-
PDF
- Split View
-
Views
-
Cite
Cite
Xinyi Liang, Ke Huang, Guangping Dong, Ruimin Chen, Shaoke Chen, Rongxiu Zheng, Chunlin Wang, Haiyan Wei, Bingyan Cao, Yan Liang, Hui Yao, Zhe Su, Mireguli Maimaiti, Feihong Luo, Pin Li, Min Zhu, Hongwei Du, Yu Yang, Lanwei Cui, Shuting Si, Guannan Bai, Yunxian Yu, Er-Gang Wang, Paul L Hofman, Junfen Fu, Current Pubertal Development in Chinese Children and the Impact of Overnutrition, Lifestyle, and Perinatal Factors, The Journal of Clinical Endocrinology & Metabolism, Volume 108, Issue 9, September 2023, Pages 2282–2289, https://doi.org/10.1210/clinem/dgad102
Close - Share Icon Share
Abstract
Age of pubertal onset has been decreasing in many countries but there have been no data on pubertal development in Chinese children over the last decade.
The primary objective of the study was to evaluate the current status of sexual maturation in Chinese children and adolescents. Secondary objectives were to examine socioeconomic, lifestyle, and auxological associations with pubertal onset.
In this national, cross-sectional, community-based health survey, a multistage, stratified cluster random sampling method was used to select a nationally representative sample, consisting of 231 575 children and adolescents (123 232 boys and 108 343 girls) between 2017 and 2019. Growth parameters and pubertal staging were assessed by physical examination.
Compared to 10 years previously, the median age of Tanner 2 breast development and menarche were similar at 9.65 years and 12.39 years respectively. However, male puberty occurred earlier with a median age of testicular volume ≥4 mL of 10.65 years. Pubertal onset did occur earlier at the extremes, with 3.3% of the girls with breast development at 6.5-6.99 years old, increasing to 5.8% by 7.5-7.99 years old. Early pubertal onset was also noted in boys, with a testicular volume ≥ 4 mL noted in 1.5% at 7.5-7.99 years, increasing to 3.5% at 8.5-8.99 years old. Obesity and overweight increased risk of developing earlier puberty relative to normal weight in both boys and girls.
Over the past decade, pubertal development is occurring earlier in Chinese children. While the cause is multifactorial, overweight and obesity are associated with earlier puberty onset. The currently used normative pubertal data of precocious puberty may not be applicable to diagnose precocious puberty.
Puberty is an important developmental milestone consisting of a complex sequence of biological events leading to the progressive maturation of sexual characteristics and ultimately attainment of full reproductive capacity (1). Numerous international studies have shown that the onset of pubertal characteristics varies with race and ethnicity, geographical location, and environmental and nutritional conditions (2). However, most data suggest a downward secular trend in the age of puberty onset, and this has been associated with better nutrition and increased obesity prevalence (3).
In 2009 and 2010, a cross-sectional study (Prevalence and Risk of Obesity and Diabetes in Youth, PRODY study) was conducted by our group in 6 medical centers with a representative sample of 9812 boys and 8895 girls aged 6 to 18 years. Data at this time indicated that for girls, breast development occurred at a median 9.69 years, and for boys, testicular volume ≥4 mL occurred at 11.25 years (4).
Whether these pubertal development parameters have changed in Chinese children and adolescents in the last decade has not been investigated. Therefore, our primary objective was to assess pubertal development in Chinese children and adolescents using the same methodology in the PRODY study in 2017 to 2019 as in 2009.
Secondary objectives were to look for associations between pubertal onset and body mass index (BMI), socioeconomic, and lifestyle factors.
Methods
This study was conducted at kindergarten, primary, secondary, and high schools in 14 provinces and 17 medical centers in China from 2017 to 2019. A multistage, stratified cluster random sampling method was used to select a nationally representative sample of children 3 to 18 years of age. The sampling process was stratified according to geographic regions (Eastern, Western, Southern, Northern, and Middle China), degree of urbanization, and economic development status (based on the gross domestic product [GDP] for each province). In each geographic region, at least one province, municipality, or autonomous region was selected. We chose Zhejiang province, Jiangxi province, and Shanghai Municipality to represent Eastern China, Chongqing Municipality and Xinjiang Uygur Autonomous Region to represent Western China, Guangdong province, Fujian province, Guangxi province to represent Southern China, Beijing Municipality, Tianjin Municipality, Jilin province, Heilongjiang province to represent Northern China, Hubei province and Henan province to represent Middle China. In each province, municipality, or autonomous region, at least one city or county was selected, and in these cities or counties, at least one school or kindergarten was selected using the whole cluster sampling method. All students at the schools were invited to participate. This multistage, stratified sampling process resulted in 34 cities, 67 counties, and 430 schools and kindergartens. In the final stage of sampling, the sample was stratified according to sex and age distribution.
We selected and invited a total of 250 000 children and adolescents to participate in the study. Among them, 231 932 (113 212 boys and 118 720 girls) completed the study. The overall response rate was 92.77%. Exclusion criteria were all conditions that could impact pubertal onset, including severe and ongoing chronic illness, relevant long-term medications such as glucocorticoid therapy, severe developmental delay, or evidence of central nervous system damage such as cerebral palsy, syndromic and genetic conditions, as well as disorders of sex development. From the initial survey, 357 children were excluded leaving a nationally representative sample of 231 575 children and adolescents (108 343 girls and 123 232 boys) aged 3 to 18 from 14 provinces, municipalities, and autonomous regions in China that participated in the study (Fig. 1). Approval was obtained by the pertinent institutional review boards. This study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants’ parents.
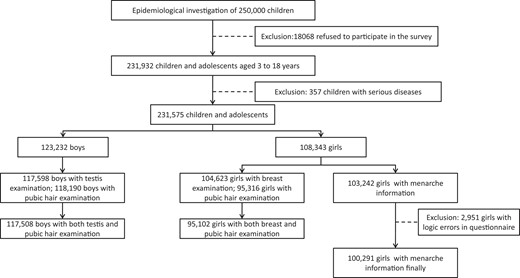
A total of 50 372 children (girls aged 6.00 to 7.99 years old and boys aged 6.00 to 8.99 years old) were included in the multivariable logistic regression models to assess the risk factors of precocious puberty. After excluding 2294 children with missing variables, 48 078 children were included for analysis. The participants were grouped by 0.5- or 1-year age intervals such that the age recorded reflected either the next 6 months or the next 12 months, respectively.
Pubertal stages were assessed by physical examination according to the 5-stage scale described by Marshall and Tanner (5). Tanner stages (breast and pubic hair stages for girls and genitalia and pubic hair stages for boys) were evaluated by pediatric endocrinologists. Breast stages were estimated by vision and palpation. Testicular volumes were estimated by palpation to the nearest 1 mL using a Prader orchidometer (6). Stages of pubic hair development were rated by visual inspection. In case the testicular volumes of the 2 testes were not equal, the larger testis measurement was recorded, and the same protocol was followed for breast measurement. The age at menarche was collected by a questionnaire survey. Standing height was measured to the nearest 0.1 cm and weight was measured to the nearest 0.1 kg. Children were measured without shoes and wearing light clothing. BMI was calculated as weight (kg) divided by height (m) squared. BMI was converted to BMI Z-scores based on the World Health Organization 2007 standards and classification (underweight, < −2; normal, −2 to 1; overweight, 1 to 2; and obesity, >2) (7).
Puberty in girls was defined as the initiation of breast development and designated Tanner stage 2 (B2). In boys, puberty was defined as an increase in testicular volume ≥4 mL (8). Precocious puberty was defined as children entering puberty more than 2 SD earlier than the median or mean age (9). In China, precocious puberty has been historically defined as the onset of secondary sexual characteristics before the age of 8 years in girls and before 9 years in boys (10). Pubarche was defined by the appearance of ≥ Tanner 2 pubic hair.
Data collected on each child by a validated questionnaire included 4 parts: 1) Informed consent; 2) Basic information (including family history and socioeconomic parameters); 3) Perinatal history/Personal history/Past history; and 4) Diet and lifestyle in the past month.
A total of 430 primary and secondary schools and kindergartens in 67 districts and counties of 34 prefecture-level cities of 14 provinces and autonomous regions were surveyed. In total, 231 932 children and adolescents participated.
Statistical Analysis
The data were collected and analyzed by the status quo method (11). Probabilistic analysis was used to estimate the secondary sexual characteristics, median age, and 95% CIs. Frequency and percentage were reported for categorical variables and Chi-square tests were used to compare the characteristic difference between children with and without precocious puberty. Multivariable logistic regressions with stepwise selection were conducted to investigate risk factors for precious puberty. Variables include gender (boy, girl), age (6 years, 7 years, 8 years), maternal education (primary school and below, high school, undergraduate and above), annual family income (<0.1 million yuan [<16 000 dollars], 0.1-0.2 million yuan [16,000-32 000 dollars], ≥ 0.2 million yuan [≥32 000 dollars]), birth weight (<2500 g, 2500-3999 g, ≥ 4000 g), BMI (normal weight, overweight, obesity), coarse grains consumption (no, yes), snacks consumption (healthy, unhealthy), midnight snacks consumption (no, yes), physical activity time (<90 minutes/week, 90-120 minutes/week, 120-150 minutes/week, ≥ 150 minutes/week) and region (Eastern, Southern, Western, Northern, Middle). A P < .05 was considered statistically significant and the analysis was conducted by R-software (version 3.6.0).
Results
Breast development was noted in 3.3% of girls aged 6.5 to 6.99 years (Fig. 2A and Supplementary Table 1 (12)). The proportion of girls with breast development increased to 5.8% of those 7.5 to 7.99 years old. Testicular volume ≥ 4 mL was noted in 1.5% of boys aged 7.5 to 7.99 years while noted in 3.5% of those 8.5 to 8.99 years old, and 17.4% in those 9.5 to 9.99 years old (Fig. 2B and Supplementary Table 1 (12)).
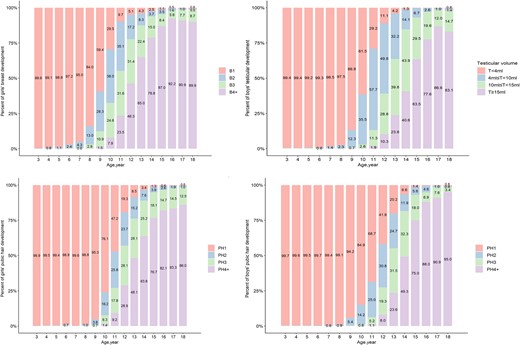
A) Breast development stages at different ages in girls. B) Testicular volume at different ages in boys. C) Pubic hair development stages at different ages in girls. D) Pubic hair development stages at different ages in boys.
The median ages and the respective percentile values for the attainment of different Tanner stages in girls and boys and menarche in girls as estimated by probabilistic analysis are summarized in Fig. 3 and Supplementary Table 2 (12). The median age for Tanner stage 2 (B2) breast development in Chinese girls was 9.65 years (95% CI, 9.63-9.68), and the 3rd and 10th percentile ages were 6.30 and 7.37 years, respectively. The median age of menarche in girls was 12.39 (95% CI, 12.37-12.42) years. The interval from B2 to menarche was 2.74 years. The median age of testicular enlargement in boys was 10.65 years (95% CI, 10.63-10.67).
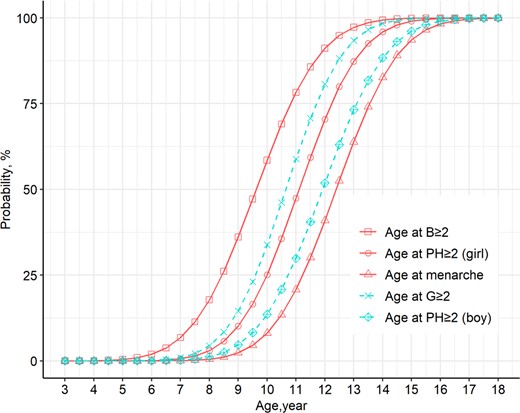
Probability curves for breast, genital, and pubic hair development, and menarche in Chinese children.
Pubic hair was noted in 3.2% of girls aged 8.5 to 8.99 years old compared with 1.4% of boys. By 9.5 to 9.99 years of age, this proportion had risen to 10.4% in girls and 7.2% in boys (see Fig. 2C and 2D and Supplementary Table 3 (12)).
Using the standard definitions of precocious puberty, 3.8% of girls 6.5 to 6.99 years old and 1.1% of the boys met the criteria. This increased to 7.0% of girls and 1.8% of boys by 7.5 to 7.99 years of age. In boys, this increased further to 3.7% by 8.5 to 8.99 years old (Supplementary Table 4 (12)).
Comparing the different regions of China, the prevalence of precocious puberty in girls residing in the Eastern, Middle, and Northern regions was higher than that in other regions, while the Western region had the highest prevalence of obesity in girls. In boys, the Northern and Southern regions had the highest rate of precocious puberty, and also the highest prevalence of obesity (Supplementary Table 5 (12)).
Obesity and overweight were associated with the development of precocious puberty relative to normal weight in both girls and boys (OR [95% CI]: 3.26 [2.80-3.79] and 2.22 [1.92-2.57], respectively) (Table 1 and Supplementary Table 6 (12)). Birth weight higher than 4000 g was associated with a higher risk of precocious puberty than normal birth weight (2500 g-3999 g) (OR [95% CI]: 1.25 [1.03-1.51]), particularly for boys (OR [95% CI]:1.43[1.13-1.82]). Eating unhealthy snacks (eg, sugary drinks, sweets, confectionery, barbecue, fried, puffed food, preserved products) was associated with a higher risk of precocious puberty than eating healthy snacks (eg, biscuits, nuts, fruits, milk, and dairy products) (OR [95% CI]: 1.44 [1.23-1.68]). Having 90 to 120 minutes physical activity per week was associated with a lower risk of precocious puberty than having physical activity less than 90 minutes per week (OR [95% CI]: 0.85 [0.74-0.99]). In addition, a higher annual family income was associated with a lower risk of early puberty in boys.
The factors associated with precocious puberty (multiple logistic regression)
| Variables . | All (n = 48 078) . | Girls (n = 17 039) . | Boys (n = 31 039) . |
|---|---|---|---|
| OR (95 CI%) . | OR (95 CI%) . | OR (95 CI%) . | |
| Gender | |||
| Boy | ref. | — | — |
| Girl | 3.36 (2.90-3.89)a | — | — |
| Age | |||
| 6 years | ref. | ref. | ref. |
| 7 years | 2.04 (1.78-2.35)a | 2.14 (1.81-2.54)a | 1.97 (1.52-2.55)a |
| 8 years | 3.14 (2.58-3.82)a | — | 3.19 (2.50-4.08)a |
| BMI | |||
| Normal weight | ref. | ref. | ref. |
| Overweight | 2.22 (1.92-2.57)a | 2.64 (2.16-3.24)a | 1.86 (1.50-2.29)a |
| Obesity | 3.26 (2.80-3.79)a | 5.58 (4.52-6.87)a | 1.91 (1.53-2.38)a |
| Birth weight | |||
| 2500-3999 g | ref. | ref. | ref. |
| <2500 g | 0.86 (.59-1.24) | 0.89 (.54-1.47) | 0.88 (.50-1.54) |
| ≥4000 g | 1.25 (1.03-1.51)a | 1.03 (.75-1.40) | 1.43 (1.13-1.82)a |
| Ate coarse grains | |||
| No | ref. | ref. | ref. |
| Yes | 0.98 (.84-1.14) | 0.93 (.75-1.15) | 1.01 (.81-1.26) |
| Ate snacks | |||
| Healthy | ref. | ref. | ref. |
| Unhealthy | 1.44 (1.23-1.68)a | 1.45 (1.15-1.83)a | 1.40 (1.12-1.74)a |
| Ate midnight snacks | |||
| No | ref. | ref. | ref. |
| Yes | 0.93 (0.77-1.12) | 0.77 (.59-1.02) | 1.04 (.81-1.34) |
| Physical time | |||
| <90 minutes/week | ref. | ref. | ref. |
| 90-120 minutes/week | 0.85 (.74-.99)a | 0.83 (.68-1.02) | 0.93 (.75-1.15) |
| 120-150 minutes/week | 0.85 (.70-1.04) | 0.80 (.61-1.05) | 0.95 (.72-1.26) |
| ≥150 minutes/week | 0.97 (.82-1.15) | 0.94 (.75-1.19) | 1.00 (.79-1.27) |
| Maternal education | |||
| Primary school and below | ref. | ref. | ref. |
| High school | 1.24 (.89-1.74) | 1.81 (.97-3.36) | 1.04 (.70-1.55) |
| Undergraduate and above | 0.89 (.63-1.26) | 1.42 (.75-2.67) | 0.65 (.42-0.99) |
| Annual family income | |||
| <0.1 million yuan (<16 000 dollars) | ref. | ref. | ref. |
| 0.1-0.2 million yuan (16,000-32,000 dollars) | 0.89 (.77-1.03) | 1.12 (.91-1.36) | 0.76 (.61-.93)a |
| ≥0.2 million yuan (≥32 000 dollars) | 0.95 (.81-1.12) | 1.24 (.99-1.56) | 0.70 (.54-.90)a |
| Region | |||
| East | ref. | ref. | ref. |
| South | 0.92 (.80-1.06) | 0.47 (.39-.57)a | 2.19 (1.75-2.74)a |
| West | 0.57 (.31-1.05) | 0.46 (.20-1.06) | 0.84 (.34-2.09) |
| North | 1.10 (.93-1.30) | 0.51 (.40-.65)a | 3.05 (2.37-3.94)a |
| Midland | 0.62 (.50-.77)a | 0.98 (.73-1.31) | 0.61 (.42-.87)a |
| Variables . | All (n = 48 078) . | Girls (n = 17 039) . | Boys (n = 31 039) . |
|---|---|---|---|
| OR (95 CI%) . | OR (95 CI%) . | OR (95 CI%) . | |
| Gender | |||
| Boy | ref. | — | — |
| Girl | 3.36 (2.90-3.89)a | — | — |
| Age | |||
| 6 years | ref. | ref. | ref. |
| 7 years | 2.04 (1.78-2.35)a | 2.14 (1.81-2.54)a | 1.97 (1.52-2.55)a |
| 8 years | 3.14 (2.58-3.82)a | — | 3.19 (2.50-4.08)a |
| BMI | |||
| Normal weight | ref. | ref. | ref. |
| Overweight | 2.22 (1.92-2.57)a | 2.64 (2.16-3.24)a | 1.86 (1.50-2.29)a |
| Obesity | 3.26 (2.80-3.79)a | 5.58 (4.52-6.87)a | 1.91 (1.53-2.38)a |
| Birth weight | |||
| 2500-3999 g | ref. | ref. | ref. |
| <2500 g | 0.86 (.59-1.24) | 0.89 (.54-1.47) | 0.88 (.50-1.54) |
| ≥4000 g | 1.25 (1.03-1.51)a | 1.03 (.75-1.40) | 1.43 (1.13-1.82)a |
| Ate coarse grains | |||
| No | ref. | ref. | ref. |
| Yes | 0.98 (.84-1.14) | 0.93 (.75-1.15) | 1.01 (.81-1.26) |
| Ate snacks | |||
| Healthy | ref. | ref. | ref. |
| Unhealthy | 1.44 (1.23-1.68)a | 1.45 (1.15-1.83)a | 1.40 (1.12-1.74)a |
| Ate midnight snacks | |||
| No | ref. | ref. | ref. |
| Yes | 0.93 (0.77-1.12) | 0.77 (.59-1.02) | 1.04 (.81-1.34) |
| Physical time | |||
| <90 minutes/week | ref. | ref. | ref. |
| 90-120 minutes/week | 0.85 (.74-.99)a | 0.83 (.68-1.02) | 0.93 (.75-1.15) |
| 120-150 minutes/week | 0.85 (.70-1.04) | 0.80 (.61-1.05) | 0.95 (.72-1.26) |
| ≥150 minutes/week | 0.97 (.82-1.15) | 0.94 (.75-1.19) | 1.00 (.79-1.27) |
| Maternal education | |||
| Primary school and below | ref. | ref. | ref. |
| High school | 1.24 (.89-1.74) | 1.81 (.97-3.36) | 1.04 (.70-1.55) |
| Undergraduate and above | 0.89 (.63-1.26) | 1.42 (.75-2.67) | 0.65 (.42-0.99) |
| Annual family income | |||
| <0.1 million yuan (<16 000 dollars) | ref. | ref. | ref. |
| 0.1-0.2 million yuan (16,000-32,000 dollars) | 0.89 (.77-1.03) | 1.12 (.91-1.36) | 0.76 (.61-.93)a |
| ≥0.2 million yuan (≥32 000 dollars) | 0.95 (.81-1.12) | 1.24 (.99-1.56) | 0.70 (.54-.90)a |
| Region | |||
| East | ref. | ref. | ref. |
| South | 0.92 (.80-1.06) | 0.47 (.39-.57)a | 2.19 (1.75-2.74)a |
| West | 0.57 (.31-1.05) | 0.46 (.20-1.06) | 0.84 (.34-2.09) |
| North | 1.10 (.93-1.30) | 0.51 (.40-.65)a | 3.05 (2.37-3.94)a |
| Midland | 0.62 (.50-.77)a | 0.98 (.73-1.31) | 0.61 (.42-.87)a |
P < .05.
The factors associated with precocious puberty (multiple logistic regression)
| Variables . | All (n = 48 078) . | Girls (n = 17 039) . | Boys (n = 31 039) . |
|---|---|---|---|
| OR (95 CI%) . | OR (95 CI%) . | OR (95 CI%) . | |
| Gender | |||
| Boy | ref. | — | — |
| Girl | 3.36 (2.90-3.89)a | — | — |
| Age | |||
| 6 years | ref. | ref. | ref. |
| 7 years | 2.04 (1.78-2.35)a | 2.14 (1.81-2.54)a | 1.97 (1.52-2.55)a |
| 8 years | 3.14 (2.58-3.82)a | — | 3.19 (2.50-4.08)a |
| BMI | |||
| Normal weight | ref. | ref. | ref. |
| Overweight | 2.22 (1.92-2.57)a | 2.64 (2.16-3.24)a | 1.86 (1.50-2.29)a |
| Obesity | 3.26 (2.80-3.79)a | 5.58 (4.52-6.87)a | 1.91 (1.53-2.38)a |
| Birth weight | |||
| 2500-3999 g | ref. | ref. | ref. |
| <2500 g | 0.86 (.59-1.24) | 0.89 (.54-1.47) | 0.88 (.50-1.54) |
| ≥4000 g | 1.25 (1.03-1.51)a | 1.03 (.75-1.40) | 1.43 (1.13-1.82)a |
| Ate coarse grains | |||
| No | ref. | ref. | ref. |
| Yes | 0.98 (.84-1.14) | 0.93 (.75-1.15) | 1.01 (.81-1.26) |
| Ate snacks | |||
| Healthy | ref. | ref. | ref. |
| Unhealthy | 1.44 (1.23-1.68)a | 1.45 (1.15-1.83)a | 1.40 (1.12-1.74)a |
| Ate midnight snacks | |||
| No | ref. | ref. | ref. |
| Yes | 0.93 (0.77-1.12) | 0.77 (.59-1.02) | 1.04 (.81-1.34) |
| Physical time | |||
| <90 minutes/week | ref. | ref. | ref. |
| 90-120 minutes/week | 0.85 (.74-.99)a | 0.83 (.68-1.02) | 0.93 (.75-1.15) |
| 120-150 minutes/week | 0.85 (.70-1.04) | 0.80 (.61-1.05) | 0.95 (.72-1.26) |
| ≥150 minutes/week | 0.97 (.82-1.15) | 0.94 (.75-1.19) | 1.00 (.79-1.27) |
| Maternal education | |||
| Primary school and below | ref. | ref. | ref. |
| High school | 1.24 (.89-1.74) | 1.81 (.97-3.36) | 1.04 (.70-1.55) |
| Undergraduate and above | 0.89 (.63-1.26) | 1.42 (.75-2.67) | 0.65 (.42-0.99) |
| Annual family income | |||
| <0.1 million yuan (<16 000 dollars) | ref. | ref. | ref. |
| 0.1-0.2 million yuan (16,000-32,000 dollars) | 0.89 (.77-1.03) | 1.12 (.91-1.36) | 0.76 (.61-.93)a |
| ≥0.2 million yuan (≥32 000 dollars) | 0.95 (.81-1.12) | 1.24 (.99-1.56) | 0.70 (.54-.90)a |
| Region | |||
| East | ref. | ref. | ref. |
| South | 0.92 (.80-1.06) | 0.47 (.39-.57)a | 2.19 (1.75-2.74)a |
| West | 0.57 (.31-1.05) | 0.46 (.20-1.06) | 0.84 (.34-2.09) |
| North | 1.10 (.93-1.30) | 0.51 (.40-.65)a | 3.05 (2.37-3.94)a |
| Midland | 0.62 (.50-.77)a | 0.98 (.73-1.31) | 0.61 (.42-.87)a |
| Variables . | All (n = 48 078) . | Girls (n = 17 039) . | Boys (n = 31 039) . |
|---|---|---|---|
| OR (95 CI%) . | OR (95 CI%) . | OR (95 CI%) . | |
| Gender | |||
| Boy | ref. | — | — |
| Girl | 3.36 (2.90-3.89)a | — | — |
| Age | |||
| 6 years | ref. | ref. | ref. |
| 7 years | 2.04 (1.78-2.35)a | 2.14 (1.81-2.54)a | 1.97 (1.52-2.55)a |
| 8 years | 3.14 (2.58-3.82)a | — | 3.19 (2.50-4.08)a |
| BMI | |||
| Normal weight | ref. | ref. | ref. |
| Overweight | 2.22 (1.92-2.57)a | 2.64 (2.16-3.24)a | 1.86 (1.50-2.29)a |
| Obesity | 3.26 (2.80-3.79)a | 5.58 (4.52-6.87)a | 1.91 (1.53-2.38)a |
| Birth weight | |||
| 2500-3999 g | ref. | ref. | ref. |
| <2500 g | 0.86 (.59-1.24) | 0.89 (.54-1.47) | 0.88 (.50-1.54) |
| ≥4000 g | 1.25 (1.03-1.51)a | 1.03 (.75-1.40) | 1.43 (1.13-1.82)a |
| Ate coarse grains | |||
| No | ref. | ref. | ref. |
| Yes | 0.98 (.84-1.14) | 0.93 (.75-1.15) | 1.01 (.81-1.26) |
| Ate snacks | |||
| Healthy | ref. | ref. | ref. |
| Unhealthy | 1.44 (1.23-1.68)a | 1.45 (1.15-1.83)a | 1.40 (1.12-1.74)a |
| Ate midnight snacks | |||
| No | ref. | ref. | ref. |
| Yes | 0.93 (0.77-1.12) | 0.77 (.59-1.02) | 1.04 (.81-1.34) |
| Physical time | |||
| <90 minutes/week | ref. | ref. | ref. |
| 90-120 minutes/week | 0.85 (.74-.99)a | 0.83 (.68-1.02) | 0.93 (.75-1.15) |
| 120-150 minutes/week | 0.85 (.70-1.04) | 0.80 (.61-1.05) | 0.95 (.72-1.26) |
| ≥150 minutes/week | 0.97 (.82-1.15) | 0.94 (.75-1.19) | 1.00 (.79-1.27) |
| Maternal education | |||
| Primary school and below | ref. | ref. | ref. |
| High school | 1.24 (.89-1.74) | 1.81 (.97-3.36) | 1.04 (.70-1.55) |
| Undergraduate and above | 0.89 (.63-1.26) | 1.42 (.75-2.67) | 0.65 (.42-0.99) |
| Annual family income | |||
| <0.1 million yuan (<16 000 dollars) | ref. | ref. | ref. |
| 0.1-0.2 million yuan (16,000-32,000 dollars) | 0.89 (.77-1.03) | 1.12 (.91-1.36) | 0.76 (.61-.93)a |
| ≥0.2 million yuan (≥32 000 dollars) | 0.95 (.81-1.12) | 1.24 (.99-1.56) | 0.70 (.54-.90)a |
| Region | |||
| East | ref. | ref. | ref. |
| South | 0.92 (.80-1.06) | 0.47 (.39-.57)a | 2.19 (1.75-2.74)a |
| West | 0.57 (.31-1.05) | 0.46 (.20-1.06) | 0.84 (.34-2.09) |
| North | 1.10 (.93-1.30) | 0.51 (.40-.65)a | 3.05 (2.37-3.94)a |
| Midland | 0.62 (.50-.77)a | 0.98 (.73-1.31) | 0.61 (.42-.87)a |
P < .05.
Being a girl was associated with a higher risk of precocious puberty compared to being a boy (OR [95% CI]: 3.36 [2.90-3.89]).
Discussion
A progressive decline in puberty onset has been described in most countries over the past 3 decades. For example, median age of breast development was 10.3 years in Poland (13), 9.7 years in Iran (14), 9.6 years in Thailand (15), 10.9 years in India (16), and 10.5 years in Nigeria (17). This corresponds to 9.65 years in the current study, which not only confirms these observations, but also adds several important findings. In particular, compared with a previous assessment a decade earlier (PRODY study 2009-2010), there was a gender difference, with male pubertal onset occurring 0.6 years earlier while there was no change in the median age of breast development in girls (4). However, the earliest age that breast development occurred in girls declined, with the 3rd percentile (6.3 years) declining by 0.5 years. While this increased prevalence of breast development at young ages in girls may reflect central pubertal onset, there are several alternative possibilities, such as younger normal pubertal variants (premature thelarche), exposure to exogenous sources of estrogen, and obesity. Unlike testicular enlargement, which is a more direct assessment of central pubertal activation, identification of puberty in girls focuses on signs of estrogen action such as breast development. Puberty is also defined as progressive signs of sex steroid exposure, and this was not possible to assess in a large cross-sectional study. Nevertheless, the earlier onset of breast development is concerning and requires further investigation.
A secular trend toward the earlier onset of breast development in girls has been confirmed by recent studies. In a meta-analysis, Camilla Eckert-Lind et al found that the age at thelarche had decreased by an average of almost 3 months per decade from 1977 to 2013 (3). Based on precocious puberty being defined as breast development occurring < 8 years in girls, an increasing proportion of normal girls would be referred for investigation and management of early puberty. However, as noted above, puberty is a progressive, sequential chain of events and cross-sectional data cannot readily define those who are in puberty. There are also practical difficulties accurately assessing breast development in overweight or obese girls. However, using the standard criteria of female precocious puberty starting before 8 years, over 10% of Chinese girls would be investigated for precocious puberty, with the vast majority likely being normal early pubertal variants. Using a cutoff of 8 years may also result in increasing missed cases of pathological causes of precocious puberty and further studies will be required to confirm whether a change in the definition of precocious puberty is appropriate.
Similar to girls, there was a similar trend in male puberty with a secular decline in the age of pubertal onset. Sørensen et al similarly, reported that pubertal onset based on testicular volume assessments in healthy Caucasian boys had declined by approximately 3 months from 1991 to 2006 (18). Previous studies have reported that boys diagnosed with central precocious puberty had a high prevalence (50%-70%) of identifiable pathological changes (19). This may be changing with the lower ages of puberty in boys and a recent study reported an increasing prevalence of idiopathic male central precocious puberty (20).
Along with the increasing prevalence of early male puberty, obesity rates have increased in parallel and the growing prevalence of obesity and overweight in Chinese boys may be accelerating pubertal onset, although this association is stronger in girls in other papers (21). Obesity has been closely linked to early sexual development. Previously, we reported a trend toward earlier pubertal development in obese compared to normal weight boys (4). Similarly, in this study, we confirmed that obesity and overweight were positively associated with precocious puberty compared to normal weight boys and girls. We also confirmed that the increased intake of high-calorie, low-nutrient, or high volumes of sugar foods increased the risk of precious puberty. In addition, children born with a birth weight higher than 4000 g had an increased risk of earlier puberty, particularly for boys, suggesting an in-utero effect. The importance of the fetal environment and early life has been highlighted previously with strong evidence suggesting prenatal overnutrition is associated with early obesity and early puberty (22). Furthermore, we estimated the prevalence of precocious puberty and obesity in different regions and found that the Northern and Southern regions had the highest rate of obesity in boys, and also the highest rate of precocious puberty.
Like many societies, Chinese families are concerned about early puberty. It is well established that earlier menarche has negative consequences for girls, including psycho-socially and later body composition, and these concerns over early puberty are not unwarranted (23). Thus, understanding the underlying environmental factors influencing sexual maturation is important.
In contrast, obesity and early pubertal onset prevalence did not correlate as well for girls, although the prevalence of overweight and obesity increased more quickly in regions where breast development occurred earlier. It is also likely that breast development did not always reflect central puberty, unlike testicular development, and thus the association between obesity and puberty onset may not have been as strong in girls (24). The reasons for these differences are not entirely clear but may reflect regional differences in attitudes toward obesity and in particular differences in gender perception of larger body size. In the North and South of China larger size in males is more acceptable, while this is not the case in Eastern areas where a more asthenic look in males is considered attractive (25, 26).
Despite the secular trend toward earlier gonadarche as reflected in the onset of breast development and testicular enlargement, the age of pubarche, in Chinese children has remained essentially stable. This is consistent with the studies published by Herman-Giddens et al and Rosenfield et al that found the onset age of pubic hair development in US girls remained stable over the last 25 years (27, 28). These findings suggest that the mechanisms underlying pubarche have not substantially changed. In this Chinese population pubic hair occurred over 1.5 years later than breast development in girls. In other studies, body hair and pubic hair were reported at occurring at a similar age (29). These differences likely reflect ethnic variation with Han Chinese having relatively less body hair than other ethnicities. They do highlight the need to examine ethnic appropriate populations for normative data.
The strengths of this study include the large sample size and wide national distribution of assessments. This was the largest population-based national cross-sectional research of pubertal development in Chinese children and adolescents, aged 3 to 18 years with over 230 000 children recruited. Moreover, by linking pubertal and auxological data together, this extremely large dataset was able to define not only pubertal development across different regions of China but also investigate whether these changes are associated with excess adiposity. The methodology was comprehensive and in particular involved clinical evaluation of puberty and auxological assessments by trained pediatric endocrinologists as opposed to self-report. Extensively validated questionnaires enabled a more detailed assessment of other socioeconomic and lifestyle factors.
There are several potential limitations of this study. Sexual maturation is a developmental process, and a cross-sectional study cannot obtain information on the rate at which a child passes through pubertal stages. As the definition of true puberty requires ongoing pubertal progression this cannot be fully confirmed by the presence of one sign. However, causes of bilateral testicular enlargement other than puberty, are uncommon and this makes the diagnosis of puberty in boys more secure than in girls. Breast development in girls is relatively common and can be due to estrogen exposure unrelated to puberty. Thus, a longitudinal study of a similar population is needed to confirm whether puberty is truly getting younger. There were also many professionals performing the assessments of this in large cohort studies. While validation between the teams was performed, there may have been differences in the assessments.
In summary, this study demonstrates that boys are developing puberty earlier while the data for girls are less clear, with the median onset of breast development and menarche not changing appreciably over the past decade. However, a small subgroup of girls were experiencing breast development earlier. Whether this is due to puberty onset or not remains unclear. These data suggest that the current normative reference age of precocious puberty may not be applicable. As expected, there was an impact of obesity and overweight on earlier puberty onset that was stronger in boys than in girls.
Acknowledgments
We would like to thank all participating medical centers, all participants, and all participants’ family members who took part in the study.
Funding
This work was supported by the National Key Research and Development Program of China (No. 2021YFC2701900, No. 2016YFC1305301), the National Natural Science Foundation of China (No. 81570759), the Fundamental Research Funds for the Central Universities (No. 2020XZZX002-22), Zhejiang Science and Technology Plan Project (No. 2020C03121).
Author Contributions
J.F., X.L., and K.H. contributed to the design and conduct of the study during all phases. X.L., S.S., and Y.Y. contributed to the processing and the statistical handling of the data to generate the results. All authors contributed to the acquisition and interpretation of the data. J.F. initiated the project, X.L. and P.H. drafted the article and J.F., K.H., and C.W. secured the funding. All authors revised the manuscript and approved the final version before submission.
Disclosures
The authors have nothing to disclose.
Data Availability
The data sets generated and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Abbreviations
Author notes
Xinyi Liang and Ke Huang equally contributed to the manuscript.



