-
PDF
- Split View
-
Views
-
Cite
Cite
Lars Sävendahl, Tadej Battelino, Michael Højby Rasmussen, Meryl Brod, Paul Saenger, Reiko Horikawa, Effective GH Replacement With Once-weekly Somapacitan vs Daily GH in Children with GHD: 3-year Results From REAL 3, The Journal of Clinical Endocrinology & Metabolism, Volume 107, Issue 5, May 2022, Pages 1357–1367, https://doi.org/10.1210/clinem/dgab928
Close - Share Icon Share
Abstract
Current GH therapy requires daily injections, which can be burdensome. Somapacitan is a long-acting GH derivative in development for treatment of GH deficiency (GHD).
Evaluate the efficacy, safety, and tolerability of once-weekly somapacitan after 3 years of treatment.
A multicenter, randomized, controlled, phase 2 study comparing somapacitan and once-daily GH for 156 weeks (NCT02616562).
Twenty-nine sites in 11 countries.
Fifty-nine children with GHD randomized (1:1:1:1) and exposed to treatment. Fifty-three children completed the 3-year period.
Patients received somapacitan (0.04 [n = 14], 0.08 [n = 15], or 0.16 [n = 14] mg/kg/wk) or daily GH (n = 14) (0.034 mg/kg/d, equivalent to 0.238 mg/kg/wk) subcutaneously during the first year, after which all patients on somapacitan received 0.16 mg/kg/wk.
Height velocity (HV) at year 3; changes from baseline in height SD score (HSDS), HVSDS, and IGF-I SDS.
The estimated treatment difference (95% CI) in HV for somapacitan 0.16/0.16 mg/kg/wk vs daily GH at year 3 was 0.8 cm/y (−0.4 to 2.1). Change in HVSDS from baseline to year 3 was comparable between somapacitan 0.16/0.16 mg/kg/wk, the pooled somapacitan groups, and daily GH. A gradual increase in HSDS from baseline was observed for all groups. At year 3, mean HSDS was similar for the pooled somapacitan groups and daily GH. Change from baseline to year 3 in mean IGF-I SDS was similar across treatments.
Once-weekly somapacitan in children with GHD showed sustained efficacy over 3 years in all assessed height-based outcomes with similar safety and tolerability to daily GH. A plain language summary (1) is available for this study.
This study has been registered at ClinicalTrials.gov, number NCT02616562 (REAL 3).
GH deficiency (GHD) in children is characterized by diminished growth velocity and adult height below the normal range (2). The condition can negatively impact children’s quality of life, affect social and emotional well-being, and lead to diminished functionality in adulthood (3,4).
GH replacement therapy can restore normal growth in most cases, allowing patients to attain an adult height within the normal range (2). Currently approved GH therapy options have a short in vivo half-life, necessitating daily subcutaneous injections (5). Such a regimen can be burdensome for patients and caregivers, reducing treatment adherence (6, 7) and leading to suboptimal clinical outcomes (8, 9). Indeed, as many as 25% of children may miss more than 2 injections per week (8-12).
To relieve the burden of once-daily injections (13-15), somapacitan, a once-weekly treatment for GHD in children is in clinical development (16), having been approved for the treatment of adults in Europe, the United States, and Japan (17-19). Other long-acting GH compounds are in clinical development or have been recently approved (20, 21).
Somapacitan is a reversible albumin-binding GH derivative in which a 1.2-kDa side chain with noncovalent albumin-binding properties is conjugated via alkylation to GH with a single amino acid substitution resulting in a 23-kDa molecule (22). Addition of a fatty acid linker to facilitate somapacitan binding to albumin and prolonging its half-life (23) has been successfully used to prolong half-life in other commercially available products within the endocrinology field (24-26). In animal models, somapacitan has been shown to directly stimulate longitudinal growth via activation of GH receptors on the peripheral tibia growth plate (23). In previous phase 3 clinical trials, somapacitan was shown to have similar efficacy to daily GH for the treatment of adult GHD, and a safety and tolerability profile in line with established evidence for daily GH (27-29).
REAL 3 (ClinicalTrials.gov identifier: NCT02616562) is a phase 2 trial investigating the efficacy, safety, and tolerability of once-weekly somapacitan compared with daily administration of daily GH (Norditropin) in prepubertal children with GHD. We have previously reported the 26-week and 1-year data; the latter showed that height velocity (HV) (estimated treatment difference [95% CI]: 1.8 cm/y [0.5-3.1]) and height SD scores (SDS) (0.35 [0.05-0.65]) were statistically significantly greater with somapacitan 0.16 mg/kg/wk than with daily GH (30). In this report, we present novel efficacy and safety results after 3 years of once-weekly somapacitan treatment in comparison to once-daily GH.
Materials and Methods
Study Design
REAL 3 is a randomized, multinational, active-controlled, double-blinded, open-label (compared with daily GH), dose-finding, 4-arm parallel group trial in GH treatment-naïve, prepubertal children with GHD. The safety and efficacy of 3 different doses of once-weekly somapacitan treatment (0.04 mg/kg, 0.08 mg/kg, or 0.16 mg/kg/wk) was investigated in comparison to an active, open-labelled control arm of daily GH (0.034 mg/kg/d, equivalent to 0.238 mg/kg/wk). The main trial period of 26 weeks was followed by a 26-week extension (up to year 1). This was further followed by a 104-week (up to year 3) safety extension, and subsequently by an ongoing 208-week safety extension trial period to evaluate the long-term safety of somapacitan 0.16 mg/kg/wk (30). In this report, we present data from the end of the 104-week (up to year 3) extension period. The trial was double-blind regarding the initial somapacitan dose; the clinical height assessments were conducted by assessors blinded to somapacitan dosing. After the double-blind of the main trial period, the sponsor was unblinded, whereas the subjects and site staff remained blinded to somapacitan dose level allocation until end of the extension trial period (week 52).
The protocol was approved by the local and national ethics committees, as appropriate, and conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. Informed consent was obtained in writing from the parents (and/or the child’s legally acceptable representative), and child assent was obtained as age appropriate before the first study procedure.
Patients
Trial eligibility, key inclusion, and exclusion criteria have been previously reported (30). Briefly, eligible patients were prepubertal children with a confirmed diagnosis of GHD within 12 months before screening, as determined by 2 different GH stimulation tests (defined as a peak GH level of ≤7.0 ng/mL with no prior exposure to GH therapy and/or IGF- I treatment). For children with 3 or more pituitary hormone deficiencies, only 1 GH stimulation test was required. A total of 60 patients were planned for enrollment.
Treatment and Randomization
Patients were randomized (1:1:1:1 ratio) to receive either once-weekly somapacitan treatment (0.04, 0.08, or 0.16 mg/kg/wk) or daily GH (0.034 mg/kg/d, equivalent to 0.238 mg/kg/wk) subcutaneously during the 26-week main trial period and the 26-week extension trial period. Body weight (measured at all visits in the treatment period) was used for calculation of the GH dose. The children were randomly assigned by investigators at the trial sites using a trial-specific, web-based, interactive response system. The randomization was stratified by region (Japan and rest of the world), age (<6 and ≥6 years), and by sex within the rest-of-the-world region.
For the 104-week safety extension, all participants who received somapacitan during the first year of the trial either continued with somapacitan 0.16 mg/kg/wk or switched to somapacitan 0.16 mg/kg/wk. Patients receiving daily GH during the first year remained on the same treatment and dose of daily GH during the 104-week safety extension. Both trial products were administered as subcutaneous injections.
Doses of somapacitan were provided as 5 mg/1.5 mL, 10 mg/1.5 mL, and 15 mg/1.5 mL all prefilled pen injectors of the FlexPro group developed by Novo Nordisk A/S. Daily GH was provided using Norditropin FlexPro 10 mg/1.5 mL.
Outcomes
Patients were seen every 13 weeks for efficacy measurements, adverse event (AE) monitoring, and safety laboratory measurements.
Efficacy
The primary objective of the trial is to evaluate the efficacy of 3 different doses of once-weekly somapacitan treatment compared with daily GH in GH treatment-naïve prepubertal children with GHD after 26 weeks of treatment (30). The following secondary outcomes collected during the extension period are the focus of this report: investigation of efficacy included HV (cm/y) at year 3 and changes from baseline to end of year 3 for: height SDS, HVSDS, IGF-I SDS, and insulin-like binding protein-3 (IGFBP-3) SDS. For standing height measurements, European Medicines Agency guidelines were followed (31).
Safety
Safety endpoints included incidence of AEs (including injection site reactions at year 3), occurrence of anti-somapacitan and anti-GH antibodies at year 3, and bone age (X-ray of left hand and wrist), centrally assessed according to Greulich and Pyle (32) progression compared with chronological age at year 3. Anti-somapacitan and anti-GH antibodies were assessed using a validated antibody binding assay. Blood samples for antibodies and IGF-I and IGFBP-3 biomarkers were taken before trial drug administration, if planned on a sampling day.
Pharmacokinetics
IGF-I and IGFBP-3 analyses were performed by a central laboratory using commercially available assay kits (Immunodiagnostic Systems Immunoassay) (33). Trough samples were collected on day 7 after dosing, and peak samples were collected on days 1 through 4 after dosing. The samples were collected to provide information on peak-to-trough fluctuations and to derive the average using population pharmacokinetic/pharmacodynamics modeling. IGF-I SDS was calculated according to the modified least mean squares model, as published by Friedrich et al (33).
Observer-reported Outcomes
The Growth Hormone Deficiency–Child Impact Measure (GHD-CIM) was developed in line with US Food and Drug Administration guidance (34) to assess the impact of GHD on physical functioning and social and emotional well-being of children with GHD. The GHD-CIM observer report (GHD-CIM ObsRO) was developed for parents and guardians of children aged 4 to <13 years (35). Change from baseline in scores on the GHD-CIM ObsRO (35), an observer-reported outcome questionnaire, were used to investigate the impact of somapacitan relative to daily GH on emotional well-being, physical functioning, and social well-being on children as observed by their parents or legally acceptable representative. The minimally important difference provides a measure of the ObsRO that observers perceive as important (36). For GHD-CIM ObsRO, minimally important difference was quantified at 5 points for the overall score, physical functioning, and social well-being, and 7 points for emotional well-being (35).
Adherence
Adherence to treatment was assessed by recording doses (date and time of each dose as well as any missed doses). Doses counted in adherence comprised all doses above 0 recorded in the diary, administered between 3 am of the current day and 3 am of the following day (daily GH) or within 2 days before or after planned date of dosing (somapacitan).
Statistical Analysis
The full analysis set (FAS) was used in the analyses of the efficacy, pharmacokinetic, and health-related quality of life (HRQoL) endpoints. The FAS was to contain all randomly assigned children who received at least 1 dose of randomized treatment. Only in exceptional cases were children excluded from the FAS. The safety analysis set (SAS) was used for analyses of the safety endpoints and included all randomly assigned children who received at least 1 dose of randomized treatment.
Efficacy and Observer-reported Outcomes
Before year 1, HV was calculated for the main trial period (weeks 0-26) and the extension trial period (weeks 26-year 1) as HV = (height at visit – height at baseline)/(time from baseline to visit in years). After year 1, HV was calculated as for the first year of the safety extension trial period (year 1-2) as HV = (height at visit – height at year 1)/(time from year 1 to visit in years), and for the second year of the safety extension trial period (year 2-3) as HV = (height at visit – height at year 2)/(time from year 2 to visit in years). HV at year 3 was calculated and analyzed using a mixed model for repeated measurements, with treatment, age group, sex, region, and sex by age group interaction term as factors and height at baseline as a covariate, all nested within the week as a factor. An unstructured covariance matrix was used to describe the variability for the repeated measurements for a child. From the mixed model for repeated measurements, the treatment differences between somapacitan and daily GH treatment arms were estimated with the corresponding 95% CIs. Changes from baseline to year 3 in height SDS, HVSDS, and IGF-I SDS were also calculated (33) and analyzed using descriptive statistics.
IGFBP-3 SDS and GHD-CIM ObsRO scores at year 3, as well as bone age progression compared with chronological age up to year 3, were analyzed using descriptive statistics. Height-based outcomes were analyzed by treatment arm, as well as for the pooled somapacitan groups (0.04/0.16 mg/kg/wk, somapacitan 0.08/0.16 mg/kg/wk, and somapacitan 0.16/0.16 mg/kg/wk combined). Post hoc-defined statistical analyses of the estimated treatment difference between somapacitan and daily GH in HV, and GHD-CIM ObsRO scores at year 3 were carried out.
Safety and Adherence
Adverse events were analyzed using descriptive statistics and summarized by treatment, Medical Dictionary for Regulatory Activities system organ class, and Medical Dictionary for Regulatory Activities preferred term. The descriptive statistics included the number and percentage of patients who experienced AEs, as well as the number and rate of events. Adverse events were listed by treatment (pooled somapacitan groups and daily GH) and by patient with information on severity, relationship to trial product, and demographics. The occurrence of anti-somapacitan and anti-GH antibodies, technical complaints and injection site reactions, and changes from baseline up to year 3 in physical signs and vital signs were analyzed using descriptive statistics. Differences in treatment adherence between the arms were not tested for statistical significance.
Results
Patient Disposition and Characteristics
A total of 59 children with GHD were randomized and exposed to treatment; this group comprised the SAS. Two children who were randomized in error and discontinued treatment early were excluded from the FAS (total of 57 children). The number of patients included in the FAS in the 0.04/0.16, 0.08/0.16, and 0.16/0.16 mg/kg/wk and daily GH groups was 14, 15, 14, and 14, respectively (Fig. 1). Eight patients discontinued treatment (2 from AEs, 2 withdrew from the study, and 4 discontinued as a result of protocol violations) before the end of year 3 (Fig. 1). In total, 53 (89.8%) children completed 3 years of the trial, of which 51 (86.4%) completed without premature discontinuation of treatment.
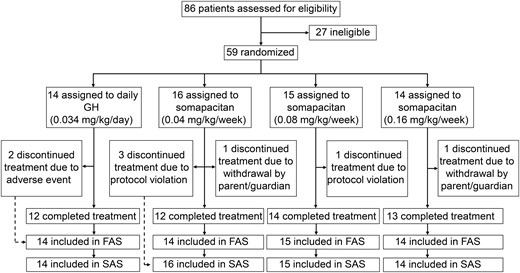
Patient disposition. All patients treated with somapacitan switched to 0.16 mg/kg/wk dose after year 1. The FAS included randomly assigned children who received at least 1 dose of treatment. The SAS included all randomly assigned children who received at least 1 dose of randomized treatment. FAS, full analysis set; GHD, GH deficiency; SAS, safety analysis set.
At baseline, patient characteristics were similar across the 4 treatment groups with no clinically relevant differences between groups (Table 1). Baseline levels of IGF-I SDS were below the normal reference range (between −2 and +2) for all treatment arms; with a mean (SD) of −2.35 (0.93) for the pooled somapacitan groups and −2.07 (0.74) for the daily GH group. Of the 57 children included in the FAS (59.6% male), median age (range) was 5.7 (2.7-9.7) years, and 93% (53/57) had idiopathic GHD.
| Characteristic . | Somapacitan (0.04 mg/kg/wk) . | Somapacitan (0.08 mg/kg/wk) . | Somapacitan (0.16 mg/kg/wk) . | Daily GH (0.034 mg/kg/d)a . |
|---|---|---|---|---|
| FAS, n | 14 | 15 | 14 | 14 |
| Male, % | 50.0 | 66.7 | 57.1 | 64.3 |
| Age, years | 5.8 (1.8) | 5.8 (1.8) | 6.1 (2.3) | 5.9 (2.0) |
| BMI, kg/m2 | 15.3 (1.1) | 14.6 (1.1) | 15.1 (1.2) | 15.6 (1.4) |
| Height SDS | −4.1 (1.9) | −3.5 (1.5) | −3.8 (2.0) | −3.4 (1.1) |
| HVSDS | −2.9 (1.9) | −1.8 (1.7) | −2.9 (1.8) | −3.1 (2.1) |
| GH peak, µg/L | 2.9 (2.2) | 3.6 (2.1) | 4.1 (2.4) | 4.0 (2.0) |
| IGF-I SDS | −2.5 (1.0) | −2.5 (0.8) | −2.0 (1.0) | −2.1 (0.7) |
| IGFBP-3 SDS | −2.3 (1.4) | −2.4 (1.2) | −1.6 (1.5) | −2.1 (1.4) |
| Bone age vs chronological age ratio | 0.47 (0.15) | 0.62 (0.18) | 0.60 (0.20) | 0.56 (0.15) |
| Characteristic . | Somapacitan (0.04 mg/kg/wk) . | Somapacitan (0.08 mg/kg/wk) . | Somapacitan (0.16 mg/kg/wk) . | Daily GH (0.034 mg/kg/d)a . |
|---|---|---|---|---|
| FAS, n | 14 | 15 | 14 | 14 |
| Male, % | 50.0 | 66.7 | 57.1 | 64.3 |
| Age, years | 5.8 (1.8) | 5.8 (1.8) | 6.1 (2.3) | 5.9 (2.0) |
| BMI, kg/m2 | 15.3 (1.1) | 14.6 (1.1) | 15.1 (1.2) | 15.6 (1.4) |
| Height SDS | −4.1 (1.9) | −3.5 (1.5) | −3.8 (2.0) | −3.4 (1.1) |
| HVSDS | −2.9 (1.9) | −1.8 (1.7) | −2.9 (1.8) | −3.1 (2.1) |
| GH peak, µg/L | 2.9 (2.2) | 3.6 (2.1) | 4.1 (2.4) | 4.0 (2.0) |
| IGF-I SDS | −2.5 (1.0) | −2.5 (0.8) | −2.0 (1.0) | −2.1 (0.7) |
| IGFBP-3 SDS | −2.3 (1.4) | −2.4 (1.2) | −1.6 (1.5) | −2.1 (1.4) |
| Bone age vs chronological age ratio | 0.47 (0.15) | 0.62 (0.18) | 0.60 (0.20) | 0.56 (0.15) |
Data are mean (SD) unless otherwise stated.
Abbreviations: BMI, body mass index; FAS, full analysis set (all children who were correctly randomized and received at least 1 dose of randomized treatment); HVSDS, height velocity SD score; IGFBP-3, IGF binding protein 3; SDS, SD score.
aEquivalent to 0.238 mg/kg/wk.
| Characteristic . | Somapacitan (0.04 mg/kg/wk) . | Somapacitan (0.08 mg/kg/wk) . | Somapacitan (0.16 mg/kg/wk) . | Daily GH (0.034 mg/kg/d)a . |
|---|---|---|---|---|
| FAS, n | 14 | 15 | 14 | 14 |
| Male, % | 50.0 | 66.7 | 57.1 | 64.3 |
| Age, years | 5.8 (1.8) | 5.8 (1.8) | 6.1 (2.3) | 5.9 (2.0) |
| BMI, kg/m2 | 15.3 (1.1) | 14.6 (1.1) | 15.1 (1.2) | 15.6 (1.4) |
| Height SDS | −4.1 (1.9) | −3.5 (1.5) | −3.8 (2.0) | −3.4 (1.1) |
| HVSDS | −2.9 (1.9) | −1.8 (1.7) | −2.9 (1.8) | −3.1 (2.1) |
| GH peak, µg/L | 2.9 (2.2) | 3.6 (2.1) | 4.1 (2.4) | 4.0 (2.0) |
| IGF-I SDS | −2.5 (1.0) | −2.5 (0.8) | −2.0 (1.0) | −2.1 (0.7) |
| IGFBP-3 SDS | −2.3 (1.4) | −2.4 (1.2) | −1.6 (1.5) | −2.1 (1.4) |
| Bone age vs chronological age ratio | 0.47 (0.15) | 0.62 (0.18) | 0.60 (0.20) | 0.56 (0.15) |
| Characteristic . | Somapacitan (0.04 mg/kg/wk) . | Somapacitan (0.08 mg/kg/wk) . | Somapacitan (0.16 mg/kg/wk) . | Daily GH (0.034 mg/kg/d)a . |
|---|---|---|---|---|
| FAS, n | 14 | 15 | 14 | 14 |
| Male, % | 50.0 | 66.7 | 57.1 | 64.3 |
| Age, years | 5.8 (1.8) | 5.8 (1.8) | 6.1 (2.3) | 5.9 (2.0) |
| BMI, kg/m2 | 15.3 (1.1) | 14.6 (1.1) | 15.1 (1.2) | 15.6 (1.4) |
| Height SDS | −4.1 (1.9) | −3.5 (1.5) | −3.8 (2.0) | −3.4 (1.1) |
| HVSDS | −2.9 (1.9) | −1.8 (1.7) | −2.9 (1.8) | −3.1 (2.1) |
| GH peak, µg/L | 2.9 (2.2) | 3.6 (2.1) | 4.1 (2.4) | 4.0 (2.0) |
| IGF-I SDS | −2.5 (1.0) | −2.5 (0.8) | −2.0 (1.0) | −2.1 (0.7) |
| IGFBP-3 SDS | −2.3 (1.4) | −2.4 (1.2) | −1.6 (1.5) | −2.1 (1.4) |
| Bone age vs chronological age ratio | 0.47 (0.15) | 0.62 (0.18) | 0.60 (0.20) | 0.56 (0.15) |
Data are mean (SD) unless otherwise stated.
Abbreviations: BMI, body mass index; FAS, full analysis set (all children who were correctly randomized and received at least 1 dose of randomized treatment); HVSDS, height velocity SD score; IGFBP-3, IGF binding protein 3; SDS, SD score.
aEquivalent to 0.238 mg/kg/wk.
Adherence
Most children were administered treatment as planned, with a mean adherence rate of 92.2% for somapacitan (somapacitan pooled) and 87.2% for daily GH (for the 57 children included in the FAS).
Efficacy Results
Height-based outcomes
Data on HV at year 1 were previously reported (30). Observed mean HV at years 1, 2, and 3 for all 4 groups, and the pooled somapacitan groups, are displayed in Fig. 2. At years 2 and 3, there were no statistically significant differences in HV between the 0.08/0.16 and 0.16/0.16 mg/kg/wk doses of somapacitan and daily GH treatment. In the post hoc analysis, the estimated treatment difference (95% CI) in HV for the 0.16/0.16 mg/kg/wk somapacitan group compared with daily GH at year 3 was 0.8 cm/y (−0.4 to 2.1). The baseline HVSDS value for the somapacitan 0.08/0.16 mg/kg/wk treatment arm was higher than the HVSDS values for the 3 other treatment arms (Table 1).
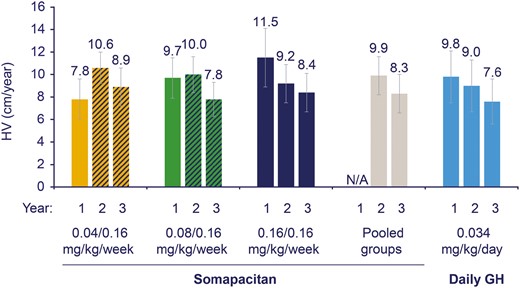
Height velocity (cm/year) by treatment group and by year. Data are observed mean (SD), FAS. All patients treated with somapacitan switched to 0.16 mg/kg/wk dose after year 1, as represented by the dashed bars at years 2 and 3 for the 0.04 and 0.08 mg/kg/wk somapacitan doses. Data for the pooled somapacitan groups are therefore not shown at year 1, indicated by N/A. FAS, full analysis set; HV, height velocity; N/A, not applicable.
A dose-related response in HVSDS was seen within the somapacitan dose groups up until year 1. By year 3, mean (SD) HVSDS was numerically higher for all somapacitan treatment arms compared with the daily GH group (Fig. 3). Change in HVSDS from baseline to year 3 was comparable between the 0.16/0.16 mg/kg/wk somapacitan group, the pooled somapacitan groups, and daily GH (Table 2).
| Characteristic . | Somapacitan (0.04/0.16 mg/kg/wk)a . | Somapacitan (0.08/0.16 mg/kg/wk)a . | Somapacitan (0.16/0.16 mg/kg/wk)a . | Somapacitan pooled . | Daily GH (0.034 mg/kg/d)b . |
|---|---|---|---|---|---|
| Change in height velocity SDS from baseline | |||||
| Year 1 | 4.7 (2.8) | 6.1 (3.4) | 8.6 (3.2) | N/A | 7.4 (4.1) |
| Year 2 | 8.0 (2.5) | 6.2 (2.9) | 6.4 (3.0) | 6.8 (2.9) | 6.6 (3.2) |
| Year 3 | 5.4 (2.5) | 4.2 (2.8) | 5.3 (3.0) | 4.9 (2.8) | 5.3 (3.9) |
| Change in height SDS from baseline | |||||
| Year 1 | 0.6 (0.5) | 1.0 (0.5) | 1.5 (0.9) | N/A | 1.0 (0.5) |
| Year 2 | 1.7 (0.8) | 1.9 (0.8) | 2.2 (1.2) | 1.9 (0.9) | 1.7 (0.7) |
| Year 3 | 2.4 (1.0) | 2.4 (1.0) | 2.7 (1.4) | 2.5 (1.1) | 2.1 (0.9) |
| Characteristic . | Somapacitan (0.04/0.16 mg/kg/wk)a . | Somapacitan (0.08/0.16 mg/kg/wk)a . | Somapacitan (0.16/0.16 mg/kg/wk)a . | Somapacitan pooled . | Daily GH (0.034 mg/kg/d)b . |
|---|---|---|---|---|---|
| Change in height velocity SDS from baseline | |||||
| Year 1 | 4.7 (2.8) | 6.1 (3.4) | 8.6 (3.2) | N/A | 7.4 (4.1) |
| Year 2 | 8.0 (2.5) | 6.2 (2.9) | 6.4 (3.0) | 6.8 (2.9) | 6.6 (3.2) |
| Year 3 | 5.4 (2.5) | 4.2 (2.8) | 5.3 (3.0) | 4.9 (2.8) | 5.3 (3.9) |
| Change in height SDS from baseline | |||||
| Year 1 | 0.6 (0.5) | 1.0 (0.5) | 1.5 (0.9) | N/A | 1.0 (0.5) |
| Year 2 | 1.7 (0.8) | 1.9 (0.8) | 2.2 (1.2) | 1.9 (0.9) | 1.7 (0.7) |
| Year 3 | 2.4 (1.0) | 2.4 (1.0) | 2.7 (1.4) | 2.5 (1.1) | 2.1 (0.9) |
Data are mean (SD), FAS.
Abbreviations: FAS, full analysis set; N/A, not applicable; SDS, SD score.
aPatients in the somapacitan groups received either 0.04, 0.08, or 0.16 mg/kg/wk for the first year before all switching to 0.16 mg/kg/wk for years 2 and 3. Data for the pooled somapacitan groups are therefore not shown at year 1, indicated by N/A.
bEquivalent to 0.238 mg/kg/wk.
| Characteristic . | Somapacitan (0.04/0.16 mg/kg/wk)a . | Somapacitan (0.08/0.16 mg/kg/wk)a . | Somapacitan (0.16/0.16 mg/kg/wk)a . | Somapacitan pooled . | Daily GH (0.034 mg/kg/d)b . |
|---|---|---|---|---|---|
| Change in height velocity SDS from baseline | |||||
| Year 1 | 4.7 (2.8) | 6.1 (3.4) | 8.6 (3.2) | N/A | 7.4 (4.1) |
| Year 2 | 8.0 (2.5) | 6.2 (2.9) | 6.4 (3.0) | 6.8 (2.9) | 6.6 (3.2) |
| Year 3 | 5.4 (2.5) | 4.2 (2.8) | 5.3 (3.0) | 4.9 (2.8) | 5.3 (3.9) |
| Change in height SDS from baseline | |||||
| Year 1 | 0.6 (0.5) | 1.0 (0.5) | 1.5 (0.9) | N/A | 1.0 (0.5) |
| Year 2 | 1.7 (0.8) | 1.9 (0.8) | 2.2 (1.2) | 1.9 (0.9) | 1.7 (0.7) |
| Year 3 | 2.4 (1.0) | 2.4 (1.0) | 2.7 (1.4) | 2.5 (1.1) | 2.1 (0.9) |
| Characteristic . | Somapacitan (0.04/0.16 mg/kg/wk)a . | Somapacitan (0.08/0.16 mg/kg/wk)a . | Somapacitan (0.16/0.16 mg/kg/wk)a . | Somapacitan pooled . | Daily GH (0.034 mg/kg/d)b . |
|---|---|---|---|---|---|
| Change in height velocity SDS from baseline | |||||
| Year 1 | 4.7 (2.8) | 6.1 (3.4) | 8.6 (3.2) | N/A | 7.4 (4.1) |
| Year 2 | 8.0 (2.5) | 6.2 (2.9) | 6.4 (3.0) | 6.8 (2.9) | 6.6 (3.2) |
| Year 3 | 5.4 (2.5) | 4.2 (2.8) | 5.3 (3.0) | 4.9 (2.8) | 5.3 (3.9) |
| Change in height SDS from baseline | |||||
| Year 1 | 0.6 (0.5) | 1.0 (0.5) | 1.5 (0.9) | N/A | 1.0 (0.5) |
| Year 2 | 1.7 (0.8) | 1.9 (0.8) | 2.2 (1.2) | 1.9 (0.9) | 1.7 (0.7) |
| Year 3 | 2.4 (1.0) | 2.4 (1.0) | 2.7 (1.4) | 2.5 (1.1) | 2.1 (0.9) |
Data are mean (SD), FAS.
Abbreviations: FAS, full analysis set; N/A, not applicable; SDS, SD score.
aPatients in the somapacitan groups received either 0.04, 0.08, or 0.16 mg/kg/wk for the first year before all switching to 0.16 mg/kg/wk for years 2 and 3. Data for the pooled somapacitan groups are therefore not shown at year 1, indicated by N/A.
bEquivalent to 0.238 mg/kg/wk.

Height velocity SDS by treatment group and by year. Data are mean (SD), FAS. All patients treated with somapacitan switched to 0.16 mg/kg/wk dose after year 1, as represented by the dashed bars at years 2 and 3 for the 0.04 and 0.08 mg/kg/wk somapacitan doses. Data for the pooled somapacitan groups are therefore not shown at year 1, indicated by N/A. FAS, full analysis set; HVSDS, height velocity SD score; N/A, not applicable; SDS, SD score.
Height SDS at years 1, 2, and 3 is shown in Fig. 4. At baseline, mean height SDS was low and similar for the 3 somapacitan dose groups and daily GH (Table 1). A gradual increase in height SDS from baseline to year 3 was observed for all somapacitan treatment arms and for daily GH. At year 3, the mean (SD) height SDS was similar for the pooled somapacitan groups and daily GH (Fig. 4). The mean (SD) change in height SDS from baseline to year 3 was similar across treatment arms (Table 2).
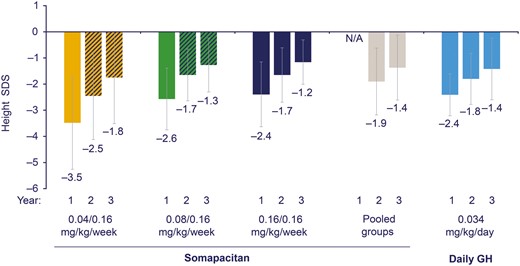
Height SDS by treatment group and by year. Data are mean (SD), FAS. All patients treated with somapacitan switched to 0.16 mg/kg/wk dose after year 1, as represented by the dashed bars at years 2 and 3 for the 0.04 and 0.08 mg/kg/wk somapacitan doses. Data for the pooled somapacitan groups are therefore not shown at year 1, indicated by N/A. FAS, full analysis set; N/A, not applicable; SDS, SD score.
IGF-I SD score
After 3 years of treatment, mean (SD) IGF-I SDS values for both the somapacitan and daily GH treatment arms had increased from baseline to within the normal range: 0.97 (1.13), 1.03 (1.32), 1.63 (0.89), and 1.22 (1.14) for somapacitan 0.04/0.16, 0.08/0.16, and 0.16/0.16 mg/kg/wk, and pooled groups, respectively, and 1.30 (0.94) for daily GH. The observed change from baseline to year 3 in mean (SD) IGF-I SDS was similar for all treatment arms: 3.26 (1.04), 3.52 (1.43), 3.66 (1.29), 3.49 (1.25), and 3.40 (1.58), respectively. The observed IGF-I SDS values at week 143 (trough) and week 156 (peak) and model-derived average IGF-I SDS values for all treatment arms are shown in Fig. 5. During the 3 years of the trial, IGF-I SDS values > 2 were occasionally recorded in 17 children (39.5%) treated with somapacitan (somapacitan 0.04/0.16 mg/kg/wk, n = 3 [21.4%]; somapacitan 0.08/0.16 mg/kg/wk, n = 6 [40%]; somapacitan 0.16/0.16 mg/kg/wk, n = 8 [57.1%]), and 4 children (28.6%) treated with daily GH.
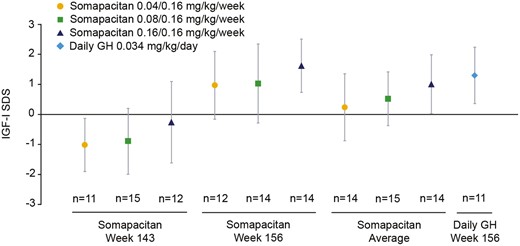
Mean IGF-I SDS: observed and derived values (FAS). Somapacitan trough values are an average from values obtained at week 143. Somapacitan peak values are an average from values obtained at week 156. Somapacitan average values were derived by population PK/PD modelling. Points and bars are means with SD. FAS, full analysis set; PK/PD, pharmacokinetic/pharmacodynamic; SDS, SD score.
IGFBP-3 SD score
IGFBP-3 SDS changes during the trial overall resembled the changes observed for IGF-I SDS. At year 3, mean (SD) IGFBP-3 SDS had increased from low baseline levels (below the normal range; Table 1) to levels within the normal range (somapacitan 0.04/0.16 mg/kg/wk: −0.39 [0.96]; somapacitan 0.08/0.16 mg/kg/wk: from −0.08 [0.71]; somapacitan 0.16/0.16 mg/kg/wk: 0.02 [0.90]; daily GH: 0.29 [0.79]. For the pooled somapacitan groups, IGFBP-3 SDS increased from −2.08 (1.35) to −0.14 (0.86).
Bone age
The bone age compared with chronological age ratio at baseline was below unity in all treatment arms (Table 1). During the 3 years of the trial, this ratio increased in all treatment arms, but remained < 1 (Fig. 6). The observed change from baseline to year 3 in mean (SD) bone age was 3.98 (1.51), 4.09 (1.42), 4.9 (1.78), and 4.34 (1.59) for somapacitan 0.04/0.16, 0.08/0.16, 0.16/0.16 mg/kg/wk, and pooled groups, respectively, and 3.06 (1.76) for daily GH.
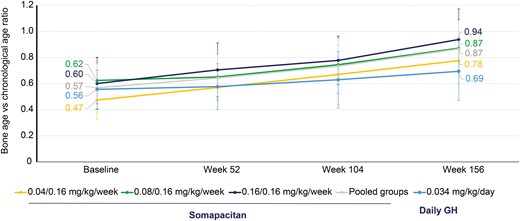
Bone age compared with chronological age per year. Data are mean (SD), FAS. FAS, full analysis set.
Observer-reported outcomes
Post hoc analysis of the estimated treatment difference in change from baseline to years 1 and 3 in GHD-CIM ObsRO scores between somapacitan and daily GH for the FAS are shown in Fig. 7. The estimated treatment difference for all 3 GHD-CIM ObsRO domains and the total score favored somapacitan treatment arms over daily GH after 3 years of treatment but were not statistically significant.
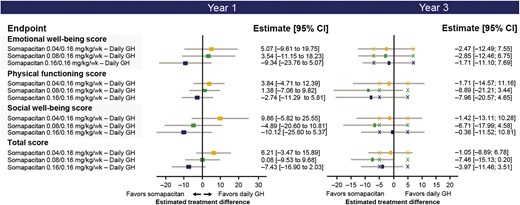
Observer-reported outcomes: estimated treatment differences in change from baseline to years 1 and 3 between somapacitan and daily GH in treatment impact (GHD-CIM ObsRO) domain scores and total score (FAS). X marks represent minimal important differences. FAS, full analysis set; GHD-CIM ObsRO, Growth Hormone Deficiency–Child Impact Measure observer report.
Safety Results
Somapacitan treatment was well tolerated throughout the 3 years of treatment, with no new clinically significant safety or local tolerability issues identified. Overall, AE rates per 100 patient-years during years 2 and 3 were similar between the treatment arms: pooled somapacitan groups, 237.7; daily GH, 224.9. The majority of AEs were classed as mild (89.5%) and deemed unlikely to be treatment related (92.5%) (Table 3). The most common AEs, nasopharyngitis and influenza, were reported in similar proportions of somapacitan-treated and daily GH-treated patients (Fig. 8). A total of 6 (10.2%) children had 11 serious AEs (SAEs) during the 3 years of treatment (3 children experienced SAEs during the first year of treatment) (30). The event rates for SAEs were similar for the pooled somapacitan groups (6.5 SAEs/100 patient-years of exposure) and daily GH (8.5 SAEs/100 patient-years of exposure) treatment arms (Table 4). Two SAEs (generalized edema and vomiting) in 1 child treated with somapacitan 0.16/0.16 mg/kg/wk were evaluated as probably related to trial product and were reported as unexpected serious adverse reactions. The child was admitted to hospital because of the edema and was treated for a suspected infection with IV fluids and antibiotics (ceftriaxone), following which the condition improved after 6 days, and the trial product was reintroduced without reoccurrence of the events. One SAE (hypopituitarism) was reported in 1 child treated with somapacitan 0.08/0.16 mg/kg/wk during the first year of the trial. The event was considered mild in severity and unlikely to be related to treatment. All other SAEs were also deemed unlikely to be treatment related.
| . | Pooled somapacitan groups . | . | . | . | Daily GH 0.034 mg/kg/da . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | N . | (%) . | E . | R . | N . | (%) . | E . | R . |
| Patients exposed | 45 | 14 | ||||||
| All events | 32 | 71.1 | 189 | 237.7 | 10 | 71.4 | 50 | 224.9 |
| Mild events | 30 | 66.7 | 170 | 213.8 | 9 | 64.3 | 44 | 197.9 |
| Moderate events | 11 | 24.4 | 19 | 23.9 | 2 | 14.3 | 5 | 22.5 |
| Severe events | 0 | - | - | - | 1 | 7.1 | 1 | 4.5 |
| Probably related | 4 | 8.9 | 7 | 8.8 | 1 | 7.1 | 1 | 4.5 |
| Possibly related | 4 | 8.9 | 5 | 6.3 | 2 | 14.3 | 5 | 22.5 |
| Unlikely related | 32 | 71.1 | 177 | 222.6 | 10 | 71.4 | 44 | 197.9 |
| . | Pooled somapacitan groups . | . | . | . | Daily GH 0.034 mg/kg/da . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | N . | (%) . | E . | R . | N . | (%) . | E . | R . |
| Patients exposed | 45 | 14 | ||||||
| All events | 32 | 71.1 | 189 | 237.7 | 10 | 71.4 | 50 | 224.9 |
| Mild events | 30 | 66.7 | 170 | 213.8 | 9 | 64.3 | 44 | 197.9 |
| Moderate events | 11 | 24.4 | 19 | 23.9 | 2 | 14.3 | 5 | 22.5 |
| Severe events | 0 | - | - | - | 1 | 7.1 | 1 | 4.5 |
| Probably related | 4 | 8.9 | 7 | 8.8 | 1 | 7.1 | 1 | 4.5 |
| Possibly related | 4 | 8.9 | 5 | 6.3 | 2 | 14.3 | 5 | 22.5 |
| Unlikely related | 32 | 71.1 | 177 | 222.6 | 10 | 71.4 | 44 | 197.9 |
Abbreviations: %, percentage of patients; E, number of events; R, event rate per 100 patient-years at risk.
aEquivalent to 0.238 mg/kg/wk.
| . | Pooled somapacitan groups . | . | . | . | Daily GH 0.034 mg/kg/da . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | N . | (%) . | E . | R . | N . | (%) . | E . | R . |
| Patients exposed | 45 | 14 | ||||||
| All events | 32 | 71.1 | 189 | 237.7 | 10 | 71.4 | 50 | 224.9 |
| Mild events | 30 | 66.7 | 170 | 213.8 | 9 | 64.3 | 44 | 197.9 |
| Moderate events | 11 | 24.4 | 19 | 23.9 | 2 | 14.3 | 5 | 22.5 |
| Severe events | 0 | - | - | - | 1 | 7.1 | 1 | 4.5 |
| Probably related | 4 | 8.9 | 7 | 8.8 | 1 | 7.1 | 1 | 4.5 |
| Possibly related | 4 | 8.9 | 5 | 6.3 | 2 | 14.3 | 5 | 22.5 |
| Unlikely related | 32 | 71.1 | 177 | 222.6 | 10 | 71.4 | 44 | 197.9 |
| . | Pooled somapacitan groups . | . | . | . | Daily GH 0.034 mg/kg/da . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | N . | (%) . | E . | R . | N . | (%) . | E . | R . |
| Patients exposed | 45 | 14 | ||||||
| All events | 32 | 71.1 | 189 | 237.7 | 10 | 71.4 | 50 | 224.9 |
| Mild events | 30 | 66.7 | 170 | 213.8 | 9 | 64.3 | 44 | 197.9 |
| Moderate events | 11 | 24.4 | 19 | 23.9 | 2 | 14.3 | 5 | 22.5 |
| Severe events | 0 | - | - | - | 1 | 7.1 | 1 | 4.5 |
| Probably related | 4 | 8.9 | 7 | 8.8 | 1 | 7.1 | 1 | 4.5 |
| Possibly related | 4 | 8.9 | 5 | 6.3 | 2 | 14.3 | 5 | 22.5 |
| Unlikely related | 32 | 71.1 | 177 | 222.6 | 10 | 71.4 | 44 | 197.9 |
Abbreviations: %, percentage of patients; E, number of events; R, event rate per 100 patient-years at risk.
aEquivalent to 0.238 mg/kg/wk.
| . | Pooled somapacitan groups . | . | . | . | Daily GH 0.034 mg/kg/da . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | N . | (%) . | E . | R . | N . | (%) . | E . | R . |
| Patients exposed | 45 | 14 | ||||||
| All events | 40 | 88.9 | 318 | 258.1 | 14 | 100.0 | 95 | 267.7 |
| SAEs | 4 | 8.9 | 8 | 6.5 | 2 | 14.3 | 3 | 8.5 |
| Probably related | 1 | 2.2 | 2 | 1.6 | 0 | - | - | - |
| Unlikely related | 3 | 6.6 | 6 | 4.9 | 2 | 14.3 | 3 | 8.5 |
| . | Pooled somapacitan groups . | . | . | . | Daily GH 0.034 mg/kg/da . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | N . | (%) . | E . | R . | N . | (%) . | E . | R . |
| Patients exposed | 45 | 14 | ||||||
| All events | 40 | 88.9 | 318 | 258.1 | 14 | 100.0 | 95 | 267.7 |
| SAEs | 4 | 8.9 | 8 | 6.5 | 2 | 14.3 | 3 | 8.5 |
| Probably related | 1 | 2.2 | 2 | 1.6 | 0 | - | - | - |
| Unlikely related | 3 | 6.6 | 6 | 4.9 | 2 | 14.3 | 3 | 8.5 |
%, percentage of patients; E, number of events; R, event rate per 100 patient-years at risk; SAE, serious adverse event.
aEquivalent to 0.238 mg/kg/wk.
| . | Pooled somapacitan groups . | . | . | . | Daily GH 0.034 mg/kg/da . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | N . | (%) . | E . | R . | N . | (%) . | E . | R . |
| Patients exposed | 45 | 14 | ||||||
| All events | 40 | 88.9 | 318 | 258.1 | 14 | 100.0 | 95 | 267.7 |
| SAEs | 4 | 8.9 | 8 | 6.5 | 2 | 14.3 | 3 | 8.5 |
| Probably related | 1 | 2.2 | 2 | 1.6 | 0 | - | - | - |
| Unlikely related | 3 | 6.6 | 6 | 4.9 | 2 | 14.3 | 3 | 8.5 |
| . | Pooled somapacitan groups . | . | . | . | Daily GH 0.034 mg/kg/da . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| . | N . | (%) . | E . | R . | N . | (%) . | E . | R . |
| Patients exposed | 45 | 14 | ||||||
| All events | 40 | 88.9 | 318 | 258.1 | 14 | 100.0 | 95 | 267.7 |
| SAEs | 4 | 8.9 | 8 | 6.5 | 2 | 14.3 | 3 | 8.5 |
| Probably related | 1 | 2.2 | 2 | 1.6 | 0 | - | - | - |
| Unlikely related | 3 | 6.6 | 6 | 4.9 | 2 | 14.3 | 3 | 8.5 |
%, percentage of patients; E, number of events; R, event rate per 100 patient-years at risk; SAE, serious adverse event.
aEquivalent to 0.238 mg/kg/wk.

Most common adverse events, occurring in 10% or more of patients in any patient group. Safety analysis set. % indicates percentage of patients in each treatment group. R, event rate per 100 patient-years at risk.
After the first year, 4 children (somapacitan, n = 3; daily GH, n = 1) experienced 6 injection site-related AEs (subcutaneous hemorrhage, n = 1; hematoma, n = 2; hip deformity, n = 1; skin atrophy, n = 1; injection site reaction, n = 1). All injection site AEs were considered mild in severity. The single hip deformity event was reported as an injection site reaction with subcutaneous atrophy and considered possibly related to trial product. The patient was instructed to rotate the injection site, and after 67 days, the outcome was reported as resolved.
During the 3 years of the trial, low titer non-neutralizing antibodies were observed in 10 children who were treated with somapacitan. For 7 children, somapacitan antibodies were observed at single time points (somapacitan 0.04/0.16 mg/kg/wk, n = 4; somapacitan 0.08/0.16 mg/kg/wk, n = 2; somapacitan 0.16/0.16 mg/kg/wk, n = 1).
For 3 children, positive samples were observed in 2 (somapacitan 0.16/0.16 mg/kg/wk), 3 (somapacitan 0.04/ 0.16 mg/kg/wk), and 4 (somapacitan 0.08/0.16 mg/kg/wk) consecutive samples, respectively. One child in the daily GH treatment arm had persistent anti-human GH antibodies of low titer from visit 4 to visit 16 (follow-up visit for the present trial period). All antibody positive samples were negative for in vitro neutralizing antibodies. Antibodies did not appear to affect the pharmacokinetic or pharmacodynamic profiles of somapacitan or annualized HV.
Across all treatment groups, an increase in fasting insulin levels was observed from baseline to year 3. The change from baseline was within expected levels for children with GHD treated with GH and was similar between the somapacitan and daily GH treatment arms. There were no apparent clinically relevant changes in fasting glucose or mean glycate hemoglobin from baseline to year 3 in any of the treatment groups. During year 2, 1 child treated with daily GH had 2 events of abnormal glucose metabolism (“impaired fasting glucose” and “blood glucose abnormal”), which were rated by the investigator as mild and possibly related to trial product; no action was deemed necessary following the AE, and the abnormalities were reversible.
Discussion
The data presented here represent the first report of a long-acting GH demonstrating a sustained efficacy over 3 years in all assessed height-based outcomes. The 3-year data showed that somapacitan is efficacious and safe in treatment-naïve prepubertal children with GHD; the data were similar to daily GH, suggesting that somapacitan may be a valuable treatment alternative to daily GH, particularly as a result of fewer injections being required (compared with daily GH). We have previously reported a dose-dependent response with somapacitan in children with GHD for HV after 1 year, where treatment with somapacitan 0.16 mg/kg/wk resulted in a statistically significant greater HV compared with daily GH (30). Now, we present novel 3-year data for the 3 height-based outcomes, showing higher or equal values for all 3 somapacitan treatment arms compared with daily GH, except for change from baseline in HVSDS for somapacitan 0.08/0.16 mg/kg/wk compared with daily GH. Owing to all children initially randomized to somapacitan being allocated to the same dose level of somapacitan (0.16 mg/kg/wk) after year 1, at year 3, all somapacitan-treated children had received the same 0.16 mg/kg/wk dose of somapacitan for at least 2 years.
IGF-I is the most accepted, widely adopted biomarker for GH response, and in children, it is monitored to ensure adherence and long-term safety of GH treatment (37). Up to year 1, a dose-dependent increase in IGF-I SDS and IGFBP-3 SDS was observed in the somapacitan treatment arms. After 3 years of treatment, the mean increase in IGF-I SDS from baseline was similar across the somapacitan-treated and daily GH groups, suggesting a similar effect of somapacitan and daily GH on IGF-I (and IGFBP-3) in children with GHD. Importantly, at year 3, mean IGF-I SDS remained within the normal range across all treatment arms. The IGF-I profile associated with long-acting GH differs from that observed following administration of daily GH. With daily GH, stable IGF-I levels are achieved within days to weeks of starting on a new dose (38). Following injection of long-acting GH, there is an increase in IGF-I concentrations over a few days, reaching a maximum that may exceed the usual defined normal reference range, before declining to trough concentrations prior to the next injection (39). The reported IGF-I SDS value therefore depends on the time of sampling, as the maximum levels are higher, and the trough values are lower with long-acting GH than those with daily GH. Observed IGF-I SDS values for daily GH are less sensitive to the time of sampling because of less weekly fluctuation. For years 1 and 3, somapacitan samples were taken near maximum value for IGF-I SDS. This should be taken into consideration when comparing the level of somapacitan- and daily GH-induced IGF-I SDS at these time points.
GH treatment of children with GHD leads to an increase in bone age that is typically appropriate for the height of the child (40). The ratio of bone age to chronological age ratio approached 1 in all treatment groups, thus indicating a normalization of the ratio after 3 years of replacement therapy.
GHD-CIM ObsRO is a validated and reliable measure to assess disease-specific functioning (35) and was used to investigate the impact of somapacitan relative to daily GH on emotional well-being, physical functioning, and social well-being. Point estimates for both the individual GHD-CIM ObsRO domains and total scores favored somapacitan 0.16 mg/kg/wk over daily GH after 1 year of treatment, and all 3 somapacitan arms over daily GH after 3 years of treatment. However, these differences did not reach statistical significance. These favorable scores are particularly encouraging considering that somapacitan was trialed against an active comparator, and therefore large differences between the comparator arms would not be expected. Treatment burden is also expected to be reduced as a result of fewer injections. Furthermore, these results may indicate a relationship between increasing height and improved HRQoL, which is supported by previous findings that have demonstrated improved HRQoL in children and adolescents with GHD after undergoing GH treatment (41, 42). It may therefore be beneficial to start treatment early and at an appropriate dose to achieve improved height during childhood, aligning with previous findings stating the importance of starting treatment as early as possible in this condition (43, 44).
Somapacitan 0.16 mg/kg/wk administered to prepubertal children with GHD was well tolerated with no new clinically significant safety or local tolerability issues identified. The AE rates (event rate per 100 patient-years at risk) were similar between the somapacitan treatment arms and the daily GH treatment arm. All injection site-related AEs reported after year 1 were considered mild in severity. Two injection site reactions reported in 1 child during the third year of treatment with somapacitan were considered nonserious and resolved following rotation of the injection site.
Overall, results at year 3 indicate that once-weekly somapacitan has a similar efficacy and safety profile to daily GH. Somapacitan may provide an alternative to daily GH treatment in children with GHD, with a reduced treatment burden as a result of fewer injections than daily GH (reduction from 365 to 52 injections per year). The observed safety profiles in the present study were consistent with a phase 1 trial with somapacitan (16, 30), the first year of this study (30), and known safety profiles for GH products in general (45). Adherence was similar between groups, and there were only a very small number of discontinuations (2 from AEs, 2 withdrew from the study, and 4 discontinued as a result of protocol violations) from the trial. However, the results of this study are limited by the small number of patients enrolled in each trial arm. Further studies are ongoing to assess the long-term safety of somapacitan.
In conclusion, these data demonstrated that treatment with once-weekly somapacitan in children with GHD resulted in a sustained efficacy over 3 years in all assessed height-based outcomes. In the third year, the observed height-based outcomes for the somapacitan groups were similar to those observed for the daily GH group. The mean change in IGF-I SDS during treatment was similar between somapacitan and daily GH. HRQoL data were favorable but did not reach significance within this time frame. In addition, a reduction in treatment burden is expected because of fewer injections. The safety profile of once-weekly somapacitan was similar to the safety profile observed for daily GH with no new safety or tolerability issues identified.
Abbreviations
- AE
adverse event
- FAS
full analysis set
- GHD-CIM
Growth Hormone Deficiency–Child Impact Measure
- GHD
GH deficiency
- HRQoL
health-related quality of life
- HV
height velocity
- HVSDS
height velocity SD score
- IGFBP-3
insulin-like binding protein-3
- ObsRO
observer report
- SAE
serious adverse event
- SAS
safety analysis set
- SDS
SD score
Acknowledgments
The authors thank the patients, their families, the nurses and study coordinators, and all investigators involved in this study, including members of the REAL 3 Study Group*, without whom the study would not have been possible. The authors also thank Kai Wai Lee, Sebastian Roehrich, Mette Suntum, Knud Vad, and Rasmus Vejrup-Hansen, of Novo Nordisk, for review of the manuscript. Statistical analyses were carried out by Charlotte Bisgaard. Medical writing and editorial support were provided by Ewan Smith and Rosalind Perrett, of Ashfield MedComms, an Ashfield Health company, supported by Novo Nordisk Health Care AG. The sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript.
Funding
This research was funded by Novo Nordisk A/S, Denmark.
Disclosures
L.S. reports consultation fees from Ascendis, Novo Nordisk, Merck, and Pfizer. T.B. reports advisory boards for Novo Nordisk, Eli Lilly, and Pfizer; speakers honoraria from Novo Nordisk, Eli. Lilly, and Pfizer; and research grants to the institution from Novo Nordisk and Sandoz. MB reports consultation fees from Novo Nordisk. M.H.R. is an employee and stockholder of Novo Nordisk. PS has nothing to disclose. R.H. reports advisory boards for Novo Nordisk, OPKO-Pfizer, Ascendis, and Lumos Pharma; lecturer fees from Novo Nordisk, Pfizer, and JCR; and a research grant from Sandoz.
*Members of the REAL 3 Study Group: Austria: Dieter Furthner, Bettina Piringer, Lorenz Auer-Hackenberg, Klaus Schmitt, Marlene Reitmayr. Brazil: Marcello Delano Bronstein, Francisco Samuel Magalhães Lima. Germany: Martin Wabitsch, Carsten Posovszky, Volker Böttcher, Alexander Mann. Israel: Eli Hershkovitz, Alon Haim, Neta Lowenthal, Orit Hamiel, Sharon Sheinvald Levin, Kineret Mazor-Aronovitch, Michal Ben-Ami, Yael Levy Shraga, Dalit Modan, Noah Gruber, Moshe Phillip, Yael Lebenthal, Ariel Tenenbaum, Alon Eliakim, Nitzan Dror, Ruby Haviv, Nehama Zuckerman-Levin, Naim Shehadeh, Liav Givon, Ameer Elemy, Miriam Marji, Vardit Gepstein. India: Praveen V.P., Aswin P, Nithiya Abraham, Rajesh Khadgawat, Yashdeep Gupta, Vaman Khadilkar, Anuradha Khadilkar, Sagar Lad. Japan: Reiko Horikawa, Yasuhiro Naiki, Yasuko Ogiwara, Yuta Chiba, Yusuke Fujisawa, Yumiko Terada, Tomoko Yoshida, Kenichi Kinjo, Atsushi Tsukamura, Shinobu Ida, Yuri Etani, Yasuko Shoji, Masanobu Kawai, Hisakazu Nakajima, Jun Mori, Shota Fukuhara, Keiichi Shigehara, Hidechika Morimoto, Yusuke Tsuma, Yasuhiro Kawabe, Takeshi Ota, Kenichi Kashimada, Ryuichi Nakagawa, Atsumi Tsuji, Risa Nomura, Kei Takasawa, Takeru Yamauchi, Kanako Ishii, Naoko Toda, Kazuhiro Ohkubo, Tohru Yorifuji, Yuki Hosokawa, Rie Kawakita, Yukiko Hashimoto, Azumi Sakakibara, Shinji Higuchi, Shun Soneda, Kenichiro Ogushi, Shuichi Yatsuga, Yasutoshi Koga, Takako Matsumoto, Miyuki Kitamura. Sweden: Lars Sävendahl, Ricard Nergårdh. Slovenia: Tadej Battelino, Mojca Zerjav Tansek. Turkey: Serap Turan, Abdullah Bereket, Zeynep Atay, Azad Akbarzade, Tülay Güran. Ukraine: Olena Bolshova, Mykola Tronko, Olga Vyshnevskaya, Natalia Sprynchuk, Iryna Lukashuk, Natalia Muz, Tatyana Marchenko, Nataliya Chorna, Marіana Konovalova, Liliya Zelinska. USA: Lawrence Silverman, Barbara Cerame, Sunita Cheruvu, Daisy Chin, Laurie Ebner-Lyon, Marie Fox, Marianna Nicolette-Gentile, Kristin Sabanosh, Harold Starkman, Ian Marshall, Mariam Gangat, Sadana Balachandar, Philippe Backeljauw, Andrew Dauber, Leah Tyzinski, Paul H. Saenger, Luis Zamora Siliezar, Jacqueline P. Velasco, Judith L. Ross, Martha Bardsley, Karen Kowal, Gad B. Kletter, Britney G. Frazier, Kathryn Garrison.
Ethical approval: Before trial initiation, the protocol, the consent form, and the subject information sheet were reviewed and approved according to local regulations by appropriate health authorities, and by an independent ethics committee/institutional review board.
Guarantor: L.S. is guarantor for integrity of data and its reporting in this manuscript.
Prior publication: Primary publication: Sävendahl L, Battelino T, Brod M, Højby Rasmussen M, Horikawa R, Juul RV, Saenger P. Once-weekly somapacitan vs daily GH in children with GH deficiency: results from a randomized phase 2 trial. J Clin Endocrinol Metab. 2020;105(4):e1847-e1861.
Data Availability
Available from corresponding author on reasonable request.



