-
PDF
- Split View
-
Views
-
Cite
Cite
Lars Sävendahl, Tadej Battelino, Michael Højby Rasmussen, Meryl Brod, Sebastian Röhrich, Paul Saenger, Reiko Horikawa, Weekly Somapacitan in GH Deficiency: 4-Year Efficacy, Safety, and Treatment/Disease Burden Results From REAL 3, The Journal of Clinical Endocrinology & Metabolism, Volume 108, Issue 10, October 2023, Pages 2569–2578, https://doi.org/10.1210/clinem/dgad183
Close - Share Icon Share
Abstract
Growth hormone deficiency (GHD) in children is currently treated with daily injections of GH, which can be burdensome for patients and their parents/guardians. Somapacitan is a GH derivative in development for once-weekly treatment of GHD.
This work aimed to assess the efficacy and safety of somapacitan, and associated disease/treatment burden, after 4 years of treatment and 1 year after switching to somapacitan from daily GH.
This long-term safety extension of a multicenter, controlled phase 2 trial (NCT02616562) took place at 29 sites in 11 countries. Patients were prepubertal, GH-naive children with GHD. Fifty patients completed 4 years of treatment. Patients in the pooled group received somapacitan (0.04, 0.08, 0.16 mg/kg/week) for 1 year, followed by the highest dose (0.16 mg/kg/week) for 3 years. Patients in the switched group received daily GH 0.034 mg/kg/day for 3 years, then somapacitan 0.16 mg/kg/week for 1 year. Main outcome measures were height velocity (HV), change from baseline in HV SD score (SDS), change from baseline in height SDS, disease burden, and treatment burden for patients and parents/guardians.
Changes from baseline in HV and HV SDS were similar and as expected in both groups. Observer-reported outcomes showed that patients and parents/guardians seem to have experienced a reduced treatment burden when switching from daily GH to somapacitan. Most parents/guardians (81.8%) strongly/very strongly preferred somapacitan over daily GH.
Somapacitan showed similar efficacy and safety in patients who continued somapacitan treatment and those who switched from daily GH to somapacitan. Once-weekly injections may lead to a reduced treatment burden relative to once-daily injections. A plain-language summary of this work is available.
Growth hormone deficiency (GHD) in children occurs with reduced circulating levels of GH. GHD is characterized by diminished growth velocity and a markedly reduced final adult height compared to that predicted (1). Additionally, children with GHD experience a reduced quality of life in terms of physical functioning, emotional well-being, and social well-being (2, 3).
GH replacement therapy is used to treat GHD (1) and, until recently, most treatments required once-daily injections. This routine can be burdensome for children and their parents/guardians, affecting adherence and leading to suboptimal clinical outcomes (4, 5). Factors contributing to poor adherence include the burden of daily injections, pain caused by the injections, storage issues, the need to reconstitute solutions, and managing injections while traveling (5, 6). There is therefore a need for GH therapies that require a reduced injection frequency while still being effective and having a tolerable safety profile.
Three questionnaires were developed and validated to assess the burden that GHD and GH treatment can have on patients and their parents/guardians. The GHD–Child Impact Measure (CIM) questionnaire can be used to assess disease burden in children with GHD (5, 7, 8). The GHD–Child Treatment Burden (CTB) and GHD–Parent Treatment Burden (PTB) questionnaires can be used to assess treatment burden in patients and their parents/guardians, respectively (5, 9).
Somapacitan (Novo Nordisk A/S) has been approved for once-weekly treatment of adult GHD (AGHD) in Europe, the United States, and Japan (10-12). In a recently published post hoc-defined analysis of data from 3 phase 3 trials, the long-term effects of somapacitan on glucose metabolism and insulin resistance were investigated in a large cohort of GH-naive and previously GH-treated patients with AGHD (13). Relative to daily GH, somapacitan was found to have no adverse effect on glucose metabolism parameters in patients with AGHD over up to 86 weeks of treatment, both for GH-naive patients and those who switched to somapacitan from daily GH. Additionally, no new cases of diabetes mellitus were reported in patients treated with somapacitan.
The use of somapacitan in children with GHD is currently being investigated in the fifth year of REAL 3 (treatment follow-up for up to 7 years) and in the extension phase of the pivotal phase 3 REAL 4 trial.
REAL 3 (NCT02616562) is an ongoing phase 2 trial comparing once-weekly somapacitan with daily GH (Norditropin; Novo Nordisk A/S) in GH-treatment-naive prepubertal children with GHD. The 26-week, 1-year, 2-year, and 3-year data from REAL 3 have already been reported (14, 15). Safety and tolerability of somapacitan were consistent with known profiles of daily GH. After 3 years, somapacitan and daily GH showed sustained efficacy in all height-based outcomes, with somapacitan having similar safety and tolerability to daily GH (15). Moreover, disease burden, as assessed by the GHD-CIM, favored somapacitan treatment compared with daily GH after 3 years of treatment, although this difference was not statistically significant (15).
In this report we present efficacy and safety results from the first year of a long-term safety extension, in which all patients were treated with somapacitan (ie, after 4 years of treatment with somapacitan, or after 3 years of treatment with daily GH followed by a switch to somapacitan for 1 year). Additionally, disease burden and treatment burden scores at years 2, 3, and 4 (weeks 104, 156, and 208) and the results of a GH–Patient Preference Questionnaire (GH-PPQ) at year 4 are reported.
The plain-language summary of this work is available at (16).
Materials and Methods
Study Design
Detailed methodology for REAL 3 has been previously published (14, 15). Briefly, the first year of REAL 3 was a randomized, multinational, active-controlled, double-blinded, dose-finding, 4-arm parallel-group trial in GH treatment-naive, prepubertal children with GHD. The REAL 3 study was conducted across 29 sites in 11 countries. The safety and efficacy of 3 different doses of once-weekly somapacitan treatment were compared with an active, open-labeled control arm of daily GH at weeks 26 and 52. This was followed by a 104-week (up to year 3) safety extension in which all patients previously on somapacitan received the same dose of somapacitan (0.16 mg/kg/week) and those on daily GH continued receiving daily GH. In the following ongoing, 208-week long-term safety extension period (years 4-7 of treatment), all patients previously on somapacitan and daily GH are treated with somapacitan 0.16 mg/kg/week. We report results from the first year of the ongoing long-term safety extension.
The primary objective of REAL 3 was to evaluate the efficacy of multiple-dose regimens of once-weekly somapacitan after 26 weeks of treatment in GH treatment-naive prepubertal children with GHD, compared with once-daily administration of GH (Norditropin FlexPro). Secondary objectives applicable to the long-term safety extension were as follows: to evaluate the efficacy and safety of once-weekly somapacitan for up to 364 weeks (7 years) of treatment; and to investigate the effect of somapacitan on well-being, psychosocial functioning, treatment burden, and user preference in this patient population.
The protocol was approved in accordance with local regulations by appropriate health authorities and by an independent ethics committee/institutional review board. The trial was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. Informed consent was obtained in writing from the parents (and/or the child's legally acceptable representative), and child assent was obtained as age-appropriate before the first study procedure.
Patients
Key inclusion and exclusion criteria have been previously reported (14). Briefly, eligible patients were prepubertal children with a confirmed diagnosis of GHD within 12 months before screening, as determined by 2 different GH stimulation tests (defined as a peak GH level of ≤ 7.0 ng/mL), with no prior exposure to GH therapy and/or insulin-like growth factor-I (IGF-I) treatment. For children with 3 or more pituitary hormone deficiencies, only 1 GH stimulation test was required.
To minimize the number of children entering puberty during the first 3 years of the trial, key inclusion criteria included the following: for boys—Tanner stage 1 for pubic hair and testis volume less than 4 mL, aged 2.5 years or older and 10.0 years or younger at screening; for girls—Tanner stage 1 for breast development (no palpable glandular breast tissue) and pubic hair, aged 2.5 years or older and 9.0 years or younger at screening (17). Puberty was defined as no longer Tanner stage 1. In the pooled group, Tanner stage was not determined for 4 patients at week 182 and 1 patient at week 208.
Treatment and Randomization
In the long-term safety extension, the pooled group consisted of all patients who were receiving somapacitan 0.16 mg/kg/week during the 104-week safety extension that began at the end of year 1. The switched group consisted of patients who were previously randomly assigned to daily GH and then switched to somapacitan 0.16 mg/kg/week at the end of year 3.
Doses of somapacitan were provided as 5 mg/1.5 mL, 10 mg/1.5 mL, and 15 mg/1.5 mL, all in prefilled pen injectors from the FlexPro group developed by Novo Nordisk A/S. Daily GH was provided using Norditropin FlexPro 10 mg/1.5 mL (14). Patients were seen every 13 weeks for efficacy measurements, adverse event (AE) monitoring, and laboratory safety measurements (14).
Efficacy
HV per year for all groups for years 1 to 4 was measured. Mean HVs in year 4 are based on height measurements from the end of year 3 to the end of year 4 (weeks 156-208), calculated as follows:
For standing height measurements, European Medicines Agency guidelines were followed (18).
Other reported efficacy outcomes include HV SDS, height SDS, IGF-I SDS, and IGF binding protein-3 (IGFBP-3) SDS, all measured at year 3, midway through year 4, and at year 4. Changes from baseline to years 3 and 4 were assessed and reported for HV SDS and height SDS, IGF-I SDS, and IGFBP-3 SDS. IGF-I and IGFBP-3 analyses were performed as described previously (14). Bone age vs chronological age ratio at years 3 to 4 was also assessed.
Observer-reported Outcomes
GHD-CIM, GHD-CTB, and GHD-PTB are disease-specific questionnaires developed to assess disease burden on patients and treatment burden on patients and their parents/guardians, respectively. GHD-CIM, developed and validated in line with US Food and Drug Administration guidance (7), was used to assess disease burden in terms of physical functioning, social well-being, and emotional well-being (8). GHD-CTB and GHD-PTB were developed and validated in line with US Food and Drug Administration guidance for parents/guardians of children aged 4 to younger than 13 years with GHD (3, 5, 19, 20). GHD-CTB measures the physical well-being, emotional well-being, and interference burdens of GH treatment on children and adolescents with GHD, as assessed by the child's parent/guardian. GHD-PTB is a self-reported measure of emotional well-being and interference burdens of GH treatment on the parent/guardian (5, 19). For all these measures, total scores range from 0 to 100. Lower scores indicate a better health state, ie, less disease or treatment burden. The results were reported descriptively.
To gauge preference for daily GH or once-weekly somapacitan, the GH-PPQ was completed by the parents/guardians of patients in the switched group 4 weeks after the child switched from daily GH to somapacitan.
Safety
Safety was assessed as the incidence of AEs in years 0 to 4 and year 4 only. AEs were analyzed using descriptive statistics and summarized by treatment, Medical Dictionary for Regulatory Activities (MedDRA) system organ class, and MedDRA preferred term.
Statistical Analysis
The full analysis set was used in the analyses of the efficacy assessments and contained all randomly assigned children who received at least one dose of randomized treatment. The safety analysis set was used for analyses of the safety assessments and included all randomly assigned children who received at least one dose of randomized treatment.
Results
Patient Disposition and Characteristics
The disposition and characteristics of patients enrolled in years 0 to 3 of REAL 3 have been previously reported (14, 15). Briefly, 59 children with GHD were randomly assigned and exposed to treatment. In total, 52 patients completed 3 years of the trial, of whom 50 completed the long-term safety extension without premature discontinuation of treatment. During year 4, the pooled group (all patients randomly assigned to somapacitan) consisted of 39 patients, while the switched group (patients who switched from daily GH to somapacitan) consisted of 11 patients (Fig. 1). Baseline characteristics at week 0 were similar for the 2 treatment groups, except that HV was greater in the pooled group (Table 1). The number of children who had entered puberty at year 3, midway through year 4, and at the end of year 4, was 2, 4, and 5 in the pooled group, and 2, 3, and 3 in the switched group, respectively. Thus, a higher proportion of children had entered puberty in the pooled group.
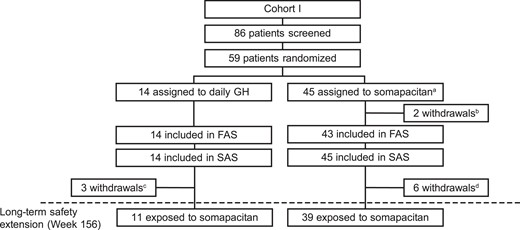
Patient disposition in REAL 3. The bottom row shows the number of patients exposed to somapacitan during the long-term safety extension, starting at week 156 (end of year 3). FAS, full analysis set; SAS, safety analysis set. aAssigned to dosing groups 0.04, 0.08, and 0.16 mg/kg/week, respectively. bDue to protocol violations. cOne due to protocol violation, one discontinued treatment before visit 5, one followed protocol version 1, for which visit 7 (week 52) was the last visit. dOne lost to follow-up, 2 withdrawn by parent/guardian, 1 discontinued treatment before visit 5, 2 discontinued treatment before visit 15.
| . | Pooled N = 43a . | Switched N = 14 . |
|---|---|---|
| Male, % | 58.1 | 64.3 |
| Race, % | ||
| White | 48.8 | 50.0 |
| Asian | 51.2 | 42.9 |
| African American | 0.0 | 7.1 |
| Age, y | 5.9 (2.0) | 5.9 (2.0) |
| Height, cm | 96.8 (13.7) | 98.3 (13.8) |
| Weight, kg | 14.4 (4.3) | 15.5 (5.0) |
| HV, cm/y | 4.2 (1.6) | 3.5 (1.6) |
| Height SDS | −3.80 (1.79) | −3.39 (1.05) |
| HV SDS | −2.50 (1.81) | −3.14 (2.14) |
| IGF-I SDS | −2.35 (0.93) | −2.07 (0.74) |
| Cause of GHD, n (%) | ||
| Idiopathic | 41 (95.3) | 12 (85.7) |
| Organic | 2 (4.7) | 2 (14.3) |
| . | Pooled N = 43a . | Switched N = 14 . |
|---|---|---|
| Male, % | 58.1 | 64.3 |
| Race, % | ||
| White | 48.8 | 50.0 |
| Asian | 51.2 | 42.9 |
| African American | 0.0 | 7.1 |
| Age, y | 5.9 (2.0) | 5.9 (2.0) |
| Height, cm | 96.8 (13.7) | 98.3 (13.8) |
| Weight, kg | 14.4 (4.3) | 15.5 (5.0) |
| HV, cm/y | 4.2 (1.6) | 3.5 (1.6) |
| Height SDS | −3.80 (1.79) | −3.39 (1.05) |
| HV SDS | −2.50 (1.81) | −3.14 (2.14) |
| IGF-I SDS | −2.35 (0.93) | −2.07 (0.74) |
| Cause of GHD, n (%) | ||
| Idiopathic | 41 (95.3) | 12 (85.7) |
| Organic | 2 (4.7) | 2 (14.3) |
Abbreviations: GHD, growth hormone deficiency; HV, height velocity; SDS, SD score.
Two patients discontinued because of protocol violation and were therefore excluded from the full analysis set. Values are mean (SD) unless otherwise stated. The pooled group consisted of 3 treatment arms previously treated with 3 different somapacitan doses (0.04, 0.08, and 0.16 mg/kg/week) during year 1. The switched group represents patients who were on daily GH for 3 years followed by somapacitan for 1 year.
| . | Pooled N = 43a . | Switched N = 14 . |
|---|---|---|
| Male, % | 58.1 | 64.3 |
| Race, % | ||
| White | 48.8 | 50.0 |
| Asian | 51.2 | 42.9 |
| African American | 0.0 | 7.1 |
| Age, y | 5.9 (2.0) | 5.9 (2.0) |
| Height, cm | 96.8 (13.7) | 98.3 (13.8) |
| Weight, kg | 14.4 (4.3) | 15.5 (5.0) |
| HV, cm/y | 4.2 (1.6) | 3.5 (1.6) |
| Height SDS | −3.80 (1.79) | −3.39 (1.05) |
| HV SDS | −2.50 (1.81) | −3.14 (2.14) |
| IGF-I SDS | −2.35 (0.93) | −2.07 (0.74) |
| Cause of GHD, n (%) | ||
| Idiopathic | 41 (95.3) | 12 (85.7) |
| Organic | 2 (4.7) | 2 (14.3) |
| . | Pooled N = 43a . | Switched N = 14 . |
|---|---|---|
| Male, % | 58.1 | 64.3 |
| Race, % | ||
| White | 48.8 | 50.0 |
| Asian | 51.2 | 42.9 |
| African American | 0.0 | 7.1 |
| Age, y | 5.9 (2.0) | 5.9 (2.0) |
| Height, cm | 96.8 (13.7) | 98.3 (13.8) |
| Weight, kg | 14.4 (4.3) | 15.5 (5.0) |
| HV, cm/y | 4.2 (1.6) | 3.5 (1.6) |
| Height SDS | −3.80 (1.79) | −3.39 (1.05) |
| HV SDS | −2.50 (1.81) | −3.14 (2.14) |
| IGF-I SDS | −2.35 (0.93) | −2.07 (0.74) |
| Cause of GHD, n (%) | ||
| Idiopathic | 41 (95.3) | 12 (85.7) |
| Organic | 2 (4.7) | 2 (14.3) |
Abbreviations: GHD, growth hormone deficiency; HV, height velocity; SDS, SD score.
Two patients discontinued because of protocol violation and were therefore excluded from the full analysis set. Values are mean (SD) unless otherwise stated. The pooled group consisted of 3 treatment arms previously treated with 3 different somapacitan doses (0.04, 0.08, and 0.16 mg/kg/week) during year 1. The switched group represents patients who were on daily GH for 3 years followed by somapacitan for 1 year.
Efficacy Results
Height-based outcomes
Data on HV at years 1, 2, and 3 were previously reported (14, 15). Observed mean HV values at years 1, 2, 3, and 4 for all treatment groups are shown in Fig. 2. HV at year 3 (week 156), midway through year 4 (week 182), and year 4 (week 208) is shown in Fig. 3A. Both groups had a lower HV at year 4 compared with year 3. HV SDS change from baseline (week 0) decreased in both groups (Fig. 3B). The observed mean height SDS showed minor increases for both treatment groups from years 3 to 4 (Fig. 3c).
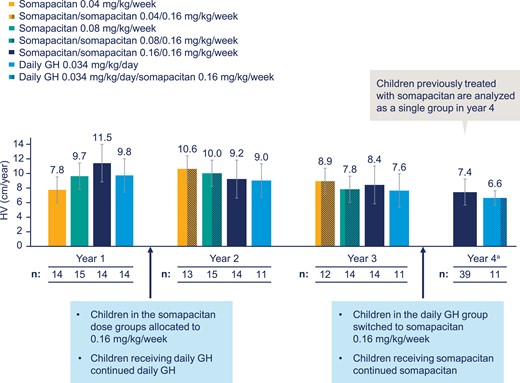
Mean (SD) height velocity (HV) by treatment group and by year. Full analysis set. Data are observed mean with SD bars. HV shown is the HV for the given year: year 0 to 1 (year 1), year 1 to 2 (year 2), year 2 to 3 (year 3), and year 3 to 4 (year 4). aIn year 4, all patients receiving somapacitan continued somapacitan; all patients receiving daily growth hormone (GH) switched to somapacitan 0.16 mg/kg/week, as represented by the dashed bars. Sample numbers are patients who were exposed to treatment.
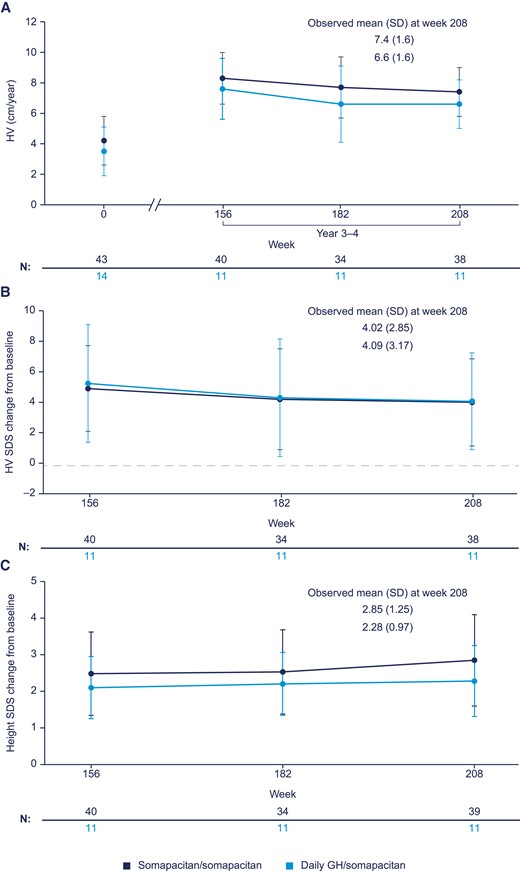
Observed values (mean, SD) for A, height velocity (HV); B, HV SD score (SDS) change from baseline to 156, 182, and 208 weeks; C, height SDS change from baseline to 156, 182, and 208 weeks.
Insulin-like growth factor-I and IGF binding protein-3 SD score
Mean (SD) IGF-I SDS levels at baseline (week 0) were consistent between the 2 groups (see Table 1). Both groups had increased to similar mean (SD [range]) IGF-I SDS levels by the end of year 3; pooled group 1.22 (1.14 [−1.98 to 2.92]) and switched group 1.30 (0.94 [0.28-3.29]). Year 4 values were 1.29 (1.23 [−1.71 to 4.33]) and 0.94 (1.60 [−2.24 to 3.90]), respectively, ie, no marked further change was observed. Mean (SD) change in IGF-I SDS from baseline (week 0) to year 4 for the pooled and switched groups was 3.61 (1.42) and 3.05 (1.96), respectively. An increase in mean (SD [range]) IGFBP-3 SDS between year 3 and year 4 was also consistent between the 2 groups, with respective scores of −0.14 (0.86 [−2.14 to 2.03]) and −0.26 (0.90 [−2.73 to 1.26]) for the pooled group, and 0.29 (0.79 [−1.05 to 1.44]) and −0.54 (1.0 [−1.94 to 1.40]) for the switched group (Supplementary Table S1) (21).
Bone age vs chronological age
The ratio of bone age to chronological age was below one in both groups. At year 4, mean (SD) bone age vs chronological age ratios were 0.88 (0.19) for the pooled group and 0.77 (0.12) for the switched group.
Observer-reported outcomes
Mean changes from baseline to years 3 and 4 in GHD-CIM scores were similar between the 2 treatment groups across emotional well-being, social well-being, physical functioning, and overall scores (Fig. 4).

Observed mean change from baseline (week 0) to years 3 and 4 in Growth Hormone Deficiency–Child Impact Measure (GHD-CIM) scores. A lower score indicates a better health state and total scores range from 0 to 100. Values are mean (SD).
GHD-CTB mean (SD) scores for years 2, 3, and 4 are shown in Fig. 5A. In patients switching from daily GH to once-weekly somapacitan, the mean (SD) overall GHD-CTB score was 6.4 (8.2) for year 2, 15.1 (16.3) for year 3, and 7.1 (9.4) for year 4. In patients remaining on somapacitan, the scores were 7.6 (9.4), 7.4 (6.8), and 5.8 (7.5) for years 2, 3, and 4, respectively. The mean (SD) GHD-PTB scores are shown in Fig. 5B. In patients switching from daily GH to once-weekly somapacitan, the mean (SD) overall GHD-PTB score was 8.7 (10.9) for year 2, 11.3 (16.4) for year 3, and 6.3 (12.2) for year 4. In patients remaining on somapacitan, the scores were 9.5 (11.2), 9.6 (12.2), and 8.7 (12.1) for years 2, 3, and 4, respectively.
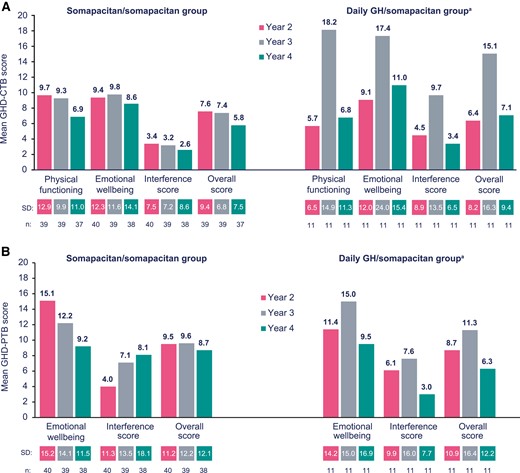
A, Growth Hormone Deficiency–Child Treatment Burden (GHD-CTB) and B, Growth Hormone Deficiency–Parent Treatment Burden (GHD-PTB) mean (SD) scores at years 2, 3, and 4 (weeks 104, 156, and 208). aThe increase in GHD-CTB and GHD-PTB mean (SD) scores in the daily growth hormone (GH)/somapacitan group between years 2 and 3 can largely be attributed to 2 patients, who may have experienced greater treatment fatigue compared with the rest of the cohort. Total scores range from 0 to 100.
The GH-PPQ results showed that most parents/guardians of patients who switched treatment at year 3 preferred somapacitan over daily GH (9/11; 81.8%), and their preference for somapacitan was either “very strong” (2/9; 22.2%) or “strong” (7/9; 77.8%). Most of these respondents (8/9; 88.9%) also answered that they would be “more adherent” to somapacitan than daily GH. The remaining 2 respondents (18.2%) reported no preference for either treatment.
Safety Results
Eight patients (72.7%) experienced 12 AEs in the switched group during year 4 while 20 patients (51.3%) experienced 84 AEs in the pooled group during year 4 (Table 2).
| . | Pooled . | Switched . | ||||
|---|---|---|---|---|---|---|
| N . | E . | R . | N . | E . | R . | |
| No. of exposed patients | 39 | — | — | 11 | — | — |
| All eventsa | 20 | 84 | 216.6 | 8 | 12 | 111.2 |
| Serious events | 0 | — | — | 0 | — | — |
| Severity | ||||||
| Mild events | 19 | 76 | 195.9 | 7 | 9 | 83.4 |
| Moderate events | 3 | 7 | 18.0 | 3 | 3 | 27.8 |
| Severe events | 1 | 1 | 2.6 | 0 | — | — |
| Relation to daily GH | ||||||
| Probably related | N/A | N/A | N/A | 0 | — | — |
| Possibly relatedb | N/A | N/A | N/A | 1 | 1 | 9.3 |
| Unlikely related | N/A | N/A | N/A | 7 | 11 | 101.9 |
| Relation to somapacitan | ||||||
| Probably relatedc | 2 | 7 | 18.0 | 0 | — | — |
| Possibly relatedd | 2 | 2 | 5.2 | 0 | — | — |
| Unlikely related | 19 | 75 | 193.3 | 8 | 12 | 111.2 |
| . | Pooled . | Switched . | ||||
|---|---|---|---|---|---|---|
| N . | E . | R . | N . | E . | R . | |
| No. of exposed patients | 39 | — | — | 11 | — | — |
| All eventsa | 20 | 84 | 216.6 | 8 | 12 | 111.2 |
| Serious events | 0 | — | — | 0 | — | — |
| Severity | ||||||
| Mild events | 19 | 76 | 195.9 | 7 | 9 | 83.4 |
| Moderate events | 3 | 7 | 18.0 | 3 | 3 | 27.8 |
| Severe events | 1 | 1 | 2.6 | 0 | — | — |
| Relation to daily GH | ||||||
| Probably related | N/A | N/A | N/A | 0 | — | — |
| Possibly relatedb | N/A | N/A | N/A | 1 | 1 | 9.3 |
| Unlikely related | N/A | N/A | N/A | 7 | 11 | 101.9 |
| Relation to somapacitan | ||||||
| Probably relatedc | 2 | 7 | 18.0 | 0 | — | — |
| Possibly relatedd | 2 | 2 | 5.2 | 0 | — | — |
| Unlikely related | 19 | 75 | 193.3 | 8 | 12 | 111.2 |
Abbreviations: AEs, adverse events; E, number of events; GH, growth hormone; N, number of patients; N/A, not applicable; R, event rate per 100 patient-years at risk. Safety analysis set.
Only AEs with an onset after the first administration of trial product and up to visit 20 (week 208) are included.
The only possibly related AE to daily GH was nonalcoholic fatty liver disease.
AEs probably related to somapacitan were 3 events of injection-site pain and 1 event each of skin atrophy, swelling of eyelid, soft tissue swelling, and hematoma.
AEs possibly related to somapacitan were 1 event each of abnormal electrocardiogram and migraine.
| . | Pooled . | Switched . | ||||
|---|---|---|---|---|---|---|
| N . | E . | R . | N . | E . | R . | |
| No. of exposed patients | 39 | — | — | 11 | — | — |
| All eventsa | 20 | 84 | 216.6 | 8 | 12 | 111.2 |
| Serious events | 0 | — | — | 0 | — | — |
| Severity | ||||||
| Mild events | 19 | 76 | 195.9 | 7 | 9 | 83.4 |
| Moderate events | 3 | 7 | 18.0 | 3 | 3 | 27.8 |
| Severe events | 1 | 1 | 2.6 | 0 | — | — |
| Relation to daily GH | ||||||
| Probably related | N/A | N/A | N/A | 0 | — | — |
| Possibly relatedb | N/A | N/A | N/A | 1 | 1 | 9.3 |
| Unlikely related | N/A | N/A | N/A | 7 | 11 | 101.9 |
| Relation to somapacitan | ||||||
| Probably relatedc | 2 | 7 | 18.0 | 0 | — | — |
| Possibly relatedd | 2 | 2 | 5.2 | 0 | — | — |
| Unlikely related | 19 | 75 | 193.3 | 8 | 12 | 111.2 |
| . | Pooled . | Switched . | ||||
|---|---|---|---|---|---|---|
| N . | E . | R . | N . | E . | R . | |
| No. of exposed patients | 39 | — | — | 11 | — | — |
| All eventsa | 20 | 84 | 216.6 | 8 | 12 | 111.2 |
| Serious events | 0 | — | — | 0 | — | — |
| Severity | ||||||
| Mild events | 19 | 76 | 195.9 | 7 | 9 | 83.4 |
| Moderate events | 3 | 7 | 18.0 | 3 | 3 | 27.8 |
| Severe events | 1 | 1 | 2.6 | 0 | — | — |
| Relation to daily GH | ||||||
| Probably related | N/A | N/A | N/A | 0 | — | — |
| Possibly relatedb | N/A | N/A | N/A | 1 | 1 | 9.3 |
| Unlikely related | N/A | N/A | N/A | 7 | 11 | 101.9 |
| Relation to somapacitan | ||||||
| Probably relatedc | 2 | 7 | 18.0 | 0 | — | — |
| Possibly relatedd | 2 | 2 | 5.2 | 0 | — | — |
| Unlikely related | 19 | 75 | 193.3 | 8 | 12 | 111.2 |
Abbreviations: AEs, adverse events; E, number of events; GH, growth hormone; N, number of patients; N/A, not applicable; R, event rate per 100 patient-years at risk. Safety analysis set.
Only AEs with an onset after the first administration of trial product and up to visit 20 (week 208) are included.
The only possibly related AE to daily GH was nonalcoholic fatty liver disease.
AEs probably related to somapacitan were 3 events of injection-site pain and 1 event each of skin atrophy, swelling of eyelid, soft tissue swelling, and hematoma.
AEs possibly related to somapacitan were 1 event each of abnormal electrocardiogram and migraine.
The most common AEs over the whole period from baseline (week 0) were pyrexia, influenza, nasopharyngitis, and gastroenteritis in the pooled group, and pyrexia and nasopharyngitis in the switched group. The most common AE during year 4 was nasopharyngitis, occurring in 4 patients (10.3%) in the pooled group and 1 patient (9.1%) in the switched group; all other AEs occurred in less than 10% of patients in either group. The AEs occurring in 5% or more of patients during year 4 are presented in Fig. 6. Most AEs occurring in year 4 were mild or moderate and unlikely related to GH treatment. One severe AE (elbow fracture) occurred in the pooled group and was deemed unlikely related to the trial product. No serious AEs were reported in year 4.
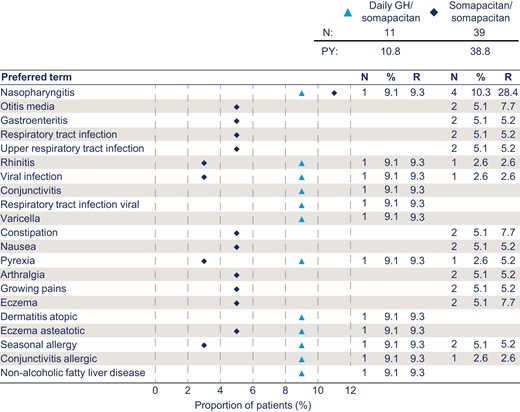
Most frequent adverse events (occurring in ≥ 5% of patients) during year 4 by preferred term. %, percentage of patients; E, number of events; GH, growth hormone; N, number of patients; R, event rate per 100 patient-years at risk; PY, total patient-years at risk. Baseline is week 0 of the trial.
Since previously reported (15), 3 patients experienced 5 injection-site reactions, collectively; none were reported in the switched group (Table 3). Three of the reported injection-site reactions occurred with pain as an associated descriptor.
| . | Pooled group . | Switched group . |
|---|---|---|
| No. of patients with a reaction | 3 | 0 |
| No. of reactions in each group | 5 | 0 |
| No. of reactions with associated pain | 3 | 0 |
| . | Pooled group . | Switched group . |
|---|---|---|
| No. of patients with a reaction | 3 | 0 |
| No. of reactions in each group | 5 | 0 |
| No. of reactions with associated pain | 3 | 0 |
Safety analysis set.
| . | Pooled group . | Switched group . |
|---|---|---|
| No. of patients with a reaction | 3 | 0 |
| No. of reactions in each group | 5 | 0 |
| No. of reactions with associated pain | 3 | 0 |
| . | Pooled group . | Switched group . |
|---|---|---|
| No. of patients with a reaction | 3 | 0 |
| No. of reactions in each group | 5 | 0 |
| No. of reactions with associated pain | 3 | 0 |
Safety analysis set.
Low-titer, non-neutralizing antibodies were detected in 10 patients (26%) in the pooled group and 1 patient (10%) in the switched group. All antibody-positive samples were negative for in vitro neutralizing antibodies.
Glucose metabolism results showed that fasting plasma glucose and glycated hemoglobin, as well as fasting insulin, remained within the normal range between years 3 and 4 of treatment for both groups (Supplementary Table S2) (21).
No new safety signals were observed during year 4. The safety profile of once-weekly somapacitan was similar to the profile observed for daily GH; the rate of AEs per 100 patient-years at risk for the switched group before the switch to somapacitan was 270.8, compared with 248.2 for the pooled group up to the end of year 4.
Discussion
The 4-year results of the REAL 3 trial support the efficacy and safety outcomes previously observed for somapacitan as a once-weekly treatment for prepubertal children with GHD. Somapacitan was well tolerated, and no new safety signals were identified.
Weekly administration of somapacitan reduces the number of GH injections required from 365 to 52 per year compared with daily administration. Thus, it is expected to reduce treatment burden, minimize disruption to patients' lives, and potentially improve treatment adherence (4). Somapacitan, to our knowledge, is 1 of only 2 long-acting GH (LAGH) derivatives with data demonstrating sustained 3-year and 4-year efficacy and safety in children. Despite REAL 3 being a phase 2 trial with relatively small patient numbers, height SDS values showed a normalization over time, and both tolerability and the favorable safety profile were maintained over 4 years of treatment. Both treatment groups had a lower HV at year 4 compared with year 3, as expected in clinical practice after a full 3 years of treatment. The numerical difference in HV between the treatment groups (observed values were greater in the pooled group) was of the same magnitude at years 3 and 4. Subsequently, the growth response from years 3 to 4 may be regarded as similar between the 2 groups. Moreover, observed changes in HV SDS and height SDS were also similar in the 2 groups. These results suggest similar efficacy between the 2 treatments. A total of 8 patients (5 in the pooled group and 3 in the switched group) entered puberty during the trial, which may have had an influence on growth outcomes, although this association was not directly assessed in the trial.
The safety profile of once-weekly somapacitan in prepubertal children with GHD was similar to the well-known safety profile of daily GH, with no new safety or tolerability issues identified after 4 years of treatment. As previously reported with the 3-year results, AE rates (per 100 patient-years at risk) were similar between the somapacitan groups and the daily GH group up to the end of year 3 (14). In the year 4 analysis, the AE rate for the switched group was notably lower compared to the pooled group; however, it should be noted that the overall treatment exposure to once-weekly somapacitan in the switched group was much lower. Most of the AEs reported were nonserious and of mild or moderate severity. Out of the 5 injection-site reactions reported in 3 patients during year 4, none were considered serious.
IGF-I SDS is commonly used to monitor GH effects in the body as a surrogate for efficacy, adherence, and safety in long-term GH treatment (22). Serum IGF-I profiles in patients receiving LAGH preparations such as somapacitan are expected to differ from those obtained with daily GH treatment. In earlier publications from the REAL 3 and REAL 4 trials, mean IGF-I SDS values were similar between somapacitan 0.16 mg/kg/week and 0.034 mg/kg/day daily GH in children with GHD (15, 23). Once-weekly injections with somapacitan are expected to result in an IGF–I SDS peak approximately 2 days after dosing with a gradual reduction to trough levels just before the next dose, whereas weekly mean IGF-I levels are best estimated with samples taken at day 4 (24, 25). Differences in the mechanisms used to prolong GH action may also cause variations in peak and trough IGF-I levels between different LAGH molecules (4, 26). Several recent reviews have highlighted that the doses needed for the clinical efficacy of LAGHs could result in higher peak IGF-I levels compared with daily GH (4, 26). Clinical guidelines recommend that IGF-I levels be maintained within the normal range of −2.0 to +2.0 SDS; however, small elevations can be clinically justified in certain cases and there is no evidence for any risks associated with transient elevations above +2 SDS (26, 27). In the REAL 3 trial, both treatment groups had an observed mean IGF-I SDS that increased from low baseline (week 0) values to year 3, and there was no marked change from year 3 to year 4. All mean IGF-I SDS values remained below the accepted upper normal threshold of +2.0.
Observer-reported outcomes were used in this trial to assess disease burden and treatment burden in the 2 treatment groups. Results of the GHD-CIM showed that disease burden was reduced similarly in the somapacitan and daily GH treatment groups and showed sustained improvement from years 3 to 4. These similar results for disease burden likely reflect the similar changes in height seen in the 2 groups. Results of the GHD-CTB showed that, between years 3 and 4, treatment burden decreased slightly for patients in the pooled group, and markedly for patients in the switched group. The slight decrease seen for the pooled group may reflect the patients' familiarity with the treatment, whereas the marked decrease in the switched group might be due to reduced injection frequency; however, it should be noted that, before the marked decrease, there was an increase in GHD-CTB mean scores in the switched group between years 2 and 3. This can largely be attributed to 2 patients, who may have experienced greater treatment fatigue compared with the rest of the cohort. The same can be said for the GHD-PTB scores for the switched group, in whom there was an increase between years 2 and 3, followed by a marked decrease by the end of year 4. Again, this decrease in treatment burden for the parents/guardians of patients in the switched group may well be due to reduced frequency of injections, or the fact that the parents/guardians of the 2 aforementioned patients may have experienced higher treatment burden from years 2 to 3 compared with years 3 to 4. The fact that only an overall minor decrease in parent/guardian treatment burden was observed in the pooled group might again reflect familiarity with the treatment for these parents/guardians.
Most caregivers of patients who switched from daily GH to somapacitan strongly preferred somapacitan and indicated that they would be more adherent to it. This would probably improve clinical outcomes, as the burden of daily GH has been shown to reduce adherence to treatment (4).
Conclusion
These year 4 data support the 3-year efficacy and safety results previously published for somapacitan in the treatment of prepubertal children with GHD. In year 4, height-related outcomes and safety profiles were similar for patients who continued treatment with somapacitan and those who switched from daily GH to somapacitan. Observer-reported outcomes suggested that once-weekly injections may pose a reduced treatment burden both for patients and their parents/guardians relative to once-daily injections, while reducing disease burden to a similar degree. Most parents/guardians preferred once-weekly somapacitan to daily GH and indicated that they would be more adherent to the weekly, rather than daily, treatment regimen.
Acknowledgments
The authors thank the patients, their families, the nurses and study coordinators, and all investigators involved in this study, including members of the REAL 3 Study Group*, without whom the study would not have been possible. The authors would like to thank Kai Wai Lee, formerly of Novo Nordisk, for his contributions to early drafts of this manuscript. The authors also thank Kristine Grønning Kongsbak and Ryan Ard of Novo Nordisk for reviewing the manuscript. Statistical analyses were carried out by Charlotte Bisgaard of Novo Nordisk. Editorial support (in the form of writing assistance, collating author comments, assembling tables/figures, grammatical editing and referencing) was provided by Emra Baker, Jessica Harris, and Jennifer Banks of Ashfield MedComms, and was funded by Novo Nordisk.
*Members of the REAL 3 Study Group: Austria: Dieter Furthner, Bettina Piringer, Lorenz Auer-Hackenberg, Klaus Schmitt, Marlene Reitmayr. Brazil: Marcello Delano Bronstein, Francisco Samuel Magalhães Lima. Germany: Martin Wabitsch, Carsten Posovszky, Volker Böttcher, Alexander Mann. Israel: Eli Hershkovitz, Alon Haim, Neta Lowenthal, Orit Hamiel, Sharon Sheinvald Levin, Kineret Mazor-Aronovitch, Michal Ben-Ami, Yael Levy Shraga, Dalit Modan, Noah Gruber, Moshe Phillip, Yael Lebenthal, Ariel Tenenbaum, Alon Eliakim, Nitzan Dror, Ruby Haviv, Nehama Zuckerman-Levin, Naim Shehadeh, Liav Givon, Ameer Elemy, Miriam Marji, Vardit Gepstein. India: Praveen V.P., Aswin P, Nithiya Abraham, Rajesh Khadgawat, Yashdeep Gupta, Vaman Khadilkar, Anuradha Khadilkar, Sagar Lad. Japan: Reiko Horikawa, Yasuhiro Naiki, Yasuko Ogiwara, Yuta Chiba, Yusuke Fujisawa, Yumiko Terada, Tomoko Yoshida, Kenichi Kinjo, Atsushi Tsukamura, Shinobu Ida, Yuri Etani, Yasuko Shoji, Masanobu Kawai, Hisakazu Nakajima, Jun Mori, Shota Fukuhara, Keiichi Shigehara, Hidechika Morimoto, Yusuke Tsuma, Yasuhiro Kawabe, Takeshi Ota, Kenichi Kashimada, Ryuichi Nakagawa, Atsumi Tsuji, Risa Nomura, Kei Takasawa, Takeru Yamauchi, Kanako Ishii, Naoko Toda, Kazuhiro Ohkubo, Tohru Yorifuji, Yuki Hosokawa, Rie Kawakita, Yukiko Hashimoto, Azumi Sakakibara, Shinji Higuchi, Shun Soneda, Kenichiro Ogushi, Shuichi Yatsuga, Yasutoshi Koga, Takako Matsumoto, Miyuki Kitamura. Sweden: Lars Sävendahl, Ricard Nergårdh. Slovenia: Tadej Battelino, Mojca Zerjav Tansek. Türkiye: Serap Turan, Abdullah Bereket, Zeynep Atay, Azad Akbarzade, Tülay Güran. Ukraine: Olena Bolshova, Mykola Tronko, Olga Vyshnevskaya, Natalia Sprynchuk, Iryna Lukashuk, Natalia Muz, Tatyana Marchenko, Nataliya Chorna, Marіana Konovalova, Liliya Zelinska. USA: Lawrence Silverman, Barbara Cerame, Sunita Cheruvu, Daisy Chin, Laurie Ebner-Lyon, Marie Fox, Marianna Nicolette-Gentile, Kristin Sabanosh, Harold Starkman, Ian Marshall, Mariam Gangat, Sadana Balachandar, Philippe Backeljauw, Andrew Dauber, Leah Tyzinski, Paul H. Saenger, Luis Zamora Siliezar, Jacqueline P. Velasco, Judith L. Ross, Martha Bardsley, Karen Kowal, Gad B. Kletter, Britney G. Frazier, Kathryn Garrison.
Funding
This work was funded by Novo Nordisk.
Disclosures
L.S. has received consulting and lecture fees and support for attending meetings from, and participated on data safety monitoring boards or advisory boards by, Ascendis, Novo Nordisk, Merck, Pfizer, and Sandoz. T.B. has served on advisory boards for Novo Nordisk, Eli Lilly, and Pfizer; has received speakers' honoraria for Novo Nordisk, Eli Lilly, and Pfizer; and has received research grants to the institution from Novo Nordisk and Sandoz. M.H.R. is an employee and stockholder of Novo Nordisk. M.B. is a paid consultant for Novo Nordisk. S.R. is an employee of Novo Nordisk. P.S. has nothing to disclose. R.H. consults for or has previously consulted for Novo Nordisk, OPKO-Pfizer, Ascendis, and Lumos Pharma; has received lecturer fees from Novo Nordisk, Pfizer, and JCR; and has received grant support from Sandoz.
Data Availability
Some or all data sets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Clinical Trial Registration
ClinicalTrials.gov registration number NCT02616562 (registered November 25, 2015).
References
Abbreviations
- AE
adverse event
- AGHD
adult growth hormone deficiency
- GH
growth hormone
- GHD
growth hormone deficiency
- GHD-CIM
Growth Hormone Deficiency–Child Impact Measure
- GHD-CTB
Growth Hormone Deficiency–Child Treatment Burden
- GHD-PTB
Growth Hormone Deficiency–Parent Treatment Burden
- GH-PPQ
GH–Patient Preference Questionnaire
- HV
height velocity
- IGFBP-3
IGF binding protein-3
- IGF-I
insulin-like growth factor-I
- LAGH
long-acting growth hormone



