-
PDF
- Split View
-
Views
-
Cite
Cite
Annika Ewert, Mirko Rehberg, Karl Peter Schlingmann, Olaf Hiort, Ulrike John-Kroegel, Oliver Metzing, Elke Wühl, Franz Schaefer, Markus J Kemper, Ute Derichs, Annette Richter-Unruh, Ludwig Patzer, Norbert Albers, Desiree Dunstheimer, Holger Haberland, Sabine Heger, Carmen Schröder, Norbert Jorch, Elmar Schmid, Hagen Staude, Marcus Weitz, Clemens Freiberg, Maren Leifheit-Nestler, Miroslav Zivicnjak, Dirk Schnabel, Dieter Haffner, on behalf of the German Society for Pediatric Nephrology (GPN) and the German Society for Pediatric Endocrinology and Diabetology (DGKED), Effects of Burosumab Treatment on Mineral Metabolism in Children and Adolescents With X-linked Hypophosphatemia, The Journal of Clinical Endocrinology & Metabolism, Volume 108, Issue 10, October 2023, Pages e998–e1006, https://doi.org/10.1210/clinem/dgad223
Close - Share Icon Share
Abstract
Burosumab has been approved for the treatment of children and adults with X-linked hypophosphatemia (XLH). Real-world data and evidence for its efficacy in adolescents are lacking.
To assess the effects of 12 months of burosumab treatment on mineral metabolism in children (aged <12 years) and adolescents (aged 12-18 years) with XLH.
Prospective national registry.
Hospital clinics.
A total of 93 patients with XLH (65 children, 28 adolescents).
Z scores for serum phosphate, alkaline phosphatase (ALP), and renal tubular reabsorption of phosphate per glomerular filtration rate (TmP/GFR) at 12 months.
At baseline, patients showed hypophosphatemia (−4.4 SD), reduced TmP/GFR (−6.5 SD), and elevated ALP (2.7 SD, each P < .001 vs healthy children) irrespective of age, suggesting active rickets despite prior therapy with oral phosphate and active vitamin D in 88% of patients. Burosumab treatment resulted in comparable increases in serum phosphate and TmP/GFR in children and adolescents with XLH and a steady decline in serum ALP (each P < .001 vs baseline). At 12 months, serum phosphate, TmP/GFR, and ALP levels were within the age-related normal range in approximately 42%, 27%, and 80% of patients in both groups, respectively, with a lower, weight-based final burosumab dose in adolescents compared with children (0.72 vs 1.06 mg/kg, P < .01).
In this real-world setting, 12 months of burosumab treatment was equally effective in normalizing serum ALP in adolescents and children, despite persistent mild hypophosphatemia in one-half of patients, suggesting that complete normalization of serum phosphate is not mandatory for substantial improvement of rickets in these patients. Adolescents appear to require lower weight-based burosumab dosage than children.
X-linked hypophosphatemia (XLH) is the most common cause of inherited rickets, with a prevalence of approximately 4 to 5/100 000 children (1, 2). It is caused by pathogenic variants in the PHEX (phosphate-regulating neutral endopeptidase homolog X-linked) gene, resulting in increased synthesis of the phosphaturic hormone fibroblast growth factor 23 (FGF23) in bone (3). Elevated circulating FGF23 concentrations inhibit phosphate (Pi) reabsorption in the proximal tubule via decreased expression of sodium-dependent Pi transporters NaPi2a and NaPi2c, suppress renal 1,25(OH)2D3 synthesis, and increase 1,25(OH)2D3 degradation, resulting in hypophosphatemia and, consecutively, rickets (4, 5). Patients usually develop clinical symptoms in the first 2 years of life, including delayed walking, a broad-based, waddling gait, progressive disproportionate short stature, thickened wrists and ankles because of widened metaphyses, and leg deformities associated with elevated serum alkaline phosphatase (ALP) levels (6, 7).
Historically, XLH treatment consisted of frequent oral supplementation of Pi salts in conjunction with active vitamin D (“conventional treatment”), which has limited efficacy in healing rickets based on normalization of serum ALP and resolution of radiological manifestations and is associated with side effects such as secondary hyperparathyroidism, intestinal problems, and nephrocalcinosis (7-12). Recently, burosumab, a humanized anti-FGF23 monoclonal antibody, was approved in the United States, Europe, and Japan for the treatment of children and adults with XLH. So far, 2 uncontrolled and 1 controlled pediatric trials were reported, including a total of 94 pediatric patients with XLH treated over periods ranging from 40 to 160 weeks (9, 13-15). Only children (aged 1-12 years) with substantial, active rickets, based on a radiological rickets severity score (RSS) of at least 1.5 or 2.0, despite conventional treatment, were included (6, 16). Adolescents (aged 12-18 years) and those with hyperparathyroidism were excluded. In these trials, burosumab was titrated to raise fasting serum Pi levels to the lower end of the normal reference range for age, which resulted in healing rickets in the vast majority of patients, as evidenced by a radiological RSS ≤1. However, the optimal burosumab dose and appropriate Pi target range to achieve normalization of mineral metabolism in pediatric patients with XLH remains unclear (6, 7, 16, 17). Real-world data on the use of burosumab in pediatric patients with XLH, except in small case series, are lacking (18-21).
We hypothesized that (1) treatment with burosumab is comparably effective in adolescents and children with XLH in normalizing serum ALP z score and (2) normalization of serum ALP z score does not necessarily require complete normalization of age-related serum Pi levels. To test this, we analyzed the effects of 12 months of burosumab treatment on mineral metabolism, based on z scores for serum Pi, tubular reabsorption of Pi per glomerular filtration rate (TmP/GFR) and ALP in 93 children and adolescents enrolled in the German XLH registry.
Material and Methods
Patients and Study Design
This is an analysis of the XLH Registry data of the German Society for Pediatric Nephrology and the German Society for Pediatric Endocrinology and Diabetology, initiated in July 2017. The registry received appropriate ethics committee approval from the institutional review board at each participating center and was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all parents/guardians, with age-appropriate consent or assent from patients. Included in this prospective registry are children and adolescents (aged 0-18 years) diagnosed with XLH based on family history and/or genetic confirmation, presence of clinical and/or radiological signs of rickets, impaired growth velocity, and serum Pi levels below the age-related reference range, associated with selective renal Pi wasting in the absence of vitamin D or calcium deficiency (7). A total of 117 pediatric patients with XLH were enrolled in Germany between July 2017 and August 2022.
The analysis presented here is restricted to 93 pediatric patients with XLH: 65 children (aged 1.0-11.9 years) and 28 adolescents (aged 12.0-18.0 years) who received treatment with burosumab for at least 12 months up to August 2022. The data set included, age, sex, family history, PHEX gene analysis, medical history including age at diagnosis, age at start of treatment (conventional therapy/burosumab), skeletal comorbidity/orthopedic interventions, weight, height, concomitant medication (eg, native vitamin D), and relevant biochemical parameters, including serum Pi, calcium, ALP, intact PTH, and creatinine. Optional, additional parameters include urinary Pi and creatinine levels to calculate TmP/GFR and fractional renal Pi reabsorption (TRP), 25-hydroxyvitamin D3 (25(OH)D) and 1,25(OH)2D3 levels, which were available in 69% of patients. Diagnosis was proven by genetic analysis of the PHEX gene in 73/93 (78%) patients, including all those with a negative XLH family history. Conventional treatment was discontinued in all patients for at least 1 week before starting burosumab treatment and data were collected at 3-month intervals.
The indication for use of burosumab, its dosage, and control in the present study were entirely up to the treating physicians on site. In February 2018, the European Medicines Agency (EMA) granted a conditional marketing authorization in the European Union for burosumab in the treatment of XLH with radiographic evidence of bone disease in children ≥1 year of age and in adolescents (22). Some of these patients in this cohort (n = 27) received compassionate burosumab treatment before marketing approval from July 2017 and April 2018. All patients showed signs of rickets on radiography before initiation of burosumab treatment. A standardized radiological follow-up (eg, radiography of both wrist and knee at baseline and after 12 months to assess RSS) was not possible because of the nature of this study and radiation protection regulations applicable in this regard. The physicians were required to comply with EMA recommendations on burosumab treatment in pediatric patients with XLH: (1) reduced fasting, age-related serum Pi levels, after a washout period of at least 7 days in patients on conventional treatment; (2) initial burosumab dose of 0.4 mg/kg body weight given every 2 weeks subcutaneously (0.8 mg/kg body weight after October 2019 according to the updated EMA recommendation); (3) titration of burosumab dose in increments of 0.4 mg/kg body weight to raise fasting serum Pi levels within the lower end of the normal age reference range, with a maximum dosage of 2.0 mg/kg body weight (maximum dose 90 mg); and (4) discontinuation of burosumab if fasting serum Pi level were above the upper normal limit (ULN). Burosumab can be restarted at approximately one-half of the previous dose if serum Pi concentration is below the normal range. Patient monitoring was done according to the EMA recommendation (23). In addition, patients were monitored for changes in 1,25(OH)2D3 serum levels, as suggested previously (7).
Methods
Reference values for height and weight of healthy children were used to calculate age and sex-related z scores (24). At each contributing center, local standard laboratory techniques were used to measure serum and/or urine concentrations of Pi, calcium, creatinine, ALP, PTH, 25(OH)D, and 1,25(OH)2D3. TRP and TmP/GFR were calculated using the following equations: TRP = 1 − ((Up/Pp) × (Pcr/Ucr)); TmP/GFR = Pp − (Up/Ucr) × Pcr, where Up = urinary Pi, Pp = plasma Pi, Pcr = plasma creatinine, and Ucr = urinary creatinine (25, 26). Reference values for TRP and serum calcium were taken from Kruse et al (27) and Soldin et al (28), respectively. Reference values from healthy children for serum Pi (28), ALP (taking into account the local methodology) (29), and TmP/GFR (26, 30, 31) were used to calculate z scores. These reference values were used for this analysis because they correspond to those used locally in the participating centers. Hypophosphatemia, hypercalcemia, elevated ALP, and reduced TmP/GFR or TRP were defined accordingly. Vitamin D insufficiency was defined as 25(OH)D between 12 and 20 ng/mL and vitamin D deficiency as <12 ng/mL (32). Age-related reference values for 1,25(OH)2D3 serum levels and urinary calcium creatinine ratios were used to define 1,25(OH)2D3 deficiency (<lower limit of normal), elevated 1,25(OH)2D3 (>ULN), and hypercalciuria (>ULN), respectively (33, 34). PTH values are given in multiples of ULN of the local assay used (usually 65 pg/mL).
Statistical Analysis
Data are expressed as mean (SD) or median (interquartile range [IQR]) depending on the presence of a normal distribution or n (%). Characteristics were analyzed by χ2, McNemar (paired) test, or Wilcoxon rank-sum tests, as appropriate. Longitudinal changes in parameters of mineral homeostasis were assessed by ANOVA, followed by the Duncan multiple range test. We used simple linear regression analyses to identify factors associated with parameters of mineral homeostasis at 12 months. P < .05 was considered statistically significant. SPSS for Windows, version 28.0 (IBM Corporation, New York, NY), was used.
Results
Patient Characteristics
Data on 65 children and 28 adolescents started on burosumab for treatment of XLH at a median age of 6.9 years (IQR, 3.4-9.7) and 13.7 years (IQR, 12.3-15.2), respectively, is given in Table 1. Patients were diagnosed with XLH at a median age of 2.1 years (IQR, 0.7-3.0); 82 patients (88%) received previous treatment with oral Pi salts and active vitamin D over a median period of 4.6 (IQR, 1.0-9.3) years, which was shorter in children (4.0 years; IQR, 1.0-7.2) compared with adolescents (10.5 years; IQR, 6.8-12.2), P < .001). The median age of the 11 children started on burosumab without prior exposure to conventional therapy was 2.5 years (IQR, 1.4-4.6). Median height z scores were equally reduced in children and adolescents, and 60% of patients showed short stature (<−2.0 z score).
Patient characteristics at initiation of burosumab treatment in patients with XLH
| Characteristics . | All patients (n = 93) . | Age <12 y (n = 65) . | Age ≥12 y (n = 28) . | P value . |
|---|---|---|---|---|
| Age, y | 9.6 (5.0-12.3) | 6.9 (3.4-9.7) | 13.7 (12.3-15.2) | <.001 |
| Female, n (%) | 59 (63.4) | 43 (66.2) | 16 (57.1) | .634 |
| Height, cm | 118.5 (99.0-137.5) | 106.8 (87.6-121.0) | 150.0 (145.3-154.9) | <.001 |
| Height, z score | −2.5 (−3.0 to −1.6)a | −2.6 (−3.0 to −1.8)a | −1.7 (−3.6 to −1.1)a | .218 |
| Body weight, kg | 24.0 (15.8-41.0) | 21.7 (13.3-29.0) | 50.0 (41.6-59.6) | <.001 |
| Body weight, z score | −0.7 (−1.3 to 0.2)b | −0.7 (−1.6 to 0.0) | −0.3 (−1.1 to 0.4) | .480 |
| Previous conventional Tx, n (%) | 82 (88.2) | 54 (83.1) | 28 (100.0) | .089 |
| Duration of conventional Tx, y | 4.6 (1.0-9.3) | 4.0 (1.0-7.2) | 10.5 (6.8-12.2) | <.001 |
| Age when conventional Tx was initiated, y | 2.1 (0.7-3.0) | 1.8 (0.4-3.0) | 2.1 (1.0-8.1) | .486 |
| Serum Pi, mmol/L | 0.70 (0.54-0.85) | 0.71 (0.58-0.90) | 0.58 (0.50-0.81) | .165 |
| Serum Pi, z score | −4.4 (−6.2 to −2.9)a | −4.3 (−5.8 to −2.9)a | −4.3 (−5.8 to −2.9)a | .418 |
| Serum ALP, U/L | 436 (384-563) | 439 (394-561) | 430 (317-577) | .532 |
| Serum ALP, z score | 2.7 (2.0-3.7)a | 2.8 (2.1-3.8)a | 2.7 (1.8-3.3)a | .361 |
| Serum iPTH, ng/mL | 52.9 ± 24.1 | 52.0 ± 22.5 | 55.4 ± 28.9 | .629 |
| Serum PTH, x-fold ULN | 0.76 ± 0.34 | 0.74 ± 0.32 | 0.79 ± 0.41 | .629 |
| Serum 25(OH)D, ng/mL | 22 (16-28) | 23 (16-28) | 20 (10-28) | .443 |
| 1,25(OH)2D, pmol/L | 131 ± 68 | 141 ± 76 | 110 ± 46 | .331 |
| TRP (%) | 0.84 (0.70-0.93) | 0.84 (0.76-0.90) | 0.83 (0.68-0.96) | .693 |
| TmP/GFR, mmol/L | 0.58 ± 0.27 | 0.58 ± 0.29 | 0.58 ± 0.23 | .983 |
| TmP/GFR, z-score | −6.5 (−7.7 to −4.5)a | −6.9 (−7.7 to −5.1)a | −5.6 (−7.6 to −3.2)a | .375 |
| Characteristics . | All patients (n = 93) . | Age <12 y (n = 65) . | Age ≥12 y (n = 28) . | P value . |
|---|---|---|---|---|
| Age, y | 9.6 (5.0-12.3) | 6.9 (3.4-9.7) | 13.7 (12.3-15.2) | <.001 |
| Female, n (%) | 59 (63.4) | 43 (66.2) | 16 (57.1) | .634 |
| Height, cm | 118.5 (99.0-137.5) | 106.8 (87.6-121.0) | 150.0 (145.3-154.9) | <.001 |
| Height, z score | −2.5 (−3.0 to −1.6)a | −2.6 (−3.0 to −1.8)a | −1.7 (−3.6 to −1.1)a | .218 |
| Body weight, kg | 24.0 (15.8-41.0) | 21.7 (13.3-29.0) | 50.0 (41.6-59.6) | <.001 |
| Body weight, z score | −0.7 (−1.3 to 0.2)b | −0.7 (−1.6 to 0.0) | −0.3 (−1.1 to 0.4) | .480 |
| Previous conventional Tx, n (%) | 82 (88.2) | 54 (83.1) | 28 (100.0) | .089 |
| Duration of conventional Tx, y | 4.6 (1.0-9.3) | 4.0 (1.0-7.2) | 10.5 (6.8-12.2) | <.001 |
| Age when conventional Tx was initiated, y | 2.1 (0.7-3.0) | 1.8 (0.4-3.0) | 2.1 (1.0-8.1) | .486 |
| Serum Pi, mmol/L | 0.70 (0.54-0.85) | 0.71 (0.58-0.90) | 0.58 (0.50-0.81) | .165 |
| Serum Pi, z score | −4.4 (−6.2 to −2.9)a | −4.3 (−5.8 to −2.9)a | −4.3 (−5.8 to −2.9)a | .418 |
| Serum ALP, U/L | 436 (384-563) | 439 (394-561) | 430 (317-577) | .532 |
| Serum ALP, z score | 2.7 (2.0-3.7)a | 2.8 (2.1-3.8)a | 2.7 (1.8-3.3)a | .361 |
| Serum iPTH, ng/mL | 52.9 ± 24.1 | 52.0 ± 22.5 | 55.4 ± 28.9 | .629 |
| Serum PTH, x-fold ULN | 0.76 ± 0.34 | 0.74 ± 0.32 | 0.79 ± 0.41 | .629 |
| Serum 25(OH)D, ng/mL | 22 (16-28) | 23 (16-28) | 20 (10-28) | .443 |
| 1,25(OH)2D, pmol/L | 131 ± 68 | 141 ± 76 | 110 ± 46 | .331 |
| TRP (%) | 0.84 (0.70-0.93) | 0.84 (0.76-0.90) | 0.83 (0.68-0.96) | .693 |
| TmP/GFR, mmol/L | 0.58 ± 0.27 | 0.58 ± 0.29 | 0.58 ± 0.23 | .983 |
| TmP/GFR, z-score | −6.5 (−7.7 to −4.5)a | −6.9 (−7.7 to −5.1)a | −5.6 (−7.6 to −3.2)a | .375 |
Data are presented as median (interquartile range) or mean ± SD depending on the presence of normal distribution or numbers (percentages). In patients with prior conventional Tx, serum samples were obtained 1 to 2 weeks after discontinuation of conventional Tx.
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D3 (calcitriol); 25(OH)D, 25 hydroxyvitamin D3 (calcidiol); ALP, alkaline phosphatase; Pi, phosphate; PTH, parathyroid hormone; TmP/GFR, tubular maximum reabsorption of phosphate per glomerular filtration rate; TRP, fractional tubular reabsorption of phosphate; Tx, treatment; ULN, upper limit of normal.
P < .001 vs healthy children.
P < .05 vs healthy children.
Patient characteristics at initiation of burosumab treatment in patients with XLH
| Characteristics . | All patients (n = 93) . | Age <12 y (n = 65) . | Age ≥12 y (n = 28) . | P value . |
|---|---|---|---|---|
| Age, y | 9.6 (5.0-12.3) | 6.9 (3.4-9.7) | 13.7 (12.3-15.2) | <.001 |
| Female, n (%) | 59 (63.4) | 43 (66.2) | 16 (57.1) | .634 |
| Height, cm | 118.5 (99.0-137.5) | 106.8 (87.6-121.0) | 150.0 (145.3-154.9) | <.001 |
| Height, z score | −2.5 (−3.0 to −1.6)a | −2.6 (−3.0 to −1.8)a | −1.7 (−3.6 to −1.1)a | .218 |
| Body weight, kg | 24.0 (15.8-41.0) | 21.7 (13.3-29.0) | 50.0 (41.6-59.6) | <.001 |
| Body weight, z score | −0.7 (−1.3 to 0.2)b | −0.7 (−1.6 to 0.0) | −0.3 (−1.1 to 0.4) | .480 |
| Previous conventional Tx, n (%) | 82 (88.2) | 54 (83.1) | 28 (100.0) | .089 |
| Duration of conventional Tx, y | 4.6 (1.0-9.3) | 4.0 (1.0-7.2) | 10.5 (6.8-12.2) | <.001 |
| Age when conventional Tx was initiated, y | 2.1 (0.7-3.0) | 1.8 (0.4-3.0) | 2.1 (1.0-8.1) | .486 |
| Serum Pi, mmol/L | 0.70 (0.54-0.85) | 0.71 (0.58-0.90) | 0.58 (0.50-0.81) | .165 |
| Serum Pi, z score | −4.4 (−6.2 to −2.9)a | −4.3 (−5.8 to −2.9)a | −4.3 (−5.8 to −2.9)a | .418 |
| Serum ALP, U/L | 436 (384-563) | 439 (394-561) | 430 (317-577) | .532 |
| Serum ALP, z score | 2.7 (2.0-3.7)a | 2.8 (2.1-3.8)a | 2.7 (1.8-3.3)a | .361 |
| Serum iPTH, ng/mL | 52.9 ± 24.1 | 52.0 ± 22.5 | 55.4 ± 28.9 | .629 |
| Serum PTH, x-fold ULN | 0.76 ± 0.34 | 0.74 ± 0.32 | 0.79 ± 0.41 | .629 |
| Serum 25(OH)D, ng/mL | 22 (16-28) | 23 (16-28) | 20 (10-28) | .443 |
| 1,25(OH)2D, pmol/L | 131 ± 68 | 141 ± 76 | 110 ± 46 | .331 |
| TRP (%) | 0.84 (0.70-0.93) | 0.84 (0.76-0.90) | 0.83 (0.68-0.96) | .693 |
| TmP/GFR, mmol/L | 0.58 ± 0.27 | 0.58 ± 0.29 | 0.58 ± 0.23 | .983 |
| TmP/GFR, z-score | −6.5 (−7.7 to −4.5)a | −6.9 (−7.7 to −5.1)a | −5.6 (−7.6 to −3.2)a | .375 |
| Characteristics . | All patients (n = 93) . | Age <12 y (n = 65) . | Age ≥12 y (n = 28) . | P value . |
|---|---|---|---|---|
| Age, y | 9.6 (5.0-12.3) | 6.9 (3.4-9.7) | 13.7 (12.3-15.2) | <.001 |
| Female, n (%) | 59 (63.4) | 43 (66.2) | 16 (57.1) | .634 |
| Height, cm | 118.5 (99.0-137.5) | 106.8 (87.6-121.0) | 150.0 (145.3-154.9) | <.001 |
| Height, z score | −2.5 (−3.0 to −1.6)a | −2.6 (−3.0 to −1.8)a | −1.7 (−3.6 to −1.1)a | .218 |
| Body weight, kg | 24.0 (15.8-41.0) | 21.7 (13.3-29.0) | 50.0 (41.6-59.6) | <.001 |
| Body weight, z score | −0.7 (−1.3 to 0.2)b | −0.7 (−1.6 to 0.0) | −0.3 (−1.1 to 0.4) | .480 |
| Previous conventional Tx, n (%) | 82 (88.2) | 54 (83.1) | 28 (100.0) | .089 |
| Duration of conventional Tx, y | 4.6 (1.0-9.3) | 4.0 (1.0-7.2) | 10.5 (6.8-12.2) | <.001 |
| Age when conventional Tx was initiated, y | 2.1 (0.7-3.0) | 1.8 (0.4-3.0) | 2.1 (1.0-8.1) | .486 |
| Serum Pi, mmol/L | 0.70 (0.54-0.85) | 0.71 (0.58-0.90) | 0.58 (0.50-0.81) | .165 |
| Serum Pi, z score | −4.4 (−6.2 to −2.9)a | −4.3 (−5.8 to −2.9)a | −4.3 (−5.8 to −2.9)a | .418 |
| Serum ALP, U/L | 436 (384-563) | 439 (394-561) | 430 (317-577) | .532 |
| Serum ALP, z score | 2.7 (2.0-3.7)a | 2.8 (2.1-3.8)a | 2.7 (1.8-3.3)a | .361 |
| Serum iPTH, ng/mL | 52.9 ± 24.1 | 52.0 ± 22.5 | 55.4 ± 28.9 | .629 |
| Serum PTH, x-fold ULN | 0.76 ± 0.34 | 0.74 ± 0.32 | 0.79 ± 0.41 | .629 |
| Serum 25(OH)D, ng/mL | 22 (16-28) | 23 (16-28) | 20 (10-28) | .443 |
| 1,25(OH)2D, pmol/L | 131 ± 68 | 141 ± 76 | 110 ± 46 | .331 |
| TRP (%) | 0.84 (0.70-0.93) | 0.84 (0.76-0.90) | 0.83 (0.68-0.96) | .693 |
| TmP/GFR, mmol/L | 0.58 ± 0.27 | 0.58 ± 0.29 | 0.58 ± 0.23 | .983 |
| TmP/GFR, z-score | −6.5 (−7.7 to −4.5)a | −6.9 (−7.7 to −5.1)a | −5.6 (−7.6 to −3.2)a | .375 |
Data are presented as median (interquartile range) or mean ± SD depending on the presence of normal distribution or numbers (percentages). In patients with prior conventional Tx, serum samples were obtained 1 to 2 weeks after discontinuation of conventional Tx.
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D3 (calcitriol); 25(OH)D, 25 hydroxyvitamin D3 (calcidiol); ALP, alkaline phosphatase; Pi, phosphate; PTH, parathyroid hormone; TmP/GFR, tubular maximum reabsorption of phosphate per glomerular filtration rate; TRP, fractional tubular reabsorption of phosphate; Tx, treatment; ULN, upper limit of normal.
P < .001 vs healthy children.
P < .05 vs healthy children.
After a washout period of conventional treatment for at least 7 days, median serum Pi (−4.4 SD) and TmP/GFR (−6.5 SD) were equally reduced in children and adolescents compared with healthy children and median serum ALP levels were elevated by approximately 2.7 SD in both groups (each P < .001 vs healthy children). The latter suggests active rickets in view of prevalent, reduced linear growth. Plasma PTH levels were above ULN in 23% of patients. Twenty-five patients (27%) received supplementation with native vitamin D (cholecalciferol) at a median dosage of 1000 IU (range, 500-2000). Vitamin D insufficiency and vitamin D deficiency were noted in 27% and 16% of patients, respectively. Calcitriol levels were decreased in 5% of patients and hypercalciuria was noted in 17% of patients.
Effects of Burosumab Treatment on Mineral Metabolism
In both children and adolescents, burosumab treatment resulted in sustained increases of median Pi, amounting to −2.0 z score and −1.7 z score at 6 months, respectively (each P < .001 vs baseline, P > .05 children vs adolescents) (Table 2, Fig. 1A). The respective increases in median absolute Pi values amounted 0.26 mmol/L (children) and 0.38 mmol/L (adolescents; each P < .001 vs baseline, P > .05 children vs adolescents). This was paralleled by sustained increases in TmP/GFR values in both groups (each P < .001 vs baseline; P > .05 children vs adolescents; Fig. 1b). At 12 months, both absolute and standardized serum Pi and TmP/GFR values did not differ between children and adolescents (Table 2, Fig. 1A and 1B) and were within the age-related reference intervals in 42% (Pi) and 27% (TmP/GFR) of patients, respectively, whereas TRP values were normalized (>0.85) in the vast majority of patients (85%) (Table 2, Fig. 1E). The final median weight-based burosumab dose was significantly lower in adolescents compared with children (0.72 vs 1.06 mg/kg body weight, P < .01) (Table 2, Fig. 1F).
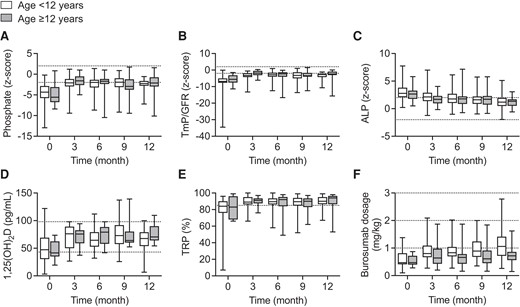
Parameters of mineral metabolism and burosumab dosage in 93 children and adolescents with XLH treated with burosumab over a period of 12 months. Standardized values of serum phosphate (A), tubular maximum reabsorption of phosphate per glomerular filtration rate (TmP/GFR) (B), and alkaline phosphatase (ALP) (C) as well as serum 1,25-dihydroxyvitamin D3 (1,25(OH)2D) (D), fractional tubular reabsorption of phosphate (TRP) (E) and (F) weight-related burosumab dose are given. Box plots indicate median, interquartile range, and min/max, respectively. In both children and adolescents, all parameters of mineral metabolism (ie, serum phosphate, TmP/GFR, and ALP) significantly differed at all time points when compared with baseline (each P < .01). Dotted horizontal lines indicate upper and lower limit of normal (A–D, F) and lower limit of normal (E), respectively.
Parameters of mineral metabolism during burosumab treatment in children and adolescents with XLH
| . | 0 mo . | 6 mo . | 12 mo . | |||
|---|---|---|---|---|---|---|
| <12 y . | ≥12 y . | <12 y . | ≥12 y . | <12 y . | ≥12 y . | |
| Phosphate, mmol/L | 0.71 (0.58-0.90)a | 0.58 (0.50-0.81)a | 1.00 (0.86-1.10)b | 1.00 (0.81-1.11)b | 0.97 (0.90-1.10)b | 0.95 (0.84-1.10)b |
| Phosphate, z score | −4.3 (−5.8 to −2.9)a | −5.6 (−6.8 to −3.1)a,## | −2.0 (−3.2 to −1.4)b | −1.7 (−2.4 to −1.1)b,## | −2.3 (−2.8 to −1.6)b | −2.1 (−3.0 to −0.7)b,## |
| TmP/GFR, mmol/L | 0.52 (0.47-0.66)a | 0.56 (0.39-0.76)a | 0.92 (0.75-1.08)b | 0.89 (0.68-1.03)b | 0.89 (0.77-0.97)b | 0.92 (0.80- 1.03)b |
| TmP/GFR, z score | −6.9 (−7.7 to −5.1)a | −5.6 (−7.6 to −3.2)a,## | −2.8 (−4.0 to −1.5)b | −2.2 (−4.1 to −1.3)b,## | −2.7 (−4.3 to −2.3)b | −2.4 (−3.0 to −0.9)b,# |
| TRP, % | 0.84 (0.76-0.90)a | 0.83 (0.68-0.96)a | 0.90 (0.86-0.94)b | 0.92 (0.84-9.5)b | 0.90 (0.87-0.95)b | 0.94 (0.86-0.96)b |
| ALP, U/L | 439 (394-561)a | 430 (317-577)a | 366 (321-449)b | 325 (232-425)b | 322 (282-384)c | 270 (205-407)b |
| ALP, z score | 2.8 (2.1-3.8)a | 2.7 (1.8-3.3)a | 1.8 (1.0-2.7)b | 1.8 (0.8-2.2)b | 1.2 (0.5-2.0)c | 1.3 (0.5-1.7)b |
| PTH, x-fold ULN | 0.8 (0.5-0.9)a | 0.7 (0.4-1.1)a | 0.7 (0.5-0.8)a | 0.9 (0.6-1.4)a | 0.7 (0.4-0.9)a | 0.7 (0.5-1.2)a |
| 25(OH)D, ng/mL | 22 (16-28)a | 20 (10-28)a | 24 (17-29)a | 23 (17-26)a | 21 (18-25)a | 19 (16-26)a |
| 1,25(OH)2D, pmol/L | 128 (90-201)a | 98 (80-155)a | 161 (112-205)b | 201 (150-224)b | 168 (132-198)b | 182 (160-243)b |
| UCa/Crea, mol/mol | 0.2 (0.1-0.4)a | 0.42 (0.16-0.74)a | 0.3 (0.1-0.5)a | 0.2 (0.1-0.4)a | 0.4 (0.2-0.5)a | 0.1 (0.1-0.6)a |
| Burosumab dose, mg/kg### | 0.47 (0.41-0.81)a | 0.47 (0.40-0.72)a | 0.83 (0.70-1.04)b | 0.66 (0.43-0.78)b | 1.06 (0.72-1.41)b | 0.72 (0.56-0.86)b |
| . | 0 mo . | 6 mo . | 12 mo . | |||
|---|---|---|---|---|---|---|
| <12 y . | ≥12 y . | <12 y . | ≥12 y . | <12 y . | ≥12 y . | |
| Phosphate, mmol/L | 0.71 (0.58-0.90)a | 0.58 (0.50-0.81)a | 1.00 (0.86-1.10)b | 1.00 (0.81-1.11)b | 0.97 (0.90-1.10)b | 0.95 (0.84-1.10)b |
| Phosphate, z score | −4.3 (−5.8 to −2.9)a | −5.6 (−6.8 to −3.1)a,## | −2.0 (−3.2 to −1.4)b | −1.7 (−2.4 to −1.1)b,## | −2.3 (−2.8 to −1.6)b | −2.1 (−3.0 to −0.7)b,## |
| TmP/GFR, mmol/L | 0.52 (0.47-0.66)a | 0.56 (0.39-0.76)a | 0.92 (0.75-1.08)b | 0.89 (0.68-1.03)b | 0.89 (0.77-0.97)b | 0.92 (0.80- 1.03)b |
| TmP/GFR, z score | −6.9 (−7.7 to −5.1)a | −5.6 (−7.6 to −3.2)a,## | −2.8 (−4.0 to −1.5)b | −2.2 (−4.1 to −1.3)b,## | −2.7 (−4.3 to −2.3)b | −2.4 (−3.0 to −0.9)b,# |
| TRP, % | 0.84 (0.76-0.90)a | 0.83 (0.68-0.96)a | 0.90 (0.86-0.94)b | 0.92 (0.84-9.5)b | 0.90 (0.87-0.95)b | 0.94 (0.86-0.96)b |
| ALP, U/L | 439 (394-561)a | 430 (317-577)a | 366 (321-449)b | 325 (232-425)b | 322 (282-384)c | 270 (205-407)b |
| ALP, z score | 2.8 (2.1-3.8)a | 2.7 (1.8-3.3)a | 1.8 (1.0-2.7)b | 1.8 (0.8-2.2)b | 1.2 (0.5-2.0)c | 1.3 (0.5-1.7)b |
| PTH, x-fold ULN | 0.8 (0.5-0.9)a | 0.7 (0.4-1.1)a | 0.7 (0.5-0.8)a | 0.9 (0.6-1.4)a | 0.7 (0.4-0.9)a | 0.7 (0.5-1.2)a |
| 25(OH)D, ng/mL | 22 (16-28)a | 20 (10-28)a | 24 (17-29)a | 23 (17-26)a | 21 (18-25)a | 19 (16-26)a |
| 1,25(OH)2D, pmol/L | 128 (90-201)a | 98 (80-155)a | 161 (112-205)b | 201 (150-224)b | 168 (132-198)b | 182 (160-243)b |
| UCa/Crea, mol/mol | 0.2 (0.1-0.4)a | 0.42 (0.16-0.74)a | 0.3 (0.1-0.5)a | 0.2 (0.1-0.4)a | 0.4 (0.2-0.5)a | 0.1 (0.1-0.6)a |
| Burosumab dose, mg/kg### | 0.47 (0.41-0.81)a | 0.47 (0.40-0.72)a | 0.83 (0.70-1.04)b | 0.66 (0.43-0.78)b | 1.06 (0.72-1.41)b | 0.72 (0.56-0.86)b |
Data are presented as median (interquartile range). Within-group changes: values not sharing common superscript letters a,b,c are significantly different from the values for other time points within 1 row for patients aged younger than 12 years and patients aged equal to or older, respectively. Between-group differences: #P < .05 vs patients aged <12 years; ##P < .01 vs patients aged <12 years. ###Starting dose is given at 0 months.
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D3 (calcitriol); 25(OH)D, 25 hydroxyvitamin D3 (calcidiol); ALP, alkaline phosphatase; Ca, calcium; Crea, creatinine; PTH, parathyroid hormone; TmP/GFR, tubular maximum reabsorption of phosphate per glomerular filtration rate; TRP, fractional tubular reabsorption of phosphate; U, urine; ULN, upper limit of normal.
Parameters of mineral metabolism during burosumab treatment in children and adolescents with XLH
| . | 0 mo . | 6 mo . | 12 mo . | |||
|---|---|---|---|---|---|---|
| <12 y . | ≥12 y . | <12 y . | ≥12 y . | <12 y . | ≥12 y . | |
| Phosphate, mmol/L | 0.71 (0.58-0.90)a | 0.58 (0.50-0.81)a | 1.00 (0.86-1.10)b | 1.00 (0.81-1.11)b | 0.97 (0.90-1.10)b | 0.95 (0.84-1.10)b |
| Phosphate, z score | −4.3 (−5.8 to −2.9)a | −5.6 (−6.8 to −3.1)a,## | −2.0 (−3.2 to −1.4)b | −1.7 (−2.4 to −1.1)b,## | −2.3 (−2.8 to −1.6)b | −2.1 (−3.0 to −0.7)b,## |
| TmP/GFR, mmol/L | 0.52 (0.47-0.66)a | 0.56 (0.39-0.76)a | 0.92 (0.75-1.08)b | 0.89 (0.68-1.03)b | 0.89 (0.77-0.97)b | 0.92 (0.80- 1.03)b |
| TmP/GFR, z score | −6.9 (−7.7 to −5.1)a | −5.6 (−7.6 to −3.2)a,## | −2.8 (−4.0 to −1.5)b | −2.2 (−4.1 to −1.3)b,## | −2.7 (−4.3 to −2.3)b | −2.4 (−3.0 to −0.9)b,# |
| TRP, % | 0.84 (0.76-0.90)a | 0.83 (0.68-0.96)a | 0.90 (0.86-0.94)b | 0.92 (0.84-9.5)b | 0.90 (0.87-0.95)b | 0.94 (0.86-0.96)b |
| ALP, U/L | 439 (394-561)a | 430 (317-577)a | 366 (321-449)b | 325 (232-425)b | 322 (282-384)c | 270 (205-407)b |
| ALP, z score | 2.8 (2.1-3.8)a | 2.7 (1.8-3.3)a | 1.8 (1.0-2.7)b | 1.8 (0.8-2.2)b | 1.2 (0.5-2.0)c | 1.3 (0.5-1.7)b |
| PTH, x-fold ULN | 0.8 (0.5-0.9)a | 0.7 (0.4-1.1)a | 0.7 (0.5-0.8)a | 0.9 (0.6-1.4)a | 0.7 (0.4-0.9)a | 0.7 (0.5-1.2)a |
| 25(OH)D, ng/mL | 22 (16-28)a | 20 (10-28)a | 24 (17-29)a | 23 (17-26)a | 21 (18-25)a | 19 (16-26)a |
| 1,25(OH)2D, pmol/L | 128 (90-201)a | 98 (80-155)a | 161 (112-205)b | 201 (150-224)b | 168 (132-198)b | 182 (160-243)b |
| UCa/Crea, mol/mol | 0.2 (0.1-0.4)a | 0.42 (0.16-0.74)a | 0.3 (0.1-0.5)a | 0.2 (0.1-0.4)a | 0.4 (0.2-0.5)a | 0.1 (0.1-0.6)a |
| Burosumab dose, mg/kg### | 0.47 (0.41-0.81)a | 0.47 (0.40-0.72)a | 0.83 (0.70-1.04)b | 0.66 (0.43-0.78)b | 1.06 (0.72-1.41)b | 0.72 (0.56-0.86)b |
| . | 0 mo . | 6 mo . | 12 mo . | |||
|---|---|---|---|---|---|---|
| <12 y . | ≥12 y . | <12 y . | ≥12 y . | <12 y . | ≥12 y . | |
| Phosphate, mmol/L | 0.71 (0.58-0.90)a | 0.58 (0.50-0.81)a | 1.00 (0.86-1.10)b | 1.00 (0.81-1.11)b | 0.97 (0.90-1.10)b | 0.95 (0.84-1.10)b |
| Phosphate, z score | −4.3 (−5.8 to −2.9)a | −5.6 (−6.8 to −3.1)a,## | −2.0 (−3.2 to −1.4)b | −1.7 (−2.4 to −1.1)b,## | −2.3 (−2.8 to −1.6)b | −2.1 (−3.0 to −0.7)b,## |
| TmP/GFR, mmol/L | 0.52 (0.47-0.66)a | 0.56 (0.39-0.76)a | 0.92 (0.75-1.08)b | 0.89 (0.68-1.03)b | 0.89 (0.77-0.97)b | 0.92 (0.80- 1.03)b |
| TmP/GFR, z score | −6.9 (−7.7 to −5.1)a | −5.6 (−7.6 to −3.2)a,## | −2.8 (−4.0 to −1.5)b | −2.2 (−4.1 to −1.3)b,## | −2.7 (−4.3 to −2.3)b | −2.4 (−3.0 to −0.9)b,# |
| TRP, % | 0.84 (0.76-0.90)a | 0.83 (0.68-0.96)a | 0.90 (0.86-0.94)b | 0.92 (0.84-9.5)b | 0.90 (0.87-0.95)b | 0.94 (0.86-0.96)b |
| ALP, U/L | 439 (394-561)a | 430 (317-577)a | 366 (321-449)b | 325 (232-425)b | 322 (282-384)c | 270 (205-407)b |
| ALP, z score | 2.8 (2.1-3.8)a | 2.7 (1.8-3.3)a | 1.8 (1.0-2.7)b | 1.8 (0.8-2.2)b | 1.2 (0.5-2.0)c | 1.3 (0.5-1.7)b |
| PTH, x-fold ULN | 0.8 (0.5-0.9)a | 0.7 (0.4-1.1)a | 0.7 (0.5-0.8)a | 0.9 (0.6-1.4)a | 0.7 (0.4-0.9)a | 0.7 (0.5-1.2)a |
| 25(OH)D, ng/mL | 22 (16-28)a | 20 (10-28)a | 24 (17-29)a | 23 (17-26)a | 21 (18-25)a | 19 (16-26)a |
| 1,25(OH)2D, pmol/L | 128 (90-201)a | 98 (80-155)a | 161 (112-205)b | 201 (150-224)b | 168 (132-198)b | 182 (160-243)b |
| UCa/Crea, mol/mol | 0.2 (0.1-0.4)a | 0.42 (0.16-0.74)a | 0.3 (0.1-0.5)a | 0.2 (0.1-0.4)a | 0.4 (0.2-0.5)a | 0.1 (0.1-0.6)a |
| Burosumab dose, mg/kg### | 0.47 (0.41-0.81)a | 0.47 (0.40-0.72)a | 0.83 (0.70-1.04)b | 0.66 (0.43-0.78)b | 1.06 (0.72-1.41)b | 0.72 (0.56-0.86)b |
Data are presented as median (interquartile range). Within-group changes: values not sharing common superscript letters a,b,c are significantly different from the values for other time points within 1 row for patients aged younger than 12 years and patients aged equal to or older, respectively. Between-group differences: #P < .05 vs patients aged <12 years; ##P < .01 vs patients aged <12 years. ###Starting dose is given at 0 months.
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D3 (calcitriol); 25(OH)D, 25 hydroxyvitamin D3 (calcidiol); ALP, alkaline phosphatase; Ca, calcium; Crea, creatinine; PTH, parathyroid hormone; TmP/GFR, tubular maximum reabsorption of phosphate per glomerular filtration rate; TRP, fractional tubular reabsorption of phosphate; U, urine; ULN, upper limit of normal.
Median ALP continuously decreased during burosumab treatment, amounting to 1.2 z score and 1.3 z score in children and adolescents at 12 months (each P < .001 vs baseline, P > .05 children vs adolescents) (Fig. 1C). ALP z scores were within the age-related reference intervals in approximately 80% of patients in both groups. Median calcitriol levels were significantly increased throughout (Fig. 1D), compared with baseline values, whereas no significant changes were noted for serum PTH concentrations and urinary calcium creatinine ratios (Table 2). Persistent mild hyperparathyroidism (PTH: 1-3 ULN) was noted in 23% of patients (Table 2). None of the patients developed hypercalcemia. Median height z scores did not change significantly during the 12 months of burosumab treatment, irrespective of age (children −2.6 [IQR, −3.0 to −1.8] vs −2.5 [IQR, −3.0 to −1.81]; adolescents −1.8 [IQR, −3.0 to −1.4] vs −1.7 [IQR, −3.6 to −1.1]; each P > .05). There was no significant difference between boys and girls with respect to the previously mentioned parameters, including burosumab dosage.
Predictors of Serum Pi and ALP During Burosumab Treatment
Final standardized Pi and ALP levels were not significantly associated with patient age, sex, weight-related burosumab dose, 25(OH)D levels or with TRP in the whole patient cohort nor in the 2 subgroups (Fig. 2, data not shown). Likewise, there was no significant association between final standardized serum Pi and standardized ALP in the entire cohort nor in the 2 subgroups (each P > .05). However, a weak association between Pi z score and PTH levels was noted after 6 months of burosumab treatment (r = 0.21; P = .06).
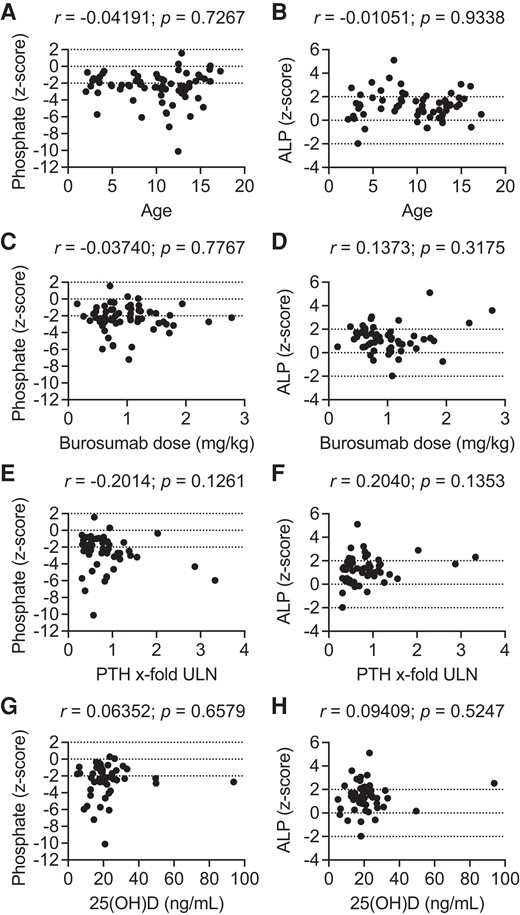
Serum phosphate and alkaline phosphatase z scores after 12 months of burosumab treatment in the whole patient cohort as a function of age (A, B), burosumab dose (C, D), parathyroid hormone (E, F), and 25-hydroxyvitamin D3 levels (G, H). 25(OH)D, 25-hydroxyvitamin D3; ALP, alkaline phosphatase; PTH, parathyroid hormone; ULN, upper limit of normal. Dotted horizontal lines indicate mean and upper and lower limit of normal.
Adverse Events
Burosumab treatment was associated with transient hyperphosphatemia in 2 siblings treated at the same center (maximum serum Pi at 3 weeks, 2.56 and 2.96 mmol/L). Treatment was discontinued and reinstated again when Pi levels returned to below normal range, using one-half of the previous dosage. Later, the dose was increased in both patients to reach low normal Pi levels, suggesting a dosage error as the cause of the hyperphosphatemia. Calcitriol levels were slightly elevated during the observation period in 3.3% of patients, which was not associated with side effects (eg, hypercalcemia or hypercalciuria [data not shown]). Otherwise, no adverse events were reported.
Discussion
This real-world study on 12 months of burosumab treatment in a large cohort of pediatric patients with XLH showed that burosumab treatment was equally effective in adolescents as well as children in normalizing ALP z scores, with adolescents requiring a lower weight-based dosage. Normalization of ALP z scores was achieved despite persistent mild hypophosphatemia in about one-half of patients. Our data thus extend the evidence for burosumab treatment in patients with XLH into the adolescent age range and suggests that complete normalization of serum Pi levels is not a prerequisite for normalization of serum ALP z scores and thus substantial improvement of rickets in these patients.
To account for the wide age range (1-18 years), mineral metabolism parameters were presented as z scores in the present study, which was not the case in the available pediatric clinical trials, where only absolute values of serum Pi, TmP/GFR, and ALP were given, which hampers comparisons with our study. After the washout period, median serum Pi values were decreased by −4.3 SD and −5.6 SD in children and adolescents, respectively, indicating profound hypophosphatemia. Of note, median absolute serum Pi values were slightly lower in our cohort compared with the pediatric clinical trials reporting mean values of 0.81 mmol/L and 0.77 mmol/L in children aged 1 to 4 years and 4 to 12 years, respectively (9, 13, 14). This discrepancy is probably the result of the older age of the patients in the present study because absolute Pi levels decrease with age and/or differences in the methods of determination (29).
Despite prior longstanding treatment with frequent doses of oral Pi and active vitamin D in the vast majority of patients, mean serum ALP levels were elevated by 2.8 SD in both patient groups, suggesting active rickets. Uday et al evaluated ALP z scores together with radiological RSS in a UK cohort of 38 pediatric patients with XLH, who were all on longstanding conventional treatment using the same ALP reference values as in our study (8). At the most recent observation, mean ALP levels were elevated by 3.8 SD and the mean total RSS amounted to 2.5 ± 1.5, indicating a considerable degree of rickets, which supports the concept that conventional treatment has limited efficacy in healing rickets in patients with XLH (7, 9, 11, 35, 36). In the present study, median ALP z scores were less elevated compared with the UK study (2.8 SD vs 2.7 SD) suggesting a lower degree of rickets. This discrepancy may be due to differences in dosage in conventional therapy and/or adherence to this medication. Indeed, adherence to conventional medication is a major challenge in the treatment of patients with XLH, especially in adolescence (37).
Burosumab treatment resulted in sustained increases in absolute (+0.26 mmol/L) and standardized (+2.3 z score) serum Pi, which did not differ between children and adolescents, and was paralleled by similar increases in TmP/GFR. These effects are comparable to those reported in pediatric trials including XLH patients aged 1 to 12 years, revealing increases in mean absolute serum Pi ranging from 0.24 to 0.31 mmol/L and from 0.27 to 0.29 mmol/L at 40 weeks and 64 weeks, respectively (9, 13, 14). Nevertheless, in the present study, persistently reduced serum Pi and TmP/GFR values (<−2.0 z score) were noted in 58% and 73% of patients, respectively. In the pediatric clinical trials, mean serum Pi and TmP/GFR levels were raised to the lower end of the normal range defined in children aged 6 to 12 years as 1.03 to 1.97 mmol/L and 0.84 to 1.42 mmol/L, respectively, suggesting normalization of serum Pi in at least 50% of patients in these studies (9, 13, 14). The apparent slightly lower efficacy of burosumab in normalizing Pi homeostasis in our study, compared with clinical trials is most likely because of differences in the reference values (eg, use of 1 reference value for serum Pi and TmP/GFR for all ages in the clinical trials but age-specific reference values in the present study) and/or use of different methods for determination of serum Pi or calculation of TmP/GFR (2, 38).
In both children and adolescents, the rise in serum Pi levels was accompanied by a sustained decrease in median serum ALP by −1.5 SD resulting in normalization of ALP values after 12 months in about 80% of patients. Normalization of serum ALP despite persistent mild hypophosphatemia in about one-half of patients suggests that normalization of serum Pi is not a conditio sine qua non for substantial improvement of rickets in patients with XLH. In this context, it should also be mentioned that based on the pharmacokinetics of burosumab in children, a steady state of serum Pi can only be assumed after 8 weeks of exposure at a stable dose (23). Therefore, our data suggest that a wait-and-see approach may be acceptable in cases of persistent, mild hypophosphatemia, as long as the patient shows clinical (signs of rickets, leg bowing, and bone pain) and biochemical (serum ALP) improvement during burosumab treatment. However, such an approach may require radiological confirmation, and should take into account that rickets healing on radiographs may take substantially longer than normalization of ALP (8).
The median final burosumab dose administered in children aged 1 to 12 years amounted to 1.06 mg/kg body weight every 2 weeks, which is comparable to the pediatric clinical trials using 0.98 mg/kg (with approximately 72% of patients remaining on their starting dose of 0.8 mg/kg and 28% requiring dose increment to 1.2 mg/kg to normalize serum Pi) (9, 13, 14). In our study, final weight-based burosumab doses varied widely with an interquartile range of 0.72 to 1.41 mg/kg in children aged 1 to 12 years, probably reflecting the wide heterogeneity of the disease severity in patients with XLH and that, unlike the clinical trials, patients with a mild degree of rickets were not excluded.
Pharmacokinetic modeling using data from available pediatric clinical trials suggests a clear dose-response relationship, with increasing proportions of children (1-12 years) attaining normal Pi levels, starting from 16.7% to 39.7% and 60.3% 2 weeks after titrating the burosumab dosage from 0.4 mg/kg to 1.0 and 2 mg/kg, respectively (39). However, in the present study, we were unable to demonstrate a significant association between the final weight-based burosumab dose and parameters of phosphate Pi homeostasis, suggesting that exhaustion of the maximum recommended dosage may not always be successful in normalizing serum Pi and TmP/GFR. This observation may also be interesting from a pharmacoeconomic point of view (40).
In clinical trials, no differences in weight-based burosumab exposure were noted in younger (1-4 years) compared with older children (4-12 years) (41). In the present study, final weight-based burosumab doses were significantly lower in adolescents compared with children, despite equivalent efficacy with respect to normalization of serum Pi, TmP/GFR, and ALP z scores. This phenomenon is also noted in patients with XLH on conventional treatment, in which adolescents usually require lower weight-related doses of Pi salts and active vitamin D compared with younger children (7).
About 23% of patients showed elevated PTH levels at the start of burosumab treatment, which was probably because of prior, longstanding treatment with Pi salts and is in line with previous reports in pediatric patients with XLH (42-44). In agreement with previous clinical trials excluding patients with hyperparathyroidism, PTH levels did not change significantly during burosumab treatment (9, 13-15). Although a weak association was noted between serum Pi and PTH levels at 6 months, this was no longer the case at 12 months, suggesting that at least mild secondary hyperparathyroidism is not an important risk factor for the failure to normalize full Pi homeostasis during burosumab treatment in children. Therefore, other factors have to be considered as well. Vitamin D deficiency/insufficiency noted in about 27% of patients was not associated with final Pi, TmP/GFR, or with ALP values. However, correction of vitamin D deficiency/insufficiency may have resulted in further improvement of these parameters in these patients. Low calcium intake is known to promote rickets in healthy children (45). This potential risk factor needs to be addressed in future studies because dietary calcium intake was not monitored in our registry.
Burosumab treatment was well tolerated. However, transient hyperphosphatemia was noted in 2 patients, which was most likely from a dosing error. Transient hyperphosphatemia was also noted in 1 of 12 children treated with burosumab over a period of 12 months in a retrospective single-center analysis (21). This underlines the need for close monitoring of serum Pi concentrations and correct administration of burosumab, which can be ensured by a nursing service. Finally, slightly elevated 1,25 vitamin D serum levels were reported in 3.3% of patients, which was not associated with any side effects (eg, hypercalcemia or hypercalciuria), but requires longer follow-up.
The present study has some limitations. First, as in any patient registry, data entry was not as tightly controllable because it is in clinical trials. This may result in underreporting of adverse events and limit the validity of our data. Second, the gold standard in assessing the degree of rickets continues to be repeated standardized radiological assessment of both wrist and knee to determine the RSS, as done in the clinical trials (6, 16). Unfortunately, this was not possible because of the nature of our study and to the national radiation protection regulations applicable in this regard. However, Thacher et al demonstrated a robust correlation between the degree of RSS and serum ALP levels in pediatric patients with XLH, which was used as a surrogate marker for rickets in our study (6). In addition, we calculated z scores to minimize potential bias related to age or sex. Also, serum Pi levels were not strictly taken in the fasting state and time intervals after the last injection were not standardized, ranging from 5 to 14 days. Maximum serum Pi values are expected 7 to 11 days after burosumab injection, whereas trough Pi levels are reached at 14 days (23). This may have biased the results of our study. However, all the factors listed here would result in an underestimation of the expected fasting phosphate levels before the next burosumab injection and thereby further supporting the finding of persisting hypophosphatemia in more than half of patients with XLH. Finally, the reference values for TmP/GFR used in local participating centers and in our statistical analysis are slightly lower when compared with recently published reference values established in the era of isotope dilution mass spectroscopy creatinine (46). However, this results in an overestimation of the true TmP/GFR in our patient cohort and thus further indicates that normalization of TmP/GFR is not mandatory for normalization of ALP in pediatric patients with XLH. On the other hand, for the first time, our analysis demonstrates the effects of burosumab over the entire pediatric age range, including adolescents and those with preexisting hyperparathyroidism who were excluded from previous clinical trials.
In conclusion, 12 months of burosumab treatment was equally effective in normalizing ALP z scores in adolescents and in children with XLH in a real-world setting, although mild hypophosphatemia persisted in approximately one-half of patients. Adolescents appear to require lower weight-based burosumab doses. Our data thus extend the evidence for burosumab treatment in patients with XLH into adolescence and suggest that complete normalization of serum Pi levels is not always necessary for substantial improvement of rickets in these patients.
Acknowledgments
The authors very much appreciate the willingness of our patients and their families for their participation in this registry. The authors are also grateful to the members of the Hypophosphatemic Rickets Study Group of the German Society for Pediatric Nephrology (GPN) and the German Society for Pediatric Endocrinology and Diabetology (DGKED) for their help in the design and realization of this project.
Funding
The XLH registry was supported by Kyowa Kirin, Germany.
Author Contributions
D.H., O.H., A.R.-U., and D.S. received speaker fees, consultation fees, and research grants from Kyowa Kirin. M.R. received speaker fees and consultation fees from Kyowa Kirin. U.J.-K., L.N., F.S., and L.P. received speaker fees from Kyowa Kirin.
Disclosures
All authors declare no conflict of interest.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in the References.
References
Abbreviations
- 25(OH)D
25 hydroxyvitamin D3
- ALP
alkaline phosphatase
- EMA
European Medicines Agency
- FGF23
hormone fibroblast growth factor 23
- IQR
interquartile range
- Pi
phosphate
- RSS
rickets severity score
- TmP/GFR
tubular maximum reabsorption of phosphate per glomerular filtration rate
- TRP
fractional tubular reabsorption of phosphate
- ULN
upper limit of normal
- XLH
X-linked hypophosphatemia



