-
PDF
- Split View
-
Views
-
Cite
Cite
Eva Kathrin Lamadé, Ferdinand Hendlmeier, Stefan A Wudy, Stephanie H Witt, Marcella Rietschel, Michaela Coenen, Maria Gilles, Michael Deuschle, Rhythm of Fetoplacental 11β-Hydroxysteroid Dehydrogenase Type 2 — Fetal Protection From Morning Maternal Glucocorticoids, The Journal of Clinical Endocrinology & Metabolism, Volume 106, Issue 6, June 2021, Pages 1630–1636, https://doi.org/10.1210/clinem/dgab113
Close - Share Icon Share
Abstract
Excess glucocorticoids impact fetal health. Maternal glucocorticoids peak in early morning. Fetoplacental 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) inactivates cortisol to cortisone, protecting the fetus from high glucocorticoids. However, time-specific alterations of human fetoplacental 11β-HSD2 have not been studied.
We hypothesized that fetoplacental 11β-HSD2 activity shows time-specific alteration and acute affective or anxiety disorders impact fetoplacental 11β-HSD2 activity.
In this observational study we investigated 78 pregnant European women undergoing amniocentesis (15.9 ± 0.9 weeks of gestation). Amniotic fluid was collected (8:00 to 16:30 hours) for analysis of fetoplacental 11β-HSD2 activity, using cortisol (F):cortisone (E) ratio in amniotic fluid, E/(E + F). Fetoplacental 11β-HSD2 rhythm and association with “acute affective or anxiety disorder” (patients with at least one of: a major depressive episode, specific phobia, panic disorder, generalized anxiety disorder, mixed anxiety and depressive disorder) and “acute anxiety disorder” (one of: panic disorder, generalized anxiety disorder, mixed anxiety, depressive disorder), assessed using Mini International Neuropsychiatric Interview, were investigated.
Activity of 11β-HSD2 correlated with time of amniocentesis, peaking in the morning (r = −0.398; P < 0.001) and increased with acute affective or anxiety disorder (mean [M] = 0.70 vs M = 0.74; P = 0.037) and acute anxiety disorder (M = 0.70 vs M = 0.75; P = 0.016). These associations remained significant when controlling for confounders. 11β-HSD2 activity correlated negatively with pre-pregnancy body mass index (r = −0.225; P = 0.047).
Our study indicates a time-specific alteration of fetoplacental 11β-HSD2 activity with peaking levels in the morning, demonstrating a mechanism of fetal protection from the morning maternal glucocorticoid surge.
Fetoplacental 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) can inactivate cortisol to its inactive metabolite, cortisone, to protect the fetus from excess glucocorticoids. While maternal glucocorticoids peak in the morning, it is not clear whether time-specific alterations of fetoplacental 11β-HSD2 exist in humans.
11β-Hydroxysteroid Dehydrogenase Type 2 and Glucocorticoids
Overactivation of the hypothalamus–pituitary–adrenal (HPA) system with excess cortisol exposure has been shown to have adverse effects on fetal outcome. Treatment of newborn rats with the synthetic glucocorticoid dexamethasone decreases growth and neurodevelopment, with adult animals displaying a depressive-like phenotype and delayed decrease of corticosterone after a stress test (1). In humans, defects of 11β-HSD2 are associated with adverse health effects such as intrauterine growth restriction (2). In humans, early excess glucocorticoids exposure is associated with reduced anthropometric measurements and health adversities including cognitive impairments (3-6) and higher incidence of mental and behavioral disorders (7), such as aggressive, distractible, and hyperkinetic behavior (8).
Cortisol (F) and cortisone (E) can be converted into each other via 11β-hydroxysteroid dehydrogenases (11β-HSDs). 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) converts the inactive cortisone to active cortisol, whereas 11β-HSD2 converts active cortisol to its inactive metabolite, cortisone. Cortisol concentrations in the fetal circulation are up to 10-fold lower than maternal cortisol concentrations (9). This gradient is achieved by 11β-HSD2, located in fetal tissues and particularly inside the syncytiotrophoblast of placental villi between maternal and fetal blood (9), inactivating most of maternal cortisol to cortisone during transfer across the placenta in early pregnancy (10) as well as near term (11). About 75% of cortisol measured in umbilical cord blood of the fetus is of fetal origin and 90% of cortisone is of maternal origin, illustrating the effect of 11β-HSD2 acting as a barrier between maternal cortisol and the fetus, with most of maternal cortisol being inactivated to cortisone once it reaches the fetus (11, 12). To assess activity of 11β-HSD2 noninvasively in mid-pregnancy, the ratio of cortisone and cortisol as E/(E + F), displaying the conversion of cortisol to cortisone, can be used to estimate fetoplacental 11β-HSD2 activity (13, 14).
Time-Specific Rhythms of 11β-HSD
Circadian systems exist in almost every organ of the body, controlling endocrine and metabolic activity via the hypothalamic suprachiasmatic nuclei which is regulated by retinal light receptors in adults (15), and molecular circadian rhythms are based upon feedback loops of peripheral proteins (16). Time-specific alterations of cortisol and cortisone levels exist during the course of pregnancy, with cortisol increasing from early toward late pregnancy and cortisone decreasing from early toward late pregnancy (17), while placental 11β-HSD1 expression significantly increases and 11β-HSD2 activity significantly decreases at the end of pregnancy, between weeks 38 to 40 (18). This allows the fetus to be protected during the first trimesters of pregnancy and fetal cortisol to increase toward term, allowing for the development of the fetus, including lung maturation.
Time-specific rhythms of maternal cortisol include a peak in the morning and consequent decrease in the course of the day. While placental 11β-HSD1 increases during the day and peaks in the evening in rats (19), the time-specific rhythm of 11β-HSD2 has, to our knowledge, never been studied in animal studies or humans before. As 11β-HSD2 and 11β-HSD1 are inversely regulated, we hypothesized a time-specific alteration of 11β-HSD2 in humans.
Prenatal Stress and 11β-HSD2
Prenatal stress affects 11β-HSD2 via epigenetic mechanisms in animal models and humans, by altering the methylation levels of 11β-HSD2. This leads to altered levels of glucocorticoids (20-22), showing mechanisms by which maternal stress may impact fetal endocrinology.
Using E/(E + F) as a proxy of fetoplacental 11β-HSD2 activity, our goal was to determine the time-specific alteration of fetoplacental 11β-HSD2 activity and to determine if acute affective or anxiety disorders impact activity of fetoplacental 11β-HSD2.
Methods
Women pregnant in their second trimester undergoing amniocentesis (15.9 ± 0.9 weeks of gestational age) from 2013 to 2015 and their endocrine measurements in amniotic fluid were studied. 125 women were contacted. Inclusion criteria were German-speaking, capacity to consent, age of majority (≥18 years). Amniotic fluid samples contaminated with blood and fetuses with human genetic anomalies of fetuses were excluded. A total of 99 subjects were originally included in the study; 20 were then excluded due to twin pregnancies, chromosomal anomalies, shortages of biomaterial and incomplete psychological interviews. Additionally, 1 extreme outlier of 11β-HSD2 activity was excluded (>5 SD). Thus, 78 women were analyzed in our study.
The time of amniocentesis was recorded between 0800 and 1630 hours. Within 1 week after amniocentesis, questionnaires, a clinical interview and the Mini International Neuropsychiatric Interview (23) were imparted, assessing acute affective and anxiety disorders, including major depressive episodes, specific phobias, generalized anxiety, panic disorders, and mixed anxiety and depressive disorder. We defined acute affective or anxiety disorder as having a major depressive episode, specific phobia, panic disorder, generalized anxiety disorder, or mixed anxiety and depressive disorder. Acute anxiety disorder was defined as having a panic disorder, generalized anxiety disorder, or mixed anxiety and depressive disorder. Perceived stress of amniocentesis was assessed using a Likert scale (0 = no stress – 10 = maximum stress). Maternal age and pre-pregnancy body mass index (BMI) were recorded.
In amniotic fluid, free cortisol (F) and free cortisone (E) were measured using turbulent flow chromatography connected with fused-core chromatography-tandem mass spectrometry (24-26) in cooperation with the Laboratory for Translational Hormone Analytics in Pediatric Endocrinology of Justus Liebig University Giessen (Germany), and E/(E + F) was calculated as a proxy for fetoplacental 11β-HSD2 activity.
All participants gave written informed consent allowing their data to be used for research purposes. The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Medical Faculty Mannheim (2013-621N-MA). The study number at the German Clinical Trials Register is DRKS-ID: DRKS00005741.
For the statistical analysis using IBM SPSS Statistics 25, we used descriptive and bivariate analyses. For the latter we used chi-squared test, t test, and Mann-Whitney U test as well as Pearson and Spearman’s rank correlations according to the type of measurement scale and data distribution. 11β-HSD2 activity and cortisone are metric normally distributed variables. Pre-pregnancy BMI, time of amniocentesis and cortisol are metric not normally distributed variables. Perceived stress of amniocentesis is an ordinal variable. Acute affective or anxiety disorder and acute anxiety disorder are dichotomous variables. Backward linear regression analyses for prediction of 11β-HSD2 activity were run. The significance level was set to P ≤ 0.05.
Results
A total of 78 women aged between 22 and 45 years (mean (M) = 36.3 ± 3.9 years) who underwent amniocentesis in their second trimester of pregnancy (M = 15.9 ± 0.9 weeks of gestational age) and endocrine measurements in amniotic fluid were analyzed (see Table 1 for descriptive statistics).
| . | Mean . | ±SD . | Range (min-max) . |
|---|---|---|---|
| Maternal variables | |||
| Maternal age (years) | 36.3 | 3.9 | 22-45 |
| Maternal pre-pregnancy BMI (kg/m2) | 24.1 | 4.6 | 17.4-41.0 |
| Acute affective or anxiety disorder (-/+) | 59/19 | ||
| Acute anxiety disorder (-/+) | 63/15 | ||
| Fetal sex (male/female) | 43/35 | ||
| Amniocentesis-related variables | |||
| Perceived stress of amniocentesis (0-10) | 5.8 | 2.4 | 1-10 |
| Gestational age at amniocentesis (weeks) | 15.9 | 0.9 | 14.3-18.9 |
| Time of amniocentesis (hours:minutes) | 11:22 | 2:19 | 8:00 to 16:30 |
| Proxy of 11β-HSD2 activity (E/E + F) | 0.71 | 0.08 | 0.54-0.86 |
| Free cortisol (F) in ng/ml | 4.7 | 1.5 | 1.3-9.4 |
| Free cortisone (E) in ng/ml | 11.5 | 2.9 | 1.5-19.6 |
| . | Mean . | ±SD . | Range (min-max) . |
|---|---|---|---|
| Maternal variables | |||
| Maternal age (years) | 36.3 | 3.9 | 22-45 |
| Maternal pre-pregnancy BMI (kg/m2) | 24.1 | 4.6 | 17.4-41.0 |
| Acute affective or anxiety disorder (-/+) | 59/19 | ||
| Acute anxiety disorder (-/+) | 63/15 | ||
| Fetal sex (male/female) | 43/35 | ||
| Amniocentesis-related variables | |||
| Perceived stress of amniocentesis (0-10) | 5.8 | 2.4 | 1-10 |
| Gestational age at amniocentesis (weeks) | 15.9 | 0.9 | 14.3-18.9 |
| Time of amniocentesis (hours:minutes) | 11:22 | 2:19 | 8:00 to 16:30 |
| Proxy of 11β-HSD2 activity (E/E + F) | 0.71 | 0.08 | 0.54-0.86 |
| Free cortisol (F) in ng/ml | 4.7 | 1.5 | 1.3-9.4 |
| Free cortisone (E) in ng/ml | 11.5 | 2.9 | 1.5-19.6 |
N = 78 women were analyzed.
Abbreviations: 11β-HSD2, 11β-hydroxysteroid dehydrogenase type 2; BMI, body mass index; E, cortisone; F, cortisol.
| . | Mean . | ±SD . | Range (min-max) . |
|---|---|---|---|
| Maternal variables | |||
| Maternal age (years) | 36.3 | 3.9 | 22-45 |
| Maternal pre-pregnancy BMI (kg/m2) | 24.1 | 4.6 | 17.4-41.0 |
| Acute affective or anxiety disorder (-/+) | 59/19 | ||
| Acute anxiety disorder (-/+) | 63/15 | ||
| Fetal sex (male/female) | 43/35 | ||
| Amniocentesis-related variables | |||
| Perceived stress of amniocentesis (0-10) | 5.8 | 2.4 | 1-10 |
| Gestational age at amniocentesis (weeks) | 15.9 | 0.9 | 14.3-18.9 |
| Time of amniocentesis (hours:minutes) | 11:22 | 2:19 | 8:00 to 16:30 |
| Proxy of 11β-HSD2 activity (E/E + F) | 0.71 | 0.08 | 0.54-0.86 |
| Free cortisol (F) in ng/ml | 4.7 | 1.5 | 1.3-9.4 |
| Free cortisone (E) in ng/ml | 11.5 | 2.9 | 1.5-19.6 |
| . | Mean . | ±SD . | Range (min-max) . |
|---|---|---|---|
| Maternal variables | |||
| Maternal age (years) | 36.3 | 3.9 | 22-45 |
| Maternal pre-pregnancy BMI (kg/m2) | 24.1 | 4.6 | 17.4-41.0 |
| Acute affective or anxiety disorder (-/+) | 59/19 | ||
| Acute anxiety disorder (-/+) | 63/15 | ||
| Fetal sex (male/female) | 43/35 | ||
| Amniocentesis-related variables | |||
| Perceived stress of amniocentesis (0-10) | 5.8 | 2.4 | 1-10 |
| Gestational age at amniocentesis (weeks) | 15.9 | 0.9 | 14.3-18.9 |
| Time of amniocentesis (hours:minutes) | 11:22 | 2:19 | 8:00 to 16:30 |
| Proxy of 11β-HSD2 activity (E/E + F) | 0.71 | 0.08 | 0.54-0.86 |
| Free cortisol (F) in ng/ml | 4.7 | 1.5 | 1.3-9.4 |
| Free cortisone (E) in ng/ml | 11.5 | 2.9 | 1.5-19.6 |
N = 78 women were analyzed.
Abbreviations: 11β-HSD2, 11β-hydroxysteroid dehydrogenase type 2; BMI, body mass index; E, cortisone; F, cortisol.
Time of amniocentesis correlated strongly with 11β-HSD2 activity (r = −0.398; P < 0.001) (see Fig. 1), free cortisol (r = 0.318; P ≤ 0.005), and cortisone (r = −0.268; P = 0.018) in amniotic fluid. 11β-HSD2 activity correlated positively with acute affective or anxiety disorder (M = 0.70 vs M = 0.74; P = 0.037) and acute anxiety disorder (M = 0.70 vs M = 0.75; P = 0.016) but not with fetal sex or perceived stress of amniocentesis. 11β-HSD2 activity correlated negatively with pre-pregnancy BMI (r = −0.225; P = 0.047).
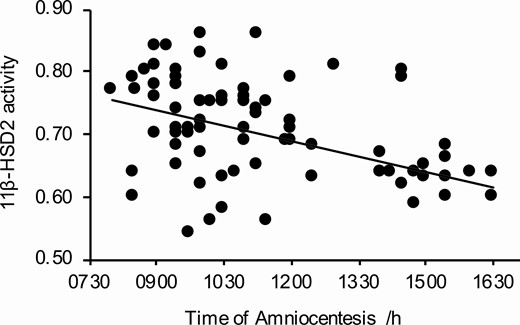
Time-specific alterations of fetoplacental 11β-HSD2 activity (P < 0.001; r = −0.398). A line of best fit is drawn using the least square method.
In multiple regression analysis for prediction of 11β-HSD2 activity, 2 regression models were run. The two models that were run, included either acute affective or anxiety disorder, or acute anxiety disorder, as the two variables intercorrelated (P < 0.001).
In the first regression model, including acute affective or anxiety disorder (see Table 2), time of amniocentesis and acute affective or anxiety disorder remained significantly associated with 11β-HSD2 activity (R2 = 0.195; P < 0.001). In the second regression model, including acute anxiety disorder (see Table 3), time of amniocentesis and acute anxiety disorder remained significantly associated with 11β-HSD2 activity (R2 = 0.224; P < 0.001). Selected covariates for the regression models did not intercorrelate (see Table 4).
Multiple regression analyses for 11β-HSD2 activity including acute affective or anxiety disorder as covariate (P < 0.001; R2= 0.195)
| Model for . | Input . | Output . | β . | P value . |
|---|---|---|---|---|
| 11β-HSD2 activity | Time of amniocentesis | Time of amniocentesis | −0.371 | 0.001 |
| Acute affective or anxiety disorder | Acute affective or anxiety disorder | +0.208 | 0.050 | |
| Maternal pre-pregnancy BMI | - |
| Model for . | Input . | Output . | β . | P value . |
|---|---|---|---|---|
| 11β-HSD2 activity | Time of amniocentesis | Time of amniocentesis | −0.371 | 0.001 |
| Acute affective or anxiety disorder | Acute affective or anxiety disorder | +0.208 | 0.050 | |
| Maternal pre-pregnancy BMI | - |
N = 78 women were analyzed. Standardized β coefficient. Bold: P ≤ 0.05.
Abbreviations: 11β-HSD2, 11β-hydroxysteroid dehydrogenase type 2; BMI, body mass index.
Multiple regression analyses for 11β-HSD2 activity including acute affective or anxiety disorder as covariate (P < 0.001; R2= 0.195)
| Model for . | Input . | Output . | β . | P value . |
|---|---|---|---|---|
| 11β-HSD2 activity | Time of amniocentesis | Time of amniocentesis | −0.371 | 0.001 |
| Acute affective or anxiety disorder | Acute affective or anxiety disorder | +0.208 | 0.050 | |
| Maternal pre-pregnancy BMI | - |
| Model for . | Input . | Output . | β . | P value . |
|---|---|---|---|---|
| 11β-HSD2 activity | Time of amniocentesis | Time of amniocentesis | −0.371 | 0.001 |
| Acute affective or anxiety disorder | Acute affective or anxiety disorder | +0.208 | 0.050 | |
| Maternal pre-pregnancy BMI | - |
N = 78 women were analyzed. Standardized β coefficient. Bold: P ≤ 0.05.
Abbreviations: 11β-HSD2, 11β-hydroxysteroid dehydrogenase type 2; BMI, body mass index.
Multiple regression analyses for 11β-HSD2 activity including acute anxiety disorder as covariate (P < 0.001; R2 = 0.224)
| Model for . | Input . | Output . | β . | P value . |
|---|---|---|---|---|
| 11β-HSD2 activity | Time of amniocentesis | Time of amniocentesis | −0.370 | 0.001 |
| Acute anxiety disorder | Acute anxiety disorder | +0.268 | 0.011 | |
| Maternal pre-pregnancy BMI | - |
| Model for . | Input . | Output . | β . | P value . |
|---|---|---|---|---|
| 11β-HSD2 activity | Time of amniocentesis | Time of amniocentesis | −0.370 | 0.001 |
| Acute anxiety disorder | Acute anxiety disorder | +0.268 | 0.011 | |
| Maternal pre-pregnancy BMI | - |
N = 78 women were analyzed. Standardized β coefficient. Bold: P ≤ 0.05.
Abbreviations: 11β-HSD2, 11β-hydroxysteroid dehydrogenase type 2; BMI, body mass index.
Multiple regression analyses for 11β-HSD2 activity including acute anxiety disorder as covariate (P < 0.001; R2 = 0.224)
| Model for . | Input . | Output . | β . | P value . |
|---|---|---|---|---|
| 11β-HSD2 activity | Time of amniocentesis | Time of amniocentesis | −0.370 | 0.001 |
| Acute anxiety disorder | Acute anxiety disorder | +0.268 | 0.011 | |
| Maternal pre-pregnancy BMI | - |
| Model for . | Input . | Output . | β . | P value . |
|---|---|---|---|---|
| 11β-HSD2 activity | Time of amniocentesis | Time of amniocentesis | −0.370 | 0.001 |
| Acute anxiety disorder | Acute anxiety disorder | +0.268 | 0.011 | |
| Maternal pre-pregnancy BMI | - |
N = 78 women were analyzed. Standardized β coefficient. Bold: P ≤ 0.05.
Abbreviations: 11β-HSD2, 11β-hydroxysteroid dehydrogenase type 2; BMI, body mass index.
Intercorrelation with P values of the selected covariates for multiple regression analyses
| . | Time of amniocentesis . | Acute affective or anxiety disorder . | Acute anxiety disorder . |
|---|---|---|---|
| Time of amniocentesis | -/- | -/- | -/- |
| Acute affective or anxiety disorder | 0.771 (Mdn = 10:30 vs 10:45)a | -/- | -/- |
| Acute anxiety disorder | 0.703 (Mdn = 11:00 vs 10:30)a | 0.000 (0% vs 79%)c | -/- |
| Maternal pre-pregnancy BMI | 0.144 (r = 0.155)b | 0.723 (Mdn = 23.1 vs 24.1)a | 0.849 (Mdn = 23.2 vs 24.1)a |
| . | Time of amniocentesis . | Acute affective or anxiety disorder . | Acute anxiety disorder . |
|---|---|---|---|
| Time of amniocentesis | -/- | -/- | -/- |
| Acute affective or anxiety disorder | 0.771 (Mdn = 10:30 vs 10:45)a | -/- | -/- |
| Acute anxiety disorder | 0.703 (Mdn = 11:00 vs 10:30)a | 0.000 (0% vs 79%)c | -/- |
| Maternal pre-pregnancy BMI | 0.144 (r = 0.155)b | 0.723 (Mdn = 23.1 vs 24.1)a | 0.849 (Mdn = 23.2 vs 24.1)a |
N = 78 women were analyzed. Bold: P ≤ 0.05. -/- indicates that this association is already shown in another part of the table or not applicable.
Abbreviations: BMI, body mass index; Mdn: median; r, correlation coefficient.aMann-Whitney U test
bSpearman’s rank correlation
cChi-squared test.
Intercorrelation with P values of the selected covariates for multiple regression analyses
| . | Time of amniocentesis . | Acute affective or anxiety disorder . | Acute anxiety disorder . |
|---|---|---|---|
| Time of amniocentesis | -/- | -/- | -/- |
| Acute affective or anxiety disorder | 0.771 (Mdn = 10:30 vs 10:45)a | -/- | -/- |
| Acute anxiety disorder | 0.703 (Mdn = 11:00 vs 10:30)a | 0.000 (0% vs 79%)c | -/- |
| Maternal pre-pregnancy BMI | 0.144 (r = 0.155)b | 0.723 (Mdn = 23.1 vs 24.1)a | 0.849 (Mdn = 23.2 vs 24.1)a |
| . | Time of amniocentesis . | Acute affective or anxiety disorder . | Acute anxiety disorder . |
|---|---|---|---|
| Time of amniocentesis | -/- | -/- | -/- |
| Acute affective or anxiety disorder | 0.771 (Mdn = 10:30 vs 10:45)a | -/- | -/- |
| Acute anxiety disorder | 0.703 (Mdn = 11:00 vs 10:30)a | 0.000 (0% vs 79%)c | -/- |
| Maternal pre-pregnancy BMI | 0.144 (r = 0.155)b | 0.723 (Mdn = 23.1 vs 24.1)a | 0.849 (Mdn = 23.2 vs 24.1)a |
N = 78 women were analyzed. Bold: P ≤ 0.05. -/- indicates that this association is already shown in another part of the table or not applicable.
Abbreviations: BMI, body mass index; Mdn: median; r, correlation coefficient.aMann-Whitney U test
bSpearman’s rank correlation
cChi-squared test.
Discussion
We investigated the time-specific alteration of fetoplacental 11β-HSD2 activity and investigated whether acute affective and anxiety disorders impact fetoplacental 11β-HSD2 activity or not. 11β-HSD2 activity correlates with time of amniocentesis, peaking in the morning and is increased in women with the affective disorders, acute affective or anxiety disorder and acute anxiety disorder. Time of amniocentesis and the affective disorders remain significantly associated in multivariate analysis when controlling for pre-pregnancy BMI.
In animal studies, the fetal circadian rhythm is influenced by the maternal circadian system but develops even in mothers lacking the suprachiasmatic nucleus, displaying normal circadian rhythms in pups in the second month after birth (27). The uterus as well as placenta are known to have circadian rhythms in animal studies (28). Placental pro-inflammatory cytokines, placental nutrient transporters, and placental 11β-HSD1, which is inversely regulated to 11β-HSD2, show time-specific rhythms of the placenta in animal studies (19). In humans, placental time-specific rhythms are scarcely studied. Yet, peripheral clock genes known to regulate circadian rhythms on a molecular level are expressed in human placenta at term (16). While maternal cortisol is known to have a stable circadian rhythm, with peaking levels in early morning (15), our results show high activity of 11β-HSD2 in the morning, decreasing during the course of the day, with the correlation staying significant when controlling for confounders. This further supports that placental proteins display time-specific rhythms in humans and shows a mechanism by which the fetus is protected from the morning maternal glucocorticoid surge, as 11β-HSD2 acts as a placental barrier to maternal glucocorticoids (9). Placental circadian rhythms are still understudied, and further studies are required to fully understand placental time-specific rhythms in humans.
In animal models, acute stress increases and chronic stress decreases levels of placental 11β-HSD2 (29, 30). In humans, acute stress increases fetoplacental 11β-HSD2 activity (31), while affective disorders during pregnancy, including depression and anxiety, decrease 11β-HSD2 levels and, in preliminary analysis, enzyme activity near term (32-34). Together, these findings indicate that the duration of pregnancy moderates the effect of psychiatric disorders on placental 11β-HSD2 activity. Our results show an increase of mid-pregnancy fetoplacental 11β-HSD2 activity in patients with acute affective or anxiety disorder and in patients with acute anxiety disorder, the correlation remaining significant when controlling for confounders (see Tables 2 and 3). Acute depression is associated with hypercortisolism (35, 36), and while studies assessing anxiety disorders or phobias show no long-term effect on cortisol levels measured in hair (37), others show a sex-specific regulation upon acute stressors (38) or even an upregulation (39). Cortisol may be acting as a confounder in our analysis. Further studies in humans investigating the effect of acute and chronic affective disorders on regulation of 11β-HSD2 are necessary.
Diet in Western societies often involves high calorie intake (40). High fat and carbohydrate diet impacts placental physiology in animals (41). In humans, high norepinephrine levels that persist in obese humans (42) decrease placental 11β-HSD2 (43). According to this, we found pre-pregnancy BMI to be associated with decreased 11β-HSD2 activity, supporting the hypothesis that women wanting to become pregnant should take precaution for their BMI even before pregnancy. However, this correlation did not stay significant when controlling for confounders (Tables 2 and 3). Disruptions of placental 11β-HSD2 during pregnancy is shown to have adverse effects on fetal outcome, such as low birthweight, delayed puberty onset, hypertension in adult rats (44, 45), and adverse outcomes in human children, including a higher BMI, lower score of intelligence quotient, memory deficits, and increased risk of attention deficit disorder. Therefore, further studies should assess the impact of altered 11β-HSD2 on long-term fetal outcome.
We did not directly measure fetoplacental 11β-HSD2 activity but used the proxy E/(E + F) to prevent invasive placental biopsies in mid-pregnancy. However, this proxy might be impacted by current fetal and maternal HPA system activity and there is no direct evidence that amniotic fluid cortisol:cortisone ratio directly parallels fetoplacental 11β-HSD2 activity in humans. A further limitation is that we measured glucocorticoids in amniotic fluid to assess fetoplacental 11β-HSD2 activity but did not directly measure maternal glucocorticoids in serum. Collection times of amniotic fluid samples were restricted to working hours during the day. Even though our data found a significant association between time of day and 11β-HSD2 activity, further studies should address timepoints during the night.
In summary, our results demonstrate time-specific alteration of increased fetoplacental 11β-HSD2 activity in the morning, showing a mechanism of fetal protection from the morning maternal glucocorticoid surge in humans. Acute affective or anxiety disorder and acute anxiety disorder both associate with increased fetoplacental 11β-HSD2 activity. Although further studies are needed to validate our results, one might speculate that long-term disruptions of placental 11β-HSD2 during pregnancy might consistently alter levels of amniotic glucocorticoids with potentially adverse effects in the unborn and in postnatal development, such as decreased growth and development. Further studies should target differentiation of acute and chronic stress of different severities in humans on fetoplacental 11β-HSD2 activity.
Abbreviations
- 11β-HSD
11β-hydroxysteroid dehydrogenase
- 11β-HSD2
11β-hydroxysteroid dehydrogenase type 2
- BMI
body mass index
- E
cortisone
- F
cortisol
- HPA
hypothalamus-pituitary-adrenal.
Acknowledgments
Financial Support: There was no external funding to this study.
Clinical Trial Information: Clinical Trial Registration Number at the German Clinical Trials Register: DRKS-ID: DRKS00005741.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References



