-
PDF
- Split View
-
Views
-
Cite
Cite
Tali Cukierman-Yaffe, Hertzel C Gerstein, Jan Basile, M Angelyn Bethel, Ernesto G Cardona-Muñoz, Ignacio Conget, Gilles Dagenais, Edward Franek, Stephanie Hall, Nicolae Hancu, Petr Jansky, Mark Lakshmanan, Fernando Lanas, Lawrence A Leiter, Patricio Lopez-Jaramillo, Valdis Pirags, Nana Pogosova, Jeffrey Probstfield, Purnima Rao-Melacini, Chinthanie Ramasundarahettige, Peter J Raubenheimer, Matthew C Riddle, Lars Rydén, Jonathan E Shaw, Wayne H-H Sheu, Theodora Temelkova-Kurktschiev, Novel Indices of Cognitive Impairment and Incident Cardiovascular Outcomes in the REWIND Trial, The Journal of Clinical Endocrinology & Metabolism, Volume 107, Issue 8, August 2022, Pages e3448–e3454, https://doi.org/10.1210/clinem/dgac200
Close - Share Icon Share
Abstract
Low cognitive scores are risk factors for cardiovascular outcomes. Whether this relationship is stronger using novel cognitive indices is unknown.
Participants in the Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) trial who completed both the Montreal Cognitive Assessment (MoCA) score and Digit Substitution Test (DSST) at baseline (N = 8772) were included. These scores were used to identify participants with baseline substantive cognitive impairment (SCI), defined as a baseline score on either the MoCA or DSST ≥ 1.5 SD below either score’s country-specific mean, or SCI-GM, which was based on a composite index of both scores calculated as their geometric mean (GM), and defined as a score that was ≥ 1.5 SD below their country’s average GM. Relationships between these measures and incident major adverse cardiovascular events (MACE), and either stroke or death were analyzed.
Compared with 7867 (89.7%) unaffected participants, the 905 (10.3%) participants with baseline SCI had a higher incidence of MACE (unadjusted hazard ratio [HR] 1.34; 95% CI 1.11, 1.62; P = 0.003), and stroke or death (unadjusted HR 1.60; 95% CI 1.33, 1.91; P < 0.001). Stronger relationships were noted for SCI-GM and MACE (unadjusted HR 1.61; 95% CI 1.28, 2.01; P < 0.001), and stroke or death (unadjusted HR 1.85; 95% CI 1.50, 2.30; P < 0.001). For SCI-GM but not SCI, all these relationships remained significant in models that adjusted for up to 10 SCI risk factors.
Country-standardized SCI-GM was a strong independent predictor of cardiovascular events in people with type 2 diabetes in the REWIND trial.
Cognitive aging is a continuum that extends from normal to cognitive impairment and dementia (1, 2). Cognitive impairment without evidence of dementia affects 6.7% of people aged 60 to 64 years and 25% of people aged 80 to 84 years (3). Affected individuals are at high risk for future dementia with reported yearly conversion rates of 4% to 17% and an estimated relative risk of 2.8 compared to individuals without cognitive impairment (4, 5). Studies in the general population (6-8), in people with diabetes, and in individuals with additional cardiovascular risk factors (9) (10) demonstrate that cognitive impairment is also a risk factor for cardiovascular events and death.
A variety of approaches have been used to measure cognitive impairment in large epidemiologic studies. These were typically based on one or more pen-and-paper tests, administered in different languages, to participants from different cultures and countries, in a variety of settings. Novel ways of defining cognitive impairment that both account for this heterogeneity and incorporate evidence from more than one test may provide additional insights into its impact and determinants.
The Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) trial (11) recruited and followed 9901 individuals with type 2 diabetes and additional cardiovascular risk factors for a median period of 5.4 years and reported that random assignment to the drug dulaglutide reduced the first occurrence of a major adverse cardiovascular event (MACE). In addition to measuring incident myocardial infarctions, strokes, and mortality, 2 different measures were used to assess cognitive function at baseline and during follow-up. These cognitive measures and the calculation of a novel index of cognitive function that combines these measures provide a unique opportunity to assess the relationship between various indices of baseline cognitive impairment and future cardiovascular events in this contemporaneous population with generally well-treated risk factors.
Methods
Participants
The REWIND trial’s design and key findings have already been published (11, 12). Men and women from 24 countries with additional cardiovascular risk factors, newly diagnosed or established diabetes, a glycated hemoglobin (HbA1c) level of ≤ 9.5% (80 mmol/mol) on 0, 1, or 2 oral glucose-lowering drugs with or without basal insulin, and a body mass index ≥ 23 kg/m² were included. Key exclusion criteria included a coronary or cerebrovascular event within the prior 2 months, severe hypoglycemia in the prior year, cancer in the prior 5 years, an estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m², plans for revascularization, or a life expectancy < 1 year. A full list of inclusion and exclusion criteria has previously been published (12). The trial was approved by Research Ethics Committee at all sites and all participants provided written informed consent.
Procedures
Participants who were adherent to weekly injections of placebo study medication during a 3-week single blind run-in period were randomly assigned to weekly subcutaneous injections of either dulaglutide 1.5 mg or an equal volume of identically appearing placebo and were followed every 6 months for the occurrence of cardiovascular and other serious health outcomes. All REWIND participants were asked to complete 2 validated pen-and-paper tests of cognitive status at baseline and at the 2-year, 5-year, and final visit. The Montreal Cognitive Assessment (MoCA) test is a 1-page, 30-item questionnaire that assesses 7 cognitive domains, and is scored by adding the number of items answered correctly with an additional point given for individuals who report ≤ 12 years of education. The Digit Symbol Substitution Test (DSST) presents 9 symbols that are each above a blank square, and a key that links each symbol to a number. Respondents are asked to write the number corresponding to each symbol in the corresponding square as quickly as possible. The score is the number of correct responses during a 2-minute period.
All MoCA scores were centrally reviewed by blinded investigators. Items left uncompleted were assigned a score of 0 and items graded above the maximum score were assigned the maximum score. As DSST results were not sent centrally, scores used were those provided by the study site on the case report form. As previously published, every participant’s raw MoCA and DSST score was converted to a country-standardized score to account for potential country differences. This was done by subtracting the country’s mean raw score from the individual’s score and dividing the result by the country’s SD of the score. This provides an average baseline standardized score of 0 with a SD of 1 for each test within each country. People whose standardized score on either test was ≤ −1.5 were classified as having country-standardized substantive cognitive impairment (SCI). In addition, a composite index that reflects information captured by both of the cognitive test scores was created by combining each person’s MoCA and DSST score into a composite cognitive score. This was achieved calculating the geometric mean (GM) of the 2 scores for each person, which is the square root of the product of the 2 scores (13). Each person’s country-standardized GM score was then estimated as above, by calculating each country’s average GM scores, subtracting it from the individual’s GM score, and dividing the result by the country’s SD of the average GM score. Thus, for each country, the average GM score was 0 with a SD of 1, and people whose GM score was ≤ −1.5 were classified as having country-standardized substantive cognitive impairment based on the GM score (SCI-GM).
Outcomes and Baseline Cognitive Impairment
The REWIND trial’s primary outcome was the first occurrence of MACE defined as a nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular or unknown causes. These and other outcomes, including deaths were adjudicated by an external, masked adjudication committee. The association between random assignment to dulaglutide and a reduced hazard of country-standardized SCI defined as the first occurrence of a postrandomization country-standardized MoCA or DSST score ≤ −1.5 (with standardization to the country’s baseline score) was also reported in an exploratory analysis (14).
This report focuses on the cardiovascular prognosis of people with SCI at baseline. It defines this in 2 different ways that are both country-standardized (ie, SCI and SCI-GM), and estimates the relationship between each of these 2 variables and the following: incident MACE; and the composite outcome of incident stroke or death. In addition to exploring SCI and SCI-GM as risk factors, it also explores the association between dulaglutide assignment and incident SCI-GM as an outcome, defined as the first occurrence of a postrandomization country-standardized geometric mean of the MoCA and DSST score ≤ −1.5 SD of the baseline country-specific mean of this composite score.
Statistical Analysis
All analyses were restricted to the 8772 participants with both a MoCA and DSST score at baseline. Continuous variables were summarized with means and SD and categorical variables with numbers and percentages. Cox proportional hazard were used to estimate the hazard of MACE in the presence versus the absence of baseline SCI defined using either both scores separately or the geometric mean, before and after adjustment for various combinations of age, sex, ≤ 12 years education, prior stroke or transient ischemic attack, systolic blood pressure, HbA1c, current tobacco use, the natural logarithm of the albumin-to-creatinine ratio, the eGFR, prior diabetic retinopathy, and dulaglutide assignment. Similar models were also used to assess the hazard of either stroke or death from any cause. Proportionality was assessed by inspection. Unadjusted Kaplan-Meier curves stratified according to the presence or absence of country-standardized SCI or SCI-GM at baseline were also constructed and compared using log rank tests.
All reported P values are 2-sided with a nominal level of significance of 0.05, with no adjustments for multiplicity. Statistical analyses were all done using SAS version 9.4. The REWIND trial was registered with clinicaltrials.gov as NCT01394952.
Results
REWIND participants with SCI at baseline (N = 905/8772; 10.3%) were more likely to be female; were older; had a longer diabetes duration; achieved less education; had a higher prevalence of cerebrovascular disease, hypertension, and diabetic retinopathy; a lower prevalence of tobacco use; a higher systolic blood pressure, HbA1c, and albumin-to-creatinine ratio; and a lower eGFR, MoCA, and DSST score (all P values < 0.045) than individuals without SCI (Table 1). Very similar patterns were noted when cognitive impairment was defined as SCI-GM, which included 529/8772 (6.0%) participants. For this latter comparison, affected participants also had a higher prevalence of previous heart failure, a similar systolic blood pressure, and lower diastolic blood pressure (Table 1).
Baseline characteristics of participants with and without country-standardized substantive cognitive impairment defined in 2 different ways
| . | Country-standardized SCI . | . | . | Country-standardized SCI-GM . | . | . |
|---|---|---|---|---|---|---|
| . | Yes . | No . | P . | Yes . | No . | P . |
| N | 905 | 7867 | 529 | 8243 | ||
| Age, years | 68.2 (6.7) | 65.7 (6.3) | <0.001 | 69.1 (6.6) | 65.8 (6.3) | <0.001 |
| Female | 530 (58.6) | 3556 (45.2%) | <0.001 | 320 (60.5) | 3766 (45.7) | <0.001 |
| Diabetes duration, years | 11.0 (7.7) | 10.3 (7.0) | 0.009 | 11.4 (8.3) | 10.3 (7.0) | 0.001 |
| Education ≤ 12 years | 685 (75.7) | 4639 (59.0) | <0.001 | 427 (80.7) | 4897 (59.4) | <0.001 |
| Current tobacco use | 95 (10.5) | 1143 (14.5) | 0.001 | 58 (11.0) | 1180 (14.3) | 0.032 |
| Prior stroke/TIA | 115 (12.7) | 659 (8.4) | <0.001 | 81 (15.3) | 693 (8.4) | <0.001 |
| Hypertension | 857 (94.7) | 7308 (92.9) | 0.043 | 504 (95.3) | 7661 (92.9) | 0.040 |
| Atrial fibrillation | 56 (6.2) | 496 (6.3) | 0.891 | 39 (7.4) | 513 (6.2) | 0.291 |
| Heart failure | 84 (9.3) | 665 (8.5) | 0.398 | 61 (11.5) | 688 (8.3) | 0.011 |
| Diabetic retinopathy | 105 (11.6) | 687 (8.7) | 0.004 | 69 (13.0) | 723 (8.8) | 0.001 |
| Prior cardiovascular diseasea | 287(31.7) | 2416 (30.7) | 0.536 | 183 (34.6) | 2520 (30.6) | 0.052 |
| Statin use | 584 (64.5) | 5227 (66.4) | 0.249 | 346 (65.4) | 5465 (66.3) | 0.674 |
| ACEi/ARB use | 759 (83.9) | 6405 (81.4) | 0.071 | 445 (84.1) | 6719 (81.5) | 0.133 |
| Body mass index, kg/m2 | 32.2 (5.8) | 32.4 (5.7) | 0.24 | 32.0(5.9) | 32.4 (5.7) | 0.12 |
| Systolic blood pressure | 139 (18) | 137 (17) | <0.001 | 138 (17) | 137 (17) | 0.077 |
| Diastolic blood pressure | 78 (11) | 79 (10) | 0.26 | 77 (10) | 79 (10) | 0.001 |
| HbA1c | 7.4 (1.0) | 7.3 (1.1) | 0.021 | 7.4 (1.0) | 7.3 (1.1) | 0.022 |
| eGFR, mL/min/1.73m2 | 74.2(24.2) | 77.8 (22.3) | <0.001 | 72.9 (23.6) | 77.7 (22.5) | <0.001 |
| LDL cholesterol, mmol/L | 2.6 (1.0) | 2.5 (1.0) | 0.17 | 2.5 (0.9) | 2.6 (1.0) | 0.61 |
| Urine ACR, mg/mmol | 2.4 (0.9, 10.2) | 1.7 (0.7, 6.1) | <0.001 | 2.2 (0.8, 8.8) | 1.7 (0.7, 6.3) | 0.011 |
| MoCA raw score | 17.4 (4.7) | 25.4 (3.2) | <0.001 | 17.9 (5.7) | 25.0 (3.6) | <0.001 |
| Standardized MoCA score | −1.9 (0.9) | 0.2 (0.7) | <0.001 | −1.7 (1.2) | 0.1 (0.9) | <0.001 |
| DSST raw score | 21.5 (17.6) | 40.2 (18.9) | <0.001 | 11.4(8.0) | 40.0 (18.9) | <0.001 |
| Standardized DSST score | −1.0 (0.9) | 0.1(0.9) | <0.001 | −1.6 (0.4) | 0.1 (0.9) | <0.001 |
| . | Country-standardized SCI . | . | . | Country-standardized SCI-GM . | . | . |
|---|---|---|---|---|---|---|
| . | Yes . | No . | P . | Yes . | No . | P . |
| N | 905 | 7867 | 529 | 8243 | ||
| Age, years | 68.2 (6.7) | 65.7 (6.3) | <0.001 | 69.1 (6.6) | 65.8 (6.3) | <0.001 |
| Female | 530 (58.6) | 3556 (45.2%) | <0.001 | 320 (60.5) | 3766 (45.7) | <0.001 |
| Diabetes duration, years | 11.0 (7.7) | 10.3 (7.0) | 0.009 | 11.4 (8.3) | 10.3 (7.0) | 0.001 |
| Education ≤ 12 years | 685 (75.7) | 4639 (59.0) | <0.001 | 427 (80.7) | 4897 (59.4) | <0.001 |
| Current tobacco use | 95 (10.5) | 1143 (14.5) | 0.001 | 58 (11.0) | 1180 (14.3) | 0.032 |
| Prior stroke/TIA | 115 (12.7) | 659 (8.4) | <0.001 | 81 (15.3) | 693 (8.4) | <0.001 |
| Hypertension | 857 (94.7) | 7308 (92.9) | 0.043 | 504 (95.3) | 7661 (92.9) | 0.040 |
| Atrial fibrillation | 56 (6.2) | 496 (6.3) | 0.891 | 39 (7.4) | 513 (6.2) | 0.291 |
| Heart failure | 84 (9.3) | 665 (8.5) | 0.398 | 61 (11.5) | 688 (8.3) | 0.011 |
| Diabetic retinopathy | 105 (11.6) | 687 (8.7) | 0.004 | 69 (13.0) | 723 (8.8) | 0.001 |
| Prior cardiovascular diseasea | 287(31.7) | 2416 (30.7) | 0.536 | 183 (34.6) | 2520 (30.6) | 0.052 |
| Statin use | 584 (64.5) | 5227 (66.4) | 0.249 | 346 (65.4) | 5465 (66.3) | 0.674 |
| ACEi/ARB use | 759 (83.9) | 6405 (81.4) | 0.071 | 445 (84.1) | 6719 (81.5) | 0.133 |
| Body mass index, kg/m2 | 32.2 (5.8) | 32.4 (5.7) | 0.24 | 32.0(5.9) | 32.4 (5.7) | 0.12 |
| Systolic blood pressure | 139 (18) | 137 (17) | <0.001 | 138 (17) | 137 (17) | 0.077 |
| Diastolic blood pressure | 78 (11) | 79 (10) | 0.26 | 77 (10) | 79 (10) | 0.001 |
| HbA1c | 7.4 (1.0) | 7.3 (1.1) | 0.021 | 7.4 (1.0) | 7.3 (1.1) | 0.022 |
| eGFR, mL/min/1.73m2 | 74.2(24.2) | 77.8 (22.3) | <0.001 | 72.9 (23.6) | 77.7 (22.5) | <0.001 |
| LDL cholesterol, mmol/L | 2.6 (1.0) | 2.5 (1.0) | 0.17 | 2.5 (0.9) | 2.6 (1.0) | 0.61 |
| Urine ACR, mg/mmol | 2.4 (0.9, 10.2) | 1.7 (0.7, 6.1) | <0.001 | 2.2 (0.8, 8.8) | 1.7 (0.7, 6.3) | 0.011 |
| MoCA raw score | 17.4 (4.7) | 25.4 (3.2) | <0.001 | 17.9 (5.7) | 25.0 (3.6) | <0.001 |
| Standardized MoCA score | −1.9 (0.9) | 0.2 (0.7) | <0.001 | −1.7 (1.2) | 0.1 (0.9) | <0.001 |
| DSST raw score | 21.5 (17.6) | 40.2 (18.9) | <0.001 | 11.4(8.0) | 40.0 (18.9) | <0.001 |
| Standardized DSST score | −1.0 (0.9) | 0.1(0.9) | <0.001 | −1.6 (0.4) | 0.1 (0.9) | <0.001 |
Data are presented as either means (SD) or counts (percentage). P values from chi-square tests, Wilcoxon rank sum tests, or t tests.
Abbreviations: ACEi/ARB, angiotensin converting enzyme inhibitor or angiotensin receptor blocker; ACR, albumin-to-creatinine ratio; DSST, Digit Symbol Substitution Test; eGFR, estimated glomerular filtration rate; GM, geometric mean; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MoCA, Montreal Cognitive Assessment; SCI, substantive cognitive impairment; TIA, transient ischemic attack.
aHistory of MI or unstable angina or ischemic stroke or need for percutaneous coronary intervention or hospitalization for unstable angina with ECG changes or myocardial ischemia by a stress test/cardiac imaging.
Baseline characteristics of participants with and without country-standardized substantive cognitive impairment defined in 2 different ways
| . | Country-standardized SCI . | . | . | Country-standardized SCI-GM . | . | . |
|---|---|---|---|---|---|---|
| . | Yes . | No . | P . | Yes . | No . | P . |
| N | 905 | 7867 | 529 | 8243 | ||
| Age, years | 68.2 (6.7) | 65.7 (6.3) | <0.001 | 69.1 (6.6) | 65.8 (6.3) | <0.001 |
| Female | 530 (58.6) | 3556 (45.2%) | <0.001 | 320 (60.5) | 3766 (45.7) | <0.001 |
| Diabetes duration, years | 11.0 (7.7) | 10.3 (7.0) | 0.009 | 11.4 (8.3) | 10.3 (7.0) | 0.001 |
| Education ≤ 12 years | 685 (75.7) | 4639 (59.0) | <0.001 | 427 (80.7) | 4897 (59.4) | <0.001 |
| Current tobacco use | 95 (10.5) | 1143 (14.5) | 0.001 | 58 (11.0) | 1180 (14.3) | 0.032 |
| Prior stroke/TIA | 115 (12.7) | 659 (8.4) | <0.001 | 81 (15.3) | 693 (8.4) | <0.001 |
| Hypertension | 857 (94.7) | 7308 (92.9) | 0.043 | 504 (95.3) | 7661 (92.9) | 0.040 |
| Atrial fibrillation | 56 (6.2) | 496 (6.3) | 0.891 | 39 (7.4) | 513 (6.2) | 0.291 |
| Heart failure | 84 (9.3) | 665 (8.5) | 0.398 | 61 (11.5) | 688 (8.3) | 0.011 |
| Diabetic retinopathy | 105 (11.6) | 687 (8.7) | 0.004 | 69 (13.0) | 723 (8.8) | 0.001 |
| Prior cardiovascular diseasea | 287(31.7) | 2416 (30.7) | 0.536 | 183 (34.6) | 2520 (30.6) | 0.052 |
| Statin use | 584 (64.5) | 5227 (66.4) | 0.249 | 346 (65.4) | 5465 (66.3) | 0.674 |
| ACEi/ARB use | 759 (83.9) | 6405 (81.4) | 0.071 | 445 (84.1) | 6719 (81.5) | 0.133 |
| Body mass index, kg/m2 | 32.2 (5.8) | 32.4 (5.7) | 0.24 | 32.0(5.9) | 32.4 (5.7) | 0.12 |
| Systolic blood pressure | 139 (18) | 137 (17) | <0.001 | 138 (17) | 137 (17) | 0.077 |
| Diastolic blood pressure | 78 (11) | 79 (10) | 0.26 | 77 (10) | 79 (10) | 0.001 |
| HbA1c | 7.4 (1.0) | 7.3 (1.1) | 0.021 | 7.4 (1.0) | 7.3 (1.1) | 0.022 |
| eGFR, mL/min/1.73m2 | 74.2(24.2) | 77.8 (22.3) | <0.001 | 72.9 (23.6) | 77.7 (22.5) | <0.001 |
| LDL cholesterol, mmol/L | 2.6 (1.0) | 2.5 (1.0) | 0.17 | 2.5 (0.9) | 2.6 (1.0) | 0.61 |
| Urine ACR, mg/mmol | 2.4 (0.9, 10.2) | 1.7 (0.7, 6.1) | <0.001 | 2.2 (0.8, 8.8) | 1.7 (0.7, 6.3) | 0.011 |
| MoCA raw score | 17.4 (4.7) | 25.4 (3.2) | <0.001 | 17.9 (5.7) | 25.0 (3.6) | <0.001 |
| Standardized MoCA score | −1.9 (0.9) | 0.2 (0.7) | <0.001 | −1.7 (1.2) | 0.1 (0.9) | <0.001 |
| DSST raw score | 21.5 (17.6) | 40.2 (18.9) | <0.001 | 11.4(8.0) | 40.0 (18.9) | <0.001 |
| Standardized DSST score | −1.0 (0.9) | 0.1(0.9) | <0.001 | −1.6 (0.4) | 0.1 (0.9) | <0.001 |
| . | Country-standardized SCI . | . | . | Country-standardized SCI-GM . | . | . |
|---|---|---|---|---|---|---|
| . | Yes . | No . | P . | Yes . | No . | P . |
| N | 905 | 7867 | 529 | 8243 | ||
| Age, years | 68.2 (6.7) | 65.7 (6.3) | <0.001 | 69.1 (6.6) | 65.8 (6.3) | <0.001 |
| Female | 530 (58.6) | 3556 (45.2%) | <0.001 | 320 (60.5) | 3766 (45.7) | <0.001 |
| Diabetes duration, years | 11.0 (7.7) | 10.3 (7.0) | 0.009 | 11.4 (8.3) | 10.3 (7.0) | 0.001 |
| Education ≤ 12 years | 685 (75.7) | 4639 (59.0) | <0.001 | 427 (80.7) | 4897 (59.4) | <0.001 |
| Current tobacco use | 95 (10.5) | 1143 (14.5) | 0.001 | 58 (11.0) | 1180 (14.3) | 0.032 |
| Prior stroke/TIA | 115 (12.7) | 659 (8.4) | <0.001 | 81 (15.3) | 693 (8.4) | <0.001 |
| Hypertension | 857 (94.7) | 7308 (92.9) | 0.043 | 504 (95.3) | 7661 (92.9) | 0.040 |
| Atrial fibrillation | 56 (6.2) | 496 (6.3) | 0.891 | 39 (7.4) | 513 (6.2) | 0.291 |
| Heart failure | 84 (9.3) | 665 (8.5) | 0.398 | 61 (11.5) | 688 (8.3) | 0.011 |
| Diabetic retinopathy | 105 (11.6) | 687 (8.7) | 0.004 | 69 (13.0) | 723 (8.8) | 0.001 |
| Prior cardiovascular diseasea | 287(31.7) | 2416 (30.7) | 0.536 | 183 (34.6) | 2520 (30.6) | 0.052 |
| Statin use | 584 (64.5) | 5227 (66.4) | 0.249 | 346 (65.4) | 5465 (66.3) | 0.674 |
| ACEi/ARB use | 759 (83.9) | 6405 (81.4) | 0.071 | 445 (84.1) | 6719 (81.5) | 0.133 |
| Body mass index, kg/m2 | 32.2 (5.8) | 32.4 (5.7) | 0.24 | 32.0(5.9) | 32.4 (5.7) | 0.12 |
| Systolic blood pressure | 139 (18) | 137 (17) | <0.001 | 138 (17) | 137 (17) | 0.077 |
| Diastolic blood pressure | 78 (11) | 79 (10) | 0.26 | 77 (10) | 79 (10) | 0.001 |
| HbA1c | 7.4 (1.0) | 7.3 (1.1) | 0.021 | 7.4 (1.0) | 7.3 (1.1) | 0.022 |
| eGFR, mL/min/1.73m2 | 74.2(24.2) | 77.8 (22.3) | <0.001 | 72.9 (23.6) | 77.7 (22.5) | <0.001 |
| LDL cholesterol, mmol/L | 2.6 (1.0) | 2.5 (1.0) | 0.17 | 2.5 (0.9) | 2.6 (1.0) | 0.61 |
| Urine ACR, mg/mmol | 2.4 (0.9, 10.2) | 1.7 (0.7, 6.1) | <0.001 | 2.2 (0.8, 8.8) | 1.7 (0.7, 6.3) | 0.011 |
| MoCA raw score | 17.4 (4.7) | 25.4 (3.2) | <0.001 | 17.9 (5.7) | 25.0 (3.6) | <0.001 |
| Standardized MoCA score | −1.9 (0.9) | 0.2 (0.7) | <0.001 | −1.7 (1.2) | 0.1 (0.9) | <0.001 |
| DSST raw score | 21.5 (17.6) | 40.2 (18.9) | <0.001 | 11.4(8.0) | 40.0 (18.9) | <0.001 |
| Standardized DSST score | −1.0 (0.9) | 0.1(0.9) | <0.001 | −1.6 (0.4) | 0.1 (0.9) | <0.001 |
Data are presented as either means (SD) or counts (percentage). P values from chi-square tests, Wilcoxon rank sum tests, or t tests.
Abbreviations: ACEi/ARB, angiotensin converting enzyme inhibitor or angiotensin receptor blocker; ACR, albumin-to-creatinine ratio; DSST, Digit Symbol Substitution Test; eGFR, estimated glomerular filtration rate; GM, geometric mean; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MoCA, Montreal Cognitive Assessment; SCI, substantive cognitive impairment; TIA, transient ischemic attack.
aHistory of MI or unstable angina or ischemic stroke or need for percutaneous coronary intervention or hospitalization for unstable angina with ECG changes or myocardial ischemia by a stress test/cardiac imaging.
The incidence of MACE in participants with and without baseline country-standardized SCI was 2.61 and 1.96 per 100 person-years respectively (Table 2). The unadjusted risk of MACE (HR 1.34; 95% CI 1.11, 1.62; P = 0.003; Fig. 1A) remained significant but was attenuated after adjusting for age, sex, education, prior stroke or transient ischemic attack (TIA), systolic blood pressure, HbA1c, and tobacco use (multivariable adjusted HR 1.22; 95% CI 1.01, 1.48; P = 0.043), and became nonsignificant after additional adjustment for albuminuria, eGFR, and retinopathy (Fig. 2A).
| . | Impairment . | . | No impairment . | . | HR or RR (95% CI) . | P . |
|---|---|---|---|---|---|---|
| . | N/at risk (%) . | N/100py . | N/at risk (%) . | N/100py . | . | . |
| MACE | ||||||
| SCI | 123/905 (13.6%) | 2.61 | 809/7867 (10.3%) | 1.96 | HR 1.34 (1.11, 1.62) | 0.003 |
| SCI-GM | 85/529 (16.1%) | 3.12 | 847/8243 (10.3%) | 1.95 | HR 1.61 (1.28, 2.01) | <0.001 |
| Stroke or Death | ||||||
| SCI | 139/905 (15.4%) | 2.89 | 764/7867 (9.7%) | 1.81 | HR1.60 (1.33, 1.91) | <0.001 |
| SCI-GM | 94/529 (17.8%) | 3.37 | 809/8243 (9.8%) | 1.83 | HR1.85 (1.50, 2.30) | <0.001 |
| . | Impairment . | . | No impairment . | . | HR or RR (95% CI) . | P . |
|---|---|---|---|---|---|---|
| . | N/at risk (%) . | N/100py . | N/at risk (%) . | N/100py . | . | . |
| MACE | ||||||
| SCI | 123/905 (13.6%) | 2.61 | 809/7867 (10.3%) | 1.96 | HR 1.34 (1.11, 1.62) | 0.003 |
| SCI-GM | 85/529 (16.1%) | 3.12 | 847/8243 (10.3%) | 1.95 | HR 1.61 (1.28, 2.01) | <0.001 |
| Stroke or Death | ||||||
| SCI | 139/905 (15.4%) | 2.89 | 764/7867 (9.7%) | 1.81 | HR1.60 (1.33, 1.91) | <0.001 |
| SCI-GM | 94/529 (17.8%) | 3.37 | 809/8243 (9.8%) | 1.83 | HR1.85 (1.50, 2.30) | <0.001 |
All hazard ratios (HRs) and relative risks (RRs) are just adjusted for the baseline value.
Abbreviations: GM, geometric mean of the Montreal Cognitive Assessment and Digit Symbol Substitution Test scores; MACE, major adverse cardiovascular event; py, person-years; SCI, substantive cognitive impairment.
| . | Impairment . | . | No impairment . | . | HR or RR (95% CI) . | P . |
|---|---|---|---|---|---|---|
| . | N/at risk (%) . | N/100py . | N/at risk (%) . | N/100py . | . | . |
| MACE | ||||||
| SCI | 123/905 (13.6%) | 2.61 | 809/7867 (10.3%) | 1.96 | HR 1.34 (1.11, 1.62) | 0.003 |
| SCI-GM | 85/529 (16.1%) | 3.12 | 847/8243 (10.3%) | 1.95 | HR 1.61 (1.28, 2.01) | <0.001 |
| Stroke or Death | ||||||
| SCI | 139/905 (15.4%) | 2.89 | 764/7867 (9.7%) | 1.81 | HR1.60 (1.33, 1.91) | <0.001 |
| SCI-GM | 94/529 (17.8%) | 3.37 | 809/8243 (9.8%) | 1.83 | HR1.85 (1.50, 2.30) | <0.001 |
| . | Impairment . | . | No impairment . | . | HR or RR (95% CI) . | P . |
|---|---|---|---|---|---|---|
| . | N/at risk (%) . | N/100py . | N/at risk (%) . | N/100py . | . | . |
| MACE | ||||||
| SCI | 123/905 (13.6%) | 2.61 | 809/7867 (10.3%) | 1.96 | HR 1.34 (1.11, 1.62) | 0.003 |
| SCI-GM | 85/529 (16.1%) | 3.12 | 847/8243 (10.3%) | 1.95 | HR 1.61 (1.28, 2.01) | <0.001 |
| Stroke or Death | ||||||
| SCI | 139/905 (15.4%) | 2.89 | 764/7867 (9.7%) | 1.81 | HR1.60 (1.33, 1.91) | <0.001 |
| SCI-GM | 94/529 (17.8%) | 3.37 | 809/8243 (9.8%) | 1.83 | HR1.85 (1.50, 2.30) | <0.001 |
All hazard ratios (HRs) and relative risks (RRs) are just adjusted for the baseline value.
Abbreviations: GM, geometric mean of the Montreal Cognitive Assessment and Digit Symbol Substitution Test scores; MACE, major adverse cardiovascular event; py, person-years; SCI, substantive cognitive impairment.
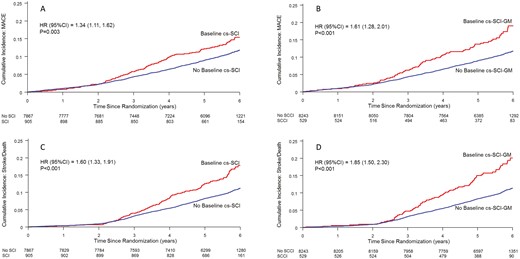
Event curves illustrating the incidence of major adverse cardiovascular events (MACE) (Panel A and B) and the composite outcome of stroke or death (Panel C and D) according to the presence or absence of country-standardized substantive cognitive impairment based on either a Montreal Cognitive Assessment or Digit Symbol Substitution Score ≤ −1.5 (cs-SCI) or the geometric mean (GM) of these scores (cs-SCI-GM).
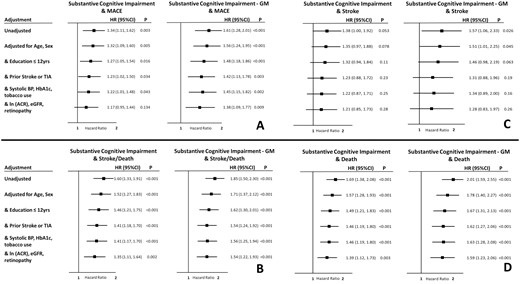
Hazard of events in the presence of country-standardized substantive cognitive impairment, defined as either a country-standardized Montreal Cognitive Assessment or Digit Symbol Substitution Score ≤ −1.5, or the country-standardized geometric mean (GM) of those 2 scores < -1.5. Panel A depicts the hazard of major adverse cardiovascular events (MACE), Panel B depicts the hazard of stroke or death, and Panels C and D depict stroke and death separately. All models are adjusted for the baseline country-standardized cognitive score. Abbreviations: ACR, albumin-to-creatinine ratio; BP, blood pressure; eGFR, estimated glomerular filtration rate; TIA, transient ischemic attack.
A somewhat stronger relationship between country-standardized SCI and MACE was noted when its definition was based on SCI-GM (Fig. 1B; Table 2) for which the incidence of MACE in those with and without SCI-GM at baseline was 3.12 and 1.95 per 100 person-years respectively (HR 1.61; 95% CI 1.28, 2.01; P < 0.001). As noted in Fig. 2A, this hazard ratio remained significant after adjustment for all the risk factors noted above in addition to albuminuria, the eGFR, and retinopathy (multivariable adjusted HR 1.38; 95% CI 1.09, 1.77; P = 0.009).
Similar but stronger relationships were noted for the incidence of either stroke or death from any cause during follow-up (Fig. 1C and 1D). Compared to people without SCI (Fig. 2B), after adjustment for age, sex, education ≤ 12 years, prior stroke or TIA, systolic blood pressure, HbA1c, tobacco use, albuminuria, eGFR, and retinopathy, country-standardized SCI was associated with an increased hazard of 35% (HR 1.35; 95% CI 1.11, 1.64; P = 0.002) and country-standardized SCI-GM was associated with an increased hazard of 54% (HR 1.54; 95% CI 1.22, 1.93; P < 0.001). Similar relationships were noted for stroke (Fig. 2C) and death (Fig. 2D).
Adjustment for either dulaglutide assignment or previous heart failure did not alter the relationship between the measures of cognitive impairment and the analyzed outcomes. Notably, similar to the previously reported association between dulaglutide and SCI (14), random assignment to dulaglutide was associated with a reduced hazard of the first occurrence of SCI-GM during follow-up (HR 0.82; 95% CI 0.74, 0.92; P = 0.0007).
Discussion
This post hoc analysis of 8772 individuals with type 2 diabetes demonstrates a relationship between cognitive dysfunction at baseline and both future MACE, and the composite outcome of stroke or death. Indeed, participants with SCI were up to 1.6 times more likely to experience MACE, and 1.8 times more like to experience a stroke or die compared to people without baseline SCI. The fact that these relationships generally persisted after adjustment for age, sex, education, prior stroke or TIA, and several other possible confounding variables (noted in Fig. 2) suggests that cognitive status provides additional pertinent information regarding an individual’s risk of future vascular outcomes.
The MoCA and the DSST are 2 commonly used cognitive tests that each assess cognitive function in different ways and reflect different aspects of cognitive function. This analysis classified an individual as having country-standardized SCI if either of these scores was 1.5 SD or more below their country’s average score for each measurement, when analyzed separately. This novel composite measure controls for socio-cultural differences in test performance between countries and has been validated by a recent epidemiologic analysis showing that known risk factors for cognitive decline were also risk factors for country-standardized SCI (14). This report describes a way of incorporating information from both tests, which is based on computing the geometric mean of each individual’s 2 scores and defining country-standardized SCI-GM as a value 1.5 SD or more below their country’s average geometric mean (13). Both country-standardized SCI based on either the MoCA or DSST score and country-standardized SCI-GM that combines the information from these 2 tests into one new composite score were associated with similar degrees of hazard for MACE and for stroke/death outcomes and the relationships for SCI-GM remain robust even after adjusting for multiple risk factors. These observations support the utility of SCI-GM as a novel predictor in this setting. The finding that random assignment to dulaglutide versus placebo was associated with a hazard of SCI-GM (ie, HR 0.82; 95% CI 0.74, 0.92) that was similar to the previously reported hazard of SCI (ie, HR 0.86; 95% CI 0.79, 0.95) (14) also supports the use of this composite measure as an outcome.
Prospective studies conducted in both the general population and in people with diabetes also reported an independent relationship between cognitive status and future cardiovascular outcomes. Thus, low cognitive test scores were associated with future all-cause and cardiovascular mortality in a random sample of 7087 middle-aged and older-aged individuals followed for 10 years (7), and lower Mini-Mental State Examination (MMSE) scores were associated with future stroke and other cardiovascular outcomes during more than 4.5 years of follow-up in 30 959 participants in 2 large cardiovascular outcomes trials (15). Comparable relationships were reported in people with diabetes. Thus, lower MMSE scores were associated with future MACE and death in an epidemiologic analysis of 11 140 participants in a diabetes cardiovascular outcomes trial followed for a median of 5 years (10), and a lower DSST score was associated with future MACE in another trial that followed 2977 individuals with type 2 diabetes for a median of 4.3 years (9). Similar relationships between cognitive status and incident vascular outcomes or death were reported in people who were followed after suffering a stroke (16). (17)
A variety of explanations may account for the relationship observed between cognitive impairment and these outcomes. First, cognitive impairment may reflect preexisting and unsuspected white matter or small vessel disease or infarctions in the brain that may also exist elsewhere. However, the observation that the relationship persisted in models that adjusted for prior cardiovascular disease and diabetes complications suggests the measure is also reflecting other aspects of health. Second, cognitive dysfunction is associated with a lower capacity for self-care and risk-avoidance, which is a cornerstone of diabetes management (18, 19). This includes measured and unmeasured self-management parameters such as adherence to prescribed medications, that may in-turn be related to outcomes (18, 19). Third, the fact that cognitive function is known to be associated with unmeasured risk factors for cardiovascular outcomes such as socioeconomic status suggests that some component of the observed relationship may be due to these other risk factors. Finally, both cognitive impairment and cardiovascular outcomes could share common antecedent causes such as mitochondrial dysfunction (20, 21), dysregulation of the sortilin pathway (22), activation of the hypothalamic-pituitary-adrenal axis, inflammation, dysglycemia, or brain and systemic insulin resistance (23-25).
These analyses are limited by the fact that they were not prespecified, and that the cognitive tests were done in a wide range of countries. Moreover, they were not based on a comprehensive assessment of cognitive function. Therefore, analyses of the relationship between a variety of different cognitive domains and vascular outcomes could not be done. Finally, they may not be generalizable to lower risk or younger people with diabetes at lower risk for cardiovascular disease. Strengths include the predetermined use of the 1.5 SD threshold for cognitive impairment based on the baseline scores of REWIND participants, and not reliance on an external threshold based on a different population. They also include the large sample size, the fact that the population was not selected for cognitive dysfunction, demographic characteristics similar to a large proportion of people with type 2 diabetes, long follow-up, and use of 2 different cognitive instruments.
In summary, these findings elucidate 2 ways of using the results from more than one cognitive test to identify cognitive impairment in large international studies. The first (country-standardized SCI), used similar cut-points from the component tests, and the second (country –standardized SCI-GM) combined them into a composite index that embeds prognostic information from the component tests. These findings also highlight the relevance of these novel indices of cognitive impairment for future vascular outcomes and suggest that the application of recent guidelines that recommend routine screening for cognitive impairment in older people with diabetes (26-30) may identify individuals most likely to benefit from proven cardioprotective therapies.
Abbreviations
- AE
adverse event
- AO-GHD
adult-onset growth hormone deficiency
- BMI
body mass index
- BP
blood pressure
- CO-GHD
childhood-onset growth hormone deficiency
- DSST
Digit Symbol Substitution Test
- eGFR
estimated glomerular filtration rate
- GH
growth hormone
- GHD
growth hormone deficiency
- GM
geometric mean
- HbA1c
glycated hemoglobin A1c
- HDL
high-density lipoprotein
- IGF-1
insulin-like growth factor 1
- KIMS
Pfizer International Metabolic Database
- LDL
low-density lipoprotein
- MACE
major adverse cardiovascular event
- MedDRA
Medical Dictionary for Regulatory Activities
- MoCA
Montreal Cognitive Assessment
- PT
preferred term (MedDRA)
- SAE
serious adverse event
- SCI
substantive cognitive impairment
- SDS
standard deviation score
- SIR
standardized incidence ratio
- TIA
transient ischemic attack
- WHO
World Health Organization
Funding
The REWIND trial was funded by Eli Lilly.
Author Contributions
H.C.G. and T.C.Y. wrote the first draft of the paper and P.R.M. did the statistical analyses. All the authors researched the data and critically revised the paper. H.C.G. is the guarantor of the study and made the final decision to submit and publish the manuscript.
Disclosures
T.C.Y. reports research grants from Medtronic, MSD; honoraria for speaking from MSD, Sanofi, Novo Nordisk, Eli Lilly, AstraZeneca, and Medtronic; H.C.G. holds the McMaster-Sanofi Population Health Institute Chair in Diabetes Research and Care. He reports research grants from Eli Lilly, AstraZeneca, Merck, Novo Nordisk, and Sanofi; honoraria for speaking from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi, DKSH, Roche and Zuellig; and consulting fees from Abbott, Covance, Eli Lilly, Novo Nordisk, Sanofi, Pfizer, Kowa and Hanmi. J.B. reports research grant support from ReCor, and Ablative Solutions, and consulting fees from Eli Lilly, ReCor, Up-to-Date, and Medtronic. M.A.B. is a current employee and shareholder of Eli Lilly and Company; I.C. received and reports honoraria for speaking from Medtronic, Sanofi, Astra Zeneca, Novo Nordisk, MSD, Boehringer Ingelheim, and Eli Lilly; G.R.D. reports honoraria for speaking from Bayer; E.F. reports honoraria for speaking from Bioton, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Polfa Tarchomin and consulting fees (advisory boards) from Bioton, Boehringer Ingelheim, and Novo Nordisk. M.L. is a Lilly retiree and owns Lilly stock; F.L. reports research grants from Bayer and Pfizer and honoraria for speaking from Boehringer Ingelheim, Eli Lilly, Sanofi, Astra Zeneca, Novartis, and Pfizer; L.A.L. has received grants from Amgen Esperion, Kowa, Lexicon The Medicines Company (all to institution); has provided CME on behalf of Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly Sanofi, Servier; and has acted as an advisor to Amarin, Amgen, AstraZeneca, Bayer, Esperion, Novartis, Novo Nordisk, Pfizer, and Sanofi; P.L.-J. has received honoraria for speaking from Menarini, Eli Lilly, and Abbott; M.C.R. reports consulting fees from Adocia, Intercept and Theracos; J.E.S. reports grants from Astra Zeneca and honoraria for speaking from Astra Zeneca, Eli Lilly, MSD, Mylan and Zuellig; and W.H.H.S. has been an advisor and/or speaker for AstraZeneca, Bayer HealthCare, Boehringer Ingelheim Pharmaceuticals., Daiichi-Sankyo, Eli Lilly Company, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma Corporation, Novartis Pharmaceuticals, Novo Nordisk, Pfizer, Sanofi Aventis, and Takeda.
Clinical Trial Information
ClinicalTrials.gov registration no. NCT01394952
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.



