-
PDF
- Split View
-
Views
-
Cite
Cite
Elena Tsourdi, M Carola Zillikens, Christian Meier, Jean-Jacques Body, Elena Gonzalez Rodriguez, Athanasios D Anastasilakis, Bo Abrahamsen, Eugene McCloskey, Lorenz C Hofbauer, Nuria Guañabens, Barbara Obermayer-Pietsch, Stuart H Ralston, Richard Eastell, Jessica Pepe, Andrea Palermo, Bente Langdahl, Fracture Risk and Management of Discontinuation of Denosumab Therapy: A Systematic Review and Position Statement by ECTS, The Journal of Clinical Endocrinology & Metabolism, Volume 106, Issue 1, January 2021, Pages 264–281, https://doi.org/10.1210/clinem/dgaa756
Close - Share Icon Share
Abstract
Denosumab discontinuation is characterized by an increase in bone turnover overriding pretreatment status, a rapid bone loss in the majority and multiple vertebral fractures (VFx) in some patients.
A working group of the European Calcified Tissue Society performed an updated systematic review of existing literature on changes of bone turnover, bone mineral density (BMD), and fracture risk after denosumab discontinuation and provided advice on management based on expert opinion.
Important risk factors for multiple VFx following denosumab cessation are prevalent VFx, longer duration off therapy, greater gain in hip BMD during therapy, and greater loss of hip BMD after therapy according to a retrospective analysis of the FREEDOM Extension Study. Case series indicate that prior bisphosphonate therapy mitigates the biochemical rebound phenomenon after denosumab discontinuation, but it is uncertain whether this attenuation prevents BMD loss and fractures. Current evidence indicates partial efficacy of subsequent antiresorptive treatment with results seemingly dependent on duration of denosumab treatment.
A careful assessment of indications to start denosumab treatment is advised, especially for younger patients. A case for long-term treatment with denosumab can be made for patients at high fracture risk already on denosumab treatment given the favorable efficacy and safety profile. In case of denosumab discontinuation, alternative antiresorptive treatment should be initiated 6 months after the final denosumab injection. Assessment of bone turnover markers may help define the optimal regimen, pending results of ongoing randomized controlled trials. Patients who have sustained VFx should be offered prompt treatment to reduce high bone turnover.
Denosumab, a monoclonal antibody against the receptor activator of nuclear factor κB ligand (RANKL), is a potent antiresorptive agent that profoundly suppresses bone turnover markers (BTMs), continuously increases bone mineral density (BMD), and reduces fracture risk, along with a good safety profile for up to 10 years (1). After first being approved for the treatment of postmenopausal osteoporosis at a dose of 60 mg subcutaneously every 6 months, it subsequently received approval at the same dose for: i) male osteoporosis, ii) glucocorticoid-induced osteoporosis, iii) women with low bone mass receiving adjuvant aromatase inhibitor therapy for breast cancer, and iv) men with low bone mass on androgen-deprivation therapy for nonmetastatic prostate cancer. Moreover, denosumab in different regimens has shown efficacy in the treatment of other conditions, including bone metastases, Paget disease of bone, fibrous dysplasia, avascular necrosis of bone, bone marrow edema, and multiple myeloma. By inhibiting osteoclast differentiation, activity, and survival, denosumab results in a profound decrease in the rate of bone remodeling (2). Bone biopsies performed in patients on long-term denosumab treatment confirmed low bone remodeling activity while ascertaining normal bone histology (3). Regarding BTMs, in the pivotal FREEDOM trial, denosumab was associated with median reductions of 86%, 72%, and 72% in concentrations of the bone resorption marker C-telopeptide of type 1 collagen (CTX) at months 1, 6, and 36, respectively. Concentrations of the bone formation marker procollagen type 1 N-terminal propeptide (PINP) decreased by 18%, 50%, and 76% at months 1, 6, and 36, respectively (4). With regard to BMD, at the completion of the 10-year FREEDOM Extension trial, the cumulative BMD increase from baseline amounted to 21.7% at the lumbar spine (LS) and 9.2% at the total hip (TH), consistent with a gradual increase in bone mass without a plateau effect for the entire duration of treatment (1). This continuous BMD increase with denosumab treatment has been attributed to an imbalance between a profusely suppressed bone remodeling and an unaffected modeling-based bone accrual, with a net effect favoring bone formation (5, 6). The persistent BMD increase also translated into a sustained fracture risk reduction, with the incidence of new vertebral fractures (VFx) and non-VFx remaining low during FREEDOM Extension (1).
Denosumab discontinuation leads to a complete and rapid reversal of its effects on BTMs and BMD. Six months after the last denosumab injection, BTMs increase rapidly with some interindividual variation, but the average values of BTMs exceed their baseline values 9 months after the last injection, and remain elevated until slowly decreasing to baseline levels approximately 30 months after the last injection (7-10). BMD gains are lost with a return to pretreatment baseline values after 1 to 2 years off treatment (7-10). The reversible effect of denosumab on bone remodeling was also confirmed by a histomorphometric study that revealed that women who discontinued treatment with denosumab for 2 years depicted bone remodeling levels similar to those of untreated postmenopausal women (11). Subsequently, there was concern in the medical community as to whether the rebound activation of bone turnover after denosumab discontinuation would translate into an increased fracture risk. Initially, a post hoc analysis of the FREEDOM phase 3 trial (4) seemed reassuring, reporting a comparable incidence of fractures in women having stopped denosumab or placebo; however, the median duration off therapy was only 8 months, an interval that appears to be too short to reveal possible fractures (12). An additional limitation of the study by Brown et al lies in the fact that VFx and nonvertebral fractures were analyzed together (12). A more thorough analysis based on patients discontinuing denosumab in the FREEDOM and FREEDOM Extension trials showed that, while the proportion of patients sustaining new VFx during the off-treatment period was similar to that in women discontinuing placebo, there was a larger proportion of patients suffering VFx who experienced multiple VFx when these patients discontinued denosumab (60.7%) than placebo (38.7%, P = .049) (13). Taken together with an increasing number of case reports and clinical case series describing multiple (≥ 2) VFx after denosumab cessation, it became clear that denosumab should not be stopped without considering an alternative treatment, and a number of scientific societies have issued position statements on this topic (14-16).
Following our previous systematic review (14), here we report new evidence regarding the pathophysiology and risk factors underlying the occurrence of multiple VFx after denosumab discontinuation and how different regimens of predenosumab and postdenosumab treatment with osteoporosis drugs affect the rebound phenomenon. We provide recommendations on how to mitigate the rebound increase in bone turnover following cessation of denosumab in the osteoporosis setting.
Materials and Methods
The systematic review was performed under the auspices of the Clinical Action Group of the Policy and Consensus Committee of the European Calcified Tissues Society (ECTS). We searched electronic databases (PubMed/MEDLINE) and ClinicalTrials.gov using MeSH terms “Denosumab” and “Osteoporosis” up to August 20, 2020. The effect of denosumab discontinuation on BMD, BTMs, bone histomorphometry, and clinical or morphometric VFx and/or nonvertebral fractures in postmenopausal women with osteopenia or osteoporosis has been extensively described in our previous review (14). Here, we included randomized controlled trials (RCTs) and observational studies that investigated both risk factors and the pathophysiology of the rebound effect after denosumab discontinuation as well as studies investigating the effects of predenosumab or postdenosumab treatment with bisphosphonates and other antiosteoporosis treatments on the decrease of BMD and/or increase of BTMs, and when available on fracture incidence. We included studies conducted in the oncology setting when these were performed using the denosumab dose approved for the treatment of osteoporosis. We also included studies in patients receiving glucocorticoid treatment. Studies conducted in cancer patients with metastatic disease, patients with multiple myeloma, giant cell tumor of bone, fibrous dysplasia, avascular necrosis, bone marrow edema, or other metabolic bone disease, such as Paget disease of bone, were not included. In view of sparse data concerning fracture incidence after denosumab discontinuation, we also included patient case series and searched for abstracts from the annual meetings of American Society for Bone and Mineral Research (ASBMR), ECTS, the European League Against Rheumatism, the American College of Rheumatology, the International Osteoporosis Foundation, the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and the Endocrine Society during 2017 to 2019 using the same terms. The ECTS Clinical Action Group of the Policy and Consensus Committee, E.T., M.C.Z., and B.L. planned this update. Three independent researchers (E.T., M.C.Z., and B.L.) reviewed all eligible studies. E.T., M.C.Z., and B.L. prepared the initial draft, and all other named authors—members of the ECTS Clinical Action Group of the Policy and Consensus Committee and the ECTS Board—participated in the interpretation and completion of the manuscript.
Results
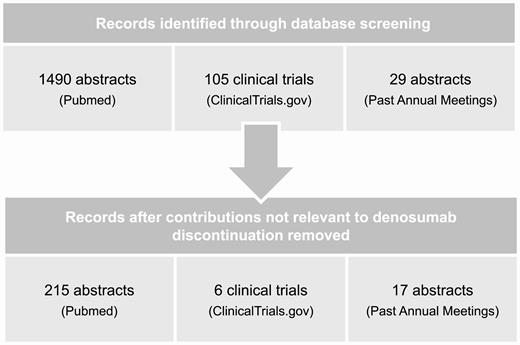
As part of the search for the systematic review, we identified 1490 abstracts on PubMed, 105 clinical trials on ClinicalTrials.gov, and 29 abstracts from past annual meetings of the societies mentioned in “Materials and Methods” using the terms stated there. After eliminating publications that were not pertinent to the subject of discontinuation of denosumab, we retained 215 abstracts on PubMed, 6 clinical trials on ClinicalTrials.gov and 17 abstracts from past annual meetings (Fig. 1).
Is the rebound effect on bone turnover pertinent only to postmenopausal osteoporosis?
Although initially described in the setting of postmenopausal osteoporosis (7-10), more recent data have confirmed rapid loss of BMD gains during 12-month off-denosumab therapy in patients receiving glucocorticoids for rheumatoid arthritis (17). Few data exist regarding the simultaneous discontinuation of denosumab and aromatase inhibitors (AIs) in patients with breast cancer. A preliminary analysis of the follow-up of the placebo-controlled ABCSG-18 trial, presented in abstract form, reported a significantly higher risk of clinical VFx and multiple clinical VFx in patients who stopped denosumab than in those who stopped placebo (hazard ratio [HR] 3.52 [0.98-12.64] and HR 2.44 [1.12-5.32]) (18). In a case series of 15 patients treated with AI for early-stage breast cancer who sustained nonmetastatic fragility VFx after denosumab discontinuation, 10 of the patients had discontinued denosumab simultaneously with AI therapy (19). The authors noted that VFx developed earlier in patients with longer denosumab treatment duration (r2 = 0.29, P = .04) and in patients without osteoporosis before denosumab (19). These results confirmed an earlier similar report (20).
Although the majority of fractures described to date after denosumab discontinuation have been VFx, which are typically multiple, recently hip fractures were also reported in a small case series in this setting (21). This is in line with previous larger observational studies that showed that loss of BMD during the off-treatment period is not localized only at the spine, but that hip BMD loss is equal to or even greater than the gain achieved during treatment (7, 9, 22). Furthermore, although initially multiple VFx as a consequence of cessation of denosumab treatment were reported only in female patients, recently a few male cases were also described (23).
What is the incidence of VFx after denosumab discontinuation?
In the post hoc analysis of the FREEDOM and FREEDOM Extension trial, which evaluated 1001 women who stopped denosumab or placebo during the FREEDOM study or its Extension and were followed for 9 to12 months after the last denosumab or placebo injection, the annualized risk of VFx was 7.1%, this rate being similar to the risk of 7.0% observed after discontinuation of placebo (13). However, the proportion of women with VFx who experienced multiple VFx was larger among those who discontinued denosumab (60.7%) compared to placebo (38.7%; P = .049), corresponding to a 3.4% and 2.2% risk of multiple VFx, respectively (13). Another observational study of 82 postmenopausal women who had been treated with denosumab for 4 to 8 years reported a similar incidence of VFx (8.5%) during the 12 months after denosumab discontinuation, even if 17 of these women were treated with bisphosphonates after stopping denosumab (9). Zanchetta et al reported an incidence of 10.5% in 38 postmenopausal women who were followed for 17 months after discontinuing long-term denosumab treatment (7 to 10 years) (10). In a different approach, Tripto-Shkolnik et al. evaluated real-world data from a large health care provider and reported on 1500 patients failing to refill their denosumab prescription for 3 months or more. The authors estimated that the incidence rate ratio of multiple VFx per 100 patient-years of follow-up was significantly higher in patients discontinuing denosumab as compared to persistent users (incidence risk ratio 14.63; 95% CI, 3.3-65.3) (24). It appears that a follow-up period shorter than the biological rebound might lead to an underestimation of the VFx risk (25). Nevertheless, it should be stated that at least part of the observed incidence of VFx is due to discontinuation of a drug with a known potent antifracture activity (ie, denosumab) in a usually high-risk population, and thus the return of the pretreatment fracture risk cannot be solely attributed to the rebound phenomenon.
Which are the risk factors for fractures on denosumab discontinuation?
The analysis of the FREEDOM Extension identified prevalent VFx, longer duration off therapy, greater gain in hip BMD during therapy, and greater loss of hip BMD off therapy as important risk factors for multiple VFx in patients having received at least 2 denosumab injections prior to discontinuation (13). An additional analysis of FREEDOM and FREEDOM Extension, presented in abstract form, showed that BMD loss during the off-treatment period was greatest among individuals who sustained multiple VFx, and greater among those who sustained a single VFx than those with no VFx (26). Thus, the rate of BMD loss per se could be a risk factor for (multiple) VFx. In a recent single-center observational study reporting on 35 patients sustaining VFx within a median of 11 months after their last denosumab injection, younger women seemed at higher risk, as the number of VFx was inversely associated with age (27). Younger age also seems to be a risk factor for bone loss, as younger patients lost more BMD at 1 year in the ZOLARMAB study investigating subsequent zoledronate treatment after denosumab discontinuation (28) as well as in the observational study by the ReoLaus cohort (29). In a large-scale population study among 1500 patients who had discontinued denosumab, patients with chronic kidney disease had a higher fracture risk (24). Anastasilakis et al identified vertebroplasty as a possible precipitating factor for further VFx during the immediate time following the procedure in a large case series of patients discontinuing denosumab (30). Subsequently, this finding was confirmed in other reports (27, 31, 32).
The concentration of the bone resorption marker CTX exceeded the upper limit of the normal range for premenopausal women by 2- to 3-fold at the time of VFx diagnosis (27, 32-34). Most likely, this increase is predominantly caused by discontinuation of denosumab; however, it cannot be ruled out that the fracture per se contributed to the increase. In a case series of 35 patients suffering 172 VFx after denosumab discontinuation, no correlation between CTX values and the number of VFx was found (27). Usually, CTX rapidly decreases on reinitiation of treatment (8); however, a persistent elevation of CTX, with suppression of bone formation markers, in a patient who suffered multiple VFx despite reinitiation of denosumab treatment has been reported recently (35). Longer denosumab treatment was associated with a higher number of VFx in the large case series by Anastasilakis and colleagues (30), and with earlier development of fractures in patients discontinuing denosumab administered for the prevention of bone loss during AI treatment (19). Complementary to these findings, CTX and BMD changes after denosumab discontinuation were also found to reflect the duration of treatment with denosumab, with CTX values increasing with the number of previously administered denosumab injections (27) and a longer on-treatment period being associated with a more pronounced bone loss after denosumab withdrawal (22). However, as mentioned earlier, the analysis of the FREEDOM Extension failed to identify denosumab treatment duration as a predictor of multiple VFx during the off-treatment period (13).
What is the influence of a pretreatment period with antiresorptive drugs on the magnitude of the rebound effect on bone after denosumab discontinuation?
There is no RCT on the putative effect of bisphosphonate administration before denosumab treatment on the rebound phenomenon, and most information derives from post hoc or retrospective analyses of clinical cohorts (Table 1). Pretreatment with bisphosphonates appeared to be associated with a diminished increase in BTMs in a small (n = 37) observational study of patients with osteoporosis who discontinued denosumab when compared to patients without bisphosphonate exposure (36). Whether these apparent effects on biochemical changes seen in patients pretreated with bisphosphonates correspond to a preservation of BMD gains after denosumab discontinuation is less certain. Prior treatment with bisphosphonates results in smaller BMD increases in patients transitioning to denosumab as compared to treatment-naive patients beginning denosumab therapy (37, 38), which may suggest that these patients have less BMD to lose. In contrast, a recent small study comparing BMD changes after denosumab discontinuation in postmenopausal women who were either treatment naive or had received bisphosphonates before starting denosumab indicated that BMD decline following cessation of denosumab treatment might not be affected by previous bisphosphonate therapy (39). Similarly, the change in BMD appeared to be unaffected by previous bisphosphonate treatment in 63 postmenopausal women during the first year after discontinuing on average 4 years of denosumab treatment in the ReoLaus cohort (29).
Effects of pretreatment on magnitude of the rebound effect on bone after denosumab discontinuation
| Design . | No. of patients . | Previous treatment used . | Duration of previous treatment, y . | Duration of Dmab treatment, y . | Duration of Dmab discontinuation, moa . | Effect on BTMs . | Effect on BMD . | Effect on VFx . | Ref. . |
|---|---|---|---|---|---|---|---|---|---|
| Retrospective analysis of patient case series | 37 (33 W/4 M) | 12: treatment-naive 25: ALN, IBN, RIS, ZOL (or combined) | 0.9-15 | 0.5-4.5 | 1-18 | Previous BP exposure prevented significant increase of CTX concentrations | NA | NA | (36) |
| Retrospective analysis of patient case series | 24 (all W) | 20: treatment-naive 1: TPTD 1: SERM 2: ALN | TPTD: 1 SERM: 5 ALN: 0.6-3 | 1-5 | 2-10 | NA | NA | Prior treatment did not prevent VFx (all 4 patients) | (30) |
| Retrospective analysis of patient case series | 14 (all W) | 4: treatment-naive 10: BP | NA | 1-4 | NA | NA | Prior BP treatment did not prevent BMD loss (all patients) | 1 treatment-naive and 1 BP pretreated patient had multiple VFx | (39) |
| Observational follow-up study after Dmab discontinuation in W with osteoporosis | 63 (all w) | 47: treatment-naive or > 2 y 12: ALN, IBN, RIS, ZOL 2: SERM 2: Strontium ranelate | BP: 0.6-11.2 SERM: 3-7 Strontium ranelate: 0.4-1 | 1-7 | 6-22 | NA | Prior BP treatment did not prevent BMD loss | (29) | |
| Retrospective analysis of large population data | 1500 (1380 W/120 M) | 120: treatment-naive 1380: ALN, RIS, ZOL | 0.5-15.8 | > 1 | > 9 | NA | NA | Prior treatment did not prevent multiple osteoporotic Fx | (24) |
| Retrospective analysis of patient case series | 9 (all W) | 2: treatment-naive 1: TPTD + BP 6: BP | TPTD: 2 BP: 3-10 | 1.5-4 | 1-15 | NA | NA | Prior treatment did not prevent VFx (all 7 patients) | (31) |
| Retrospective analysis of patient case series | 9 (all W) | 8: treatment-naive 1: BP | 1.5-4 | 3-10 | NA | NA | Prior BP treatment did not prevent VFx (all 8 patients) | (34) |
| Design . | No. of patients . | Previous treatment used . | Duration of previous treatment, y . | Duration of Dmab treatment, y . | Duration of Dmab discontinuation, moa . | Effect on BTMs . | Effect on BMD . | Effect on VFx . | Ref. . |
|---|---|---|---|---|---|---|---|---|---|
| Retrospective analysis of patient case series | 37 (33 W/4 M) | 12: treatment-naive 25: ALN, IBN, RIS, ZOL (or combined) | 0.9-15 | 0.5-4.5 | 1-18 | Previous BP exposure prevented significant increase of CTX concentrations | NA | NA | (36) |
| Retrospective analysis of patient case series | 24 (all W) | 20: treatment-naive 1: TPTD 1: SERM 2: ALN | TPTD: 1 SERM: 5 ALN: 0.6-3 | 1-5 | 2-10 | NA | NA | Prior treatment did not prevent VFx (all 4 patients) | (30) |
| Retrospective analysis of patient case series | 14 (all W) | 4: treatment-naive 10: BP | NA | 1-4 | NA | NA | Prior BP treatment did not prevent BMD loss (all patients) | 1 treatment-naive and 1 BP pretreated patient had multiple VFx | (39) |
| Observational follow-up study after Dmab discontinuation in W with osteoporosis | 63 (all w) | 47: treatment-naive or > 2 y 12: ALN, IBN, RIS, ZOL 2: SERM 2: Strontium ranelate | BP: 0.6-11.2 SERM: 3-7 Strontium ranelate: 0.4-1 | 1-7 | 6-22 | NA | Prior BP treatment did not prevent BMD loss | (29) | |
| Retrospective analysis of large population data | 1500 (1380 W/120 M) | 120: treatment-naive 1380: ALN, RIS, ZOL | 0.5-15.8 | > 1 | > 9 | NA | NA | Prior treatment did not prevent multiple osteoporotic Fx | (24) |
| Retrospective analysis of patient case series | 9 (all W) | 2: treatment-naive 1: TPTD + BP 6: BP | TPTD: 2 BP: 3-10 | 1.5-4 | 1-15 | NA | NA | Prior treatment did not prevent VFx (all 7 patients) | (31) |
| Retrospective analysis of patient case series | 9 (all W) | 8: treatment-naive 1: BP | 1.5-4 | 3-10 | NA | NA | Prior BP treatment did not prevent VFx (all 8 patients) | (34) |
Abbreviations: ALN, alendronate; BMD, bone mineral density; BP, bisphosphonate; BTMs, bone turnover markers; CTX, C-terminal-cross-linking telopeptide of type 1; Dmab, denosumab; Fx, fractures; RIS, risedronate; NA, not available; SERM, selective estrogen receptor modulator; TPTD, teriparatide; VFx, vertebral fractures; ZOL, zoledronate.
aDuration of discontinuation in months is calculated from the time point the next injection of Dmab would be due.
Effects of pretreatment on magnitude of the rebound effect on bone after denosumab discontinuation
| Design . | No. of patients . | Previous treatment used . | Duration of previous treatment, y . | Duration of Dmab treatment, y . | Duration of Dmab discontinuation, moa . | Effect on BTMs . | Effect on BMD . | Effect on VFx . | Ref. . |
|---|---|---|---|---|---|---|---|---|---|
| Retrospective analysis of patient case series | 37 (33 W/4 M) | 12: treatment-naive 25: ALN, IBN, RIS, ZOL (or combined) | 0.9-15 | 0.5-4.5 | 1-18 | Previous BP exposure prevented significant increase of CTX concentrations | NA | NA | (36) |
| Retrospective analysis of patient case series | 24 (all W) | 20: treatment-naive 1: TPTD 1: SERM 2: ALN | TPTD: 1 SERM: 5 ALN: 0.6-3 | 1-5 | 2-10 | NA | NA | Prior treatment did not prevent VFx (all 4 patients) | (30) |
| Retrospective analysis of patient case series | 14 (all W) | 4: treatment-naive 10: BP | NA | 1-4 | NA | NA | Prior BP treatment did not prevent BMD loss (all patients) | 1 treatment-naive and 1 BP pretreated patient had multiple VFx | (39) |
| Observational follow-up study after Dmab discontinuation in W with osteoporosis | 63 (all w) | 47: treatment-naive or > 2 y 12: ALN, IBN, RIS, ZOL 2: SERM 2: Strontium ranelate | BP: 0.6-11.2 SERM: 3-7 Strontium ranelate: 0.4-1 | 1-7 | 6-22 | NA | Prior BP treatment did not prevent BMD loss | (29) | |
| Retrospective analysis of large population data | 1500 (1380 W/120 M) | 120: treatment-naive 1380: ALN, RIS, ZOL | 0.5-15.8 | > 1 | > 9 | NA | NA | Prior treatment did not prevent multiple osteoporotic Fx | (24) |
| Retrospective analysis of patient case series | 9 (all W) | 2: treatment-naive 1: TPTD + BP 6: BP | TPTD: 2 BP: 3-10 | 1.5-4 | 1-15 | NA | NA | Prior treatment did not prevent VFx (all 7 patients) | (31) |
| Retrospective analysis of patient case series | 9 (all W) | 8: treatment-naive 1: BP | 1.5-4 | 3-10 | NA | NA | Prior BP treatment did not prevent VFx (all 8 patients) | (34) |
| Design . | No. of patients . | Previous treatment used . | Duration of previous treatment, y . | Duration of Dmab treatment, y . | Duration of Dmab discontinuation, moa . | Effect on BTMs . | Effect on BMD . | Effect on VFx . | Ref. . |
|---|---|---|---|---|---|---|---|---|---|
| Retrospective analysis of patient case series | 37 (33 W/4 M) | 12: treatment-naive 25: ALN, IBN, RIS, ZOL (or combined) | 0.9-15 | 0.5-4.5 | 1-18 | Previous BP exposure prevented significant increase of CTX concentrations | NA | NA | (36) |
| Retrospective analysis of patient case series | 24 (all W) | 20: treatment-naive 1: TPTD 1: SERM 2: ALN | TPTD: 1 SERM: 5 ALN: 0.6-3 | 1-5 | 2-10 | NA | NA | Prior treatment did not prevent VFx (all 4 patients) | (30) |
| Retrospective analysis of patient case series | 14 (all W) | 4: treatment-naive 10: BP | NA | 1-4 | NA | NA | Prior BP treatment did not prevent BMD loss (all patients) | 1 treatment-naive and 1 BP pretreated patient had multiple VFx | (39) |
| Observational follow-up study after Dmab discontinuation in W with osteoporosis | 63 (all w) | 47: treatment-naive or > 2 y 12: ALN, IBN, RIS, ZOL 2: SERM 2: Strontium ranelate | BP: 0.6-11.2 SERM: 3-7 Strontium ranelate: 0.4-1 | 1-7 | 6-22 | NA | Prior BP treatment did not prevent BMD loss | (29) | |
| Retrospective analysis of large population data | 1500 (1380 W/120 M) | 120: treatment-naive 1380: ALN, RIS, ZOL | 0.5-15.8 | > 1 | > 9 | NA | NA | Prior treatment did not prevent multiple osteoporotic Fx | (24) |
| Retrospective analysis of patient case series | 9 (all W) | 2: treatment-naive 1: TPTD + BP 6: BP | TPTD: 2 BP: 3-10 | 1.5-4 | 1-15 | NA | NA | Prior treatment did not prevent VFx (all 7 patients) | (31) |
| Retrospective analysis of patient case series | 9 (all W) | 8: treatment-naive 1: BP | 1.5-4 | 3-10 | NA | NA | Prior BP treatment did not prevent VFx (all 8 patients) | (34) |
Abbreviations: ALN, alendronate; BMD, bone mineral density; BP, bisphosphonate; BTMs, bone turnover markers; CTX, C-terminal-cross-linking telopeptide of type 1; Dmab, denosumab; Fx, fractures; RIS, risedronate; NA, not available; SERM, selective estrogen receptor modulator; TPTD, teriparatide; VFx, vertebral fractures; ZOL, zoledronate.
aDuration of discontinuation in months is calculated from the time point the next injection of Dmab would be due.
Whether previous exposure to bisphosphonates influences the occurrence of fractures after denosumab discontinuation is even more difficult to assess. Using health insurance data in a real-life setting, Tripto-Shkolnik et al. reported that pretreatment with bisphosphonates was not associated with reduced fracture risk following denosumab discontinuation (24). Furthermore, in patient case series reporting multiple VFx after cessation of denosumab, a variable number of patients had been treated with bisphosphonates before denosumab (27, 30-32). It should be noted that these studies were highly heterogeneous regarding the type of bisphosphonate, the duration of pretreatment, and the interval between discontinuation of bisphosphonate and denosumab introduction. Thus, studies specifically designed to investigate the potential protective effect of bisphosphonate prior treatment are warranted (40-42).
What is the evidence for postdenosumab treatment with antiresorptive drugs to prevent the rebound effect on bone after denosumab discontinuation?
Interestingly, current data suggest that longer denosumab treatment periods are associated with a more pronounced rebound in bone metabolism and subsequent bone loss after denosumab withdrawal (22, 28). However, it should be noted that in addition to duration of treatment, another factor that varies among studies is the definition of BMD reduction reported, that is, some studies use BMD reductions tailored on least significant change and others thresholds that are more arbitrary. A number of studies have investigated the effects of antiresorptive drugs after denosumab treatment for up to 2.5 years (ie, short duration) (Table 2). The effects of alendronate after denosumab were assessed in the DAPS (Denosumab Adherence Preference Satisfaction) study (43). In this 24-month, randomized, open-label crossover study, 250 postmenopausal women with low BMD were randomly assigned to either alendronate for 1 year followed by denosumab for 1 year or vice versa. Patients who received 1 year of alendronate after 1 year of denosumab maintained the BMD gained with denosumab during the first year (43). Further investigations of participant characteristics and the BMD response after transitioning from denosumab to alendronate revealed that patients experiencing larger BMD increases in year 1 on denosumab were more likely to lose BMD in year 2 on alendronate, while other participant characteristics were not predictive of the response in year 2 (44). The authors noted that this finding could be due to regression to the mean phenomenon, so more studies are warranted to confirm this finding. Moreover, there is a growing body of data regarding the subsequent use of zoledronate to mitigate bone loss after cessation of denosumab. In general, zoledronate was more effective in maintaining BMD when denosumab treatment did not exceed 2.5 years (see Table 2). An observational study of 11 women who received zoledronate with a median delay of 65 days after presumed cessation of denosumab activity (ie, 245 days after the last denosumab injection) reported minimal loss of BMD gained during denosumab treatment at all skeletal sites (45). It should be noted that these patients had initially been treated with romosozumab for 1 year before being switched to denosumab as part of the ARCH protocol (46). Similarly, a retrospective observational study of 18 postmenopausal women who had been treated with denosumab for an average of 1.5 years and were switched to zoledronate at an average of 274 days after the last denosumab injection showed a preservation of BMD gained during the denosumab treatment period (47). Furthermore, the initial results of the DATA-HD Extension trial, indicated that a single dose of zoledronate, given 5 to 7 months after the last denosumab injection, effectively maintains the BMD gains achieved with a combination of teriparatide/denosumab therapy followed by denosumab therapy alone at all skeletal sites (48).
Effects of subsequent treatment on the magnitude of the rebound effect after denosumab discontinuation (short duration of denosumab treatment of 2.5 years or less)
| Design . | No. of patients . | Duration of Dmab treatment, y . | Duration of Dmab discontinuation, mob . | Subsequent treatment used . | Effect on BTMs . | Effect on BMD . | Effect on VFx . | Ref. . |
|---|---|---|---|---|---|---|---|---|
| Randomized, open-label, cross-over study in W with postmenopausal osteoporosis/osteopenia | 126 (all W) | 1 | 0 | ALN | Smaller increase in comparison with end of Dmab period | Stabilization of BMD gained on Dmab | NA | (43) |
| Retrospective analysis of case series | 11 (all W) | 2 | 2 | ZOL | BTMs remained suppressed with ZOL treatment | Minimal loss of BMD gain during Dmab treatment at all 3 skeletal sites | NA | (45) |
| Retrospective observational study | 18 (all W) | 1.5 | 3 | ZOL | BTMs remained suppressed with ZOL treatment | Maintenance of BMD at all skeletal sites | None | (47) |
| Randomized, open-label study in W with postmenopausal osteoporosis | 53 (all W) | 1a | –1 to +1 | ZOL | NA | Maintenance of BMD at all skeletal sites | NA | (48) |
| Randomized, open-label, parallel-assignment study in postmenopausal W with osteopenia | 27 (all W) | 2.4 | 0 | ZOL | Small increase of BTMs during y 1 post ZOL and stabilization thereafter | Maintenance of BMD at LS and FN | 1/27 patients with VFx | (49) |
| Design . | No. of patients . | Duration of Dmab treatment, y . | Duration of Dmab discontinuation, mob . | Subsequent treatment used . | Effect on BTMs . | Effect on BMD . | Effect on VFx . | Ref. . |
|---|---|---|---|---|---|---|---|---|
| Randomized, open-label, cross-over study in W with postmenopausal osteoporosis/osteopenia | 126 (all W) | 1 | 0 | ALN | Smaller increase in comparison with end of Dmab period | Stabilization of BMD gained on Dmab | NA | (43) |
| Retrospective analysis of case series | 11 (all W) | 2 | 2 | ZOL | BTMs remained suppressed with ZOL treatment | Minimal loss of BMD gain during Dmab treatment at all 3 skeletal sites | NA | (45) |
| Retrospective observational study | 18 (all W) | 1.5 | 3 | ZOL | BTMs remained suppressed with ZOL treatment | Maintenance of BMD at all skeletal sites | None | (47) |
| Randomized, open-label study in W with postmenopausal osteoporosis | 53 (all W) | 1a | –1 to +1 | ZOL | NA | Maintenance of BMD at all skeletal sites | NA | (48) |
| Randomized, open-label, parallel-assignment study in postmenopausal W with osteopenia | 27 (all W) | 2.4 | 0 | ZOL | Small increase of BTMs during y 1 post ZOL and stabilization thereafter | Maintenance of BMD at LS and FN | 1/27 patients with VFx | (49) |
Abbreviations: ALN, alendronate; BMD, bone mineral density; BTMs, bone turnover markers; Dmab, denosumab; FN, femoral neck; LS, lumbar spine; M, men; NA, not available; RIS, risedronate; TH, total hip; TPTD, teriparatide; VFx, vertebral fractures; W, women; ZOL, zoledronate.
aThese patients had received combination therapy with Dmab and 2 different regimens of TPTD.
bDuration of discontinuation in months is calculated from the time point the next injection of Dmab would be. This was also the time point when subsequent treatment was administered.
Effects of subsequent treatment on the magnitude of the rebound effect after denosumab discontinuation (short duration of denosumab treatment of 2.5 years or less)
| Design . | No. of patients . | Duration of Dmab treatment, y . | Duration of Dmab discontinuation, mob . | Subsequent treatment used . | Effect on BTMs . | Effect on BMD . | Effect on VFx . | Ref. . |
|---|---|---|---|---|---|---|---|---|
| Randomized, open-label, cross-over study in W with postmenopausal osteoporosis/osteopenia | 126 (all W) | 1 | 0 | ALN | Smaller increase in comparison with end of Dmab period | Stabilization of BMD gained on Dmab | NA | (43) |
| Retrospective analysis of case series | 11 (all W) | 2 | 2 | ZOL | BTMs remained suppressed with ZOL treatment | Minimal loss of BMD gain during Dmab treatment at all 3 skeletal sites | NA | (45) |
| Retrospective observational study | 18 (all W) | 1.5 | 3 | ZOL | BTMs remained suppressed with ZOL treatment | Maintenance of BMD at all skeletal sites | None | (47) |
| Randomized, open-label study in W with postmenopausal osteoporosis | 53 (all W) | 1a | –1 to +1 | ZOL | NA | Maintenance of BMD at all skeletal sites | NA | (48) |
| Randomized, open-label, parallel-assignment study in postmenopausal W with osteopenia | 27 (all W) | 2.4 | 0 | ZOL | Small increase of BTMs during y 1 post ZOL and stabilization thereafter | Maintenance of BMD at LS and FN | 1/27 patients with VFx | (49) |
| Design . | No. of patients . | Duration of Dmab treatment, y . | Duration of Dmab discontinuation, mob . | Subsequent treatment used . | Effect on BTMs . | Effect on BMD . | Effect on VFx . | Ref. . |
|---|---|---|---|---|---|---|---|---|
| Randomized, open-label, cross-over study in W with postmenopausal osteoporosis/osteopenia | 126 (all W) | 1 | 0 | ALN | Smaller increase in comparison with end of Dmab period | Stabilization of BMD gained on Dmab | NA | (43) |
| Retrospective analysis of case series | 11 (all W) | 2 | 2 | ZOL | BTMs remained suppressed with ZOL treatment | Minimal loss of BMD gain during Dmab treatment at all 3 skeletal sites | NA | (45) |
| Retrospective observational study | 18 (all W) | 1.5 | 3 | ZOL | BTMs remained suppressed with ZOL treatment | Maintenance of BMD at all skeletal sites | None | (47) |
| Randomized, open-label study in W with postmenopausal osteoporosis | 53 (all W) | 1a | –1 to +1 | ZOL | NA | Maintenance of BMD at all skeletal sites | NA | (48) |
| Randomized, open-label, parallel-assignment study in postmenopausal W with osteopenia | 27 (all W) | 2.4 | 0 | ZOL | Small increase of BTMs during y 1 post ZOL and stabilization thereafter | Maintenance of BMD at LS and FN | 1/27 patients with VFx | (49) |
Abbreviations: ALN, alendronate; BMD, bone mineral density; BTMs, bone turnover markers; Dmab, denosumab; FN, femoral neck; LS, lumbar spine; M, men; NA, not available; RIS, risedronate; TH, total hip; TPTD, teriparatide; VFx, vertebral fractures; W, women; ZOL, zoledronate.
aThese patients had received combination therapy with Dmab and 2 different regimens of TPTD.
bDuration of discontinuation in months is calculated from the time point the next injection of Dmab would be. This was also the time point when subsequent treatment was administered.
A recent RCT investigated the efficacy of a single intravenous infusion of zoledronate to prevent bone loss in women with postmenopausal osteoporosis following discontinuation of denosumab treatment after attaining a nonosteoporotic BMD (NCT02499237). Fifty-seven postmenopausal women who had been treated with denosumab for a mean duration of 2.4 years and achieved osteopenia were randomly assigned to receive either a single 5 mg infusion of zoledronate (n = 27) or 2 additional 60 mg injections of denosumab (n = 30); both groups were subsequently followed for a period of 24 months. At 24 months, BMD at the LS did not differ from baseline in the group treated with zoledronate, but decreased in the denosumab group by 4.8% (P < .001) from the 12-month value and the difference in BMD changes between the 2 groups was statistically significant (P = .025). Similar results were observed at the femoral neck. Patients treated with zoledronate had small but significant increases in PINP and CTX during the first year and stabilization thereafter. In the denosumab group, BTMs did not change during the first 12 months but increased significantly at 15 months and remained elevated until the end of the study. Regarding fractures, 3 patients sustained VFx in the denosumab group, whereas 1 patient experienced a VFx 12 months after zoledronate infusion. The authors concluded that in women treated with denosumab for on average 2.4 years, a single infusion of zoledronate administered 6 months after the last denosumab injection prevents bone loss for at least 2 years independently of the rate of bone turnover, but recommended follow-up because these results might not be generalizable to all patients (49). Recently, the 1-year follow-up results of this RCT were published, showing that gains in BMD with denosumab treatment are maintained for 3 years through a single zoledronate infusion. The authors stated that this appears to be true for women who have been treated with denosumab for an average of 2.4 years and have attained a nonosteoporotic BMD during denosumab treatment (50).
A number of studies have investigated the effects of antiresorptive drugs after denosumab treatment for more than 2.5 years (ie, long duration) (Table 3). The observational study by McClung and colleagues reported attenuation of bone loss after denosumab discontinuation only in the 17 out of 82 patients who were either treated with various oral bisphosphonates or continued denosumab therapy, while demonstrating rapid bone loss in all other patients (9). Similar results were reported in the DATA-Follow-up study, in which only recipients of prompt antiresorptive treatment maintained the large BMD gains achieved by denosumab (51). In another recent observational study, 98 women were followed for 1 year after discontinuing denosumab (at least 2 doses); of those, 65 received no further treatment whereas 33 were switched to bisphosphonates (24 zoledronate, 2 oral risedronate, and 7 oral ibandronate). As expected, a significant BMD loss was observed at all sites in the nontreatment group. In the treatment group, BMD at the LS was significantly lower than baseline (ie, 6 months after last denosumab injection), whereas BMD at the TH was nonsignificantly lower. Moreover, although BTMs increased in both groups, this increase was less pronounced in bisphosphonate users and no patients in the treatment group experienced fragility fractures (52). Furthermore, in a group of 64 women who were treated with either selective estrogen receptor modulator (SERM) (n = 17), oral bisphosphonate (n = 35), or intravenous zoledronate (n = 12) after discontinuing denosumab (information about treatment duration not provided) in a real-world setting, treatment with a SERM could not prevent bone loss, whereas zoledronate therapy over 1 year led to BMD increases of a magnitude of 1.7% at the LS and 0.6% at the TH (53). Consistently, a recent case report described spontaneous multiple VFx in a patient discontinuing denosumab after a 6-year treatment period despite preventive treatment with raloxifene (54). Similarly, multiple VFx were reported in 2 postmenopausal women who had been treated with denosumab for 3 and 3.5 years despite preventive treatment with alendronate at denosumab discontinuation (55). Moreover, an observational study of 18 postmenopausal women who were treated with weekly risedronate for 3 months after 3-year denosumab therapy (mean duration) showed that this short-term risedronate treatment was unable to prevent bone loss (56).
Effects of subsequent treatment on magnitude of the rebound effect after denosumab discontinuation (long duration of denosumab treatment of more than 2.5 years
| Design . | No. of patients . | Duration of Dmab treatment, y . | Duration of Dmab discontinuation, moc . | Subsequent treatment used . | Effect on BTMs . | Effect on BMD . | Effect on VFx . | Ref . |
|---|---|---|---|---|---|---|---|---|
| Randomized, open-label, study in postmenopausal W and M with osteopenia | 60 (W and M) | 4.6 | 0-3 (and variable according to BTM) | ZOL | Increase of BTMs More pronounced with delayed ZOL administration | Subsequent loss of BMD at LS and TH more rapidly with delayed ZOL administration | NA | (28) |
| Observational follow-up study after 8 y of Dmab treatment for postmenopausal osteoporosis | 82 (all W) | 8 | 0a | ALN: 7 Dmab: 5 RIS: 4 IBN: 2 TPTD: 2 | NA | Smaller decreases at LH and TH in patients on subsequent treatment | 6/8 patients with VFx had no treatment | (9) |
| Observational follow-up study of Dmab treatment for postmenopausal osteoporosis (real-world setting) | 120 (all W) | 2-5 | 0 | ZOL | BTMs within upper normal range after ZOL treatmentb | Subsequent partial loss of BMD gain during Dmab treatment (more pronounced at TH than LS) | 3 singular VFx 4 peripheral VFx | (57) |
| Observational follow-up study of Dmab treatment for postmenopausal osteoporosis or concomitant antiaromatase (real-world setting) | 63 (all W) | 1-7 | 5-16 | ALN: 9 ZOL: 33 (1-3 infusions according to BTMs) ALN + ZOL: 10 IBN: 1 SERM-ALN: 1 No treatment: 9 | Variable response necessitating replacement of ALN by ZOL, or repeated ZOL infusions, mostly because of BTM increase | Dependent on BTMs values | 1 VFx with no treatment, 1 VFx with ZOL, 1 multiple VFx with ALN | (29) |
| Observational follow-up study after 4 y of osteoporosis treatment (sequence of Dmab, TPTD, or combination of both) for postmenopausal osteoporosis | 50 (all W) | 2-4 | 1-7 | Dmab: 10 Oral BP: 10 ZOL: 8 | NA | Stabilization of BMD gained on Dmab when subsequent treatment but significant loss at LS, TH, and FN when no treatment | NA | (51) |
| Observational follow-up study after at least 1 y of Dmab treatment for postmenopausal osteoporosis | 98 (all W) | > 1 | 0-12 | ZOL: 24 RIS: 2 IBN: 7 | Increase in all patients but less pronounced when subsequent BP treatment | Significant loss at all sites when no treatment. In patients with BP therapy less pronounced bone loss (especially at TH) | 2 patients with VFx in nontreated group/none in treated | (52) |
| Observational follow-up study of Dmab treatment for postmenopausal osteoporosis (real-world setting) | 64 (all W) | NA | NA | SERM: 17 Oral BP: 35 ZOL: 12 | More pronounced with SERM and less pronounced with ZOL treatment | SERM treatment could not prevent bone loss, smaller BMD increase at LS with oral BP, more pronounced BMD increase at LS and TH with ZOL | VFx in 17% of patients on SERM vs 3% of patients on oral BP vs 0% of patients on ZOL | (53) |
| Observational follow-up study after at least 1 y of Dmab treatment for postmenopausal osteoporosis | 18 (all W) | 1-4 | 0 | RIS | Increase of BTMs | RIS treatment could not prevent bone loss at all sites | 1 VFx | (56) |
| Case series | 6 (all W) | 7 | 0 | ZOL | NA | Subsequent loss of BMD gain during Dmab treatment (more pronounced at TH than LS) | None | (58) |
| Case report | 1 (W) | 6 | 7 | SERM | BTMs remained suppressed with SERM treatment | NA | Multiple VFx while on SERM | (54) |
| Case series | 2 (W) | 3-3.5 | 1-8 | ALN | Case 1: increase after ALN initiation Case 2: no significant increase | Case 1: rapid loss at LS despite ALN Case 2: BMD stabilization gained on Dmab | ALN did not prevent multiple VFx | (55) |
| Retrospective analysis of case series | 22 (all W) | 2.5 | 0 | ZOL | NA | Subsequent loss of BMD gain during Dmab treatment (more pronounced at LS and FN than TH) | None | (59) |
| Retrospective review of patients records | 35 | 1-5 | 6-9 | Oral BP: 20 ZOL: 15 | NA | Subsequent loss of BMD gain during Dmab in 11/20 patients on oral BP and 8/15 on ZOL | None | (60) |
| Retrospective analysis of case series | 20 (all ♀) | > 1 | 8-12 | ZOL | BTMs decreased at mo 3 after ZOL, but reaugmented at mo 12 after ZOL | NA | NA | (61) |
| Design . | No. of patients . | Duration of Dmab treatment, y . | Duration of Dmab discontinuation, moc . | Subsequent treatment used . | Effect on BTMs . | Effect on BMD . | Effect on VFx . | Ref . |
|---|---|---|---|---|---|---|---|---|
| Randomized, open-label, study in postmenopausal W and M with osteopenia | 60 (W and M) | 4.6 | 0-3 (and variable according to BTM) | ZOL | Increase of BTMs More pronounced with delayed ZOL administration | Subsequent loss of BMD at LS and TH more rapidly with delayed ZOL administration | NA | (28) |
| Observational follow-up study after 8 y of Dmab treatment for postmenopausal osteoporosis | 82 (all W) | 8 | 0a | ALN: 7 Dmab: 5 RIS: 4 IBN: 2 TPTD: 2 | NA | Smaller decreases at LH and TH in patients on subsequent treatment | 6/8 patients with VFx had no treatment | (9) |
| Observational follow-up study of Dmab treatment for postmenopausal osteoporosis (real-world setting) | 120 (all W) | 2-5 | 0 | ZOL | BTMs within upper normal range after ZOL treatmentb | Subsequent partial loss of BMD gain during Dmab treatment (more pronounced at TH than LS) | 3 singular VFx 4 peripheral VFx | (57) |
| Observational follow-up study of Dmab treatment for postmenopausal osteoporosis or concomitant antiaromatase (real-world setting) | 63 (all W) | 1-7 | 5-16 | ALN: 9 ZOL: 33 (1-3 infusions according to BTMs) ALN + ZOL: 10 IBN: 1 SERM-ALN: 1 No treatment: 9 | Variable response necessitating replacement of ALN by ZOL, or repeated ZOL infusions, mostly because of BTM increase | Dependent on BTMs values | 1 VFx with no treatment, 1 VFx with ZOL, 1 multiple VFx with ALN | (29) |
| Observational follow-up study after 4 y of osteoporosis treatment (sequence of Dmab, TPTD, or combination of both) for postmenopausal osteoporosis | 50 (all W) | 2-4 | 1-7 | Dmab: 10 Oral BP: 10 ZOL: 8 | NA | Stabilization of BMD gained on Dmab when subsequent treatment but significant loss at LS, TH, and FN when no treatment | NA | (51) |
| Observational follow-up study after at least 1 y of Dmab treatment for postmenopausal osteoporosis | 98 (all W) | > 1 | 0-12 | ZOL: 24 RIS: 2 IBN: 7 | Increase in all patients but less pronounced when subsequent BP treatment | Significant loss at all sites when no treatment. In patients with BP therapy less pronounced bone loss (especially at TH) | 2 patients with VFx in nontreated group/none in treated | (52) |
| Observational follow-up study of Dmab treatment for postmenopausal osteoporosis (real-world setting) | 64 (all W) | NA | NA | SERM: 17 Oral BP: 35 ZOL: 12 | More pronounced with SERM and less pronounced with ZOL treatment | SERM treatment could not prevent bone loss, smaller BMD increase at LS with oral BP, more pronounced BMD increase at LS and TH with ZOL | VFx in 17% of patients on SERM vs 3% of patients on oral BP vs 0% of patients on ZOL | (53) |
| Observational follow-up study after at least 1 y of Dmab treatment for postmenopausal osteoporosis | 18 (all W) | 1-4 | 0 | RIS | Increase of BTMs | RIS treatment could not prevent bone loss at all sites | 1 VFx | (56) |
| Case series | 6 (all W) | 7 | 0 | ZOL | NA | Subsequent loss of BMD gain during Dmab treatment (more pronounced at TH than LS) | None | (58) |
| Case report | 1 (W) | 6 | 7 | SERM | BTMs remained suppressed with SERM treatment | NA | Multiple VFx while on SERM | (54) |
| Case series | 2 (W) | 3-3.5 | 1-8 | ALN | Case 1: increase after ALN initiation Case 2: no significant increase | Case 1: rapid loss at LS despite ALN Case 2: BMD stabilization gained on Dmab | ALN did not prevent multiple VFx | (55) |
| Retrospective analysis of case series | 22 (all W) | 2.5 | 0 | ZOL | NA | Subsequent loss of BMD gain during Dmab treatment (more pronounced at LS and FN than TH) | None | (59) |
| Retrospective review of patients records | 35 | 1-5 | 6-9 | Oral BP: 20 ZOL: 15 | NA | Subsequent loss of BMD gain during Dmab in 11/20 patients on oral BP and 8/15 on ZOL | None | (60) |
| Retrospective analysis of case series | 20 (all ♀) | > 1 | 8-12 | ZOL | BTMs decreased at mo 3 after ZOL, but reaugmented at mo 12 after ZOL | NA | NA | (61) |
Abbreviations: ALN, alendronate; BMD, bone mineral density; BP, bisphosphonate; BTMs, bone turnover markers; Dmab, denosumab; FN, femoral neck; IBN, ibandronate; LS, lumbar spine; M, men; NA, not available; RIS, risedronate; SERM, selective estrogen receptor modulator; TH, total hip; TPTD, teriparatide; VFx, vertebral fractures; W, women; ZOL, zoledronate.
aOnly 17 of the 82 patients received antiresorptive treatment after denosumab discontinuation.
bBTMs were available for only 27 of the 120 patients of the study.
cDuration of discontinuation in months is calculated from the time point the next injection of denosumab would be. This was also the time point when subsequent treatment was administered.
Effects of subsequent treatment on magnitude of the rebound effect after denosumab discontinuation (long duration of denosumab treatment of more than 2.5 years
| Design . | No. of patients . | Duration of Dmab treatment, y . | Duration of Dmab discontinuation, moc . | Subsequent treatment used . | Effect on BTMs . | Effect on BMD . | Effect on VFx . | Ref . |
|---|---|---|---|---|---|---|---|---|
| Randomized, open-label, study in postmenopausal W and M with osteopenia | 60 (W and M) | 4.6 | 0-3 (and variable according to BTM) | ZOL | Increase of BTMs More pronounced with delayed ZOL administration | Subsequent loss of BMD at LS and TH more rapidly with delayed ZOL administration | NA | (28) |
| Observational follow-up study after 8 y of Dmab treatment for postmenopausal osteoporosis | 82 (all W) | 8 | 0a | ALN: 7 Dmab: 5 RIS: 4 IBN: 2 TPTD: 2 | NA | Smaller decreases at LH and TH in patients on subsequent treatment | 6/8 patients with VFx had no treatment | (9) |
| Observational follow-up study of Dmab treatment for postmenopausal osteoporosis (real-world setting) | 120 (all W) | 2-5 | 0 | ZOL | BTMs within upper normal range after ZOL treatmentb | Subsequent partial loss of BMD gain during Dmab treatment (more pronounced at TH than LS) | 3 singular VFx 4 peripheral VFx | (57) |
| Observational follow-up study of Dmab treatment for postmenopausal osteoporosis or concomitant antiaromatase (real-world setting) | 63 (all W) | 1-7 | 5-16 | ALN: 9 ZOL: 33 (1-3 infusions according to BTMs) ALN + ZOL: 10 IBN: 1 SERM-ALN: 1 No treatment: 9 | Variable response necessitating replacement of ALN by ZOL, or repeated ZOL infusions, mostly because of BTM increase | Dependent on BTMs values | 1 VFx with no treatment, 1 VFx with ZOL, 1 multiple VFx with ALN | (29) |
| Observational follow-up study after 4 y of osteoporosis treatment (sequence of Dmab, TPTD, or combination of both) for postmenopausal osteoporosis | 50 (all W) | 2-4 | 1-7 | Dmab: 10 Oral BP: 10 ZOL: 8 | NA | Stabilization of BMD gained on Dmab when subsequent treatment but significant loss at LS, TH, and FN when no treatment | NA | (51) |
| Observational follow-up study after at least 1 y of Dmab treatment for postmenopausal osteoporosis | 98 (all W) | > 1 | 0-12 | ZOL: 24 RIS: 2 IBN: 7 | Increase in all patients but less pronounced when subsequent BP treatment | Significant loss at all sites when no treatment. In patients with BP therapy less pronounced bone loss (especially at TH) | 2 patients with VFx in nontreated group/none in treated | (52) |
| Observational follow-up study of Dmab treatment for postmenopausal osteoporosis (real-world setting) | 64 (all W) | NA | NA | SERM: 17 Oral BP: 35 ZOL: 12 | More pronounced with SERM and less pronounced with ZOL treatment | SERM treatment could not prevent bone loss, smaller BMD increase at LS with oral BP, more pronounced BMD increase at LS and TH with ZOL | VFx in 17% of patients on SERM vs 3% of patients on oral BP vs 0% of patients on ZOL | (53) |
| Observational follow-up study after at least 1 y of Dmab treatment for postmenopausal osteoporosis | 18 (all W) | 1-4 | 0 | RIS | Increase of BTMs | RIS treatment could not prevent bone loss at all sites | 1 VFx | (56) |
| Case series | 6 (all W) | 7 | 0 | ZOL | NA | Subsequent loss of BMD gain during Dmab treatment (more pronounced at TH than LS) | None | (58) |
| Case report | 1 (W) | 6 | 7 | SERM | BTMs remained suppressed with SERM treatment | NA | Multiple VFx while on SERM | (54) |
| Case series | 2 (W) | 3-3.5 | 1-8 | ALN | Case 1: increase after ALN initiation Case 2: no significant increase | Case 1: rapid loss at LS despite ALN Case 2: BMD stabilization gained on Dmab | ALN did not prevent multiple VFx | (55) |
| Retrospective analysis of case series | 22 (all W) | 2.5 | 0 | ZOL | NA | Subsequent loss of BMD gain during Dmab treatment (more pronounced at LS and FN than TH) | None | (59) |
| Retrospective review of patients records | 35 | 1-5 | 6-9 | Oral BP: 20 ZOL: 15 | NA | Subsequent loss of BMD gain during Dmab in 11/20 patients on oral BP and 8/15 on ZOL | None | (60) |
| Retrospective analysis of case series | 20 (all ♀) | > 1 | 8-12 | ZOL | BTMs decreased at mo 3 after ZOL, but reaugmented at mo 12 after ZOL | NA | NA | (61) |
| Design . | No. of patients . | Duration of Dmab treatment, y . | Duration of Dmab discontinuation, moc . | Subsequent treatment used . | Effect on BTMs . | Effect on BMD . | Effect on VFx . | Ref . |
|---|---|---|---|---|---|---|---|---|
| Randomized, open-label, study in postmenopausal W and M with osteopenia | 60 (W and M) | 4.6 | 0-3 (and variable according to BTM) | ZOL | Increase of BTMs More pronounced with delayed ZOL administration | Subsequent loss of BMD at LS and TH more rapidly with delayed ZOL administration | NA | (28) |
| Observational follow-up study after 8 y of Dmab treatment for postmenopausal osteoporosis | 82 (all W) | 8 | 0a | ALN: 7 Dmab: 5 RIS: 4 IBN: 2 TPTD: 2 | NA | Smaller decreases at LH and TH in patients on subsequent treatment | 6/8 patients with VFx had no treatment | (9) |
| Observational follow-up study of Dmab treatment for postmenopausal osteoporosis (real-world setting) | 120 (all W) | 2-5 | 0 | ZOL | BTMs within upper normal range after ZOL treatmentb | Subsequent partial loss of BMD gain during Dmab treatment (more pronounced at TH than LS) | 3 singular VFx 4 peripheral VFx | (57) |
| Observational follow-up study of Dmab treatment for postmenopausal osteoporosis or concomitant antiaromatase (real-world setting) | 63 (all W) | 1-7 | 5-16 | ALN: 9 ZOL: 33 (1-3 infusions according to BTMs) ALN + ZOL: 10 IBN: 1 SERM-ALN: 1 No treatment: 9 | Variable response necessitating replacement of ALN by ZOL, or repeated ZOL infusions, mostly because of BTM increase | Dependent on BTMs values | 1 VFx with no treatment, 1 VFx with ZOL, 1 multiple VFx with ALN | (29) |
| Observational follow-up study after 4 y of osteoporosis treatment (sequence of Dmab, TPTD, or combination of both) for postmenopausal osteoporosis | 50 (all W) | 2-4 | 1-7 | Dmab: 10 Oral BP: 10 ZOL: 8 | NA | Stabilization of BMD gained on Dmab when subsequent treatment but significant loss at LS, TH, and FN when no treatment | NA | (51) |
| Observational follow-up study after at least 1 y of Dmab treatment for postmenopausal osteoporosis | 98 (all W) | > 1 | 0-12 | ZOL: 24 RIS: 2 IBN: 7 | Increase in all patients but less pronounced when subsequent BP treatment | Significant loss at all sites when no treatment. In patients with BP therapy less pronounced bone loss (especially at TH) | 2 patients with VFx in nontreated group/none in treated | (52) |
| Observational follow-up study of Dmab treatment for postmenopausal osteoporosis (real-world setting) | 64 (all W) | NA | NA | SERM: 17 Oral BP: 35 ZOL: 12 | More pronounced with SERM and less pronounced with ZOL treatment | SERM treatment could not prevent bone loss, smaller BMD increase at LS with oral BP, more pronounced BMD increase at LS and TH with ZOL | VFx in 17% of patients on SERM vs 3% of patients on oral BP vs 0% of patients on ZOL | (53) |
| Observational follow-up study after at least 1 y of Dmab treatment for postmenopausal osteoporosis | 18 (all W) | 1-4 | 0 | RIS | Increase of BTMs | RIS treatment could not prevent bone loss at all sites | 1 VFx | (56) |
| Case series | 6 (all W) | 7 | 0 | ZOL | NA | Subsequent loss of BMD gain during Dmab treatment (more pronounced at TH than LS) | None | (58) |
| Case report | 1 (W) | 6 | 7 | SERM | BTMs remained suppressed with SERM treatment | NA | Multiple VFx while on SERM | (54) |
| Case series | 2 (W) | 3-3.5 | 1-8 | ALN | Case 1: increase after ALN initiation Case 2: no significant increase | Case 1: rapid loss at LS despite ALN Case 2: BMD stabilization gained on Dmab | ALN did not prevent multiple VFx | (55) |
| Retrospective analysis of case series | 22 (all W) | 2.5 | 0 | ZOL | NA | Subsequent loss of BMD gain during Dmab treatment (more pronounced at LS and FN than TH) | None | (59) |
| Retrospective review of patients records | 35 | 1-5 | 6-9 | Oral BP: 20 ZOL: 15 | NA | Subsequent loss of BMD gain during Dmab in 11/20 patients on oral BP and 8/15 on ZOL | None | (60) |
| Retrospective analysis of case series | 20 (all ♀) | > 1 | 8-12 | ZOL | BTMs decreased at mo 3 after ZOL, but reaugmented at mo 12 after ZOL | NA | NA | (61) |
Abbreviations: ALN, alendronate; BMD, bone mineral density; BP, bisphosphonate; BTMs, bone turnover markers; Dmab, denosumab; FN, femoral neck; IBN, ibandronate; LS, lumbar spine; M, men; NA, not available; RIS, risedronate; SERM, selective estrogen receptor modulator; TH, total hip; TPTD, teriparatide; VFx, vertebral fractures; W, women; ZOL, zoledronate.
aOnly 17 of the 82 patients received antiresorptive treatment after denosumab discontinuation.
bBTMs were available for only 27 of the 120 patients of the study.
cDuration of discontinuation in months is calculated from the time point the next injection of denosumab would be. This was also the time point when subsequent treatment was administered.
Regarding zoledronate, most data show that this potent antiresorptive agent was less effective in maintaining BMD when previous denosumab treatment exceeds 2.5 years compared to a shorter duration of denosumab therapy (see Table 3). In a case series of 6 women receiving a single infusion of 5 mg zoledronate 6 months after the last denosumab injection, a partial BMD preservation was observed at the LS, whereas a complete loss of bone gain was noted at the TH 2 years after zoledronate infusion (58). Conversely, Lehmann et al reported a 38% loss of BMD gain at the LS and 43% loss of BMD gain at the TH in a group of 22 patients in whom BMD was measured 2.5 years after zoledronate infusion (59). None of these 28 postmenopausal women experienced fractures (58, 59). Recently, the same group reported on 120 women with postmenopausal osteoporosis who had been treated with denosumab for 2 to 5 years and subsequently received one infusion of 5 mg zoledronate 6 months after the last denosumab injection. The authors of this study found that 66% of BMD gained with denosumab was retained at the LS and 49% at the TH at a median of 2.5 years after denosumab discontinuation (ie, 2 years after zoledronate infusion). In this cohort, previous antiresorptive treatment or prevalent fractures had no impact on the decrease of BMD, and all bone loss occurred within the first 18 months after zoledronate infusion (57). Importantly, a recent retrospective review of 35 patients who received either zoledronate or oral bisphosphonates after stopping denosumab indicated that prescribing oral bisphosphonates an average of 182 days after the last denosumab injection resulted in a greater decline in BMD, whereas administering zoledronate an average of 275 days after the last denosumab injection showed a trend toward preserving or increasing BMD (60). However, the exact timing of BMD evaluation was not stated in this retrospective case series, which has been presented only in abstract form (60).
Results of the first year of an ongoing RCT investigating subsequent zoledronate treatment after denosumab discontinuation were recently published (NCT03087851) (28). Sixty postmenopausal women and elderly men who had been treated with denosumab for a mean duration of 4.6 years were randomly assigned to receive a single 5-mg zoledronate infusion either at 6 months after the last denosumab injection (6M group, n = 20), at 9 months (9M group, n = 20), or when BTMs were increased (observation group, n = 20). The patients in the 2 latter groups were monitored monthly and promptly received an infusion of zoledronate in case CTX increased to a level representing 50% above the range for postmenopausal women and elderly men or if they sustained a fragility fracture. The authors report that 12 months after zoledronate infusion, BMD had decreased significantly at the LS, TH, and FN in all 3 groups without differences between the groups. The decline in BMD was more rapid in the 9M and the observation group, which may at least in theory lead to a higher fracture risk. Two women, both from the 9M group, experienced a VFx. In addition, 10 patients from the 6M group needed retreatment with zoledronate 6 or 12 months after the initial treatment compared to 5 patients in each of the other 2groups. The authors of this RCT conclude that treatment with zoledronate irrespective of the timing did not fully prevent BMD loss in patients with osteopenia who had been treated for 4.6 years with denosumab (28). Concurrent to these results, an observational study of 20 postmenopausal women receiving a single infusion of 5-mg zoledronate when BTMs exceeded the upper limit of the premenopausal reference ranges showed a secondary increase in BTMs after an initial decrease after 3 months. The authors of this study suggested closer BTMs monitoring, for example, every 3 months, to identify patients experiencing a relapse in rebound bone turnover increase who could be eligible for a targeted early readministration of zoledronate (61).
A number of ongoing RCTs are further investigating the following topics: i) which antiresorptive drug to administer after denosumab discontinuation, ii) at what interval after denosumab discontinuation, iii) at what dose and at what frequency, and iv) for which duration (Table 4). These studies are expected to provide more specific information that will guide clinical action.
Future and ongoing randomized controlled studies on discontinuation of denosumab treatment
| Study No. . | Setting . | No. of patients, estimated . | Design . | Primary outcome . | Further outcomes . |
|---|---|---|---|---|---|
| NCT03755193 | Postmenopausal osteoporosis | 90 | Treatment with BP, SERM, or calcitriol after 2 y of denosumab | BMD changes (LS, TH) | NA |
| NCT04177940 | GIO | 45 | Treatment with ALN, or early ZOLa, or late ZOLb | BTM changes at various time points after random assignment | NA |
| NCT03868033 | Postmenopausal osteoporosis | 100 | Continuous treatment with denosumab for 2 y, or 1 y ZOL followed by 1 y denosumab, or continuous treatment with ZOL for 2 y, or treatment with ZOL for 1 y followed by close observation | BMD changes (TH, FN) | i) BMD changes (LS) ii) BTMs changes iii) incidence of clinical osteoporotic Fx |
| NCT03396315 | Idiopathic osteoporosis | 34 | Treatment with ALN for 1 y or 1 infusion of ZOL after 1-3 y of denosumab | BMD changes (LS) | NA |
| NCT03623633 | Postmenopausal osteoporosis | 50 | Treatment with ALN or raloxifene after 2 y of denosumab | BTM changes at various time points after random assignment | NA |
| Study No. . | Setting . | No. of patients, estimated . | Design . | Primary outcome . | Further outcomes . |
|---|---|---|---|---|---|
| NCT03755193 | Postmenopausal osteoporosis | 90 | Treatment with BP, SERM, or calcitriol after 2 y of denosumab | BMD changes (LS, TH) | NA |
| NCT04177940 | GIO | 45 | Treatment with ALN, or early ZOLa, or late ZOLb | BTM changes at various time points after random assignment | NA |
| NCT03868033 | Postmenopausal osteoporosis | 100 | Continuous treatment with denosumab for 2 y, or 1 y ZOL followed by 1 y denosumab, or continuous treatment with ZOL for 2 y, or treatment with ZOL for 1 y followed by close observation | BMD changes (TH, FN) | i) BMD changes (LS) ii) BTMs changes iii) incidence of clinical osteoporotic Fx |
| NCT03396315 | Idiopathic osteoporosis | 34 | Treatment with ALN for 1 y or 1 infusion of ZOL after 1-3 y of denosumab | BMD changes (LS) | NA |
| NCT03623633 | Postmenopausal osteoporosis | 50 | Treatment with ALN or raloxifene after 2 y of denosumab | BTM changes at various time points after random assignment | NA |
Abbreviations: ALN, alendronate; BMD, bone mineral density; BP, bisphosphonate; BTMs, bone turnover markers; CTX, C-terminal-cross-linking telopeptide of type 1; FN, femoral neck; Fx, fractures; GIO, glucocorticoid-induced osteoporosis; LS, lumbar spine; NA, not available; SERM, selective estrogen receptor modulator; TH, total hip; ZOL, zoledronate.
aEarly ZOL: zoledronate infusion (5 mg; 6 mo after last denosumab dose).
bLate ZOL: zoledronate infusion (5 mg; 9 mo after last denosumab dose).
Future and ongoing randomized controlled studies on discontinuation of denosumab treatment
| Study No. . | Setting . | No. of patients, estimated . | Design . | Primary outcome . | Further outcomes . |
|---|---|---|---|---|---|
| NCT03755193 | Postmenopausal osteoporosis | 90 | Treatment with BP, SERM, or calcitriol after 2 y of denosumab | BMD changes (LS, TH) | NA |
| NCT04177940 | GIO | 45 | Treatment with ALN, or early ZOLa, or late ZOLb | BTM changes at various time points after random assignment | NA |
| NCT03868033 | Postmenopausal osteoporosis | 100 | Continuous treatment with denosumab for 2 y, or 1 y ZOL followed by 1 y denosumab, or continuous treatment with ZOL for 2 y, or treatment with ZOL for 1 y followed by close observation | BMD changes (TH, FN) | i) BMD changes (LS) ii) BTMs changes iii) incidence of clinical osteoporotic Fx |
| NCT03396315 | Idiopathic osteoporosis | 34 | Treatment with ALN for 1 y or 1 infusion of ZOL after 1-3 y of denosumab | BMD changes (LS) | NA |
| NCT03623633 | Postmenopausal osteoporosis | 50 | Treatment with ALN or raloxifene after 2 y of denosumab | BTM changes at various time points after random assignment | NA |
| Study No. . | Setting . | No. of patients, estimated . | Design . | Primary outcome . | Further outcomes . |
|---|---|---|---|---|---|
| NCT03755193 | Postmenopausal osteoporosis | 90 | Treatment with BP, SERM, or calcitriol after 2 y of denosumab | BMD changes (LS, TH) | NA |
| NCT04177940 | GIO | 45 | Treatment with ALN, or early ZOLa, or late ZOLb | BTM changes at various time points after random assignment | NA |
| NCT03868033 | Postmenopausal osteoporosis | 100 | Continuous treatment with denosumab for 2 y, or 1 y ZOL followed by 1 y denosumab, or continuous treatment with ZOL for 2 y, or treatment with ZOL for 1 y followed by close observation | BMD changes (TH, FN) | i) BMD changes (LS) ii) BTMs changes iii) incidence of clinical osteoporotic Fx |
| NCT03396315 | Idiopathic osteoporosis | 34 | Treatment with ALN for 1 y or 1 infusion of ZOL after 1-3 y of denosumab | BMD changes (LS) | NA |
| NCT03623633 | Postmenopausal osteoporosis | 50 | Treatment with ALN or raloxifene after 2 y of denosumab | BTM changes at various time points after random assignment | NA |
Abbreviations: ALN, alendronate; BMD, bone mineral density; BP, bisphosphonate; BTMs, bone turnover markers; CTX, C-terminal-cross-linking telopeptide of type 1; FN, femoral neck; Fx, fractures; GIO, glucocorticoid-induced osteoporosis; LS, lumbar spine; NA, not available; SERM, selective estrogen receptor modulator; TH, total hip; ZOL, zoledronate.
aEarly ZOL: zoledronate infusion (5 mg; 6 mo after last denosumab dose).
bLate ZOL: zoledronate infusion (5 mg; 9 mo after last denosumab dose).
Discussion
Discontinuation of denosumab treatment is characterized by rapid reversal of its favorable skeletal effects attributed to a transient increase in bone turnover overriding pretreatment status, a response commonly described as the rebound phenomenon. It has been postulated that osteoclast precursors remaining dormant during the treatment period retain their activity and/or that a high RANKL/osteoprotegerin ratio ensues after denosumab is cleared from the patient’s circulation (62). Anastasilakis et al found a significant increase in the expression of genes characterizing osteoclast activity as well as a reduced expression of microRNAs that downregulate osteoclastogenesis and osteoclast activity in patients having sustained clinical VFx after denosumab was stopped (63). An increase in serum RANKL concentrations was identified by another group supporting the hypothesis of a sudden loss of osteoclast inhibition after denosumab clearance (64). In the latter study, serum levels of the Wnt inhibitors sclerostin and dickkopf-1 were decreased, although the pathophysiological significance of this finding needs further investigation (64). In another recent case report, elevated levels of circulating fibroblast growth factor 23 and hypercalcemia were noted in a patient with breast cancer stopping high-dose denosumab treatment after 5 years, although it remains to be clarified whether fibroblast growth factor 23 concentrations could be related to increased bone resorption triggered by discontinuation of long-term denosumab treatment (65). In an experimental approach using intravital imaging technology to visualize osteoclast dynamics on the endocortical surface of tibiae in live mice, McDonald and colleagues found that 3 to 4 weeks after withdrawal of denosumab treatment, multinucleated osteoclasts had formed. This increase in active osteoclasts resulted in increased concentrations of serum bone resorption markers and subsequent deterioration in bone microarchitecture (66). In an elegant recent study, Jähn-Rickert et al assessed bone osteocyte morphology in iliac crest bone biopsies from patients during denosumab treatment and after its discontinuation (67). They found a reduction in viable osteocytes and a higher number of micropetrotic osteocyte lacunae during active treatment. Twelve months post–denosumab discontinuation the number of empty osteocyte lacunae did not change, nor was there a direct effect on osteocyte viability as assessed by apoptosis assays (67).
Although the exact pathophysiology of the rebound phenomenon is yet to be fully elucidated, all existing data point to the fact that denosumab discontinuation results in an augmented fracture risk compared to continuation of the drug (68). Thus, the following questions arise in clinical practice: i) what is the optimal treatment duration with denosumab for patients at high risk for fracture, ii) what to do after denosumab discontinuation, and iii) how to manage VFx occurring after denosumab discontinuation.
What is the optimal treatment duration with denosumab for patients at high risk for fracture?
The treat to target concept for osteoporosis suggests that treatment decisions should be made with the goal of achieving an acceptable level of fracture risk (69, 70). In recent years, BMD has been propagated as a useful treatment target, and an ASBMR–National Osteoporosis Foundation working group on goal-directed treatment for osteoporosis has suggested a hip T score target of greater than –2.5, with a higher level of confidence for a T score target of greater than –2.0 (71), although even higher thresholds, that is, a T score target of greater than –1.5, have been proposed (72). Nevertheless, this BMD-only–based concept is not universally embraced and more data are needed until consensus is reached (73). It is also important to realize that the concept of a limited treatment duration for osteoporosis was developed based on the retention of bisphosphonates in bone and therefore cannot automatically be applied to denosumab. Until consensus is reached, it appears prudent to consider additional risk factors to define a high fracture–risk profile as has been delineated from various international societies (15, 74). These risk factors are mainly linked to prevalent osteoporotic fractures and concomitant comorbidities (eg, continuous use of glucocorticoids or aromatase inhibitors, diabetes, inflammatory diseases, frailty) (15, 74). Thus, in patients considered to be at high risk for fracture, the efficacy and safety profile of denosumab allows for long-term treatment, with existing data supporting a duration of up to 10 years (1). Regarding serious adverse effects, the recently published data based on up to 7 to 10 years of denosumab therapy reported one atypical femur fracture in the long term-group during year 4 of the extension (year 7 of denosumab treatment) and one in the crossover group during year 4 of the extension (year 3 of denosumab treatment). Moreover, 7 cases of osteonecrosis of the jaw were reported in the long-term group and 6 cases in the crossover group (1). Of the 13 cases, one affected patient discontinued the study (long-term group) and one withdrew consent (crossover group). Pending longer data, whether the 10-year treatment threshold can be superseded in a number of patients (eg, with renal insufficiency, limited life expectancy, explicit patient wish.) remains to be decided on an individual basis. However, taking into consideration that a longer duration of treatment also involves a risk of unplanned discontinuation, a very careful assessment of the indications to start denosumab treatment in the first place should be performed, especially in younger patients, who may be at higher risk of fractures or bone loss following discontinuation (22, 27-29). Regarding elective dental procedures for which treatment discontinuation is demanded by the dentist, it seems prudent to perform the procedure preferably approximately 5 months after the last denosumab injection and resume treatment as soon as the lesion is healed, although this is based on expert opinion only.
Which is the optimal treatment strategy after denosumab discontinuation?
Should a patient on denosumab treatment be no longer considered at high risk for fragility fractures (according to the parameters mentioned earlier), discontinuation of denosumab treatment may be considered. Existing data underline the necessity of subsequently prescribing a potent antiresorptive agent to prevent/limit the rebound phenomenon. Current evidence on using a SERM is not very promising, although as mentioned previously, the quality of data on this agent is very low and there are 2 ongoing RCTs that are expected to shed more light on the efficacy of SERMs (Table 4). To minimize the high bone turnover following denosumab discontinuation, it seems therefore preferable to prescribe the more potent bisphosphonates. Current data point to the fact that the duration of denosumab treatment is an important determinant of the extent of the rebound phenomenon, which may have potential implications on the type and duration of subsequent treatment with bisphosphonates (see Tables 2 and 3). Although the optimal bisphosphonate regimen is also not certain yet (see Table 4), in case of an oral bisphosphonate it seems prudent to perform a dual-energy x-ray absorptiometry (DXA) around the time the next denosumab injection would be due and start treatment at that point. Subsequently, we suggest measuring BTMs at 3 months after initiation of the oral bisphosphonate to monitor adherence and efficacy. With regard to bisphosphonate treatment, an adequate response is defined as a level below the mean found in healthy premenopausal women. Specifically, the CTX concentration should be below 280 ng/L and the PINP concentration below 35 µg/L (75). This is supported by data from the ReoLaus cohort, in which patients with stable BMD at the LS at 1 year follow-up had mean CTX values close to this threshold (336 ng/mL), significantly different from the mean CTX in patients who lost BMD (537 ng/mL), even though both are in the premenopausal range (29). In case of adequate response and low fracture risk, treatment with an oral bisphosphonate can be pursued for 1 to 2 years (ie, the estimated time of the rebound upregulation of bone turnover), although it is recommended to continue the assessment of BTMs initially after 3 months and if stable every 6 months to ensure that they remain within the lower half of the premenopausal reference range (75). At 1 to 2 years, a reevaluation including DXA should be performed and the decision to discontinue oral bisphosphonate treatment should align with current guidelines (73).
In case of poor gastrointestinal tolerability of oral bisphosphonates, which often leads to lack of adherence to this therapy, inadequate response to oral bisphosphonates, as well as in all patients with a long duration of denosumab treatment, it is recommended to administer zoledronate intravenously. The timing of this infusion is of great importance and ongoing RCTs seek to clarify this issue. After the initial observational studies (58, 59), it was speculated that zoledronate might be more efficient if administered when the rebound effect had already begun (45). However, caution is needed because existing case series have reported multiple VFx at very early time points after one missed denosumab injection (19), or even second rebound-associated VFx (76, 77). In addition, the study by Sølling et al demonstrated very rapid increases in CTX and rapid bone loss in patients with delayed infusions of zoledronate (28). Pending additional robust data, a pragmatic approach is to begin treatment with zoledronate 6 months after the last denosumab injection and monitor the effect with BTMs, for example, 3 and 6 months after the zoledronate infusion; in case of increased BTMs (ie, above the mean found in age- and sex-matched cohorts) repeated infusion of zoledronate should be considered (Fig. 2). In case BTMs are not available for monitoring the patients, a pragmatic approach could be administrating a second infusion of zoledronate 6 months after the first infusion. Similar to oral bisphosphonates, and in case of otherwise low fracture risk, the duration of zoledronate treatment should be tailored to the duration of increased bone turnover, although as mentioned earlier, the optimal duration of zoledronate treatment will have to be defined through novel prospective data.
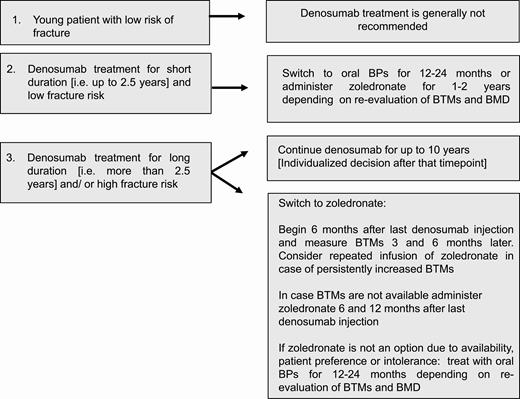
Options for osteoporosis management with denosumab. BMD, bone mineral density; BPs, bisphosphonates; BTMs, bone turnover markers.
If treatment with zoledronate after discontinuation of longer-term denosumab treatment is not possible because of unavailability, intolerance, or patient preferences, treatment with oral bisphosphonates can be attempted. Owing to sparse available data on the effectiveness of oral bisphosphonates in preventing increase in bone turnover and loss of bone mass after discontinuation of denosumab after a longer duration of treatment (9, 51, 53, 60), it is recommended to monitor the response with BTMs and DXA.
How to manage vertebral fractures occurring after denosumab discontinuation?
There is currently no recommendation for the management of (multiple) VFx occurring after denosumab discontinuation. As mentioned previously, existing evidence discourages the use of vertebroplasty because it has been identified as a possible precipitating risk factor for subsequent VFx. Thus, it is important to quickly counteract the increased bone turnover, and since denosumab is a very potent antiresorptive agent with the ability to suppress BTMs in a matter of days (78), a case for prompt reinitiation of denosumab treatment can be made (Fig. 3). Attention should be drawn to the fact that reinitiation of denosumab therapy does not fully obliterate the risk of further VFx (76, 77). Alternatively, zoledronic acid or oral bisphosphonates could be prescribed given their ability to rapidly suppress bone turnover (79, 80). Another option is a combination of denosumab with an osteoanabolic agent (eg, teriparatide) that can be prescribed to stimulate bone formation and at the same time avoid the transient but rapid decrease of BMD, especially at cortical sites, reported with teriparatide subsequent to denosumab therapy (81). As a consequence, monotherapy with teriparatide after denosumab at the present time should be discouraged, especially during the immediate period after fracture. Indeed, the combination of denosumab with teriparatide, although currently off label in most European countries, has been recently shown to be a highly efficacious concept at least concerning BMD (82); however, the combination treatment period should be followed by a potent antiresorptive therapy, such as zoledronate, to consolidate BMD gains (48). Moreover, novel data report that romosozumab following denosumab treatment also results in BMD gains, although more modest than with romosozumab treatment in treatment-naive patients (83). Whether this will translate into fracture prevention remains to be proven, since recently a patient experiencing multiple VFx under romosozumab treatment as a sequential therapy to denosumab was reported (84). Pending tailored RCTs for men, recommendations deriving from postmenopausal osteoporosis are currently used also for male osteoporosis.
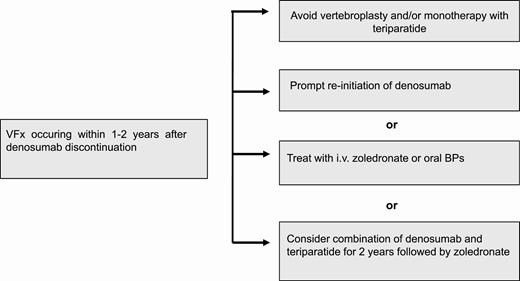
Options for management of vertebral fractures occurring after denosumab discontinuation. BPs, bisphosphonates; VFx, vertebral fractures.
Conclusion
Discontinuation of denosumab following at least 2 denosumab injections carries a significant risk of a rebound effect, manifesting as considerable loss of bone mass gained during the period of denosumab treatment and an augmented risk for (multiple) VFx. To limit this risk, it is currently recommended either to continue denosumab therapy or to prescribe a potent bisphosphonate when denosumab is stopped. A shorter duration of denosumab treatment (ie, up to 2.5 years) in patients with otherwise low fracture risk could justify treatment with an oral bisphosphonate for 1 to 2 years. In case of previous intolerance to oral bisphosphonates, expected poor adherence, or polypharmacy, zoledronate can be given once and repeated if bone turnover is still inappropriately high. Patients who have been treated with denosumab for a longer period (ie, > 2.5 years) or who are at persistently high risk for fracture should receive zoledronate. Pending results of ongoing RCTs on the optimal bisphosphonate regimen, BTMs can provide clinical guidance on the timing and duration of zoledronate treatment. It is currently not recommended to start treatment with teriparatide after denosumab discontinuation. Patients sustaining multiple VFx after stopping denosumab need prompt treatment to reduce the high bone turnover.
Abbreviations
- AIs
aromatase inhibitors
- BMD
bone mineral density
- BTMs
Bone turnover markers
- CTX
C-telopeptide of type 1 collagen
- ECTS
European Calcified Tissue Society
- LS
lumbar spine
- PINP
procollagen type 1 N-terminal propeptide
- RANKL
receptor activator of nuclear factor κB ligand
- RTC
randomized controlled trials
- SERM
selective estrogen receptor modulator
- TH
total hip
- VFx
vertebral fractures
Acknowledgments
The authors would like to acknowledge Giulia Furesi for her help with the preparation of the figures.
Financial Support: This work was not supported by any grant.
Author Contributions: Literature research, data collection and drafting first version of the manuscript: E.T., M.C.Z., and B.L.. Revising manuscript content: E.T., M.C.Z., C.M., J.J.B., E.G.R., A.D.A., B.A., E.M., L.C.H., N.G., B.O.P., S.H.R., R.E., J.P., A.P., and B.L. Approval of final version of manuscript: E.T., M.C.Z., C.M., J.J.B, E.G.R., A.D.A., B.A., E.M., L.C.H., N.G., B.O.P., S.H.R., R.E., J.P., A.P., and B.L.
Additional Information
Disclosure Summary: Dr Tsourdi has received research funding from MSD; honoraria for lectures from Amgen, UCB, Shire, and Kyowa Kirin; and educational grants from Shire and UCB. Prof Zillikens has received honoraria for lectures or advice from Amgen, Kyowa Kirin, Eli Lilly, Shire, and UCB. Prof Meier has received consultancy/research funding from Amgen, Eli Lilly, Gedeon Richter, Roche Diagnostics, and UCB. Prof Body has received consultancy fees from Amgen and Sandoz. Dr Gonzalez Rodriguez has nothing to disclose. Dr Anastasilakis has received lecture fees from Amgen, Eli Lilly, and VIANEX. Prof Abrahamsen has received institutional research grants from UCB, Novartis, and Kyowa Kirin UK; and consulting or speaker fees from UCB, Kyowa Kirin UK, Amgen, and Eli Lilly. Prof McCloskey has received research funding from Amgen, Consilient Healthcare, GSK, Hologic, I3 Innovus, Internis, IOF, Lilly, Merck, MRC, Novartis, Pfizer, Roche, Sanofi-Aventis, Servier, UCB, Unilever, and Versus Arthritis, as well as advisory board or speakers fees from Amgen, ActiveSignal, AstraZeneca, Consilient Healthcare, Fresenius Kabi, GSK, Hologic, Lilly, Synexus, and UCB. Prof Hofbauer has received consultancy fees from Alexion, Amgen, Shire, and UCB and support for clinical studies for his institution from Amgen, Alexion, Ascendis, and Shire. Dr Guañabens has received honoraria for advisory boards or lectures from Amgen, Eli Lilly, and UCB. Prof Obermayer-Pietsch has received research funding from IDS and ViennaLab; and educational grants and lecture and advisory board honoraria from Gedeon Richter, IDS, Shire, and Kyowa Kirin. Prof Ralston has received research funding to his institution from Eli Lilly, Amgen/UCB, and Kyowa Kirin. Prof Eastell has received consultancy funding from IDS, Roche Diagnostics, GSK Nutrition, FNIH, Mereo, Lilly, Sandoz, Nittobo, Abbvie, Samsung, Haoma Medica, CL Bio, Biocon, Lyramid, and Viking; and grant funding from Nittobo, IDS, Roche, Amgen, and Alexion. Dr Pepe has nothing to disclose. Dr Palermo has received lecture fees and research funding from Amgen. Prof Langdahl has received research funding from Amgen and Novo Nordisk; and honoraria for advisory board and lectures from Amgen, UCB, Eli Lilly, Gedeon-Richter, and Gilead.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.



