-
PDF
- Split View
-
Views
-
Cite
Cite
Daniel J Green, Lauren C Chasland, Louise H Naylor, Bu B Yeap, New Horizons: Testosterone or Exercise for Cardiometabolic Health in Older Men, The Journal of Clinical Endocrinology & Metabolism, Volume 108, Issue 9, September 2023, Pages 2141–2153, https://doi.org/10.1210/clinem/dgad175
Close - Share Icon Share
Abstract
Middle-aged and older men have typically accumulated comorbidities, are increasingly sedentary, and have lower testosterone concentrations (T) compared to younger men. Reduced physical activity (PA) and lower T both are associated with, and may predispose to, metabolically adverse changes in body composition, which contribute to higher risks of cardiometabolic disease. Exercise improves cardiometabolic health, but sustained participation is problematic. By contrast, rates of T prescription have increased, particularly in middle-aged and older men without organic diseases of the hypothalamus, pituitary, or testes, reflecting the unproven concept of a restorative hormone that preserves health. Two recent large randomized trials of T, and meta-analyses of randomized trials, did not show a signal for adverse cardiovascular (CV) events, and T treatment on a background of lifestyle intervention reduced type 2 diabetes by 40% in men at high risk. Men with both higher endogenous T and higher PA levels have lower CV risk, but causality remains unproven. Exercise training interventions improve blood pressure and endothelial function in middle-aged and older men, without comparable benefits or additive effects of T treatment. Therefore, exercise training improves cardiometabolic health in middle-aged and older men when effectively applied as a supervised regimen incorporating aerobic and resistance modalities. Treatment with T may have indirect cardiometabolic benefits, mediated via favorable changes in body composition. Further evaluation of T as a pharmacological intervention to improve cardiometabolic health in aging men could consider longer treatment durations and combination with targeted exercise programs.
Physical inactivity is now considered a key risk factor for noncommunicable diseases and the fourth leading cause of death worldwide (1). There is clear evidence of an inverse linear dose-response relationship between physical activity (PA) volume and all-cause mortality (2), with the greatest improvement in cardiovascular (CV) risk occurring when moving from being sedentary to engaging in low levels of activity (3). One prospective cohort study (n = 416 175) reported a 14% decrease in all-cause mortality and 3-year longer life expectancy in those who engaged in 15 minutes of daily PA, compared to being inactive (4). Moreover, the lower all-cause mortality associated with moderate levels of PA may be more apparent in older (aged 60-74 years, −34%) than younger adults (aged 35-59 years, −26%) (5), and recent studies using wearable technologies suggest a greater decrease in all-cause mortality than historical studies that relied on self-report (6). The beneficial influence of PA may be mediated by improvements in cardiorespiratory fitness and indices of vascular health such as blood pressure (BP) and endothelial function, and via exercise-induced increases in muscle mass, strength, or function. Nonetheless, increasing PA remains a population health challenge (7), and pharmacological strategies that emulate the effects of exercise have recently been mooted.
Testosterone (T) plays an important role in sexual development, behavior, and body composition, and symptoms of androgen deficiency include decreased energy levels, low sexual desire, low mood, irritability, poor concentration, and reduced muscle mass and strength (8, 9). T is the classic anabolic hormone that, like resistance exercise, acts to increase muscle mass (10). Greater muscle mass protects against the development of sarcopenia and frailty, and also confers metabolic benefits. Transgenic mice expressing a constitutively active protein kinase, Akt1, which stimulates muscle hypertrophy via growth of type IIb muscle fibers, exhibit reduced fat accumulation and increased metabolic rate, hepatic fatty acid oxidation, and ketone body production (11). Furthermore, mice with a null mutation of myostatin, an endogenous inhibitor of muscle growth, are resistant to diet-induced obesity and have markedly improved insulin sensitivity (12). Highlighting the metabolic importance of muscle mass, a monoclonal antibody targeting the activin type II receptor to facilitate skeletal muscle growth, increased lean mass, reduced fat mass, and improved glycemia in a phase 2 trial of people with diabetes (13).
T treatment has become increasingly prevalent in recent decades (14), but the safety of its use beyond men with pathological androgen deficiency remains unclear. Nonetheless, T prescription has increased 11-fold in recent decades, partly due to the unproven premise of a restorative health-preserving hormone (14). Nonmedically prescribed and off-label use may also occur, with some middle-aged and older men considering T supplementation an antiaging strategy (15). Interestingly, although T concentrations can decrease with age (16, 17), they are higher in older men who engage in a healthy lifestyle, inclusive of regular exercise, compared to men who have less healthy lifestyles (18). The influence of higher endogenous circulating T concentrations, and the effects of pharmacological intervention with exogenous T treatment, on cardiometabolic health in middle-aged and older men are pivotal to better understanding of the endocrinology of male aging (19). An equally important question is whether a combination of T treatment with exercise training might achieve optimal cardiometabolic outcomes in aging men.
This review compares the independent, and possible combined, effects of T and exercise on cardiometabolic health and longevity in men. It considers the effects of these factors from an epidemiological perspective, and in terms of their effects on physiological outcomes such as BP and vascular function, as demonstrated in randomized trials. The consequences of anabolic steroid abuse are beyond the scope of this review.
Is Endogenous Testosterone Associated With Cardiometabolic Health?
Older men on average have lower T concentrations compared to younger and middle-aged men, in conjunction with greater accumulation of medical comorbidities (20-22). Lower T concentrations have been inversely associated with CV risk factors such as body mass index (BMI), waist circumference, diabetes, and hypertension (18, 20, 23, 24). Men with lower T concentrations are also more likely to have central adiposity and insulin resistance, and to develop metabolic syndrome or type 2 diabetes (25-27). In a study of 195 men aged 70 years and older, calculated free T values (but not total T concentrations) were inversely associated with carotid intima media thickness (CIMT) progression (28). However, in another study of 1101 men aged 59 years, total T concentrations were inversely associated with carotid plaque area in a cross-sectional analysis, but there were no prospective associations with changes in plaque area or CIMT (29). While different studies have produced contrasting results (for review, see (19)), in the Osteoporotic Fractures in Men (MrOS) Sweden study of 2416 men aged 69 to 81 years, lower baseline T concentrations (from blood samples mostly collected in the morning) were associated with increased risk of CV events (30). In the Health In Men Study (HIMS) of 3690 men aged 70 to 89 years (with early-morning blood sampling), lower baseline T concentration was associated with higher risk of incident stroke but not myocardial infarction (MI) (31, 32). However, in 210 700 men from the UK Biobank aged 40 to 69 years followed for 9 years, baseline T concentrations (from blood samples collected throughout the day) were not associated with incident CV events, nor with incidence of MI, stroke, or heart failure when analyzed as distinct outcomes (33). Therefore, associations of lower endogenous T concentrations with CV events may be present in older but not middle-aged men, and driven more by stroke rather than MI.
Some studies suggest that lower T concentrations are associated with increased risk of CV and all-cause mortality (34-38), whereas others do not (39, 40) (for detailed review, see (19)). Of note, an analysis of 149 436 men aged 40 to 69 years from the UK Biobank followed for 11 years showed no association of baseline T concentrations with CV mortality (41). In that analysis, men with lower baseline T concentrations had higher all-cause mortality, but the association was nonlinear. A similar nonlinear (U-shaped) association of baseline T concentrations with all-cause mortality was seen in the HIMS study of men aged 70 years and older (34). Thus, lower endogenous T concentrations may be related to all-cause rather than CV mortality risk, but the nonlinear nature of the association merits consideration and causality remains unproven.
Does Exogenous Testosterone Treatment Affect Cardiometabolic Health?
The Testosterone in Older Men with Mobility Limitations (TOM) trial of 209 men aged 65 years and older with mobility limitations and baseline T concentration 100 to 350 ng/dL (3.5-12.1 nmol/L) or calculated free T greater than 173 pmol/L was discontinued in response to a higher rate of loosely defined adverse CV events in the T (n = 23) vs the placebo group (n = 5) (42). However, another randomized controlled trial (RCT) in 274 men aged 65 years and older, who were frail or intermediate-frail with baseline T less than 12 nmol/L (346 ng/dL) or calculated free T less than 250 pmol/L, found no increase in cardiovascular adverse events in T-treated men and reported an improvement in physical function (assessed using gait, balance, and submaximal walking tests) (43). The Testosterone Trials (T Trials) randomly assigned 790 men aged 65 years and older with baseline T concentration less than 275 ng/dL (9.54 nmol/L) and symptoms of decreased libido, difficulty walking or climbing stairs, or low vitality, to 12 months’ T treatment vs placebo, finding modest improvement in sexual function but no benefit for walking distance or vitality (44). T treatment compared to placebo was associated with improved sexual desire, erectile function, and overall sexual activity, volumetric bone mineral density and estimated bone strength, and correction of unexplained anemia (44, 45). In T Trials 7 men in each arm had major CV adverse events (MACE: comprising myocardial infarction, stroke, or death from CV causes) during the treatment period.
In the T Trials cardiovascular substudy, which analyzed 73 men receiving T and 65 receiving placebo, T treatment was associated with a greater increase in noncalcified coronary artery plaque volume, and no change in coronary artery calcium score (46). No MACE events occurred in either group. The groups were unbalanced: Placebo-treated men had greater plaque volumes at baseline and at the end of the 1-year study, thus larger and longer duration studies of this end point would be important (47). By comparison, in the Testosterone Effects on Atherosclerosis Progression in Aging Men (TEAAM) trial involving 308 men aged 60 years and older, T treatment for 3 years did not alter the rate of change in coronary artery calcium score, nor the rate of progression of CIMT, compared with placebo (48).
Testosterone for the Prevention of type 2 Diabetes Mellitus (T4DM) enrolled 1007 men aged 50 to 74 years, waist 95 cm or greater, and baseline T concentration of less than or equal to 14 nmol/L (404 ng/dL), with impaired glucose tolerance or newly diagnosed type 2 diabetes (49, 50). All men received a lifestyle program (Weight Watchers) and were randomly assigned to T treatment or placebo, for 2 years. Men in the T arm had a 40% lower risk of diabetes at 2 years, defined using oral glucose tolerance testing (OGTT) (50). T treatment was associated with favorable changes in body composition: Men in the placebo group lost 1.9 kg of fat but also lost 1.3 kg of muscle mass, whereas men in the T arm lost 4.6 kg of fat and gained 0.4 kg of muscle. There was no signal for excess CV adverse events in T4DM, with 17 men in the placebo and 12 in the T arms experiencing a MACE during the trial (50). But as in the T Trials, and all other T RCTs reported to date, the number of men experiencing such events was too low to permit definitive conclusions regarding safety of T treatment.
In T4DM, T treatment resulted in lower fasting glucose and a reduced glucose increment at 2 hours following OGTT, with no difference in glycated hemoglobin A1c (HbA1c) (50). In T Trials T treatment reduced insulin resistance (assessed using the homeostatic model assessment, HOMA-IR) with no changes in fasting glucose nor HbA1c (51). The TIMES2 Study using transdermal T treatment vs placebo in 220 men with type 2 diabetes and/or metabolic syndrome also showed improvement in HOMA-IR and stable HbA1c values at 6 and 12 months (52). The lack of change in HbA1c (despite improved fasting glucose concentrations and OGTT responses in T4DM) may relate to T effects on red blood cell stability, or possibly on deglycation mechanisms (53-55). There are key differences between participants in T Trials and T4DM: Men in T4DM were drawn from a wider age range, had central adiposity (reflected in measured waist circumference), and impaired glucose tolerance or newly diagnosed type 2 diabetes, with a higher cutoff for baseline T concentrations. Importantly, T4DM involved a longer (2-year) intervention duration, and all T4DM participants received a background lifestyle intervention, not a feature of T Trials or TIMES2.
One meta-analysis by Xu et al (56) of 27 RCTs with 2994 participants associated T with increased risk of CV-related events. However, contemporary meta-analyses (57-63) do not associate T treatment with increased CV risk. For example, Corona et al (57) in a meta-analysis of 93 RCTs with 4653 participants receiving active treatment and 3826 placebo found no clear effect of T on the incidence of CV events, with an odds ratio for MACE of 0.97 (95% CI, 0.64-1.46). Hudson et al (58) performed a 2-stage individual participant data meta-analysis, including 35 primary studies with 5601 participants, of which 17 provided individual participant data on 3431 participants, finding no evidence that T increased short- to medium-term CV risks, while noting the need for studies to evaluate long-term safety (58).
Retrospective case-control studies, typically using large health insurance databases comparing outcomes in men receiving T prescriptions vs those not prescribed T, have major limitations including missing data on indications for prescribing, absence of randomization, and other possible biases (64). Such studies have yielded contrasting results (65-75). Some have associated T prescription with CV events (66, 67, 73), others reported neutral associations or reduced risk of CV events and/or mortality (65, 68-70, 72, 74, 75). Some studies found differential associations with CV risk based on duration of therapy (70) and whether or not “normal” T concentrations were reported on treatment (68, 69, 74).
While not the primary focus of this review, erythrocytosis and elevation of hematocrit are recognized adverse events related to T treatment (9, 59, 61, 76, 77). The presence of prostate cancer is a contraindication to T therapy (8, 9). Evidence to date suggests T treatment does not increase the risk of developing prostate cancer beyond that expected in eugonadal men, but it may be associated with detection of subclinical prostate cancer via prostate-specific antigen testing and prostate biopsy (8, 9, 59, 61, 63, 77). The overall frequency of serious adverse events including prostate cancer events and MACE in large RCTs has been reassuringly low, although such trials, including T Trials and T4DM, generally excluded men at very high risk (44, 49, 50, 63). The only T RCT powered to examine CV events as a primary end point, the TRAVERSE study, recruited hypogonadal men aged 45 to 80 years with T less than 300 ng/dL (10.4 nmol/L) with evidence of CV disease (CVD) or at increased risk for CVD (78). It commenced in 2018 and was completed in November 2022 with 5246 participants (79). Its results will provide vital information both for CV and prostate safety of T therapy.
Are Higher Levels of Physical Activity and/or Exercise Associated With Better Cardiovascular Outcomes?
Inverse associations between PA levels and CV risk factors (80), risk of major vascular events (81-83), and mortality (84-88) are well established. Landmark studies in the 1960s and 1970s highlighted the inverse association between job-related PA and CVD risk by demonstrating that those in more active roles (eg, bus conductors and railroad switchmen) had a lower risk of CVD than their more sedentary counterparts (bus drivers and railroad clerks) (89, 90). The subsequent Harvard Alumni Study (91) was one of the first to suggest a dose-response between PA and risk of heart attack, while a recent prospective cohort study in 437 378 participants demonstrated that moderate to high occupational PA contributed to longevity in men after a 28-year follow-up (92). By assessing 44 studies, Lee and Skerrett (2) concluded there was clear evidence of an inverse linear dose-response relationship between the volume of PA and all-cause mortality. Collectively, these results highlight the effect of PA on CV risk and all-cause mortality into middle and older age. The feasibility of implementing lifestyle interventions in sedentary older adults was demonstrated in the LIFE Study, in which 818 participants randomly assigned to a moderate-intensity structured PA program had lower risk of major mobility disability compared to 817 who received a health education program, over a mean follow-up of 2.6 years (93).
Do Testosterone and Exercise Have Additive Effects on Cardiometabolic Health in Aging Men?
Few epidemiological or interventional studies have simultaneously assessed the effect of T and PA levels on measures of CV risk and/or mortality, a considerable gap in the literature. A large study using data from the UK Biobank (n = 208 677) recently evaluated the effect of sociodemographic, lifestyle, and medical factors on T levels in men aged 40 to 69 years (94). This study reported that T was lower in those with a less favorable combination of sociodemographic, lifestyle, and behavioural factors, including insufficient physical activity (to the order of 2 nmol/L [58 ng/dL]). Men with a diagnosis of CVD or diabetes also had lower T concentrations (∼ 0.3-1.0 nmol/L lower). This study suggests that lower PA, as well as chronic conditions such as CVD and diabetes, may be associated with lower T levels, highlighting their interrelated nature.
Two epidemiological studies have indicated that men with the combination of higher endogenous T concentrations and higher self-reported PA levels tend to have more favorable cardiometabolic outcomes. In a study of 1649 men with a mean age of 49.8 years (95), higher T concentrations and higher PA levels were consistently associated with lower BMI, waist circumference, and odds of metabolic syndrome. This is congruent with previous work assessing T concentrations and PA levels independently (18, 20, 23, 24, 80). However, improvements in CV risk factors did not translate into risk reduction of CVD events or death. Another epidemiological study (96) using a larger data set of older men (HIMS, n = 3351 age = 77 years) reported that men with higher circulating androgens and higher PA levels had the lowest BMI, waist circumference, and risk of metabolic syndrome, reinforcing earlier findings. In this study, which had twice the number of CVD events and almost 4 times the number of CVD deaths compared to the previous study, men with higher circulating androgens (T or dihydrotestosterone, DHT) and higher PA levels had the lowest risk of dying of CVD (96). These results provided the rationale for the design and implementation of a 2 × 2 factorial randomized, double-blind, placebo-controlled trial investigating the effects of combining T treatment with supervised, center-based EXercise training (the TEX trial) in middle to older-aged men with low-normal serum T levels (97-99) (Fig. 1).
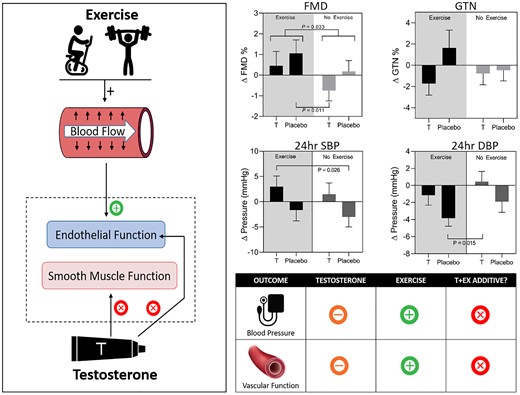
Summary of our 2 × 2 factorial, randomized, double-blind, placebo-controlled TEX (testosterone and exercise) trial. Left: Hypothesized synergistic impacts of T and Ex on vascular function: Ex has endothelium-dependent effects on vascular function, while some evidence suggests that T affects smooth muscle function. Right upper: Changes from baseline following the 12-week intervention in flow-mediated dilation (FMD), glyceryl trinitrate (GTN)-mediated dilation, and 24-hour blood pressure (BP) responses. Right lower: Infographic summary of results: exercise benefits BP and vascular function, T does not provide additive benefit. **P less than .001, *P less than .05 for week 12 change from baseline compared with placebo + no exercise group change. “+” indicates improvement, “-” indicates no significant change, “x” indicates no synergistic benefit.
Collectively, epidemiological studies suggest that men with lower endogenous T concentrations have poorer health outcomes, whereas men engaging in healthy lifestyle behaviors are more likely to have higher T concentrations and better outcomes. This raises the question as to whether T may be a modifiable risk factor for poor health in aging men. Epidemiological studies that assess the interaction between androgens and PA levels, and their association with CVD risk factors, are limited, but suggest that higher circulating androgens (T or DHT) combined with higher PA levels may be associated with favorable cardiometabolic outcomes. While outside the scope of this review, it is also possible that higher T concentrations or treatment with T, combined with higher PA levels, may have additional potential benefits on sexual and physical function, and well-being (61, 63). Important gaps exist in our understanding of the interrelationships between T concentrations, physical in/activity, and CV risk. We now consider what is known about the effect of T, exercise, and their combination on key measures of CV risk, namely BP and vascular health and function.
What Are the Effects of Testosterone, Exercise, and Their Combination on Blood Pressure?
High BP is recognized as a leading risk factor for mortality, responsible for 13% of deaths globally (1). The prevalence of hypertension increases with age, and it is forecast to affect more than 1.5 billion people worldwide by 2025 (100, 101). A 2-mm Hg lower office systolic BP (SBP) has been associated with a 10% lower risk of stroke and 7% lower risk of ischemic heart disease, in primarily middle-aged adults (102).
What Effect Does Testosterone Have on Blood Pressure?
Traditional single-measurement “office” blood pressure
The majority of cross-sectional studies in men have reported inverse associations between T concentrations and office BP and/or associated lower T concentrations with prevalent or incident hypertension (103). In contrast, 2 studies that assessed the relationship between T and components of metabolic syndrome did not find a relationship between T and hypertension (104, 105). However, a meta-analysis of 20 observational studies examining T concentrations and risk of metabolic syndrome reported an association of lower baseline T concentrations with prevalent but not incident hypertension (25).
Although many T intervention trials assess office BP at baseline, only 4 have reported BP as an outcome (106-109). These studies all report no significant difference in BP following T treatment compared to placebo over durations from 16 to 52 weeks. In contrast, a study conducted by Zitzmann and Nieschlag (110), designed to assess the safety of a new intramuscular injection of long-acting ester T undecanoate (1000 mg at 10-to 14-week intervals) in 66 men aged 17 to 66 years, reported that SBP and diastolic BP (DBP) decreased by 6 and 7 mm Hg, respectively. No placebo group was included, so results must be interpreted with caution.
A meta-analysis of 30 trials comprising 1642 men concluded that T treatment in men with low T concentrations resulted in modest changes in BP (SBP: +0.8 and DBP: +2 mm Hg) (111). However, it is important to note that this meta-analysis also included populations with chronic health conditions (such as chronic obstructive pulmonary disease and cancer) and only 6 of 30 trials reported allocation concealment.
Ambulatory (24-hour) blood pressure
Measurement of 24-hour ambulatory BP (ABP) provides a rich BP profile, inclusive of night-time readings. ABP has greater prognostic accuracy (112), with higher levels of ABP significantly associated with increased risk of CV events (113, 114) and mortality (115, 116).
Studies assessing the association of circulating T with 24-hour ABP are scant and cross-sectional. Malan et al (117) assessed 194 African and White men (aged 25-65 years) stratified into low- (mean 11.7 nmol/L [337 ng/dL]) and high- (mean 22.2 nmol/L [640 ng/dL]) T groups, finding that lower T concentrations were associated with higher (+15 mm Hg SBP) ABP in African, but not White men. In 106 normotensive men, Jimenez et al (118) reported that T concentrations displayed a significant inverse association both with office and 24-hour ABP. These studies suggest that endogenous T concentrations are inversely associated with ABP, although additional larger studies are required.
Few T intervention studies have assessed the effect of T on 24-hour ABP (119, 120). Gittelman et al (119) assessed the safety of a new subcutaneous T enanthate autoinjector and reported ABP outcomes in 133 men aged 18 to 75 years. At week 12, while 24-hour SBP and DBP were higher (3.7 and 1.3 mm Hg, respectively), as were office BPs at week 26 (3.4 and 1.8 mm Hg, respectively), there was no correlation between serum T concentrations and changes in BP. Swerdloff et al (120) compared a new oral formulation to topical T in a randomized open-label study (n = 222; aged 18-65 years). The oral formulation resulted in a mean SBP increase in ABP of 4.9 mm Hg after 16 weeks, which was also reflected in the office SBP (+2.8 mm Hg). There were no significant BP changes in the topical group. These results suggest that short-term (12- to 16-week) T use may increase SBP when measured over a 24-hour period. These results are consistent with studies involving other oral T formulations, one study (n = 138; 26-75 years) associating T treatment with increases in 24-hour SBP (+3.8 mm Hg) and DBP (+1.2 mm Hg) after 4 months’ therapy (121); the other (n = 155; mean age 51.2 years) with increases in 24-hour SBP (+1.7 and +1.8 mm Hg) at 120 and 180 days (122).
What Effect Does Exercise Training Have on Blood Pressure?
Traditional office blood pressure
The evidence supporting the BP-lowering effect of exercise is well established (123-126), and peak bodies such as the American College of Sports Medicine and American Heart Association strongly promote exercise for the prevention, and treatment, of hypertension (127, 128). A meta-analysis comprising 2419 participants concluded that regular aerobic exercise decreased SBP by 3.8 mm Hg and DBP by 2.6 mm Hg over 12 weeks (126). Consequently, PA men have a relative risk of developing hypertension that is 35% to 70% lower than their sedentary peers (80, 129, 130). For those with hypertension, exercise is recommended as initial lifestyle therapy capable of decreasing SBP by 5 to 7 mm Hg (131). Furthermore, a recent meta-analysis including 391 RCTs (n = 39 742) reported the SBP-lowering effects of aerobic or resistance exercise were similar to that of antihypertensive medication (132).
Ambulatory (24-hour) blood pressure
Palatini et al (133) assessed 602 men aged 19 to 45 years and reported that 24-hour SBP was significantly higher in nonexercisers than their exercising (> 1 aerobic session per week) counterparts. Kokkinos et al (134) assessed 407 men (aged 30-79 years) and reported prehypertensive men (resting SBP 120-139 mm Hg or DBP 80-89 mm Hg) with moderate fitness levels had 8/4, 7/3, and 6/4 mm Hg lower daytime, night-time, and 24-hour ABP (SBP/DBP) values compared to men with low fitness levels (134).
In terms of the effect of distinct forms of exercise, Lima et al (135) (n = 44, aged 60-75 years) reported greater decreases in ABP with combined (aerobic and resistance) training than aerobic alone. Saco-Ledo et al (136) conducted a meta-analysis that assessed the effect of exercise on ABP in individuals with hypertension and concluded that aerobic exercise provided the most significant benefits for all measures of ABP compared to resistance or “multicomponent” training. However, only 4 of 15 studies in that analysis included a combined (aerobic and resistance training) program with one involving water exercise (137), another designed to compare continuous vs interval training (138). This highlights the paucity of data surrounding the effects of distinct forms of exercise training on ABP, even more so in healthy middle- and older-aged men.
What Effect Does the Combination of Testosterone Treatment and Exercise Training Have on Blood Pressure?
Few studies combining a T and an exercise intervention have reported BP outcomes, with varied results (139-141). Twelve weeks of supraphysiological T treatment (weekly injection of 3.5 mg/kg body weight) combined with resistance training in men aged 19 to 45 years (n = 21) resulted in an average 10-mm Hg increase in office SBP (140). In contrast, Pasiakos et al (142) reported no significant differences in office BP between male patients (n = 50 aged 18-39 years) receiving T (200 mg weekly injection) or placebo in addition to a 28-day live-in, 55% exercise- and diet-induced energy deficit phase. Broadly in line with these results, Heufelder et al (139) reported decreases in office BP following a 12-month diet and exercise intervention, independent of whether the men (n = 32, aged 57 years) newly diagnosed with type 2 diabetes mellitus also received T (50 mg gel daily) or placebo treatment. Despite supervised exercise interventions being employed in all 3 of the aforementioned studies, none incorporated a nonexercising control group to ascertain the relative effects of T and exercise in isolation. In contrast, Hildreth et al (141) included a nonexercising control group over 12 months to assess the effect of T and/or progressive resistance exercise on supine (office) BP measures. No significant changes within or between groups were reported in the cohort of 167 men aged 60 years and older (141). None of these T + Ex studies assessed ABP.
We recently completed a direct 2 × 2 factorial randomized placebo-controlled trial in which 80 men aged 50 to 70 years with waist circumference of 95 cm or greater and T concentration 6 to 14 nmol/L (173-403 ng/dL) were randomly assigned to daily transdermal T or matching placebo (double-blind), and to supervised exercise (Ex) or no additional exercise (NEx) for 12 weeks (the TEX trial; see Fig. 1) (97). T treatment was with transdermal T cream, 2 mL (100 mg) administered to the upper body daily, a standard therapeutic dose (77). The exercise intervention was personalized and supervised, and comprised a mix of aerobic and resistance stations with gym attendance for 1 hour 3 times a week. There was a main effect of T treatment to increase 24-hour SBP, primarily seen when T was combined with Ex (97). Given that treatment-induced changes in ABP tend to be smaller than for office BP (143), the 3-mm Hg increase in 24-hour SBP observed in the T and exercise group may be clinically meaningful. We also observed that exercise decreased night-time DBP, and that this effect was attenuated by the addition of T treatment (97). This finding is of interest given that smaller nocturnal declines in BP have been associated with CV mortality risk (144).
Collectively, cross-sectional studies indicate that BP measurements (office and ABP) are inversely related to T concentrations; however, T-intervention studies contradict these results. In contrast, there is general consensus that exercise training reduces BP (office and ABP), although which type of exercise (aerobic, resistance, combined) produces more favorable BP outcomes remains a matter of debate. Few studies assessed the effect of combining T and exercise interventions on office BP measurements with heterogenous results. Our data (97) from a head-to-head comparison of the effects of exercise training and T treatment on BP outcomes suggest that T treatment in isolation does not have beneficial effects on BP in middle to older aged men with low-normal baseline T concentrations. BP should be carefully assessed and monitored when prescribing T treatment to middle-aged and older men. Conversely, middle-aged and older men with low-normal T concentrations with a higher-than-optimal BP could benefit from a suitable exercise program.
What Are the Effects of Testosterone, Exercise, and Their Combination on Vascular Function?
What Is Vascular Function and How Is it Assessed?
The vascular endothelium is integral to the maintenance of vascular health in humans, and arterial dysfunction precedes clinical manifestation of CVD (145). Flow-mediated dilation (FMD) measures the dilator response of a conduit artery to reactive hyperemia after temporary distal occlusion and ischemia (146). FMD is a validated surrogate measure for vascular health and a powerful predictor of future CVD events (147, 148), with a 1% increase in FMD translating to a 9% to 13% improvement in CVD outcomes (148, 149). In men, FMD decreases with age in a curvilinear fashion as a function of baseline arterial diameter. FMD is largely, though not solely, mediated by endothelial release of nitric oxide (NO), which has vasodilator and other antiatherogenic effects (146, 148). To specifically test vascular smooth muscle sensitivity to NO (ie, NO-mediated but endothelium-independent dilation), NO donors such as glyceryl trinitrate can be administered. Given that advancing age (150) and low T concentrations (151-153) both are associated with endothelial dysfunction and reduced FMD in men, whether T treatment has beneficial effects on artery function in aging men is a relevant question.
What Impact Does Testosterone Have on Vascular Function?
Endothelium-dependent dilation
Epidemiological studies associate higher circulating T concentrations with enhanced endothelial function, typically assessed as brachial artery FMD, in men across the age span (151-153). In contrast, interventional studies assessing the effect of T treatment on FMD in middle-aged and older men have provided inconsistent results (154-160). For example, in eugonadal men with coronary artery disease, intravenous T resulted in a dose-dependent improvement in FMD, 1 hour after the infusion (155) and in 55 obese men with type 2 diabetes and T less than 11 nmol/L (317 ng/dL) and/or calculated free T less than 220 pmol/L, intramuscular T undecanoate resulted in increased FMD over a 2-year period (156). However, another study in men with coronary artery disease and T of 12 nmol/L (346 ng/dL) or less reported no change in FMD following oral T treatment for 8 weeks (161), and other studies have reported decreased FMD following administration of T (158, 160). A meta-analysis by Sansone et al (162) concluded that T does not significantly alter vascular function in hypogonadal men, but this meta-analysis included only a total of 5 studies, highlighting the paucity of data concerning the effects of T intervention on endothelial function. Given the limited and inconsistent findings from studies to date, further studies using validated FMD techniques (163, 164) are needed in men at risk of cardiometabolic disease.
Endothelium-independent dilation
T-induced relaxation primarily involves endothelium-independent dilation in experimental studies in animals (eg, canine, porcine), where T induces relaxation in coronary, mesenteric, iliac, renal, and femoral arteries, in both in vivo and in vitro conditions (165-168). In comparison, few studies have sought to assess the effect of T on endothelium-independent dilation in humans. Supraphysiological doses of T were inversely associated with function of the NO-dilator system in a case study (169), while physiological T doses may confer beneficial vasodilator effects on vascular smooth muscle and myocardial perfusion in middle-aged and older-aged men with coronary artery disease (170-173). Other studies have reported no benefit (156, 158, 160, 161). It is likely that differences in study populations (especially comorbidities), T doses, route of administration, and study duration have contributed to the heterogeneous endothelium-independent results reported.
What Effect Does Exercise Training Have on Vascular Health and Function?
Endothelium-dependent dilation
In contrast to the vascular effects of T, the beneficial effect of exercise training on endothelial-dependent dilation is well established (174-183). A review and meta-analysis of 51 RCTs (n = 2260) by Ashor et al (184) reported that exercise training (4-52 weeks) improved FMD by 2% to 2.8%, which may translate into a reduction in CVD risk of 26% to 36% (149). The authors also assessed different exercise modalities and reported that aerobic, resistance, and combined (aerobic and resistance) exercise training improved FMD by a weighted mean difference of 2.79%, 2.52%, and 2.07%, respectively (184). Although a significant positive relationship was reported between aerobic intensity and endothelial function, for resistance exercise, greater frequency (as opposed to intensity) proved more influential (184).
Endothelium-independent dilation
In comparison to studies assessing endothelium-dependent dilation, fewer have reported results concerning endothelium-independent dilation. In the Ashor et al (184) meta-analysis mentioned previously, only 26 of 51 trials (1159 participants) included data on endothelial-independent dilation, measured in response to nitroglycerin. When compared to control groups, there was a trend toward improvement in endothelial-independent dilation following exercise training (weighted mean difference = 0.47%), but this was not statistically significant (P = .055). This suggests the effect of exercise on vascular function is predominantly endothelium mediated. However, it is important to note that the trials reporting endothelium-independent dilation primarily consisted of populations with substantial CV risk factors (hypertension, diabetes, hyperlipidemia) or documented CVD (coronary artery disease, chronic heart failure).
Does the Combination of Exercise Training and Testosterone Have Additive Effects on Vascular Health and Function?
As previous exercise studies have demonstrated improvements in endothelial function (184), and others indicated that T may confer beneficial vasodilatory effects on smooth muscle (170), it is surprising that very few studies have sought to assess the effects of this combination on vascular health and function. In a 54-week, open-label study in which participants could choose their own study arm, Francomano et al (157) reported that the addition of T to a diet and exercise intervention significantly improved endothelial function compared to placebo in obese, hypogonadal men (n = 24, aged 40-65 years). However, the exercise intervention was not supervised, nor was adherence recorded. Furthermore, endothelial function was measured using peripheral artery tonometry, which may be less reliable and reproducible compared to the FMD technique (185-188).
In addition to their main outcomes paper (discussed earlier in this review) (189), Hildreth et al separately published FMD results (141). Despite 12 months of supervised exercise training in 132 older men ± T treatment, Hildreth et al (141) reported no main effects of T or exercise, and no differences between exercise and no-exercise groups, alone or with T treatment. Differences in results between Hildreth et al (141) and Francomano et al (157) may be attributed not only to the assessment type (peripheral artery tonometry vs FMD) but also to the modality of exercise training. The exercise intervention conducted by Hildreth et al (141) was primarily resistance based, in contrast to the intervention employed by Francomano et al (157), which consisted of aerobic exercise. The dose-response relationship between aerobic exercise intensity and FMD may also influence results (184, 190).
In our recent 2 × 2 factorial randomized placebo-controlled trial, the TEX study (n = 80, aged 50-70 years) of T treatment and exercise training over 12 weeks (99), exercise training improved endothelium-dependent vasodilator function, whereas administration of T at therapeutic doses did not affect FMD or add to the benefit of exercise (see Fig. 1). Vascular smooth muscle sensitivity to NO was not modified by exercise, T, or their combination. Thus our study, using state-of-the-art methodology for assessing endothelium-dependent and -independent dilation and supervised exercise to which participants were highly compliant, found no evidence that T had a beneficial effect on endothelial function beyond those associated with exercise.
In summary, the effect of T treatment alone on vascular function in middle-aged and older men is unclear, with inconsistent results reported both for endothelium-dependent and -independent dilation. In contrast, there is a mature body of evidence supporting the effect of exercise on vascular health and function (174, 175). Whether the combination of T treatment and an exercise program provides benefit beyond that experienced with exercise alone has not been widely studied. However, the evidence that is available indicates that exercise is beneficial for vascular, and in particular endothelial, function whereas short-term T administration does not confer benefit or provide any additional effect in addition to the effect of exercise per se.
Conclusions
Often in parallel with declining T concentrations, aging men may experience decreases in fitness, strength, and vascular function; all of which are associated with increased mortality (191-193). From an epidemiological perspective, having higher endogenous T concentrations and engaging in higher levels of PA are associated with more favorable cardiometabolic outcomes in men. From a clinical perspective, exercise interventions are of undoubted benefit, but require effective implementation and sustained adherence. Effects of T pharmacotherapy in men who do not have organic hypogonadism on cardiometabolic outcomes require further study. Such T intervention may be more effective if sustained over a period of time and if administered in conjunction with lifestyle interventions (as in T4DM) or tailored exercise programs. It is possible that cardiometabolic benefits of T may be indirect, reflecting changes in body composition, rather than directly improving BP or endothelial function. Exercise remains a first-line strategy for aging men to improve their cardiometabolic health.
Funding
Some research cited in this article was supported by the Heart Foundation of Australia (Vanguard Grant No. 100578). Supplementary research funding was received from Lawley Pharmaceuticals, Western Australia, which provided testosterone medication and placebo for a study completed by the listed authors. D.J.G. was supported by an National Health and Medical Research Council Principal Research Fellowship (No. APP1080914). L.C. was supported by an Australian Postgraduate Award and a REDiMED PhD scholarship.
Disclosures
B.B.Y. has received speaker honoraria and conference support from Bayer, Lilly, and Besins Healthcare; research support from Bayer, Lilly, and Lawley Pharmaceuticals; and participated in advisory roles for Lilly, Besins Healthcare, Ferring, and Lawley Pharmaceuticals. The other authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
Abbreviations
- ABP
ambulatory blood pressure
- BMI
body mass index
- BP
blood pressure
- CIMT
carotid intima media thickness
- CV
cardiovascular
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- DHT
dihydrotestosterone
- Ex
exercise
- FMD
flow-mediated dilation
- HbA1c
glycated hemoglobin A1c
- HIMS
Health In Men Study
- HOMA-IR
homeostatic model assessment of insulin resistance
- MACE
major cardiovascular adverse event
- MI
myocardial infarction
- NO
nitric oxide
- OGTT
oral glucose tolerance testing
- PA
physical activity
- RCT
randomized controlled trial
- SBP
systolic blood pressure
- T
testosterone
- T4DM
Testosterone for the Prevention of type 2 Diabetes Mellitus
- T Trials
Testosterone Trials



