces the asymmetrical cortisol secretion, mitigating the interference of cortisol asymmetry when A/C LI is used. On the other side, in some patients with UPA, cosyntropin infusion stimulates aldosterone production from the contralateral gland, reducing A/M LI and A/C LI with incorrect BiPA diagnosis.
In summary, the use of metanephrine normalization improves the diagnostic accuracy of unstimulated AVS, avoiding the effects of asymmetrical cortisol secretion and identifying some UPA that are missed with cortisol normalization. On the other hand, the diagnosis of UPA may be missed in few specific cases of cosyntropin-sensitive APA. Cosyntropin administration allows the correct identification of these cases, both with metanephrine and cortisol normalization. However, with cosyntropin administration, some UPA may be missed, due to the stimulation of aldosterone production from the contralateral gland.
Since none of the described methods are flawless, the evaluation of both, cortisol and metanephrine normalization with unstimulated and cosyntropin-stimulated procedures, would be ideal to allow the most accurate definition of subtype diagnosis. Nevertheless, the performance of AVS in 2 different conditions, would increase the length of the procedure and the complexity of the interpretation.
The main limitation of this study is the retrospective design and consequently the absence of a post-adrenalectomy outcome in some of the patients who had a diagnosis of UPA with A/M LI only. A prospective study, in which every patient with UPA diagnosis (defined either with A/C LI or A/M LI) undergoes unilateral adrenalectomy, would be ideal to confirm our results, allowing the identification of true sensitivity and specificity of each normalization method. A second limit of our study is the absence of a systematic screening for autonomous cortisol secretion before AVS.
Conclusion
In this study we reported for the first time the presence of asymmetrical cortisol secretion in 18% of patients with PA. We showed that even a mild asymmetrical cortisol secretion can modify the subtype diagnosis using cortisol normalization. The absence of autonomous cortisol secretion or the absence adrenal nodules at CT scanning cannot rule out the presence of asymmetrical cortisol secretion. The use of metanephrine normalization avoids the effect of asymmetrical cortisol secretion, allowing a more accurate definition of PA subtype in the majority of cases.
Acknowledgments
The TESEO-Biobank of Department of Medical Sciences is ISO 9001:2015 certified and GDPR compliant, ensuring that pseudonymized samples obtained from consented participants are appropriately identified, and tracked to eliminate the risks of sample misidentification and loss.
Disclosures
P.M. received fees for educational speech from DIASORIN. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request. Supplemental materials (17) are available at the following link: https://github.com/CentroIpertenUnito/MetaAVS/raw/main/Supplemental%20Data.pdf
References
Abbreviations
- A/C LI
aldosterone to cortisol lateralization index
- A/M LI
aldosterone to metanephrine lateralization index
- APA
aldosterone-producing adenoma
- AVS
adrenal vein sampling
- BiPA
bilateral primary aldosteronism
- C/M LI
cortisol to metanephrine lateralization index
- LI
lateralization index
- PA
primary aldosteronism
- UPA
unilateral primary aldosteronism
Author notes
These authors contributed equally to this work and should be considered as joint second authors.
These authors contributed equally to this work and should be considered as joint last authors.



