-
PDF
- Split View
-
Views
-
Cite
Cite
Hong Lian, Siqian Gong, Meng Li, Xirui Wang, Fang Wang, Xiaoling Cai, Wei Liu, Yingying Luo, Simin Zhang, Rui Zhang, Lingli Zhou, Yu Zhu, Yumin Ma, Qian Ren, Xiuying Zhang, Jing Chen, Ling Chen, Jing Wu, Leili Gao, Xianghai Zhou, Yufeng Li, Liyong Zhong, Xueyao Han, Linong Ji, Prevalence and Clinical Characteristics of PDX1 Variant Induced Diabetes in Chinese Early-Onset Type 2 Diabetes, The Journal of Clinical Endocrinology & Metabolism, Volume 108, Issue 12, December 2023, Pages e1686–e1694, https://doi.org/10.1210/clinem/dgad303
Close - Share Icon Share
Abstract
Maturity-onset diabetes of the young 4 (MODY4) is caused by mutations of PDX1; its prevalence and clinical features are not well known.
This study aimed to investigate the prevalence and clinical characteristics of MODY4 in Chinese people clinically diagnosed with early-onset type 2 diabetes (EOD), and to evaluate the relationship between the PDX1 genotype and the clinical phenotype.
The study cohort consisted of 679 patients with EOD. PDX1 mutations were screened by DNA sequencing, and their pathogenicity was evaluated by functional experiments and American College of Medical Genetics and Genomics guidelines. MODY4 was diagnosed in individuals with diabetes who carry a pathogenic or likely pathogenic PDX1 variant. All reported cases were reviewed for analyzing the genotype–phenotype relationship.
4 patients with MODY4 were identified, representing 0.59% of this Chinese EOD cohort. All the patients were diagnosed before 35 years old, either obese or not obese. Combined with previously reported cases, the analysis revealed that the carriers of homeodomain variants were diagnosed earlier than those with transactivation domain variants (26.10 ± 11.00 vs 41.85 ± 14.66 years old, P < .001), and the proportions of overweight and obese individuals with missense mutation were higher than those with nonsense or frameshift mutations (27/34 [79.4%] vs 3/8 [37.5%], P = .031).
Our study suggested that MODY4 was prevalent in 0.59% of patients with EOD in a Chinese population. It was more difficult to identify clinically than other MODY subtypes owning to its clinical similarity to EOD. Also, this study revealed that there is some relationship between genotype and phenotype.
Maturity-onset diabetes of the young (MODY) is a rare form of diabetes caused by mutations in specific genes, accounting for about 1% to 2% of the total prevalence of diabetes (1). MODY4 is a less common type of MODY and is an autosomal dominant disease caused by mutations in the PDX1 gene. PDX1, the pancreas and duodenum homeobox-1, also known as insulin promoter factor 1 (IPF1), works as an important transcription factor during pancreatic growth and development (2).
PDX1 has a simple gene structure, only 2 exons, encoding for a 283 amino acid protein (3). The protein's functional domains mainly include the transactivation domain (amino acids 1-79) and the homeodomain (amino acids 146-206). The former contains binding sites for transcriptional coregulators. The latter, the homeodomain, is highly conserved during evolution and contains the nuclear localization signal as well as a DNA binding site, which mediates DNA binding to TAAT-rich motifs in PDX1 target genes (4). PDX1 is mainly expressed in the pancreas and gastrointestinal tract, regulating the transcription of pancreatic endocrine genes, such as INS, GLUT2, GCK, and PAX4 (5, 6).
Previous research has reported that homozygous inactivating variants of PDX1 cause neonatal diabetes and pancreatic agenesis. Those neonates present with intrauterine growth retardation and hyperglycemia within the first 6 months of life, requiring insulin to achieve a good glucose control target (7). Heterozygous inactivating variants of PDX1 often lead to MODY4, which demonstrates that PDX1/IPF1 expression is essential for glucose-sensing regulation and insulin production in β cells (8). Thus, decreased expression or activity of PDX1 may result in impaired GLUT2 and insulin expression, which contributes to the development of hyperglycemia.
The current reports on MODY4 are mostly single family or sporadic cases. In many cases, mutations in PDX1 have not been functionally studied or even evaluated for pathogenicity according to American College of Medical Genetics and Genomics (ACMG) guidelines. It is doubtful whether these cases can be diagnosed as MODY4 (9-11). A review from Japan summarized all reported MODY4 cases and found the clinical manifestations were heterogeneous and lack defined characteristics (12). However, that study included a number of previous cases lacking functional validation and assessment of ACMG pathogenicity, so the conclusions may not be accurate, and they did not delve into the relationship between PDX1 mutant genotypes and patient phenotypes.
The broad phenotypic spectrum of disease makes it difficult to distinguish MODY4 from type 2 diabetes, which will eventually lead to misdiagnosis and incorrect clinical treatment; their parents and siblings may subsequently miss out on genetic counseling and early diagnosis (13). Several studies have found that a small percentage of young-onset type 2 diabetes patients actually suffered from MODY (14). The accuracy of clinical diagnosis of MODY was very low among the participants in the ProDiGY study. That study found that 5 patients with diabetes with pathogenic or possibly pathogenic PDX1 mutations and the detection rate of MODY4 in the youth-onset type 2 diabetes population was 0.15% (15). The ProDiGY study mainly included Hispanic and non-Hispanic Whites, and the Asian population accounted for a small proportion. Meanwhile, the study identified 33 PDX1 variants of uncertain significance due to a lack of functional validation and some of those mutations may contain pathogenic mutations, so the prevalence of MODY4 may be underestimated. Because of the low prevalence of MODY4, most large-scale genomic studies did not screen or detect PDX1 variants in populations (16-18). So far, only 2 cases carrying a rare variant of PDX1 have been reported in China, of whom only 1 had been evaluated as likely pathogenic based on ACMG. As a rare monogenetic disease, no study has yet calculated the actual prevalence of MODY4 in the Chinese population (19, 20).
This study aimed to screen for pathogenic variants of PDX1 in our cohort of clinically diagnosed early-onset type 2 diabetes mellitus (EOD) to obtain prevalence estimates for representative Chinese populations and to summarize and characterize the clinical manifestations of MODY4. We hope that our research will provide more definitive evidence and recommendations for accurate diagnosis and screening of these monogenic diseases. In order to accurately diagnose MODY4 patients in the EOD population, we set clear MODY4 diagnostic criteria and performed functional verification for mutations evaluated as “uncertain significance” by ACMG. Also, our study explored the specific clinical features of MODY4 and the relationship between the PDX1 genotype and the phenotype.
Materials and Methods
Study Population
This study cohort population consisted of 679 patients who were diagnosed with EOD in the Endocrinology and Metabolism Department of the Peking University People's Hospital from September 2013 to June 2019. Criteria for selecting the participants were as follows: (1) the diagnosis of diabetes was made according to the criteria of the 1999 World Health Organization: fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) or 2-hour plasma glucose ≥200 mg/dL (11.1 mmol/L) during the oral glucose tolerance test (21); (2) age at diagnosis was under 40 years; (3) antiglutamic acid decarboxylase antibodies, anti-islet cell antibodies, and anti-insulin antibodies were confirmed to be negative; (4) patients with pancreatitis and typical clinical manifestations of type 1 diabetes (eg, complete lack of endogenous insulin secretion) were excluded. Studies involving human participants were reviewed and approved by the Ethics Committee of Peking University People's Hospital. All patients provided their written informed consent to participate in this study.
Clinical and Biochemical Indicators
Participants received a physical examination and a questionnaire survey to gather information on demographic data at the time of enrollment as described previously (22). Venous blood was collected for laboratory tests after overnight fasting for at least 8 hours. The National Glycoprotein Standardization Program/International Union of Clinical Chemistry and Laboratory Medicine (NGSP/IFCC) for HbA1c conversion is as follows: NGSP (%) = IFCC (mmol/mol) × 0.0915 + 2.152 (23). Estimated glomerular filtration rate (mL/min/1.73 m2) = 175 × serum creatine (mg/dL)−1.234 × age (years)−0.179 × 0.79 (if female) (24). The BMI obesity classification standard was as follows: for East Asia, underweight <18.5 kg/m2, normal weight 18.5-23.9 kg/m2, overweight 24-27.9 kg/m2, obesity ≥28 kg/m2; for Caucasians: underweight <18.5 kg/m2, normal weight 18.5-24.9 kg/m2, overweight 25-29.9 kg/m2, obesity ≥30 kg/m2 (25, 26).
Detection and Screening of PDX1 Variants
The patient's genomic DNA was extracted from peripheral blood. Whole-exome sequencing or target sequencing was performed respectively on the Illumina Hiseq2500 system and Hiseq4000 platform using Roche NimbleGen human exon V2 capture kit or customized Agilent capture kit with data coverage (∼100×) >99% and depth >200 bp. Frequency filtering was performed in the publicly available population databases (The Genome Aggregation Database, GnomAD, and 1000Genome) to screen for variants with minor allele frequencies less than 0.001 and remove synonymous variation. All detected mutations were amplified by polymerase chain reaction using specific primers for Sanger sequencing validation. The primers used for Sanger sequencing are shown elsewhere (Table S1 (27)).
Bioinformatics Analysis and ACMG Classification
Variant pathogenicity was assessed according to 2015 ACMG guidelines (28). In silico prediction software was used to predict the structure, function, and evolutionary conservation of proteins encoded by missense mutations. The prediction websites used in the study were as follows: SIFT (https://www.jcvi.org/research/provean), MutationTaster (http://www.mutationtaster.org), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2), and CADD (http://cadd.gs.washington.edu). Population frequencies of variants were obtained by searching public databases: Genome Aggregation Database, GnomAD (http://gnomad-sg.org/), 1000 Genomes Project (https://asia.ensembl.org/); dbSNP (http://www.ncbi.nlm.nih.gov/snp), and China Metabolic Analytics Project (ChinaMAP) database (http://www.mBiobank.com). The diagnostic criteria for MODY4 were patients with diabetes who carried a pathogenic or likely pathogenic variant of PDX1.
Functional Analysis of Rare Variants of PDX1
A full-length human PDX1 transcript 1 was cloned into the pcDNA3.1 expression plasmid to construct a wild-type PDX1 overexpression plasmid. The plasmids of PDX1 variants (P33A, P46L, H128Q, Q133H) were constructed using a point mutation kit and identified through Sanger sequencing. E178G variants confirmed in previous studies were selected as positive controls (29). At the same time, the human insulin promoter sequence (NG_007114.1 chr11: 2182452-2182809) was inserted into the PGL4 plasmid to construct the reporter plasmid. The complete plasmid sequencing is shown elsewhere (Supplementary materials (27)). HEK293T cells were cultured in 6-well plates to a confluence of about 70%, then cotransfected wild-type or PDX1 variant expression plasmid (1 μg) and INS luciferase reporter plasmid (1 μg) per well using lipo3000 (Thermofisher, USA). The pRL-TK Renilla luciferase (RLuc) reporter vector (Promega, USA) was transfected at a ratio of 1:50 as internal reference. The transfection efficiency was tested by quantitative real-time polymerase chain reaction (data not shown). Thirty-six to 48 hours after transfection, cells were collected for detection of the luciferase level (Promega GloMax, USA). The Dual-Luciferase Reporter Assay System (Promega, USA, E1910) was used to measure the effect of PDX1 on INS promoter activity according to the manufacturer's instructions. The plasmid insertion sequence and Sanger sequencing results are shown elsewhere (Fig. S1 (27)).
Review of Previous MODY4 Studies
We conducted a literature review of all MODY4-related studies in the PubMed database up to July 31, 2022. We started with the search keywords “Maturity-onset diabetes of the young, MODY,” “MODY4,” “Pancreas and duodenum homeobox-1, PDX1,” “Insulin promoter factor 1, IPF1,” and “Diabetes.” A total of 37 studies published in English were included, consisting of 7 case reports and 30 articles. We also reviewed the PDX1 mutations involved in the Human Gene Mutation Database (www.hgmd.cf.ac.uk). The variants of PDX1 and clinical data of related cases were collected and summarized for subsequent analysis.
Statistical Analysis
Continuous variables that satisfy a normal distribution are described as mean ± SD, and a t-test was used to compare the means between the 2 groups. One way analysis of variance was used for comparing the means among multiple groups, and post hoc tests with Fisher's least significant difference method for pair by pair comparisons. Variables that do not satisfy a normal distribution are described as median and interquartile range. We performed the Mann–Whitney U test for comparison tests to compare the differences in significance between the 2 groups. Categorical variables are presented as numbers (proportions) and differences between groups were measured using the χ2 test or Fisher's exact test. Statistical analysis of this study was performed using SPSS 25. A 2-sided test with P < .05 was considered to be statistically significant.
Results
Screening and Identification of PDX1 Rare Mutation Carriers in Early-Onset Diabetes Cohort
In our cohort of 679 EOD patients, a total of 6 rare PDX1 variants were identified by whole-exome sequencing or target sequencing (Table 1), including 4 missense mutations (P33A, P46L, H128Q, Q133H) and 2 frameshift mutations (G246Rfs*21, A251Efs*49). The P33A and G246Rfs*21 variants were previously reported and the remaining variants were newly discovered in our study. Each mutation was verified by Sanger sequencing for bidirectional detection (Fig. S2 (27)). All variants showed minor allele frequencies <0.001 in ALFA, GnomAD, and 1000G (Table 1). PDX1 works as a transcription factor to regulate the expression of the INS gene, thereby affecting insulin secretion. In order to further clarify the pathogenicity of the mutation, we performed functional assays to examine the effect of PDX1 mutations on the transcriptional activity of the insulin promoter. We constructed overexpression plasmids of those variants (P33A, P46L, H128Q, Q133H). The dual-luciferase reporter assay showed that H128Q and Q133H variants significantly reduce the transcriptional activity of the INS promoter compared with PDX1 wild type (Fig. 1). After characterization of those genetic variants associated with Mendelian disease according to ACMG guidelines, P33A and P46L variants were classified as mutations of “Uncertain significance,” H128Q, Q133H, and 2 frameshift mutations (G246Rfs*21, A251Efs*49) were classified as “Likely pathogenic” (Table 1). The clinical features of those mutation carriers are shown in Table 2. Patients with diabetes who carry pathogenic or likely pathogenic PDX1 variants were diagnosed with MODY4. In this study, the detection rate of MODY4 in the Chinese population with early onset diabetes was 0.59%.
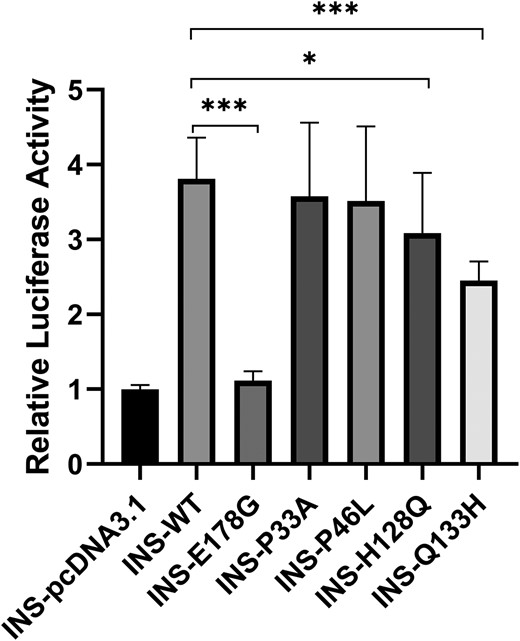
Dual-luciferase reporter assay of constitutive PDX1 variants. As described in “Materials and Methods,” 293 T cells were transfected with wild-type and rare variants of PDX1 expression vectors and INS reporter plasmid. The luciferase activity was relative to the INS-pcDNA3.1 group.
Rare variants of PDX1 identified in Chinese patients with early-onset type 2 diabetes
| . | . | Protein change . | . | ACMGa classification . | . | MAF (%)b . | |||
|---|---|---|---|---|---|---|---|---|---|
| Position . | Base changes . | Rs number . | ALFA . | GnomAD _exome . | GnomAD _genome . | 1000G . | ChinaMAP . | ||
| 28494372 | c.97C>G | P33A | rs192902098 | Uncertain significance (PM2 PP3) | 0.00006 (2/34692) | 0.0003445 (49/142222) | 0.0001913 (6/31358) | NA | 0.00401625 (85/21164) |
| 28494412 | c.137C>T | P46L | rs1334735144 | Uncertain significance (PM2) | 0.00000 (0/14050) | 0.000 (0/134122) | NA | NA | NA |
| 28494659 | c.384C>G | H128Q | rs764675125 | Likely pathogenic (PS3 PM2 PP3) | 0.00000 (0/14050) | 0.000024 (6/245608) | NA | NA | 0.000425009 (9/21176) |
| 28494674 | c.399G>T | Q133H | rs750762696 | Likely pathogenic (PS3 PM2 PP3) | 0.00000 (0/14708) | 0.00003259 (8/245444) | NA | NA | 0.0000472233 (1/21176) |
| 28498720 | c.735dupA | G246Rfs*21 | rs1566025744 | Likely pathogenic (PVS1 PM2) | 0.00000 (0/10678) | 0.000009873 (1/101282) | NA | NA | NA |
| 28498729 | c.743_761delCTGCCCCCGTTGCCGCCCG | A251Efs*49 | rs1566025750 | Likely pathogenic (PVS1 PM2) | 0.0003 (2/7026) | 0.00001 (1/72078) | NA | NA | NA |
| . | . | Protein change . | . | ACMGa classification . | . | MAF (%)b . | |||
|---|---|---|---|---|---|---|---|---|---|
| Position . | Base changes . | Rs number . | ALFA . | GnomAD _exome . | GnomAD _genome . | 1000G . | ChinaMAP . | ||
| 28494372 | c.97C>G | P33A | rs192902098 | Uncertain significance (PM2 PP3) | 0.00006 (2/34692) | 0.0003445 (49/142222) | 0.0001913 (6/31358) | NA | 0.00401625 (85/21164) |
| 28494412 | c.137C>T | P46L | rs1334735144 | Uncertain significance (PM2) | 0.00000 (0/14050) | 0.000 (0/134122) | NA | NA | NA |
| 28494659 | c.384C>G | H128Q | rs764675125 | Likely pathogenic (PS3 PM2 PP3) | 0.00000 (0/14050) | 0.000024 (6/245608) | NA | NA | 0.000425009 (9/21176) |
| 28494674 | c.399G>T | Q133H | rs750762696 | Likely pathogenic (PS3 PM2 PP3) | 0.00000 (0/14708) | 0.00003259 (8/245444) | NA | NA | 0.0000472233 (1/21176) |
| 28498720 | c.735dupA | G246Rfs*21 | rs1566025744 | Likely pathogenic (PVS1 PM2) | 0.00000 (0/10678) | 0.000009873 (1/101282) | NA | NA | NA |
| 28498729 | c.743_761delCTGCCCCCGTTGCCGCCCG | A251Efs*49 | rs1566025750 | Likely pathogenic (PVS1 PM2) | 0.0003 (2/7026) | 0.00001 (1/72078) | NA | NA | NA |
RefSeq: GRCh37. NC_000013.10 (28494137..28500450) NM_000209.4, NM_000209 ENST00000381033
aThe classification of rare variants identified in this study according to the standards and guidelines recommended by the American College of Medical Genetics (ACMG).
bNA indicates that the variant has no minor allele frequency (MAF) in the GnomAD_genome, 1000 Genomes Project databases, and ChinaMAP database.
Rare variants of PDX1 identified in Chinese patients with early-onset type 2 diabetes
| . | . | Protein change . | . | ACMGa classification . | . | MAF (%)b . | |||
|---|---|---|---|---|---|---|---|---|---|
| Position . | Base changes . | Rs number . | ALFA . | GnomAD _exome . | GnomAD _genome . | 1000G . | ChinaMAP . | ||
| 28494372 | c.97C>G | P33A | rs192902098 | Uncertain significance (PM2 PP3) | 0.00006 (2/34692) | 0.0003445 (49/142222) | 0.0001913 (6/31358) | NA | 0.00401625 (85/21164) |
| 28494412 | c.137C>T | P46L | rs1334735144 | Uncertain significance (PM2) | 0.00000 (0/14050) | 0.000 (0/134122) | NA | NA | NA |
| 28494659 | c.384C>G | H128Q | rs764675125 | Likely pathogenic (PS3 PM2 PP3) | 0.00000 (0/14050) | 0.000024 (6/245608) | NA | NA | 0.000425009 (9/21176) |
| 28494674 | c.399G>T | Q133H | rs750762696 | Likely pathogenic (PS3 PM2 PP3) | 0.00000 (0/14708) | 0.00003259 (8/245444) | NA | NA | 0.0000472233 (1/21176) |
| 28498720 | c.735dupA | G246Rfs*21 | rs1566025744 | Likely pathogenic (PVS1 PM2) | 0.00000 (0/10678) | 0.000009873 (1/101282) | NA | NA | NA |
| 28498729 | c.743_761delCTGCCCCCGTTGCCGCCCG | A251Efs*49 | rs1566025750 | Likely pathogenic (PVS1 PM2) | 0.0003 (2/7026) | 0.00001 (1/72078) | NA | NA | NA |
| . | . | Protein change . | . | ACMGa classification . | . | MAF (%)b . | |||
|---|---|---|---|---|---|---|---|---|---|
| Position . | Base changes . | Rs number . | ALFA . | GnomAD _exome . | GnomAD _genome . | 1000G . | ChinaMAP . | ||
| 28494372 | c.97C>G | P33A | rs192902098 | Uncertain significance (PM2 PP3) | 0.00006 (2/34692) | 0.0003445 (49/142222) | 0.0001913 (6/31358) | NA | 0.00401625 (85/21164) |
| 28494412 | c.137C>T | P46L | rs1334735144 | Uncertain significance (PM2) | 0.00000 (0/14050) | 0.000 (0/134122) | NA | NA | NA |
| 28494659 | c.384C>G | H128Q | rs764675125 | Likely pathogenic (PS3 PM2 PP3) | 0.00000 (0/14050) | 0.000024 (6/245608) | NA | NA | 0.000425009 (9/21176) |
| 28494674 | c.399G>T | Q133H | rs750762696 | Likely pathogenic (PS3 PM2 PP3) | 0.00000 (0/14708) | 0.00003259 (8/245444) | NA | NA | 0.0000472233 (1/21176) |
| 28498720 | c.735dupA | G246Rfs*21 | rs1566025744 | Likely pathogenic (PVS1 PM2) | 0.00000 (0/10678) | 0.000009873 (1/101282) | NA | NA | NA |
| 28498729 | c.743_761delCTGCCCCCGTTGCCGCCCG | A251Efs*49 | rs1566025750 | Likely pathogenic (PVS1 PM2) | 0.0003 (2/7026) | 0.00001 (1/72078) | NA | NA | NA |
RefSeq: GRCh37. NC_000013.10 (28494137..28500450) NM_000209.4, NM_000209 ENST00000381033
aThe classification of rare variants identified in this study according to the standards and guidelines recommended by the American College of Medical Genetics (ACMG).
bNA indicates that the variant has no minor allele frequency (MAF) in the GnomAD_genome, 1000 Genomes Project databases, and ChinaMAP database.
Clinical characteristics of patients with rare variants of PDX1 in Chinese with early-onset type 2 diabetes
| Proband . | P1 . | P2 . | P3 . | P4 . | P5 . | P6 . | P7 . |
|---|---|---|---|---|---|---|---|
| Gender | M | M | M | F | M | F | M |
| Age (years) | 42 | 35 | 35 | 34 | 30 | 28 | 38 |
| Age at diagnosis (years) | 30 | 35 | 35 | 25 | 25 | 27 | 33 |
| Family history of diabetes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Variant | P33A | P33A | P46L | H128Q | Q133H | G246Rfs*21 | A251Efs*49 |
| ACMGa classification | Uncertain significance | Uncertain significance | Uncertain significance | Likely pathogenic | Likely pathogenic | Likely pathogenic | Likely pathogenic |
| BMI (kg/m2) | 31.7 | 23.5 | 30.2 | 26.4 | 33.7 | 19.1 | 26.9 |
| SBP (mmHg) | 138 | 110 | 132 | 142 | 109 | 114 | 116 |
| DBP (mmHg) | 94 | 60 | 96 | 102 | 62 | 73 | 68 |
| HbA1c (%) | 8.4 | 12.3 | 6.8 | 8.8 | 6.1 | 6.7 | — |
| FPG (mmol/L) | 7.4 | 10.22 | 6.4 | 9.14 | 4.75 | 6.09 | 5.58 |
| FINS (µU/mL) | — | — | 23.4 | — | — | 5.62 | — |
| FCP (ng/mL) | 2.87 | 1.25 | — | — | 3.04 | — | 1.77 |
| TCb (mmol/L) | 3.54 | 5.7 | 5.1 | 5.04 | 3.23 | 3.8 | 3.18 |
| LDLb (mmol/L) | 2.15 | 4.04 | 3.49 | 3.19 | 1.97 | 2.02 | 2.19 |
| HDLb (mmol/L) | 0.74 | 1.02 | 1.02 | 1.22 | 0.82 | 1.32 | 0.66 |
| TGb (mmol/L) | 4.71 | 1.01 | 2.1 | 2.2 | 1.6 | 1.08 | 0.97 |
| BUN (µmol/L) | 4.13 | 6.23 | — | — | 1.93 | 3.45 | 2.78 |
| UACR (mg/g) | 11.07 | 12.25 | 5.84 | 12.45 | 2.37 | — | 5.59 |
| eGFR (mL/min × 1.73 m2) | 118.7 | 137.96 | 162.67 | 156.24 | 98.49 | 185.29 | 121.41 |
| Treatment | Metformin + insulin analog | Insulin analog | Metformin +αGI | Metformin +αGI + Insulin | Metformin + insulin analog | Diet | Metformin + insulin |
| Proband . | P1 . | P2 . | P3 . | P4 . | P5 . | P6 . | P7 . |
|---|---|---|---|---|---|---|---|
| Gender | M | M | M | F | M | F | M |
| Age (years) | 42 | 35 | 35 | 34 | 30 | 28 | 38 |
| Age at diagnosis (years) | 30 | 35 | 35 | 25 | 25 | 27 | 33 |
| Family history of diabetes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Variant | P33A | P33A | P46L | H128Q | Q133H | G246Rfs*21 | A251Efs*49 |
| ACMGa classification | Uncertain significance | Uncertain significance | Uncertain significance | Likely pathogenic | Likely pathogenic | Likely pathogenic | Likely pathogenic |
| BMI (kg/m2) | 31.7 | 23.5 | 30.2 | 26.4 | 33.7 | 19.1 | 26.9 |
| SBP (mmHg) | 138 | 110 | 132 | 142 | 109 | 114 | 116 |
| DBP (mmHg) | 94 | 60 | 96 | 102 | 62 | 73 | 68 |
| HbA1c (%) | 8.4 | 12.3 | 6.8 | 8.8 | 6.1 | 6.7 | — |
| FPG (mmol/L) | 7.4 | 10.22 | 6.4 | 9.14 | 4.75 | 6.09 | 5.58 |
| FINS (µU/mL) | — | — | 23.4 | — | — | 5.62 | — |
| FCP (ng/mL) | 2.87 | 1.25 | — | — | 3.04 | — | 1.77 |
| TCb (mmol/L) | 3.54 | 5.7 | 5.1 | 5.04 | 3.23 | 3.8 | 3.18 |
| LDLb (mmol/L) | 2.15 | 4.04 | 3.49 | 3.19 | 1.97 | 2.02 | 2.19 |
| HDLb (mmol/L) | 0.74 | 1.02 | 1.02 | 1.22 | 0.82 | 1.32 | 0.66 |
| TGb (mmol/L) | 4.71 | 1.01 | 2.1 | 2.2 | 1.6 | 1.08 | 0.97 |
| BUN (µmol/L) | 4.13 | 6.23 | — | — | 1.93 | 3.45 | 2.78 |
| UACR (mg/g) | 11.07 | 12.25 | 5.84 | 12.45 | 2.37 | — | 5.59 |
| eGFR (mL/min × 1.73 m2) | 118.7 | 137.96 | 162.67 | 156.24 | 98.49 | 185.29 | 121.41 |
| Treatment | Metformin + insulin analog | Insulin analog | Metformin +αGI | Metformin +αGI + Insulin | Metformin + insulin analog | Diet | Metformin + insulin |
Abbreviations: αGI, α-glucosidase inhibitor; BMI, body mass index; BUN, blood urea nitrogen; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate. F, female; FCP, fasting c-peptide; FINS, fasting serum insulin; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; M, Male; P, patient; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; UACR, urinary albumin/creatinine ratio.
aThe classification of rare variants identified in this study according to the standards and guidelines recommended by the American College of Medical Genetics (ACMG), detailed elsewhere (Table S2 (27)).
bThe blood lipid level was determined without taking lipid-lowering drugs.
Clinical characteristics of patients with rare variants of PDX1 in Chinese with early-onset type 2 diabetes
| Proband . | P1 . | P2 . | P3 . | P4 . | P5 . | P6 . | P7 . |
|---|---|---|---|---|---|---|---|
| Gender | M | M | M | F | M | F | M |
| Age (years) | 42 | 35 | 35 | 34 | 30 | 28 | 38 |
| Age at diagnosis (years) | 30 | 35 | 35 | 25 | 25 | 27 | 33 |
| Family history of diabetes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Variant | P33A | P33A | P46L | H128Q | Q133H | G246Rfs*21 | A251Efs*49 |
| ACMGa classification | Uncertain significance | Uncertain significance | Uncertain significance | Likely pathogenic | Likely pathogenic | Likely pathogenic | Likely pathogenic |
| BMI (kg/m2) | 31.7 | 23.5 | 30.2 | 26.4 | 33.7 | 19.1 | 26.9 |
| SBP (mmHg) | 138 | 110 | 132 | 142 | 109 | 114 | 116 |
| DBP (mmHg) | 94 | 60 | 96 | 102 | 62 | 73 | 68 |
| HbA1c (%) | 8.4 | 12.3 | 6.8 | 8.8 | 6.1 | 6.7 | — |
| FPG (mmol/L) | 7.4 | 10.22 | 6.4 | 9.14 | 4.75 | 6.09 | 5.58 |
| FINS (µU/mL) | — | — | 23.4 | — | — | 5.62 | — |
| FCP (ng/mL) | 2.87 | 1.25 | — | — | 3.04 | — | 1.77 |
| TCb (mmol/L) | 3.54 | 5.7 | 5.1 | 5.04 | 3.23 | 3.8 | 3.18 |
| LDLb (mmol/L) | 2.15 | 4.04 | 3.49 | 3.19 | 1.97 | 2.02 | 2.19 |
| HDLb (mmol/L) | 0.74 | 1.02 | 1.02 | 1.22 | 0.82 | 1.32 | 0.66 |
| TGb (mmol/L) | 4.71 | 1.01 | 2.1 | 2.2 | 1.6 | 1.08 | 0.97 |
| BUN (µmol/L) | 4.13 | 6.23 | — | — | 1.93 | 3.45 | 2.78 |
| UACR (mg/g) | 11.07 | 12.25 | 5.84 | 12.45 | 2.37 | — | 5.59 |
| eGFR (mL/min × 1.73 m2) | 118.7 | 137.96 | 162.67 | 156.24 | 98.49 | 185.29 | 121.41 |
| Treatment | Metformin + insulin analog | Insulin analog | Metformin +αGI | Metformin +αGI + Insulin | Metformin + insulin analog | Diet | Metformin + insulin |
| Proband . | P1 . | P2 . | P3 . | P4 . | P5 . | P6 . | P7 . |
|---|---|---|---|---|---|---|---|
| Gender | M | M | M | F | M | F | M |
| Age (years) | 42 | 35 | 35 | 34 | 30 | 28 | 38 |
| Age at diagnosis (years) | 30 | 35 | 35 | 25 | 25 | 27 | 33 |
| Family history of diabetes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Variant | P33A | P33A | P46L | H128Q | Q133H | G246Rfs*21 | A251Efs*49 |
| ACMGa classification | Uncertain significance | Uncertain significance | Uncertain significance | Likely pathogenic | Likely pathogenic | Likely pathogenic | Likely pathogenic |
| BMI (kg/m2) | 31.7 | 23.5 | 30.2 | 26.4 | 33.7 | 19.1 | 26.9 |
| SBP (mmHg) | 138 | 110 | 132 | 142 | 109 | 114 | 116 |
| DBP (mmHg) | 94 | 60 | 96 | 102 | 62 | 73 | 68 |
| HbA1c (%) | 8.4 | 12.3 | 6.8 | 8.8 | 6.1 | 6.7 | — |
| FPG (mmol/L) | 7.4 | 10.22 | 6.4 | 9.14 | 4.75 | 6.09 | 5.58 |
| FINS (µU/mL) | — | — | 23.4 | — | — | 5.62 | — |
| FCP (ng/mL) | 2.87 | 1.25 | — | — | 3.04 | — | 1.77 |
| TCb (mmol/L) | 3.54 | 5.7 | 5.1 | 5.04 | 3.23 | 3.8 | 3.18 |
| LDLb (mmol/L) | 2.15 | 4.04 | 3.49 | 3.19 | 1.97 | 2.02 | 2.19 |
| HDLb (mmol/L) | 0.74 | 1.02 | 1.02 | 1.22 | 0.82 | 1.32 | 0.66 |
| TGb (mmol/L) | 4.71 | 1.01 | 2.1 | 2.2 | 1.6 | 1.08 | 0.97 |
| BUN (µmol/L) | 4.13 | 6.23 | — | — | 1.93 | 3.45 | 2.78 |
| UACR (mg/g) | 11.07 | 12.25 | 5.84 | 12.45 | 2.37 | — | 5.59 |
| eGFR (mL/min × 1.73 m2) | 118.7 | 137.96 | 162.67 | 156.24 | 98.49 | 185.29 | 121.41 |
| Treatment | Metformin + insulin analog | Insulin analog | Metformin +αGI | Metformin +αGI + Insulin | Metformin + insulin analog | Diet | Metformin + insulin |
Abbreviations: αGI, α-glucosidase inhibitor; BMI, body mass index; BUN, blood urea nitrogen; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate. F, female; FCP, fasting c-peptide; FINS, fasting serum insulin; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; M, Male; P, patient; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; UACR, urinary albumin/creatinine ratio.
aThe classification of rare variants identified in this study according to the standards and guidelines recommended by the American College of Medical Genetics (ACMG), detailed elsewhere (Table S2 (27)).
bThe blood lipid level was determined without taking lipid-lowering drugs.
Summarizing the Previously Reported Cases of Rare PDX1 Variants
A total of 26 PDX1 missense mutations, 2 nonsense mutations, and 7 frameshift mutations have been reported previously. According to ACMG guidelines, there were 10 missense mutations, and all of the nonsense mutations and frameshift mutations were classified as “likely pathogenic” or “pathogenic” (Tables S4-S6 (27)). Based on the diagnostic criteria above, we sorted out 64 MODY4 cases and 12 probands of neonatal diabetes; the clinical characteristics are summarized elsewhere (Tables S7 and S8 (27)). The mean age of the 64 MODY4 patients diagnosed with diabetes was 36 years, about 88% had a family history of diabetes, 71.4% were overweight or obese, and 44.7% required insulin or insulin analog therapy (Table 3). We drew a schematic diagram of the PDX1 domain structure and location of the pathogenic or potentially pathogenic mutant variants identified in previous studies and in our EOD cohort (Fig. 2). At the same time, we searched for rare variations of PDX1 contained in the China Metabolic Analytics Project (ChinaMAP) database, which contains samples of 450 000 participants covering all regions in China. A total of 31 rare mutations were detected, all of which were missense mutations with minor allele frequency <0.001. Four variants were evaluated as “likely pathogenic” or “pathogenic” based on ACMG pathogenicity evaluation (D76N, H128Q, Q133H, E178K). The frequency of PDX1 rare pathogenic variation in the whole population of China was calculated to be 0.12% (Table S9 (27)).
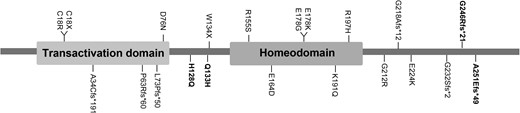
Schematic representation of the PDX1 domain structure and position of the pathogenic variants identified in previous studies and this study. Pathogenic variants described in the EOD cohort are marked in bold.
Clinical characteristics of Chinese cohort of early-onset type 2 diabetes mellitus and previously reported patients with MODY4
| . | . | Variant location . | . | Mutation type . | . | ||
|---|---|---|---|---|---|---|---|
| . | MODY4 . | Trans-D . | Homo-D . | P valuea . | Missense mutations . | Nonsense and frameshift mutations . | P valuea . |
| Sample, n | 64 | 42 | 22 | 55 | 9 | ||
| Male, n (%)b | 33 (33/64, 51.6%) | 21 (21/42, 50%) | 12 (12/22, 54.5%) | .73 | 27 (27/55, 49.1%) | 6 (6/9, 66.7%) | .476 |
| Geographical originb | <.001 | <.001 | |||||
| EuroCaucasian, n (%) | 38 (38/62, 61.3%) | 35 (35/40, 87.5%) | 3 (3/22, 13.6%) | 38 (38/55, 69.1%) | 0 | ||
| South American, n (%) | 4 (4/62, 6.5%) | 1 (1/40, 2.5%) | 3 (3/22, 13.6%) | 2 (2/55, 3.6%) | 2 (2/7, 28.6%) | ||
| South Asia and Indian Ocean, n (%) | 10 (10/62, 16.1%) | 0 | 10 (10/22, 45.5%) | 10 (10/55, 18.2%) | 0 | ||
| East Asia, n(%) | 10 (10/62, 16.1%) | 4 (4/40, 10%) | 6 (6/22, 27.3%) | 5 (5/55, 9.1%) | 5 (5/7, 71.4%) | ||
| Age at diagnosis (years)b | 36.51 ± 15.40 | 41.85 ± 14.66 | 26.10 ± 11.00 | <.001 | 37.70 ± 14.91 | 26.00 ± 17.03 | .096 |
| Sample, n | 59 | 39 | 20 | 53 | 6 | ||
| Family history of diabetesb | 32 (32/36, 88.9%) | 15(15/17, 88.2%) | 17 (17/19, 89.5%) | 1.000 | 26 (26/30, 86.7%) | 6 (6/6, 100%) | 1.000 |
| Classification by BMIb | .734 | .080 | |||||
| underweight, n (%) | 3 (3/42, 7.1%) | 2 (2/25, 8%) | 0 | 1 (1/34, 2.9%) | 1 (1/8, 12.5%) | ||
| normal weight, n (%) | 9 (9/42, 21.4%) | 5 (5/25, 20%) | 5 (5/17, 29.4%) | 6 (6/34, 17.6%) | 4 (4/8, 50%) | ||
| overweight, n (%) | 24 (24/42, 57.1%) | 14 (14/25, 56%) | 10 (10/17, 58.8%) | 22 (22/34, 64.7%) | 2 (2/8, 25%) | ||
| obesity, n (%) | 6 (6/42, 14.3%) | 4 (4/25, 16%) | 2 (2/17, 11.8%) | 5 (5/34, 14.7%) | 1 (1/8, 12.5%) | ||
| Overweight and Obesity, n(%) | 30 (30/42, 71.4%) | 18 (18/25, 72%) | 12 (12/17, 70.6%) | 1.000 | 27 (27/34, 79.4%) | 3 (3/8, 37.5%) | .031 |
| HbA1c (%)b | 7.20 (6.33, 9.38) | 6.65 (6.18, 8.50) | 9.45 (6.48, 11.43) | .108 | 7.45 (5.70, 9.88) | 7.20 (6.55, 9.28) | .573 |
| Sample, n | 14 | 8 | 6 | 6 | 8 | ||
| FPG (mmol/L)b | 8.99 (6.70, 11.95) | 6.90(6.21, 12.98) | 9.70(6.89, 12.55) | .312 | 9.70(7.35, 12.80) | 6.70(6.09, 8.83) | .115 |
| Sample, n | 20 | 6 | 14 | 13 | 7 | ||
| FCP (ng/mL)b | 0.99 (0.62, 1.6) | 1.40(1.01, 2.22) | 0.92(0.52, 1.35) | .497 | 0.80(0.23, 1.10) | 1.50(0.98, 1.73) | .164 |
| Sample, n | 11 | 3 | 8 | 7 | 4 | ||
| Insulin or insulin analog therapy, n(%)b | 17 (17/38, 44.7%) | 9 (9/18, 50%) | 8 (8/20, 40%) | .745 | 14 (14/31, 45.2%) | 3 (3/7, 42.9%) | 1.000 |
| . | . | Variant location . | . | Mutation type . | . | ||
|---|---|---|---|---|---|---|---|
| . | MODY4 . | Trans-D . | Homo-D . | P valuea . | Missense mutations . | Nonsense and frameshift mutations . | P valuea . |
| Sample, n | 64 | 42 | 22 | 55 | 9 | ||
| Male, n (%)b | 33 (33/64, 51.6%) | 21 (21/42, 50%) | 12 (12/22, 54.5%) | .73 | 27 (27/55, 49.1%) | 6 (6/9, 66.7%) | .476 |
| Geographical originb | <.001 | <.001 | |||||
| EuroCaucasian, n (%) | 38 (38/62, 61.3%) | 35 (35/40, 87.5%) | 3 (3/22, 13.6%) | 38 (38/55, 69.1%) | 0 | ||
| South American, n (%) | 4 (4/62, 6.5%) | 1 (1/40, 2.5%) | 3 (3/22, 13.6%) | 2 (2/55, 3.6%) | 2 (2/7, 28.6%) | ||
| South Asia and Indian Ocean, n (%) | 10 (10/62, 16.1%) | 0 | 10 (10/22, 45.5%) | 10 (10/55, 18.2%) | 0 | ||
| East Asia, n(%) | 10 (10/62, 16.1%) | 4 (4/40, 10%) | 6 (6/22, 27.3%) | 5 (5/55, 9.1%) | 5 (5/7, 71.4%) | ||
| Age at diagnosis (years)b | 36.51 ± 15.40 | 41.85 ± 14.66 | 26.10 ± 11.00 | <.001 | 37.70 ± 14.91 | 26.00 ± 17.03 | .096 |
| Sample, n | 59 | 39 | 20 | 53 | 6 | ||
| Family history of diabetesb | 32 (32/36, 88.9%) | 15(15/17, 88.2%) | 17 (17/19, 89.5%) | 1.000 | 26 (26/30, 86.7%) | 6 (6/6, 100%) | 1.000 |
| Classification by BMIb | .734 | .080 | |||||
| underweight, n (%) | 3 (3/42, 7.1%) | 2 (2/25, 8%) | 0 | 1 (1/34, 2.9%) | 1 (1/8, 12.5%) | ||
| normal weight, n (%) | 9 (9/42, 21.4%) | 5 (5/25, 20%) | 5 (5/17, 29.4%) | 6 (6/34, 17.6%) | 4 (4/8, 50%) | ||
| overweight, n (%) | 24 (24/42, 57.1%) | 14 (14/25, 56%) | 10 (10/17, 58.8%) | 22 (22/34, 64.7%) | 2 (2/8, 25%) | ||
| obesity, n (%) | 6 (6/42, 14.3%) | 4 (4/25, 16%) | 2 (2/17, 11.8%) | 5 (5/34, 14.7%) | 1 (1/8, 12.5%) | ||
| Overweight and Obesity, n(%) | 30 (30/42, 71.4%) | 18 (18/25, 72%) | 12 (12/17, 70.6%) | 1.000 | 27 (27/34, 79.4%) | 3 (3/8, 37.5%) | .031 |
| HbA1c (%)b | 7.20 (6.33, 9.38) | 6.65 (6.18, 8.50) | 9.45 (6.48, 11.43) | .108 | 7.45 (5.70, 9.88) | 7.20 (6.55, 9.28) | .573 |
| Sample, n | 14 | 8 | 6 | 6 | 8 | ||
| FPG (mmol/L)b | 8.99 (6.70, 11.95) | 6.90(6.21, 12.98) | 9.70(6.89, 12.55) | .312 | 9.70(7.35, 12.80) | 6.70(6.09, 8.83) | .115 |
| Sample, n | 20 | 6 | 14 | 13 | 7 | ||
| FCP (ng/mL)b | 0.99 (0.62, 1.6) | 1.40(1.01, 2.22) | 0.92(0.52, 1.35) | .497 | 0.80(0.23, 1.10) | 1.50(0.98, 1.73) | .164 |
| Sample, n | 11 | 3 | 8 | 7 | 4 | ||
| Insulin or insulin analog therapy, n(%)b | 17 (17/38, 44.7%) | 9 (9/18, 50%) | 8 (8/20, 40%) | .745 | 14 (14/31, 45.2%) | 3 (3/7, 42.9%) | 1.000 |
Abbreviations: BMI, body mass index; FCP, fasting c-peptide; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c.
aP values are for the t-test, Mann–Whitney U test and χ2 analyses or Fisher's exact test across the groups. A 2-sided test with P < .05 was considered to be statistically significant.
bData are means ± SD or medians (interquartile ranges) for skewed variables or numbers (proportions) for categorical variables.
Clinical characteristics of Chinese cohort of early-onset type 2 diabetes mellitus and previously reported patients with MODY4
| . | . | Variant location . | . | Mutation type . | . | ||
|---|---|---|---|---|---|---|---|
| . | MODY4 . | Trans-D . | Homo-D . | P valuea . | Missense mutations . | Nonsense and frameshift mutations . | P valuea . |
| Sample, n | 64 | 42 | 22 | 55 | 9 | ||
| Male, n (%)b | 33 (33/64, 51.6%) | 21 (21/42, 50%) | 12 (12/22, 54.5%) | .73 | 27 (27/55, 49.1%) | 6 (6/9, 66.7%) | .476 |
| Geographical originb | <.001 | <.001 | |||||
| EuroCaucasian, n (%) | 38 (38/62, 61.3%) | 35 (35/40, 87.5%) | 3 (3/22, 13.6%) | 38 (38/55, 69.1%) | 0 | ||
| South American, n (%) | 4 (4/62, 6.5%) | 1 (1/40, 2.5%) | 3 (3/22, 13.6%) | 2 (2/55, 3.6%) | 2 (2/7, 28.6%) | ||
| South Asia and Indian Ocean, n (%) | 10 (10/62, 16.1%) | 0 | 10 (10/22, 45.5%) | 10 (10/55, 18.2%) | 0 | ||
| East Asia, n(%) | 10 (10/62, 16.1%) | 4 (4/40, 10%) | 6 (6/22, 27.3%) | 5 (5/55, 9.1%) | 5 (5/7, 71.4%) | ||
| Age at diagnosis (years)b | 36.51 ± 15.40 | 41.85 ± 14.66 | 26.10 ± 11.00 | <.001 | 37.70 ± 14.91 | 26.00 ± 17.03 | .096 |
| Sample, n | 59 | 39 | 20 | 53 | 6 | ||
| Family history of diabetesb | 32 (32/36, 88.9%) | 15(15/17, 88.2%) | 17 (17/19, 89.5%) | 1.000 | 26 (26/30, 86.7%) | 6 (6/6, 100%) | 1.000 |
| Classification by BMIb | .734 | .080 | |||||
| underweight, n (%) | 3 (3/42, 7.1%) | 2 (2/25, 8%) | 0 | 1 (1/34, 2.9%) | 1 (1/8, 12.5%) | ||
| normal weight, n (%) | 9 (9/42, 21.4%) | 5 (5/25, 20%) | 5 (5/17, 29.4%) | 6 (6/34, 17.6%) | 4 (4/8, 50%) | ||
| overweight, n (%) | 24 (24/42, 57.1%) | 14 (14/25, 56%) | 10 (10/17, 58.8%) | 22 (22/34, 64.7%) | 2 (2/8, 25%) | ||
| obesity, n (%) | 6 (6/42, 14.3%) | 4 (4/25, 16%) | 2 (2/17, 11.8%) | 5 (5/34, 14.7%) | 1 (1/8, 12.5%) | ||
| Overweight and Obesity, n(%) | 30 (30/42, 71.4%) | 18 (18/25, 72%) | 12 (12/17, 70.6%) | 1.000 | 27 (27/34, 79.4%) | 3 (3/8, 37.5%) | .031 |
| HbA1c (%)b | 7.20 (6.33, 9.38) | 6.65 (6.18, 8.50) | 9.45 (6.48, 11.43) | .108 | 7.45 (5.70, 9.88) | 7.20 (6.55, 9.28) | .573 |
| Sample, n | 14 | 8 | 6 | 6 | 8 | ||
| FPG (mmol/L)b | 8.99 (6.70, 11.95) | 6.90(6.21, 12.98) | 9.70(6.89, 12.55) | .312 | 9.70(7.35, 12.80) | 6.70(6.09, 8.83) | .115 |
| Sample, n | 20 | 6 | 14 | 13 | 7 | ||
| FCP (ng/mL)b | 0.99 (0.62, 1.6) | 1.40(1.01, 2.22) | 0.92(0.52, 1.35) | .497 | 0.80(0.23, 1.10) | 1.50(0.98, 1.73) | .164 |
| Sample, n | 11 | 3 | 8 | 7 | 4 | ||
| Insulin or insulin analog therapy, n(%)b | 17 (17/38, 44.7%) | 9 (9/18, 50%) | 8 (8/20, 40%) | .745 | 14 (14/31, 45.2%) | 3 (3/7, 42.9%) | 1.000 |
| . | . | Variant location . | . | Mutation type . | . | ||
|---|---|---|---|---|---|---|---|
| . | MODY4 . | Trans-D . | Homo-D . | P valuea . | Missense mutations . | Nonsense and frameshift mutations . | P valuea . |
| Sample, n | 64 | 42 | 22 | 55 | 9 | ||
| Male, n (%)b | 33 (33/64, 51.6%) | 21 (21/42, 50%) | 12 (12/22, 54.5%) | .73 | 27 (27/55, 49.1%) | 6 (6/9, 66.7%) | .476 |
| Geographical originb | <.001 | <.001 | |||||
| EuroCaucasian, n (%) | 38 (38/62, 61.3%) | 35 (35/40, 87.5%) | 3 (3/22, 13.6%) | 38 (38/55, 69.1%) | 0 | ||
| South American, n (%) | 4 (4/62, 6.5%) | 1 (1/40, 2.5%) | 3 (3/22, 13.6%) | 2 (2/55, 3.6%) | 2 (2/7, 28.6%) | ||
| South Asia and Indian Ocean, n (%) | 10 (10/62, 16.1%) | 0 | 10 (10/22, 45.5%) | 10 (10/55, 18.2%) | 0 | ||
| East Asia, n(%) | 10 (10/62, 16.1%) | 4 (4/40, 10%) | 6 (6/22, 27.3%) | 5 (5/55, 9.1%) | 5 (5/7, 71.4%) | ||
| Age at diagnosis (years)b | 36.51 ± 15.40 | 41.85 ± 14.66 | 26.10 ± 11.00 | <.001 | 37.70 ± 14.91 | 26.00 ± 17.03 | .096 |
| Sample, n | 59 | 39 | 20 | 53 | 6 | ||
| Family history of diabetesb | 32 (32/36, 88.9%) | 15(15/17, 88.2%) | 17 (17/19, 89.5%) | 1.000 | 26 (26/30, 86.7%) | 6 (6/6, 100%) | 1.000 |
| Classification by BMIb | .734 | .080 | |||||
| underweight, n (%) | 3 (3/42, 7.1%) | 2 (2/25, 8%) | 0 | 1 (1/34, 2.9%) | 1 (1/8, 12.5%) | ||
| normal weight, n (%) | 9 (9/42, 21.4%) | 5 (5/25, 20%) | 5 (5/17, 29.4%) | 6 (6/34, 17.6%) | 4 (4/8, 50%) | ||
| overweight, n (%) | 24 (24/42, 57.1%) | 14 (14/25, 56%) | 10 (10/17, 58.8%) | 22 (22/34, 64.7%) | 2 (2/8, 25%) | ||
| obesity, n (%) | 6 (6/42, 14.3%) | 4 (4/25, 16%) | 2 (2/17, 11.8%) | 5 (5/34, 14.7%) | 1 (1/8, 12.5%) | ||
| Overweight and Obesity, n(%) | 30 (30/42, 71.4%) | 18 (18/25, 72%) | 12 (12/17, 70.6%) | 1.000 | 27 (27/34, 79.4%) | 3 (3/8, 37.5%) | .031 |
| HbA1c (%)b | 7.20 (6.33, 9.38) | 6.65 (6.18, 8.50) | 9.45 (6.48, 11.43) | .108 | 7.45 (5.70, 9.88) | 7.20 (6.55, 9.28) | .573 |
| Sample, n | 14 | 8 | 6 | 6 | 8 | ||
| FPG (mmol/L)b | 8.99 (6.70, 11.95) | 6.90(6.21, 12.98) | 9.70(6.89, 12.55) | .312 | 9.70(7.35, 12.80) | 6.70(6.09, 8.83) | .115 |
| Sample, n | 20 | 6 | 14 | 13 | 7 | ||
| FCP (ng/mL)b | 0.99 (0.62, 1.6) | 1.40(1.01, 2.22) | 0.92(0.52, 1.35) | .497 | 0.80(0.23, 1.10) | 1.50(0.98, 1.73) | .164 |
| Sample, n | 11 | 3 | 8 | 7 | 4 | ||
| Insulin or insulin analog therapy, n(%)b | 17 (17/38, 44.7%) | 9 (9/18, 50%) | 8 (8/20, 40%) | .745 | 14 (14/31, 45.2%) | 3 (3/7, 42.9%) | 1.000 |
Abbreviations: BMI, body mass index; FCP, fasting c-peptide; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c.
aP values are for the t-test, Mann–Whitney U test and χ2 analyses or Fisher's exact test across the groups. A 2-sided test with P < .05 was considered to be statistically significant.
bData are means ± SD or medians (interquartile ranges) for skewed variables or numbers (proportions) for categorical variables.
Analysis of the Relationship Between PDX1 Gene Variation and Clinical Phenotype of Patients
Carriers were grouped according to the location of mutations, and the clinical characteristics between the transactivation domain (including the variants between amino acids 79 and 146) and the homeodomain (including the variants after amino acid 206) were compared (Table 3). The results showed that 87.5% of PDX1 transactivation domain mutations were found in Eurasian Caucasian populations, and 45.5% of PDX1 homeodomain mutations were found in South Asian and Indian Ocean populations. Compared with the transactivation domain, we found that variant carriers of the homeodomain mutations had a significantly earlier age of diagnosis (26.10 ± 11.00 vs 41.85 ± 14.66 years, P < .001) and higher glycated hemoglobin (9.45 [6.48, 11.43] vs 6.65 [6.18,8.50], limited by the number of samples, there was no statistical difference with P = .108). We then compared patients in groups based on the type of mutation (Table 3). Nonsense and frameshift mutations mainly came from East Asian (5/7, 71.4%) and South American (2/7, 28.6%) populations, while missense mutations were mostly found in EuroCaucasian (38/55, 69.1%) and South Asia and Indian Ocean (10/55, 18.2%). The proportion of overweight and obese individuals in the missense mutation group was significantly higher than that in the nonsense and frameshift mutation groups (27/34 [79.4%] vs 3/8 [37.5%], P = .031).
Discussion
In this study, 4 PDX1 variants (H128Q, Q133H, G246Rfs*21, A251Efs*49) were assessed as “likely pathogenic” mutations based on ACMG guidelines, and 4 patients diagnosed with MODY4 were identified in a EOD cohort of 679 patients with a 0.59% prevalence of MODY4. We summarized cases reported in the previous literature and compared the relationship between mutant genotypes and clinical features, and found that patients with MODY4 were diagnosed with diabetes at an early age and most had a family history; 71.4% of them were overweight or obese, and 44.7% required insulin or insulin analog therapy. We also found the type of PDX1 mutation was geographically specific and carriers of the homeodomain variants had an earlier age of diagnosis than carriers of the transactivation domain. Patients with missense mutations have significantly higher BMI and rates of overweight and obesity than nonsense or frameshift mutations.
The diagnosis of MODY is often ignored, and most patients lack evidence of a genetic diagnosis, which leads to an underestimation of the actual prevalence of MODY. Data on the prevalence of MODY4 in the whole population are still lacking and have been calculated only in specific cohorts. Only 1 patient with the PDX1 pathogenic mutation was found from 198 patients with clinical suspicion of MODY, and the diagnostic rate of MODY4 was about 0.5% in a Brazilian cohort (10). In another study, 230 children from Anatolia with hyperglycemia or glycosuria were screened for MODY, and only 1 person was diagnosed with the PDX1 mutation. The detection rate of MODY4 was about 0.43% (30). In a study using exome sequencing from 77 184 individuals older than 40 years, including 38 618 multi-ancestral individuals from a case–control study of type 2 diabetes mellitus and 38 566 participants from the UK Biobank, only 2 diabetic patients carrying the PDX1 pathogenic variant were detected, and the detection rate of MODY4 in this population was 0.003% (31). The ProDiGY study included a total of 3333 multiethnic participants and evaluated whole-exome sequence data for these clinically diagnosed adolescents with type 2 diabetes before 20 years of age. Five cases were found to have pathogenic or likely pathogenic variants of PDX1, and the overall prevalence of MODY4 was 0.15% (14, 32). Other large cohorts were either not screened for PDX1 or have not found PDX1 gene mutations (16-18). The diagnostic criteria for MODY4 in the above data were all based on patients with diabetes carrying a pathogenic or likely pathogenic PDX1 mutation. These results indicated that there were significant differences in the prevalence of MODY4 that depended on race and clinical features. In our study, we identified 4 patients with the PDX1 mutation in a cohort of 679 individuals with EOD, and the prevalence of MODY4 was approximately 0.59%. The rare pathogenic variation of PDX1 occurred at a frequency of 0.12% in the whole Chinese population from ChinaMAP. The prevalence of MODY4 in the diabetic population in China was first reported. These results suggest that MODY4 may not be as rare as previously thought in patients with EOD.
Clinical manifestations of MODY4 are markedly heterogeneous. In previous studies, patients with MODY4 were diagnosed with diabetes between the ages of 10 and 65 years, include nonobese and obese phenotypes, lack the specificity required for a definitive clinical diagnosis, and treatment varies from oral medications to insulin interventions. Clinical features varied even among patients with the same mutation site, which makes it difficult for doctors to distinguish patients with MODY4 from EOD based on clinical manifestations alone (33, 34). Effects and consequences associated with heterozygous variants in PDX1 may be influenced by genetic and environmental background, presenting with a broad phenotypic spectrum (12). However, previous studies have not analyzed the relationship between PDX1 gene mutation and clinical phenotype. Our study is the first to analyze the clinical manifestations of patients with mutations in different PDX1 domains and different mutation types. The results of our investigation show that carriers of mutations in the homeodomain had earlier age of diagnosis than carriers of mutations in the transactivation domain. At the same time, we found the clinical characteristics of variant carriers in different mutation types were slightly different. Surprisingly, 79.4% of missense mutation carriers were overweight and obese, compared with just 37.5% of nonsense and frameshift mutation carriers. The influence of missense mutation on PDX1 activity was not as obvious as frameshift or nonsense mutations, while environmental factors may play an important role in PDX1 mutations leading to MODY. Therefore, it is reasonable to speculate that overweight or obesity may be necessary to prompt the development of diabetes in missense mutation carriers compared with frameshift or nonsense carries.
To date, only a few mutations in the PDX1 gene were thought to be the cause of monogenic diabetes. We summarized previously reported cases of MODY4 and assessed the pathogenicity of the mutation based on ACMG guidelines. Only a fraction of mutations was classified as “likely pathogenic” or “pathogenic” variants and most of them were classified as “uncertain significance,” which lack pedigree cosegregation, and reliable functional experimental verification. Therefore, it was still doubtful whether those cases reported as MODY4 can be diagnosed. In order to make a more accurate estimate of the prevalence of MODY4 in our EOD population, we set specific diagnostic criteria and performed functional tests on uncertain significance mutations selected from the EOD cohort. Our results indicated that the carriers of missense mutations who were overweight or obese were often ignored.
The 2 potentially pathogenic missense mutations (H128Q, Q133H) identified for the first time in our study were located in the middle area between the transactivation domain and the homeodomain. There is less research on the function of this region. Our study showed that the transcription of the INS promoter catalyzed by the H128Q and Q133H variants in this region was significantly inhibited. However, the specific role of this intermediate region in PDX1 as a transcription factor and how it affects the expression of insulin-related genes remains unknown. Following the homeodomain is the C-terminus. Little is currently known about the role of the C-terminus. There were indications that the C-terminus is required for full transfer activation of the PDX1 target gene promoter (35, 36). In our study, we found novel frameshift mutations (G232Sfs*2, G246Rfs*21, A251Efs*49) at the C-terminus and demonstrated that C-terminal mutations can also cause the MODY4 phenotype.
The main biological function of PDX1 is to influence the development of the pancreas during the embryonic period. Neonates with homozygous inactivating mutations in PDX1 have neonatal diabetes and concomitant pancreatic agenesis (37, 38). In mature pancreatic β-cells, PDX1 affects β-cell maintenance and regeneration, while in PDX1 knockout heterozygous mice the compensatory proliferative response of pancreatic islets in the insulin-resistant state was limited. These results suggest that PDX-1 is required for the compensatory response to insulin resistance in the postdevelopmental state of β-cell growth, and mutations in PDX1 will lead to poor islet function (39). Most of the patients with MODY4 reported in previous literature had poor β-cell function and nearly half of them required insulin therapy. While the clinical manifestations of some cases were similar to those of type 2 diabetes mellitus, only oral hypoglycemic drugs, such as metformin, pioglitazone, dipeptidyl peptidases inhibitor inhibitors, were required (12, 19, 40). Dapagliflozin, a sodium–glucose transport protein 2 inhibitor, enhanced β-cell proliferation and PDX1 expression in streptozotocin and high-fat diet–induced diabetic mice (41, 42). Early liraglutide treatment increased PDX1 expression and β-cell function improvement in db/db mice (43, 44). Therefore, new hypoglycemic drugs such as sodium–glucose transport protein 2 inhibitor and glucagon like peptide-1 receptor agonists may be new treatment options for MODY4.
Our study had the following limitations. First, the majority of the study population came from patients with EOD in northern China and had a limited sample size. Therefore, the prevalence of MODY4 estimated by the study cannot be reliably extrapolated to other races or age groups. Second, functional studies of PDX1 variants were incomplete. Although dual-luciferase reporter assays were able to demonstrate reduced transcriptional activation of the insulin promoter catalyzed by the PDX1 variants, more experiments such as chromatin immunoprecipitation assay or electrophoretic mobility shift assay and Western blot need to be conducted for further verification to explore whether variants regulate transcription by affecting PDX1 protein expression or protein–protein contacts. Third, we did not perform functional validation of all previously identified PDX1 mutations classified as “uncertain significance.” Therefore, whether these previously reported cases can be diagnosed as MODY4 remains unknown. Our study suggested that the pathogenicity of mutations with uncertain significance needs to be clarified in the future, which would be helpful to deeply characterize MODY4. Fourth, as zinc transporter 8 antibodies (ZnT8A) was not detected in this study, a small fraction of patients with type 1 diabetes diagnosed only according to ZnT8A might have been wrongly included (45, 46). In this study, we ruled out patients with typical clinical manifestations of type 1 diabetes (complete lack of endogenous insulin secretion) regardless of the result of autoantibodies of type 1 diabetes, which might reduce the effect derived from lack of Zn8TA detection. Fifth, owing to the limitation of DNA sequencing, the large fragment deletions could not be identified in this study, which might have underestimated the prevalence of MODY4.
Conclusion
In conclusion, this study first reported that the prevalence of MODY4 in EOD in China was 0.59%. Our study found the mutant genotypes had geographic specificity. Patients with missense variants had higher rates of overweight or obesity, suggesting overweight or obesity contributed to the development of MODY4. Clinically, it is more difficult to distinguish MODY4 from EOD because they had similar features with EOD compared with other MODY subtypes.
Acknowledgments
We would like to extend our sincere thanks to the participants of this study who volunteered their time to participate in the research. We would also like to thank our colleagues and our nursing staff of the Department of Endocrinology and Metabolism for their assistance in the collection of clinical samples.
Funding
This study was supported by the National Key Research and Development Program of China (2016YFC1304901), the Beijing Municipal Science and Technology Commission (Z141100007414002 and D131100005313008).
Disclosures
The authors declares no conflicts of interest relevant to this publication.
Data Availability
Data supporting the results of this study are available on request from the corresponding authors. The data cannot be made public because of privacy or ethical restrictions. The relevant public databases and resources involved in the results can be obtained online.
References
Abbreviations
- ACMG
American College of Medical Genetics and Genomics
- BMI
body mass index
- EOD
early-onset type 2 diabetes
- MODY
maturity-onset diabetes of the young
Author notes
Hong Lian and Siqian Gong contributed equally to this work.
Xueyao Han and Linong Ji contributed equally to this work.



