-
PDF
- Split View
-
Views
-
Cite
Cite
Anela Blažević, Anand M Iyer, Marie-Louise F van Velthuysen, Johannes Hofland, Lindsey Oudijk, Wouter W de Herder, Leo J Hofland, Richard A Feelders, Sexual Dimorphism in Small-intestinal Neuroendocrine Tumors: Lower Prevalence of Mesenteric Disease in Premenopausal Women, The Journal of Clinical Endocrinology & Metabolism, Volume 107, Issue 5, May 2022, Pages e1969–e1975, https://doi.org/10.1210/clinem/dgac001
Close - Share Icon Share
Abstract
Small-intestinal neuroendocrine tumors (SI-NETs) have a modest but significantly higher prevalence and worse prognosis in male patients.
This work aims to increase understanding of this sexual dimorphism in SI-NETs.
Retrospectively, SI-NET patients treated in a single tertiary center were included and analyzed for disease characteristics. Estrogen receptor 1 (ESR1) and 2 (ESR2), progesterone receptor (PGR), and androgen receptor (AR) messenger RNA (mRNA) expression was assessed in primary tumors and healthy intestine. Estrogen receptor alpha (ERα) and AR protein expression were analyzed by immunohistochemistry in primary tumors and mesenteric metastases.
Of the 559 patients, 47% were female. Mesenteric metastasis/fibrosis was more prevalent in men (71% / 46%) than women (58% / 37%; P = 0.001 and P = 0.027, respectively). In women, prevalence of mesenteric metastases increased gradually with age from 41.1% in women <50 years to 71.7% in women >70 years. Increased expression of ESR1 and AR mRNA was observed in primary tumors compared to healthy intestine (both P < 0.001). ERα staining was observed in tumor cells and stroma with a strong correlation between tumor cells of primary tumors and mesenteric metastases (rho = 0.831, P = 0.02), but not in stroma (rho = −0.037, P = 0.91). AR expression was only found in stroma.
Sexual dimorphism in SI-NETs was most pronounced in mesenteric disease, and the risk of mesenteric metastasis in women increased around menopause. The combination of increased ERα and AR expression in the SI-NET microenvironment suggests a modulating role of sex steroids in the development of the characteristic SI-NET mesenteric metastasis and associated fibrosis.
Small-intestinal neuroendocrine tumors (SI-NETs) arise from the enterochromaffin cells in the intestinal tract and have an incidence of approximately 1 per 100 000 (1). SI-NETs predominantly metastasize to the liver and mesenteric lymph nodes (2). SI-NET mesenteric metastases are distinct as they are often surrounded by hallmark mesenteric fibrosis, which can cause severe complications such as bowel obstruction and ischemia. These mesenteric metastases are generally slow-growing. However, our group has recently shown that sex is an important factor in predicting growth in SI-NET mesenteric metastases (3).
While biological sex differences affecting incidence, prognosis, and therapeutic response are well established in many cancer types, sex disparities have been scarcely investigated in NETs. However, there is accumulating data on the presence of relevant sex differences in prevalence and prognosis of NETs. Women are more likely to have a primary NET in the lung or stomach, whereas men are more likely to have a primary tumor in the small intestine or pancreas (4,5). Men are also more likely to present with metastasized disease at diagnosis and have a worse prognosis, even after correction for age and disease characteristics such as type of primary tumor, tumor grade, and disease stage (4,5).
In addition to sexual dimorphism in prevalence and prognosis, hormonal influences on SI-NETs are further suggested by the studies showing efficacy of tamoxifen in SI-NETs (6-9). Tamoxifen is a synthetic nonsteroidal selective estrogen receptor modulator primarily used in the treatment of breast cancer. However, due to its antifibrotic effect it is also used in fibrotic diseases such as retroperitoneal fibrosis (10). In SI-NETs, tumor growth control and amelioration of carcinoid syndrome symptoms has been described after treatment with tamoxifen (6-9). However, these results were not replicated in a larger case series (11). These discrepancies could be due to variable hormone receptor expression in tumor cells and tumor microenvironment (6,7). Unfortunately, little is known about sex hormone receptor expression in SI-NETs. Most studies analyzing sex hormone receptor expression in NETs focused on immunohistochemical expression of estrogen receptor alpha (ERα) or progesterone receptor (PR) in pancreatic NETs, although some studies include a subset of SI-NET patients. These studies showed that SI-NETs can express ERα, while PR expression was minimal or absent (12,13).
The objectives of this study were to (1) asses sex differences in SI-NET disease characteristics such as tumor grade, age, and tumor stage at diagnosis; metastatic pattern; and mesenteric fibrosis and (2) evaluate expression of sex steroid hormone receptors in SI-NET primary tumors and mesenteric metastases, both in tumor cells and the surrounding tumor microenvironment.
Methods and Materials
Patients
Patients were included if they were treated at our center between 1993 and 2016 with a histologically proven SI-NET and had at least 2 contrast-enhanced computed tomography scans. The study was performed retrospectively and did not require approval from an ethics committee in the Netherlands according to the Central Committee on Research involving Human Subjects guidelines. Age, sex, disease characteristics, tumor grade, tumor stage, presence of hepatic and/or mesenteric metastases and associated fibrosis and serum chromogranin A (upper limit of normal, 94 μg/L) were recorded at the time of diagnosis or at the first available moment. For correlation with messenger RNA (mRNA) expression levels, urinary 5-hydroxyindoleacetic acid (5-HIAA) excretion (upper limit of normal, 50 μmol/24 hours) excretion was recorded at the time of resection and collection of the tissue sample. Urinary 5-HIAA excretion was determined by measuring the mean urinary 5-HIAA levels in two 24-hour urine samples. Tumor grading and staging was performed in accordance with the European Neuroendocrine Tumor Society (ENETS) guideline (14).
Imaging
Radiological features of mesenteric disease were assessed on routinely performed contrast-enhanced computed tomography scans in accordance with RECIST 1.1 criteria (15). A mesenteric node of ≥10 mm on the short axis was considered a pathological mesenteric metastases. Mesenteric fibrosis was defined as radiating strands of soft tissue in the mesentery (16).
Gene Expression of Sex Steroid Receptors
Frozen tissue of 24 primary SI-NETs and adjacent normal intestine was obtained from the Erasmus MC Tissue Bank. Tissue samples were included if the tumor sample consisted of at least 80% tumor tissue and normal intestine sample contained no tumor cells or necrosis. Table 1 shows clinical data of patients included (n = 24)
Clinical information patients included for gene expression (messenger RNA) analysis
| . | All patients (n = 24) . |
|---|---|
| Median age, years | 65 (53-76) |
| Female | 12 (50) |
| Tumor grade | |
| Grade 1 | 17 (71) |
| Grade 2 | 7 (29) |
| Median urinary 5-HIAA, µmol/24 hours | 190 (54-602) |
| . | All patients (n = 24) . |
|---|---|
| Median age, years | 65 (53-76) |
| Female | 12 (50) |
| Tumor grade | |
| Grade 1 | 17 (71) |
| Grade 2 | 7 (29) |
| Median urinary 5-HIAA, µmol/24 hours | 190 (54-602) |
Data are given as median (interquartile range) or n (%).
Abbreviations: 5-HIAA, urinary 5-hydroxyindoleacetic acid excretion; IQR, interquartile range
Clinical information patients included for gene expression (messenger RNA) analysis
| . | All patients (n = 24) . |
|---|---|
| Median age, years | 65 (53-76) |
| Female | 12 (50) |
| Tumor grade | |
| Grade 1 | 17 (71) |
| Grade 2 | 7 (29) |
| Median urinary 5-HIAA, µmol/24 hours | 190 (54-602) |
| . | All patients (n = 24) . |
|---|---|
| Median age, years | 65 (53-76) |
| Female | 12 (50) |
| Tumor grade | |
| Grade 1 | 17 (71) |
| Grade 2 | 7 (29) |
| Median urinary 5-HIAA, µmol/24 hours | 190 (54-602) |
Data are given as median (interquartile range) or n (%).
Abbreviations: 5-HIAA, urinary 5-hydroxyindoleacetic acid excretion; IQR, interquartile range
RNA was extracted from 20 cryostat sections of 20 μm. For histological confirmation of the inclusion criteria, hematoxylin-eosin staining was performed on a sequential 5-μm section. Total RNA was isolated from the specimens using the High Pure RNA Tissue Kit (Roche) according to the manufacturer’s protocol. To synthesize complimentary DNA (cDNA), the RevertAid First Strand cDNA Synthesis Kit (Thermofisher Scientific, The Netherlands) was used according to manufacturer’s protocol with 500 ng of input RNA. The samples were analyzed using Taqman gene expression assays (Applied Biosystems) for expression of sex hormone receptor genes: estrogen receptor 1 (ESR1, gene coding for ERα), estrogen receptor 2 (ESR2, gene coding for ERβ), progresterone receptor (PGR), and androgen receptor (AR), and 3 reference genes [hypoxanthine phosphoribosyl transferase (HPRT1), β-Actin (ACTB), and β-glucuronidase (GUSB)] (Table 2). For each sample, quantitative polymerase chain reaction (PCR) reactions were performed in duplicates in a 384-well plate with 4 μL cDNA, 0.5 μL Taqman primers (45 nM final concentration, both forward and reverse) and probes (12.5 nM final concentration), and 5 μL TaqMan Universal PCR Master Mix (Applied Biosystems) in a total reaction volume of 10 μL. The quantitative PCR reaction was performed in a QuantStudio 7 Flex real time PCR system thermocycler (Applied Biosystems, Foster City, CA, USA). The expression of the genes of interest was normalized using the geometric mean of the expression of the 3 reference genes (17).
Primers and probes used for real-time quantitative polymerase chain reaction
| Gene . | Assay ID . | EF . |
|---|---|---|
| ACTB | Hs01060665_g1 | 1.96 |
| AR | Hs00171172_m1 | 1.98 |
| ERS1 | Hs01046816_m1 | 1.95 |
| ESR2 | Hs01100353_m1 | 2.51 |
| GUSB | Hs00939627_m1 | 1.95 |
| HPRT1 | Hs02800695_m1 | 1.92 |
| PGR | Hs01556702_m1 | 2.00 |
| Gene . | Assay ID . | EF . |
|---|---|---|
| ACTB | Hs01060665_g1 | 1.96 |
| AR | Hs00171172_m1 | 1.98 |
| ERS1 | Hs01046816_m1 | 1.95 |
| ESR2 | Hs01100353_m1 | 2.51 |
| GUSB | Hs00939627_m1 | 1.95 |
| HPRT1 | Hs02800695_m1 | 1.92 |
| PGR | Hs01556702_m1 | 2.00 |
All used primers are commercially available (Thermo Fisher Scientific, Breda, The Netherlands).
Abbreviations: ACTB, beta-actin; AR, androgen receptor; EF, efficiency factor; ESR1, estrogen receptor 1; ESR2, estrogen receptor 2; GUSB, glucuronidase beta; HRPT, hypoxanthine phosphoribosyltransferase 1; PGR, progesterone receptor.
Primers and probes used for real-time quantitative polymerase chain reaction
| Gene . | Assay ID . | EF . |
|---|---|---|
| ACTB | Hs01060665_g1 | 1.96 |
| AR | Hs00171172_m1 | 1.98 |
| ERS1 | Hs01046816_m1 | 1.95 |
| ESR2 | Hs01100353_m1 | 2.51 |
| GUSB | Hs00939627_m1 | 1.95 |
| HPRT1 | Hs02800695_m1 | 1.92 |
| PGR | Hs01556702_m1 | 2.00 |
| Gene . | Assay ID . | EF . |
|---|---|---|
| ACTB | Hs01060665_g1 | 1.96 |
| AR | Hs00171172_m1 | 1.98 |
| ERS1 | Hs01046816_m1 | 1.95 |
| ESR2 | Hs01100353_m1 | 2.51 |
| GUSB | Hs00939627_m1 | 1.95 |
| HPRT1 | Hs02800695_m1 | 1.92 |
| PGR | Hs01556702_m1 | 2.00 |
All used primers are commercially available (Thermo Fisher Scientific, Breda, The Netherlands).
Abbreviations: ACTB, beta-actin; AR, androgen receptor; EF, efficiency factor; ESR1, estrogen receptor 1; ESR2, estrogen receptor 2; GUSB, glucuronidase beta; HRPT, hypoxanthine phosphoribosyltransferase 1; PGR, progesterone receptor.
Immunohistochemistry of ERα and AR
Immunohistochemistry (IHC) for ERα and AR was performed on formalin-fixed, paraffin-embedded whole sections of primary SI-NETs and paired mesenteric metastases obtained from the Erasmus MC Tissue Bank. Samples were selected based on histopathologic review of the mesenteric metastases. Using hematoxylin-eosin–stained sections of the mesenteric metastases, the degree of fibrosis was graded based on the width of intratumoral fibrous tissue bands: no fibrosis (<1 mm), intermediate (1-2 mm), and severe (>2 mm) (16). Patients were included if there was no mesenteric fibrosis (n = 6) or severe fibrosis (n = 6) (Table 3) Sequential 4-µm thick formalin-fixed, paraffin-embedded sections were stained for ERα (antibody ID: AB_2335977, rabbit monoclonal, dilution 1 µg/mL, clone SP1, Ventana) and AR (antibody ID:AB_2893478, rabbit monoclonal, dilution 1.55 µg/mL, clone SP107, Cell Marque) by automated IHC using the Ventana Benchmark ULTRA (Ventana Medical Systems Inc.). In brief, following deparaffinization and heat-induced antigen retrieval with CC1 (#950-500, Ventana) for 64 minutes, the tissue samples were incubated with the antibody of interest for 32 minutes at 37°C. The staining was developed using Optiview universal DAB detection Kit (#760-700, Ventana), followed by hematoxylin II counterstain for 8 minutes and then a blue coloring reagent for 8 minutes according to the manufacturer’s instructions (Ventana). Positive controls were used on every slide. Sections were scored independently by 2 experienced pathologists (M.F.V. and L.O.). The mean percentage of staining positive cells was used for analysis. In case of a discrepancy of ≥25%, a consensus score was reached.
Clinical information patients included for protein expression (immunohistochemistry) analysis
| . | Mesenteric fibrosis (n = 6) . | No mesenteric fibrosis (n = 6) . | P-value . |
|---|---|---|---|
| Median age, years | 56 (49-65) | 56 (49-61) | 0.99 |
| Female | 3 (50) | 3 (50) | 1.00 |
| Tumor grade 1 | 6 (100) | 6 (100) | 1.00 |
| Median urinary 5-HIAA, µmol/24 hours | 150 (68-1299) | 60 (49-103) | 0.57 |
| ENETS disease stage | 0.25 | ||
| Stage III | 2 (33) | 4 (67) | |
| Stage IV | 4 (67) | 2 (33) | |
| Preoperative treatment | 0.22 | ||
| None | 3 (50) | 5 (83) | |
| SSA | 3 (50) | 1 (17) |
| . | Mesenteric fibrosis (n = 6) . | No mesenteric fibrosis (n = 6) . | P-value . |
|---|---|---|---|
| Median age, years | 56 (49-65) | 56 (49-61) | 0.99 |
| Female | 3 (50) | 3 (50) | 1.00 |
| Tumor grade 1 | 6 (100) | 6 (100) | 1.00 |
| Median urinary 5-HIAA, µmol/24 hours | 150 (68-1299) | 60 (49-103) | 0.57 |
| ENETS disease stage | 0.25 | ||
| Stage III | 2 (33) | 4 (67) | |
| Stage IV | 4 (67) | 2 (33) | |
| Preoperative treatment | 0.22 | ||
| None | 3 (50) | 5 (83) | |
| SSA | 3 (50) | 1 (17) |
Data are given as median (interquartile range) or n (%).
Abbreviations: 5-HIAA, urinary 5-hydroxyindoleacetic acid excretion, normal range <50 μmol/24 hour; ENETS, European Neuroendocrine Tumor Society; SSA, somatostatin analogue.
Clinical information patients included for protein expression (immunohistochemistry) analysis
| . | Mesenteric fibrosis (n = 6) . | No mesenteric fibrosis (n = 6) . | P-value . |
|---|---|---|---|
| Median age, years | 56 (49-65) | 56 (49-61) | 0.99 |
| Female | 3 (50) | 3 (50) | 1.00 |
| Tumor grade 1 | 6 (100) | 6 (100) | 1.00 |
| Median urinary 5-HIAA, µmol/24 hours | 150 (68-1299) | 60 (49-103) | 0.57 |
| ENETS disease stage | 0.25 | ||
| Stage III | 2 (33) | 4 (67) | |
| Stage IV | 4 (67) | 2 (33) | |
| Preoperative treatment | 0.22 | ||
| None | 3 (50) | 5 (83) | |
| SSA | 3 (50) | 1 (17) |
| . | Mesenteric fibrosis (n = 6) . | No mesenteric fibrosis (n = 6) . | P-value . |
|---|---|---|---|
| Median age, years | 56 (49-65) | 56 (49-61) | 0.99 |
| Female | 3 (50) | 3 (50) | 1.00 |
| Tumor grade 1 | 6 (100) | 6 (100) | 1.00 |
| Median urinary 5-HIAA, µmol/24 hours | 150 (68-1299) | 60 (49-103) | 0.57 |
| ENETS disease stage | 0.25 | ||
| Stage III | 2 (33) | 4 (67) | |
| Stage IV | 4 (67) | 2 (33) | |
| Preoperative treatment | 0.22 | ||
| None | 3 (50) | 5 (83) | |
| SSA | 3 (50) | 1 (17) |
Data are given as median (interquartile range) or n (%).
Abbreviations: 5-HIAA, urinary 5-hydroxyindoleacetic acid excretion, normal range <50 μmol/24 hour; ENETS, European Neuroendocrine Tumor Society; SSA, somatostatin analogue.
Statistics
SPSS software (version 21 for Windows, SPSS Inc.) was used for statistical analysis. Data were presented as median and interquartile range (IQR; 25th-75th percentiles) or count with percentage. Continuous data were compared by using a Mann-Whitney U test or Wilcoxon signed-rank test for paired data. For correlation analysis, the Spearman correlation coefficient was calculated. A Chi-square test was performed for comparison of categorical data. Odds ratios (OR) for development of mesenteric metastases and fibrosis were determined by logistic regression and shown with 95% CIs. Significant predictors were further analyzed in multinomial logistic regression with interaction terms. To aid interpretation of the interaction term, we divided our patient cohort in 5 equally large age categories, with group 1, <50 years; group 2, 50-57 years; group 3, 57-63 years; group 4, 63-70 years, and group 5 >70 years. A P-value of < 0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 559 SI-NET patients were included, of which 47% female. As shown in Table 4, there were no statistically significant differences between male and female patients in tumor grade, serum chromogranin A (CgA) level, or ENETS disease stage. At baseline, the majority of included patients had a SI-NET grade 1 (48%) and metastatic disease (ENETS disease stage IV, 76%). There was no difference in the percentage of patients with hepatic metastases (72% in male vs. 70% in female patients). Men more frequently had mesenteric metastases (71%) and fibrosis (46%) compared to women (58% and 37%, respectively). In case of mesenteric metastases, the dominant mesenteric metastases was larger in men (median diameter of 30 mm; IQR 24-40 mm) than in women (median diameter of 27 mm, IQR 20-36 mm). Finally, men had a higher baseline 24-hour urinary 5-HIAA excretion (median 142 μmol/24 hours vs median 97 μmol/24 hours in female patients, P = 0.001) and more often had a 24-hour urinary 5-HIAA excretion above the upper limit of normal (78% vs 71% in female patients, P = 0.037).
| Characteristic . | Men (n = 296) . | Women (n = 263) . | P-value . |
|---|---|---|---|
| Median age, years | 61 (52-68) | 60 (52-69) | 0.93 |
| Age groups, years | 0.41 | ||
| <50 | 57 (50) | 56 (50) | |
| 50-57 | 59 (52) | 54 (48) | |
| 57-63 | 66 (59) | 46 (41) | |
| 63-70 | 61 (56) | 48 (44) | |
| >70 | 53 (47) | 60 (53) | |
| Tumor grade | 0.69 | ||
| Grade 1 | 137 (46) | 134 (51) | |
| Grade II | 79 (27) | 66 (25) | |
| Grade III | 7 (2) | 8 (3) | |
| Unknown | 73 (25) | 55 (21) | |
| ENETS disease stage | 0.29 | ||
| Stage I/II | 6 (2) | 13 (5) | |
| Stage III | 60 (20) | 53 (20) | |
| Stage IV | 229 (78) | 198 (75) | |
| Hepatic metastases | 216 (72) | 185 (70) | 0.525 |
| Mesenteric metastases | 212 (71) | 153 (58) | 0.001 |
| Size of largest mesenteric metastasis, mm | 30 (24-40) | 27 (20-36) | 0.005 |
| Mesenteric fibrosis | 135 (46) | 97 (37) | 0.027 |
| Median CgA, μg/L | 215 (94-763) | 206 (79-825) | 0.516 |
| Median 5-HIAA, μmol/24 hours | 142 (53-549) | 97 (36-373) | 0.001 |
| Characteristic . | Men (n = 296) . | Women (n = 263) . | P-value . |
|---|---|---|---|
| Median age, years | 61 (52-68) | 60 (52-69) | 0.93 |
| Age groups, years | 0.41 | ||
| <50 | 57 (50) | 56 (50) | |
| 50-57 | 59 (52) | 54 (48) | |
| 57-63 | 66 (59) | 46 (41) | |
| 63-70 | 61 (56) | 48 (44) | |
| >70 | 53 (47) | 60 (53) | |
| Tumor grade | 0.69 | ||
| Grade 1 | 137 (46) | 134 (51) | |
| Grade II | 79 (27) | 66 (25) | |
| Grade III | 7 (2) | 8 (3) | |
| Unknown | 73 (25) | 55 (21) | |
| ENETS disease stage | 0.29 | ||
| Stage I/II | 6 (2) | 13 (5) | |
| Stage III | 60 (20) | 53 (20) | |
| Stage IV | 229 (78) | 198 (75) | |
| Hepatic metastases | 216 (72) | 185 (70) | 0.525 |
| Mesenteric metastases | 212 (71) | 153 (58) | 0.001 |
| Size of largest mesenteric metastasis, mm | 30 (24-40) | 27 (20-36) | 0.005 |
| Mesenteric fibrosis | 135 (46) | 97 (37) | 0.027 |
| Median CgA, μg/L | 215 (94-763) | 206 (79-825) | 0.516 |
| Median 5-HIAA, μmol/24 hours | 142 (53-549) | 97 (36-373) | 0.001 |
Data are presented as median (interquartile range) or n (%).
Abbreviations: 5-HIAA: urinary 5-HIAA excretion, normal range <50 μmol/24 hours; CgA, serum chromogranin A, normal range <94 μg/L; ENETS, European Neuroendocrine Tumor Society.
| Characteristic . | Men (n = 296) . | Women (n = 263) . | P-value . |
|---|---|---|---|
| Median age, years | 61 (52-68) | 60 (52-69) | 0.93 |
| Age groups, years | 0.41 | ||
| <50 | 57 (50) | 56 (50) | |
| 50-57 | 59 (52) | 54 (48) | |
| 57-63 | 66 (59) | 46 (41) | |
| 63-70 | 61 (56) | 48 (44) | |
| >70 | 53 (47) | 60 (53) | |
| Tumor grade | 0.69 | ||
| Grade 1 | 137 (46) | 134 (51) | |
| Grade II | 79 (27) | 66 (25) | |
| Grade III | 7 (2) | 8 (3) | |
| Unknown | 73 (25) | 55 (21) | |
| ENETS disease stage | 0.29 | ||
| Stage I/II | 6 (2) | 13 (5) | |
| Stage III | 60 (20) | 53 (20) | |
| Stage IV | 229 (78) | 198 (75) | |
| Hepatic metastases | 216 (72) | 185 (70) | 0.525 |
| Mesenteric metastases | 212 (71) | 153 (58) | 0.001 |
| Size of largest mesenteric metastasis, mm | 30 (24-40) | 27 (20-36) | 0.005 |
| Mesenteric fibrosis | 135 (46) | 97 (37) | 0.027 |
| Median CgA, μg/L | 215 (94-763) | 206 (79-825) | 0.516 |
| Median 5-HIAA, μmol/24 hours | 142 (53-549) | 97 (36-373) | 0.001 |
| Characteristic . | Men (n = 296) . | Women (n = 263) . | P-value . |
|---|---|---|---|
| Median age, years | 61 (52-68) | 60 (52-69) | 0.93 |
| Age groups, years | 0.41 | ||
| <50 | 57 (50) | 56 (50) | |
| 50-57 | 59 (52) | 54 (48) | |
| 57-63 | 66 (59) | 46 (41) | |
| 63-70 | 61 (56) | 48 (44) | |
| >70 | 53 (47) | 60 (53) | |
| Tumor grade | 0.69 | ||
| Grade 1 | 137 (46) | 134 (51) | |
| Grade II | 79 (27) | 66 (25) | |
| Grade III | 7 (2) | 8 (3) | |
| Unknown | 73 (25) | 55 (21) | |
| ENETS disease stage | 0.29 | ||
| Stage I/II | 6 (2) | 13 (5) | |
| Stage III | 60 (20) | 53 (20) | |
| Stage IV | 229 (78) | 198 (75) | |
| Hepatic metastases | 216 (72) | 185 (70) | 0.525 |
| Mesenteric metastases | 212 (71) | 153 (58) | 0.001 |
| Size of largest mesenteric metastasis, mm | 30 (24-40) | 27 (20-36) | 0.005 |
| Mesenteric fibrosis | 135 (46) | 97 (37) | 0.027 |
| Median CgA, μg/L | 215 (94-763) | 206 (79-825) | 0.516 |
| Median 5-HIAA, μmol/24 hours | 142 (53-549) | 97 (36-373) | 0.001 |
Data are presented as median (interquartile range) or n (%).
Abbreviations: 5-HIAA: urinary 5-HIAA excretion, normal range <50 μmol/24 hours; CgA, serum chromogranin A, normal range <94 μg/L; ENETS, European Neuroendocrine Tumor Society.
Sex Dimorphism in Mesenteric Metastasis and Fibrosis
Male patients had an increased risk for mesenteric metastasis [OR 1.83 (95% CI 1.29-2.59)] and mesenteric fibrosis [OR 1.47 (95% CI 1.05-2.06)]. Next, we analyzed whether the sex difference in prevalence of mesenteric metastases and fibrosis was influenced by other clinical characteristics. We performed univariate analysis for age, tumor grade, CgA serum level, and 5-HIAA urinary excretion. Significant predictors for the presence of mesenteric metastases were age [OR 1.04 (95% CI 1.03-1.06)] and urinary 5-HIAA excretion [OR per 100 μmol/L 1.06 (95% CI 1.02-1.10)]. For the presence of mesenteric fibrosis, significant predictors were also age [OR 1.03 (95% CI 1.01-1.05)] and urinary 5-HIAA excretion [OR per 100 μmol/L 1.04 (95% CI 1.01-1.07)]. Tumor grade and CgA serum level were not significant predictors for mesenteric metastases or fibrosis.
When age, sex, and 5-HIAA were added to a multinomial regression model, only the interaction term for female sex and age remained a significant predictor for mesenteric metastases [OR 1.04 (95% CI 1.02-1.07) and fibrosis [OR 1.04 (95% CI 1.02-1.07)]. To aid interpretation of the interaction term, we divided our patient cohort in 5 equally large age groups and used these age groups to show the prevalence of mesenteric metastasis and fibrosis (Fig. 1). In men, there is no significant difference between the age groups in prevalence of mesenteric metastases (P = 0.80) or fibrosis (P = 0.428). In contrast, in women, there is a significant difference between the age groups in the prevalence of mesenteric metastases (P = 0.009) and fibrosis (P = 0.035) with an increase of both in older patients.
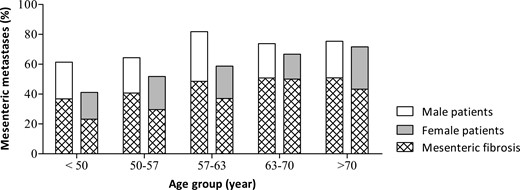
Sexual dimorphism in prevalence of mesenteric metastasis in small-intestinal neuroendocrine tumors. The patient cohort (n = 559) was divided into 5 equal age groups: <50 years, 50-57 years, 57-63 years, 63-70 years, and >70 years.
Next, we focused on mesenteric fibrosis in patients with mesenteric metastases. The percentage of patients with mesenteric metastases that develop mesenteric fibrosis was not influenced by age or sex. Mesenteric fibrosis was present in 61.3% (n = 130) of men with mesenteric metastases and this was equal to the percentage of mesenteric fibrosis (60.1%, n = 92, P = 0.82) in women with mesenteric metastases. When assessed across the 5 age groups, there was no significant difference in the percentage of mesenteric fibrosis in patients with mesenteric metastases neither in men (P = 0.874) or women (P = 0.539), as can be appreciated in Figure 1.
Expression of Sex Steroid Hormone Receptors in SI-NETs
As there was a sex difference in metastatic pattern of SI-NETs, we analyzed gene expression of four sex steroid hormone receptors AR, ESR1, ESR2, and PGR in 24 primary SI-NETs and in adjacent normal intestine (Fig. 2). There was an equal distribution of male (n = 12) and female patients (n = 12). Tumors were classified as grade 1 (71%, n = 17) or grade 2 (29%, n = 7). All 4 receptors (AR, ESR1, ESR2, and PGR) were expressed in primary SI-NET and normal intestine. Expression levels did not differ between men and women or between tumor grade 1 and grade 2. ESR1 and AR showed a significantly increased mRNA expression in primary SI-NETs compared to adjacent normal intestinal tissue.
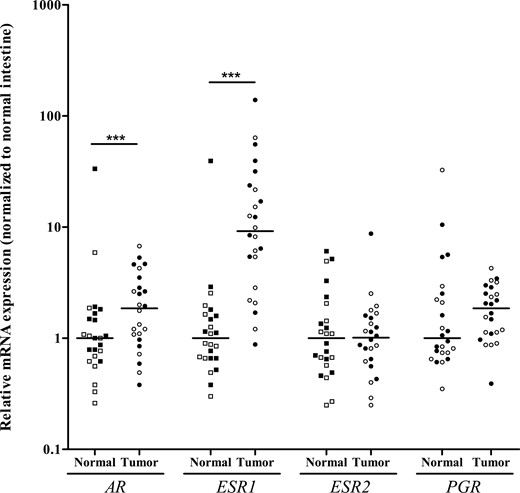
AR, ESR1, ESR2, and PGR messenger RNA expression in 24 primary small-intestinal neuroendocrine tumors (SI-NETs; tumor) and adjacent normal intestinal tissue (normal). Scatter plot with individual data points shown with median (line). Clear symbols represent male patients; black symbols female patients. ***P < 0.001, primary SI-NET vs normal intestinal tissue.
As urinary 5-HIAA excretion was significantly different between male and female patients (Table 4), we analyzed whether there was a correlation with ESR1 and AR expression and found no correlation between urinary 5-HIAA excretion and expression of ESR1 (rho = 0.179, P = 0.44), nor AR (rho = 0.305, P = 0.18). Finally, we assessed the ratio of ESR1 and AR expression and found no differences between men and women or between tumor grade or degree of fibrosis. There was also no correlation between ESR1/AR ratio and urinary 5-HIAA excretion (rho = −0.003, P = 0.99).
Immunohistochemistry
As ESR1 and AR showed significantly increased mRNA expression in primary SI-NETs, ERα and AR expression was further analyzed by IHC. IHC was performed on 12 primary tumors and paired mesenteric metastases. All primary tumors were classified as tumor grade 1, and there was an equal distribution of male (n = 6) and female (n = 6) patients. The results are shown in Figure 3. ERα expression was found both in tumor and stromal cells. Especially in tumor cells, there was a large variability in ERα expression level as can be appreciated in Figure 3. There was a strong correlation between ERα expression in tumor cells of primary tumors and tumor cells of the paired mesenteric metastases (rho = 0.769, P = 0.006). In contrast, there was no correlation between ERα expression in the stromal compartment of primary tumors and of the paired mesenteric metastases (rho = −0.198, P = 0.56). Generally, ERα expression tended to be higher in tumor cells than in stromal cells. In primary tumors, ERα expression was significantly higher in tumor cells compared to stromal cells (Fig. 3A, P = 0.03). The same trend could be observed in the mesenteric metastases (P = 0.12). There were no sex differences in ERα expression in primary tumors (tumor cells: P = 0.82, stromal cells: P = 0.33), nor in mesenteric metastases (tumor cells: P = 0.79, stromal cells: P = 0.43).
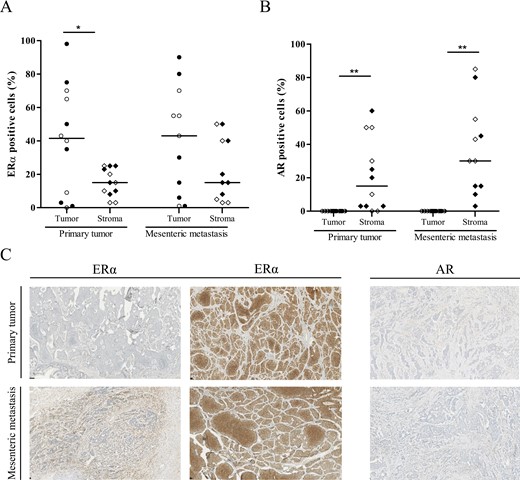
Immunohistochemical analysis of estrogen receptor alpha (ERα) and androgen receptor (AR) expression in primary tumors and paired mesenteric metastases. Percentage of ERα (A) and AR (B) positive tumor and stromal cells is shown by scatter plot of individual data points with median (line). Clear symbols represent male patients; black symbols represent female patients. *P < 0.05, **P < 0.01, tumor vs stromal cells. (C) Photomicrographs of representative tissue slides of immunohistochemical staining for ERα and AR. Upper row shows primary tumors; lower row shows mesenteric metastases. The columns show, respectively, high ERα expression in stromal cells, high ERα expression in tumor cells, and AR expression in stromal cells.
In contrast to ERα, AR expression was only found in stromal cells (Fig. 3B). Tumor cells showed no AR positivity. To understand the significant higher mRNA expression in primary SI-NETs compared to healthy intestine (Fig. 2), we also assessed the healthy intestine and found no AR expression in glandular cells and sparse AR positivity in stromal cells. The level of stromal AR expression in primary tumors did not correlate with stromal AR expression of paired mesenteric metastases (rho = −0.187, P = 0.58). Seven primary tumors and 10 mesenteric metastases had ≥10% AR-positive stromal cells. However, there was no significant difference between stromal AR expression in primary tumors and paired mesenteric metastases (P = 0.18). Also, there was no sex difference in AR expression in primary tumors (P = 0.74) or in mesenteric metastases (P = 0.24).
Discussion
It has been demonstrated that SI-NETs have a modest but significantly higher prevalence and worse prognosis in male patients (4,5). In this study, we aimed to examine this sexual dimorphism in greater detail. In our cohort of SI-NET patients, we also found a slight predominance of men. However, there were no sex differences in tumor grade and disease stage, the 2 most established prognostic factors for SI-NETs (14). There was a sexual dimorphism in the metastatic pattern. SI-NETs predominantly metastasize to the liver and mesentery, and while there was no sex difference in percentage of hepatic metastases, men significantly more often had mesenteric metastases. As mesenteric metastases are associated with a worse prognosis, even independently from the presence of mesenteric fibrosis, this could contribute to the worse prognosis in male SI-NET patients (18). Interestingly, the protective effect of female sex was most pronounced in women younger than 50 years and dissipated with increasing age. As this is in line with sex hormone changes during the lifetime of women, it might suggest a mesentery-specific effect of sex hormones, particularly estradiol.
To gain insight in the possible underlying mechanism of the sexual dimorphism in mesenteric metastatic risk, we assessed sex steroid hormone receptor expression. In accordance with previous studies, we found that SI-NETs have a highly variable ERα expression in tumor cells that strongly correlates between primary tumor and metastases (12,13). The role of ERα signaling on tumor growth and fibrogenesis in SI-NETs is scarcely investigated. However, earlier studies showing clinical effect of tamoxifen on tumor growth and hormonal secretion symptoms suggest involvement of ERα signaling in these processes. Moreover, the highly variable expression of ERα in SI-NETs could explain the inconsistent results of tamoxifen treatment in SI-NET (6-9,11).
However, to understand the sexual dimorphism in mesenteric metastatic potential, we also need to look at the tumor microenvironment. The tumor microenviroment is essential for supporting tumor growth and metastasis and can contribute to treatment efficiency or resistance (19). We found ERα and AR protein expression in stromal cells of SI-NETs. The effect of ERα and AR on the tumor microenvironment and risk of developing metastasis is most studied in cancers of the reproductive system such as breast and prostate cancer. In general, ERα signaling attenuates metastatic potential by reducing epithelial-to-mesenchymal transition. This is effected by inhibition of regulators such as transforming growth factor beta, which is also an important proliferative and profibrotic growth factor in SI-NETs (20,21). On the other hand, AR signaling is known to stimulate metastatic potential by inducting epithelial-to-mesenchymal transition and stimulating angiogenesis (22-24). It might be hypothesized that the ratio of ERα and AR activation, determined by exposure to estrogens and androgens, may in part explain sex differences in mesenteric metastatic potential.
Interestingly, in stromal cells of SI-NETs, the expression levels of both ERα and AR not correlated between primary tumors and paired mesenteric metastases. There was a trend to increased expression of both receptors in the stromal cells of mesenteric metastases. This may suggest that the mesentery is more sensitive to differences in sex hormone levels than other organs, making the protective effects of ERα signaling on SI-NET metastasis most noticeable in the mesentery. In previous research, we showed that men have a higher risk of mesenteric metastases growth compared to women (3). The gradual increase in prevalence of mesenteric metastases over the years in women, instead of a sharp increase after menopause shown in this study, may be explained by the very slow growth rate of mesenteric metastases (3).
The sexual dimorphism in metastatic pattern and the reduced rate of mesenteric metastases in premenopausal women with SI-NETs are important findings as it could help understand sex-specific risk and guide personalized treatment management. However, this study has several limitations. The study is based on data from a single tertiary center. Therefore, validation in other cohorts is needed. Further, the number of tumor samples used for IHC analysis was limited, affecting the observation of possible significant differences. Therefore, further research is necessary to determine the effects of estrogen and androgen signaling in tumorigenic processes and the potential of hormonal treatments such as tamoxifen in SI-NETs.
Conclusion
In this study we examined sex differences between SI-NET patients and found a pronounced difference in mesenteric disease. Women have a lower risk of mesenteric metastases, and this difference is most pronounced in premenopausal women. There was no sex difference in the prevalence of hepatic metastases or in the overall percentage of metastasized disease. Nor was there a sex difference in prevalence of mesenteric fibrosis in patients with mesenteric metastases. We showed that SI-NETs have increased ESR1 and AR gene expression compared to healthy intestinal tissue. SI-NET tumor cells only had ERα protein expression, while in stromal cells both ERα and AR protein expression was found. The expression level of ERα and AR in stroma of mesenteric metastases tended to be higher and did not correlate to expression in the primary tumor. This suggests that sex hormone signaling pathways might be new and important players involved in modulating metastatic processes in SI-NETs.
Funding
This work was supported by Ipsen Fund via an unrestricted research fund.
Disclosures
A.B., A.I., M.V., L.O., L.H., and R.F. have nothing to declare. J.H. has received speaker and travel fees from Ipsen and Novartis and has joined the advisory board for Novartis. W.H. received research support from Ipsen and AAA/Novartis and received speaker fees from AAA/Novartis, Pfizer, and Ipsen.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.



