-
PDF
- Split View
-
Views
-
Cite
Cite
Dorina Ylli, Leonard Wartofsky, Kenneth D Burman, Evaluation and Treatment of Amiodarone-Induced Thyroid Disorders, The Journal of Clinical Endocrinology & Metabolism, Volume 106, Issue 1, January 2021, Pages 226–236, https://doi.org/10.1210/clinem/dgaa686
Close - Share Icon Share
Abstract
Amiodarone is a class III antiarrhythmic drug containing 37% iodine by weight, with a structure similar to that of thyroid hormones. Deiodination of amiodarone releases large amounts of iodine that can impair thyroid function, causing either hypothyroidism or thyrotoxicosis in susceptible individuals reflecting ~20% of patients administered the drug. Not only the excess iodine, but also the amiodarone (or its metabolite, desethylamiodarone) itself may cause thyroid dysfunction by direct cytotoxicity on thyroid cells. We present an overview of the epidemiology and pathophysiology of amiodarone-induced thyroid disorders, with a focus on the various forms of clinical presentation and recommendations for personalized management of each form.
Amiodarone is a widely prescribed benzofuranic antiarrhythmic drug rich in iodine that may affect both thyroid function tests and thyroid function itself, inducing hypothyroidism or thyrotoxicosis. Hypothyroidism is most likely to occur in individuals with underlying Hashimoto disease, but it may also occur in patients with no morphological or serological thyroid abnormalities. Thyrotoxicosis can present in 2 forms, either as an iodine-induced thyrotoxicosis (type 1) or as a destructive thyroiditis (type 2). In this article, we present a patient with amiodarone-induced thyrotoxicosis (AIT) and discuss its underlying pathophysiology. The biochemical and imaging tools used for an appropriate diagnosis and differentiation between type 1 and type 2 AIT are discussed, as is a personalized approach to management of a critically ill patient with AIT.
Case presentation
A 54-year-old man with a history of coronary artery disease and myocardial infarction presented to the emergency department with sustained monomorphic ventricular tachycardia resulting in frequent shocks from his implantable cardioverter defibrillator. His condition quickly deteriorated, and after admission to the intensive care unit, he was intubated and extracorporeal membrane oxygenation administered as required. Among other medications, he had been treated for ventricular arrythmia with amiodarone for the previous 2 years at a dose of 200 mg/d, and that dose had recently been increased to 150 mg twice daily. His cardiac rhythm converted to normal sinus rhythm, but frequent episodes of ventricular tachycardia/fibrillation compromising hemodynamic stability necessitated readmission to the intensive care unit. Two weeks after admission to the hospital, thyroid function tests included an elevated free T4 (FT4) of 2.42 ng/dL (normal range 0.76-1.46 ng/dL), a normal T3 of 90 ng/dL (normal range 60-180 ng/dL), and a low TSH of 0.026 uIU/mL (normal range 0.4-4 uIU/mL). There was no medical history of personal or familial thyroid disease.
Epidemiology of thyroid dysfunction in amiodarone-treated patients
Amiodarone is a class III antiarrhythmic drug used in the management of ventricular and atrial arrhythmias. It is used both in life-threatening emergencies such as ventricular fibrillation/pulseless ventricular tachycardia unresponsive to defibrillation (1) and for maintenance therapy in patients who have had sustained ventricular tachyarrhythmias, particularly those with left ventricular dysfunction (2). In selected patients, amiodarone is indicated in atrial tachyarrhythmias, particularly in those patients who need rhythm control or who are refractory to atrioventricular nodal blockade and in whom other antiarrhythmic drugs are contraindicated because of a reduced ejection fraction (2). Apart from its potential benefits, amiodarone possesses a relatively high incidence of adverse side effects, such as pulmonary toxicity, cardiac QT prolongation, photosensitivity, blue skin discoloration, corneal microdeposits, optic neuropathy, hepatic enzyme elevation, and thyroid disorders (3). Amiodarone liposolubility enables prolonged storage in high concentrations in fat and muscle tissue. This characteristic of amiodarone, combined with a very long plasma half-life (about 60-142 days), explains why adverse effects can occur and persist long after discontinuation of the drug (4).
The incidence of amiodarone-induced thyroid disorders is estimated to vary from 2% to 24% (5, 6). Typically, hypothyroidism will manifest within a short time (< 3 months) after amiodarone initiation in 10% to 20% of cases, but can present later (> 1 year) in 5% to 10% of cases (4). Kinoshita et al. described a cohort of 1279 patients under treatment with amiodarone and observed a median time to onset of amiodarone induced thyroid disease of 183 days for hypothyroidism and 720 days for thyrotoxicosis (7). Thyrotoxicosis occurs less often than hypothyroidism with an incidence of 5% to 10% (4, 5, 8), with higher incidence in areas of iodine deficiency (9). Either hypothyroidism or thyrotoxicosis also can present in a subclinical form. In a cohort of 267 patients treated with amiodarone, subclinical hypothyroidism was observed in 26% of patients. In the same cohort, thyrotoxicosis was observed in 5% of the patients and its main presentation was subclinical (10).
Although amiodarone-induced hypothyroidism (AIH) may occur in patients with an apparently normal thyroid gland and absent evidence of thyroid autoimmunity, most frequently it develops in patients with underlying chronic autoimmune thyroiditis, with a higher prevalence in women and in subjects in iodine-replete areas (2, 11, 12). Women taking amiodarone are more predisposed to develop AIH at a female:male ratio of 1.5:1 (8), as was noted in a large metanalysis of 1972 patients in whom AIH occurred in 19.2% of women and 13.3% of men (13). However, underlying Hashimoto thyroiditis is the most common risk factor for the development and persistence of AIH and is the likely explanation for the female preponderance (14). AIT instead appears to be more common in men (~3.9%-8.5% males vs 0.4% females) (15, 16) but may reflect the underlying sex difference in propensity to cardiac arrhythmias.
The incidence of AIT has been reported to be higher in iodine-depleted areas (10%), whereas AIH is more frequent in iodine-sufficient areas (22%) (17). Among AIT patients, AIT type 2 is more prevalent in iodine-sufficient areas (11, 18).
The risk of developing thyroid or liver dysfunction appears similar in those on low-dose amiodarone compared with those on high-dose amiodarone and suggests that amiodarone-related thyroid toxicity is not dose-dependent (3, 12, 13, 18). Last, it is vital to appreciate that AIT may carry significant morbidity and mortality because 33% of patients with AIT-related thyroid storm admitted to an intensive care unit had a mortality rate reaching 23% (19).
Mechanisms of amiodarone-induced thyroid disorder
The effect of amiodarone on thyroid function can present as the result of either its iodine-rich content or by non-iodine-related mechanisms (20, 21), although neither presentation appears related to the daily or cumulative dose (12, 13, 18).
Iodine-related mechanisms.
Each 200-mg amiodarone tablet contains 75 mg of iodine, with ~7 mg of free iodine released by deiodination. This amount, which is notably higher than the suggested normal iodine daily requirement (0.15-0.30 mg), results in iodine overload (20, 22). The pathophysiologic response of the thyroid gland will vary as follows depending on whether the gland is normal, affected by Hashimoto thyroiditis, or has some underlying functional autonomy (Fig. 1).
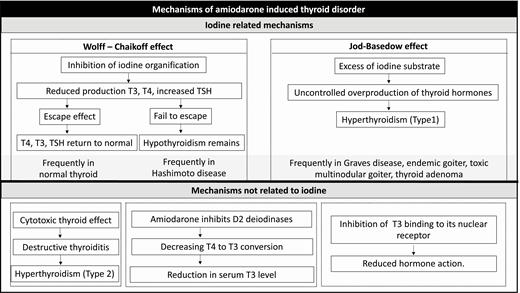
Physiologic thyroidal iodine organification does not proceed in the presence of iodine overload. Instead, as a function of the Wolff-Chaikoff effect, inhibition of organification is observed and thereby diminishing hormone biosynthesis (23). As a result, T3 and T4 production decreases, and if sufficiently reduced, serum TSH will subsequently increase. The resumption of normal iodine organification and hormone synthesis has been termed “escape from the acute Wolff-Chaikoff effect” and is marked by decreased sodium/iodide symporter function and return of serum TSH, free T3 (FT3), and FT4 levels to baseline values (20). Hypothyroidism will persist in those patients in whom the thyroid fails to escape from the Wolff-Chaikoff effect.
Presentation of an iodine overload to the thyroid gland can result in the so-called “Jod-Basedow phenomenon” or iodine-induced thyrotoxicosis. Generally, it occurs in subjects with underlying predisposition to autonomous thyroid function, and particularly those who reside in iodine-deficient areas (20). In this circumstance, the surfeit of iodine substrate is responsible for uncontrolled overproduction of the thyroid hormones (20).
Several studies indicate that exposure to excess iodine can inhibit pituitary D2 activity, causing a decrease in negative feedback of the hypothalamic-pituitary-thyroid axis and inducing an elevation of serum TSH levels. However, the mechanism has not been fully elucidated and the increase in TSH conceivably may come as a response to thyroid hormone reduction (T4, T3) resulting from the Wolff-Chaikoff effect (24, 25).
Mechanism not related to iodine.
Amiodarone can also cause direct toxicity to the thyroid gland. Amiodarone inhibits deiodinases thereby decreasing conversion of T4 to T3 and rT3 to T2 (18, 26, 27). As a result, there will be a reduction in serum T3 level and an increase in rT3. Furthermore, either amiodarone itself or its metabolite, desethylamiodarone, can inhibit T3 binding to its nuclear receptor, thus reducing the expression of thyroid hormone-related genes and hormone action (Fig. 1). Finally, amiodarone or desethylamiodarone may have a direct cytotoxic effect and thereby mediate apoptosis in thyroid cells resulting in gland alterations histologically consistent with a destructive thyroiditis (28–30).
Thus, changes in thyroid hormone levels resulting from amiodarone therapy can come as the result of a direct effect of amiodarone on the thyroid gland and/or the inhibitory effect of amiodarone on deiodinases (Fig. 1).
Clinical manifestations and diagnosis of thyroid disorders
In a euthyroid subject with a normal thyroid gland, amiodarone administration will result in slight elevations of TSH, T4, FT4, and rT3 for about the first month of therapy, only to normalize in the following 3 to 6 months (31). Because of deiodinase inhibition, serum T3 levels will remain reduced and typically will be below the reference range.
Hypothyroidism is usually the result of the inability to escape from the Wolff-Chaikoff effect and/or the presence of underlying Hashimoto thyroiditis or positive antithyroperoxidase (TPO) antibodies. The degree of hypothyroidism may be either subclinical or overt. Sixty percent of AIH is transient, resolving on its own 2 to 4 months after amiodarone cessation. In patients with no preexisting or concomitant thyroid abnormality, the transient form is more common (32).
Although thyrotoxicosis is less frequent than hypothyroidism in patients taking amiodarone, the potential clinical consequences are more severe. The latter patients often have already compromising cardiac conditions, and a rise in thyroid hormone levels further increase the risk of atrial and ventricular arrhythmias. Understanding the underlying pathophysiology and attaining a specific diagnosis of the type of AIT is crucial to delivery of the most appropriate treatment.
There are 2 main mechanisms by which amiodarone causes thyrotoxicosis: excessive thyroid hormone production and thyroid destruction (33).
Type 1 AIT occurs when the thyroid gland increases the production of thyroid hormones in response to exposure to an iodine overload (18, 34). Predisposing underlying conditions include presence of thyroid tissue with autonomous function such as in multinodular goiters or Graves’ disease, as well as low iodine intake and/or residence in an area with iodine deficiency rendering greater vulnerability to a Jod-Basedow effect when exposed to excess iodine. Even though the time of onset of AIT is unpredictable, type 1 usually occurs within two to six months from initiation of amiodarone therapy (35).
Because type 2 AIT is marked by thyroid tissue destruction with subsequent release of the thyroid hormones into the circulation, it is believed to be due to a direct toxic effect of the amiodarone drug itself and to not be directly related to the iodine overload. It can occur years after initiation of amiodarone therapy (27-32 months) or after the drug is discontinued, with 23% developing thyrotoxicosis after amiodarone withdrawal (35). In the latter patients, the course is usually that of subacute thyroiditis with an initial phase of thyrotoxicosis followed by euthyroidism and/or hypothyroidism.
Type 2 AIT is more frequent than type 1 AIT (11) and it progresses to subsequent hypothyroidism more frequently than is seen after non-amiodarone-related subacute thyroiditis (17% vs 5%) (36).
AIT is designated to be a mixed type 1 and type 2 form when characteristics of both type 1 and type 2 are present concurrently, which serves to make both diagnosis and treatment more complex.
Diagnostic tools
Effective diagnosis of the type of amiodarone-induced disorder is facilitated by the availability of various laboratory tests and imaging modalities that require interpretation and integration within the clinical context of a given patient.
Biochemical laboratory studies.
Most commonly, it is the discovery of an abnormal blood thyroid function test in a patient taking amiodarone that results in a request for endocrine consultation. In euthyroid individuals with a normal thyroid gland, inhibition of T4 to T3 conversion by amiodarone will reduce serum T3 levels, raise TSH levels, and secondarily increase serum T4 levels.
In AIH, there is an elevated TSH with either low or normal FT4, and given the likelihood of underlying Hashimoto thyroiditis in these patients, positive TPO antibodies and/or thyroglobulin antibodies may be present.
Although the patient with either type 1 or type 2 thyrotoxicosis will have a low TSH with high FT4 levels and inappropriately normal or high FT3/T3, further laboratory tests may help to differentiate between the 2 types such as measurement of thyroid-stimulating immunoglobulin, TPO antibodies, and thyroglobulin antibodies. One clue to making the distinction between type 1 or type 2 thyrotoxicosis is that patients with type 1 frequently have underlying thyroid pathology such as diffuse or nodular goiter, or Graves’ disease (with elevated TSH receptor antibodies). However, presence of positive tests for antithyroglobulin and/or antithyroperoxidase antibodies does not rule out a diagnosis of type 2 AIT (37).
The measurement of beta-glucuronidase, a lysosomal enzyme released into the extracellular fluid during inflammation, has been suggested as a marker to distinguish type 1 from type 2. Markantes et al. observed that plasma beta-glucuronidase activity in type 2 AIT was higher compared with type 1, albeit similar to levels seen in subacute thyroiditis (38). The utility of other serum biochemical markers such as C-reactive protein and IL-6 has been studied in relation to their ability to discriminate between type 1 and type 2 but found to be of limited clinical value. Levels of serum high-sensitivity C-reactive protein were not significantly different between patients with type 1 and type 2 (39) and IL-6 results found to be discriminatory between type 1 and type 2 disease at 1 center (40) were not replicated by other workers (41).
Thyroid ultrasound and color flow Doppler sonography.
Because a goitrous or structural thyroid abnormality is a common antecedent in type 1 AIT, thyroid ultrasound (US) can be useful in the discrimination between type 1 and type 2 AIT. Before initiation of amiodarone therapy, the thyroid in the subject with type 2 AIT is more likely to be normal in size and structure. Moreover, the thyroid gland in type 1 AIT should demonstrate normal or increased vascularization on Echo color Doppler, whereas the type 2 gland will be marked by distorted architecture due to the inflammatory thyroiditis and will demonstrate normal/reduced vascularity (41). However, in iodine-deficient or borderline sufficient areas, a small goiter and even a nodular goiter, may also be present in type 2 AIT. In this case, the diagnostic clue at US in differentiating the 2 main forms of AIT remains the normal/reduced vascularity (18). Because some subjectivity is present in US interpretation, the role of an experienced sonographer is fundamental.
Nuclear imaging.
Nuclear imaging techniques may be of limited utility in the distinction between type 1 and type 2 AIT. For radionuclide uptake or imaging studies, several different tracers are available, including radioiodine (RAI), 99m pertechnetate (99mTcO4–), and 99mTcO4 2-methoxy-isobutyl-isonitrile (MIBI).
Absent exposure to iodine excess, measurement of thyroidal RAI uptake provides insight into thyroid functional state, with high levels of uptake (> 30% at 24 hours) indicative of thyrotoxicosis. However, in AIT, a lower RAI uptake is seen because of the elevated serum iodine derived from amiodarone (18, 31). And the low RAI uptake seen in both type 1 and type 2 does not permit any distinction between the 2 types. Similarly, attempts to make this distinction using 99mTcO4– in patients with AIT showed mostly very low/no uptake in both type 1 and type 2 cases (42).
The MIBI uptake is reduced or absent in tissues marked by apoptotic processes, with potential collapse of mitochondrial membranes (43). Although MIBI scans have shown promising preliminary results in differentiating type 1 from type 2 or mixed cases, the number of reports is still limited (44). One encouraging report from Piga et al. of MIBI scans in 20 consecutive patients with type 1 and type 2 AIT led the authors to conclude that the MIBI scan was superior to currently used biochemical and other imaging diagnostic tools used for diagnosis. They concluded that MIBI uptake should be increased in type 1 AIT, reduced in type 2, and intermediate in mixed type 1/type 2 AIT (43), notwithstanding the limitations in interpretation because characterization of the different forms of AIT was based on MIBI results. One advantage of the utility of MIBI scans is their common accessibility in most nuclear medicine departments. However, further studies are needed to fully characterize their utility in differentiating between types of AIT.
Management of amiodarone-induced thyroid disorders
In the management of amiodarone-induced thyroid disorders, a multidisciplinary approach to care is often required with coordination of management between the endocrinologist, cardiologist, intensivist, and on occasion the thyroid surgeon. Several aspects of the individual patient must be taken into account, such as his or her general underlying condition, the contribution of the altered thyroid hormone levels to the arrhythmia, the benefits and risks of amiodarone discontinuation, and the inherent delays in the time for amiodarone to be eliminated from the body.
Management of amiodarone-induced hypothyroidism.
In the management of AIH, treatment with levothyroxine can be initiated in a starting dose as appropriate, and the dose adjusted during the follow-up according to published guidelines of the American Thyroid Association (45) based upon levels of FT4, FT3/total triiodothyronine (TT3), and TSH, and the patient’s cardiac status. Too rapid dose acceleration to supraphysiologic levothyroxine levels may precipitate or worsen cardiac arrhythmias. It is preferable, in most circumstances, to cautiously and gradually increase the levothyroxine dose with frequent clinical and laboratory monitoring. Amiodarone can be continued if deemed necessary for the treatment of the cardiac arrhythmia. If amiodarone is discontinued, hypothyroidism may resolve thereafter and levothyroxine treatment be no longer required, especially if the patient had no preexisting thyroid disease (Fig. 2).
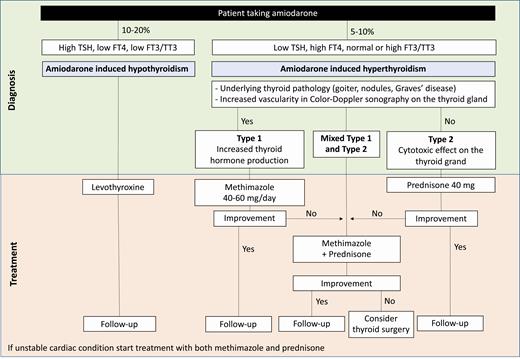
Algorithm for the suggested management of amiodarone-induced thyroid disorders. The figure illustrates a proposed flowchart for the management of a patient with amiodarone-induced hypothyroidism or thyrotoxicosis. These are general suggestions and should be tailored to the patient needs. The doses of medications may vary depending on the clinical presentation and characteristics. If the patient presents with hypothyroidism, levothyroxine treatment can be used to restore normal thyroid hormone levels. Amiodarone may be continued if needed for the cardiac arrythmia. The patient should be monitored for levothyroxine dose adjustment based on TSH, FT4, and FT3/TT3 levels and clinical status. In general, levothyroxine should be initiated and increased gradually in an attempt to mitigate any adverse cardiac effects. However, of course, clinical judgment should be incorporated into this algorithm. In case of thyrotoxicosis, treatment depends on the type of amiodarone-induced thyrotoxicosis; methimazole is used for type 1 and prednisone is used for type 2. When a mixed type 1/type 2 is present or the patient is in critical condition, both methimazole and prednisone may be started simultaneously. After patient improvement, methimazole/prednisone can be carefully tapered down. In case medical therapy is not effective, surgery can be considered upon in a multidisciplinary manner. In all cases, the patient should be followed up to monitor TSH and FT4 levels and adjust management accordingly.
Management of type 1 amiodarone-induced thyrotoxicosis.
In the case of AIT, the treatment is tailored based on whether the patient has type 1 or type 2 disease. Because type 1 is characterized by overproduction of thyroid hormones, the most effective treatment is the institution of a blockade to ongoing hormone synthesis by initiating thionamide therapy (methimazole, carbimazole, or propylthiouracil) (Fig. 2). High doses of methimazole in the range of 40 to 60 mg/d are typically needed (18), with more aggressive dosage sometimes required (eg, 120 mg/d in divided dosage [30 mg every 6 hours]). Usually methimazole is preferred for its lower hepatotoxicity compared with propylthiouracil. Although potassium perchlorate may be added to the therapy in case of methimazole failure (46), it is not available or commonly used in the United States. Because potassium perchlorate is no longer available, sodium perchlorate is available as an alternative option.
In the event of methimazole therapy failure (presence of thyrotoxicosis despite high doses of methimazole applied for 3-4 weeks) a mixed form of type 1 and type 2 AIT should be suspected (Fig. 2). However, it should be recognized that it may take methimazole a longer time to decrease thyroid hormone levels before concluding that a mixed type 1 type 2 AIT is present.
In view of the inhibitory action of lithium on thyroid hormone secretion, its use in addition to antithyroid drugs can be beneficial in type 1 and has been reported to speed recovery (47), but its possible effectiveness may be limited by adverse side effects (18). Although the potential salutary effect of amiodarone discontinuation will not be immediate because of its long biologic half-life, terminating the drug should be considered. The possible benefit of doing so is controversial because of limited data, and the decision should be individualized in relation to cardiovascular risk stratification and made jointly by cardiologists and endocrinologists (18, 48). According to the European Thyroid Association (ETA) guidelines for the “Management of amiodarone associated thyroid dysfunction,” amiodarone continuation is suggested for patients with life-threatening arrhythmias and critical illness with poor prognosis (18). Nevertheless, if amiodarone is withdrawn, methimazole should be continued until urine iodine levels return to normal, which may take 6 to 18 months. If in the future amiodarone needs to be reintroduced, close monitoring is fundamental as 75% of patients restarting amiodarone treatment risk having another episode of AIT (49). Consideration may be also given to RAI treatment of the thyrotoxicosis when the patient can be weaned off amiodarone and urinary iodine levels have normalized.
Type 2 amiodarone-induced thyrotoxicosis.
Because of the different underlying pathological mechanism in type 2 compared to type 1, a different approach to the patient is required (Fig. 2). Glucocorticoid treatment is the pillar of type 2 therapy (50, 51). The amount of thyroid hormone released into the bloodstream and consequent severity of the thyrotoxicosis depends on the scale of thyroid damage. Often the disease is self-limited, and when the patient is in stable cardiac condition, and suffering only mild thyrotoxicosis, follow-up without pharmacological therapy may be an option. But when overt thyrotoxicosis is clinically evident, or when a quick restoration of euthyroidism is required because of the underlying cardiac condition, glucocorticoid treatment should be initiated (52).
The principal glucocorticoid used is prednisone in a starting dose of 30 to 40 mg once daily, for 2 to 4 weeks, followed by a gradual tapering over 2 to 3 months, depending on the patient’s clinical and biochemical response (20, 53). When a salutary response to this approach is not seen, Campi et al. have proposed a change to combined intravenous and oral steroid therapy (oral prednisone range: 5-12.5 mg/d; IV methylprednisolone range 250-500 twice a week) (54). Although this regimen was successful in rapidly improving thyroid function, the patient group studied was limited to 4 patients; further external validation of this approach will be required. Another recent study of only 3 patients showed that IV glucocorticoids do not offer any advantage over oral glucocorticoids in the management of type 2 AIT (55). A longer duration of prednisone therapy may be required for adequate therapy of type 2 AIT as determined by FT4 levels and thyroid gland volume (56). Furthermore, as indicated previously, the possibility of a mixed type1/type2 AIT should be entertained when glucocorticoids fail to improve thyrotoxicosis.
Continuation of amiodarone treatment would appear to be feasible in type 2 disease as the thyroid destructive nature of the disorder is often self-limited (18, 48). The recurrence rate of type 2 AIT in patients continuing on amiodarone is variable and has ranged from 6% to 75% (57, 58).
Management of mixed type 1 and type 2 amiodarone-induced thyrotoxicosis.
In certain cases, both type 1 and type 2 overlap making the diagnosis and management more complex. A mixed type 1 and type 2 AIT is usually diagnosed after therapy with prednisone or methimazole alone has failed in improving the thyrotoxicosis. Mixed type 1/type 2 forms should be treated with a combination of antithyroid drug and glucocorticoids (eg, methimazole 40 mg daily and prednisone 40 mg daily) (20). Alternatively, a combined treatment (thionamide and glucocorticoids) can be instituted from the very beginning for a duration of 2 weeks (59). However, the possible risk of methimazole side effects given the high doses usually needed in AIT treatment must be considered. When levels of FT3 or TT3 are noted to decline by more than 50% compared with pretreatment levels, a diagnosis of type 2 AIT is most likely, and the dosage of thionamides can be tapered (42, 59). This approach is useful when the thyrotoxicosis is relatively severe and/or in otherwise medically compromised patients when rapid thyroid hormone normalization is sought. Once a response is seen, one of the drugs can be slowly tapered down (Fig. 2). However, if no improvement is observed, definitive treatment such as thyroidectomy should be considered.
Thyroidectomy in amiodarone-induced thyrotoxicosis.
In selected cases, undergoing thyroidectomy may be an effective option. According to the ETA guidelines, thyroidectomy should be considered in the following conditions: (1) patients with progressive deterioration of cardiac function or patients with a reduced left ventricular ejection fraction (LVEF) who have an increased mortality risk because of their cardiac condition; (2) patients with a severe underlying cardiac disease (eg, arrhythmogenic right ventricular dysplasia) or patients with malignant arrhythmias; and (3) patients with AIT who are unresponsive to aggressive medical therapy with methimazole and corticosteroids (18, 48).
A recent study by Capellani et al. retrospectively analyzed a cohort of 207 patients with AIT who had undergone either total thyroidectomy or medical therapy. Overall mortality and cardiac-specific mortality after 5 and 10 years were lower in the surgery group than in the medical therapy group. Also, postthyroidectomy improvement of the ejection fraction was observed in the total thyroidectomy group. However, the lower mortality rate was observed only in the subgroup with moderate to severely compromised LVEF. Mortality of patients with normal or mildly reduced LVEF was not different between the group who underwent total thyroidectomy and the group treated with medical therapy (60).
ETA guidelines recommend that salvage thyroidectomy can be considered for type 1, mixed type 1/type 2, and type 2 AIT for patients who present as an emergency (deterioration of cardiac function, reduced LVEF, hemodynamic instability) (18).
The decision for total thyroidectomy should be made by a multidisciplinary team that includes an endocrinologist, cardiologist, anesthesiologist, and a high-volume thyroid surgeon (18, 48).
When definitive treatment is not urgent, euthyroidism should be restored if possible, before proceeding with total thyroidectomy (18). The option of a plasma exchange can be considered as a temporizing measure to normalize thyroid hormones before surgery (61, 62).
Radioactive iodine therapy.
The use of RAI treatment as a therapy for AIT is not very practical in the short term because RAI uptake is usually low. After discontinuation of amiodarone, appropriate timing of RAI treatment can be based on iodine depletion indicated by measurements of urinary iodine. Some studies have proposed the use of RAI therapy in type 2 AIT, but the likelihood of success can be questioned because approximately 15% of the patients have been reported to still be thyrotoxic after 2 years of follow-up (63).
The use of recombinant TSH has been proposed for type 1 patients to stimulate the thyroid gland and overcome the low RAI uptake (64). However, an increase in TSH may be hazardous because it may worsen thyrotoxicosis by stimulating a greater degree of thyroid hormone release and the use of recombinant TSH is not recommended by the guidelines (18, 20).
Back to our case
Our patient presented with onset of thyrotoxicosis almost 2 years after amiodarone initiation without a previous history of a thyroid disorder. Thyrotropin receptor antibodies were negative. US did not reveal thyroid nodules or either increase or decrease in vascularization based upon Echo color Doppler. However, type 2 AIT was diagnosed on the basis of epidemiologic data and the known characteristics of AIT. Prednisone treatment was started with a dose of 40 mg/d. Seven days after receiving prednisone treatment the patient was still hyperthyroid with TSH 0.023 uIU/mL, FT4 2.42 ng/mL, and T3 97 ng/dL (normal range 60-180). Because little to no improvement was observed, a mixed type 1 and type 2 of AIT was deemed more probable, and methimazole therapy, 10 mg/d was added to the prednisone therapy. The possibility of a type 2 AIT with a delayed response to glucocorticoids remained, especially because the patient’s baseline characteristics more likely indicated a type 2 AIT. However, the patient’s critical cardiac condition necessitated prompt normalization of thyroid function and thus treatment with both prednisone and methimazole was initiated.
After 10 days of treatment with both methimazole and prednisone, an improvement in thyroid function was observed (TSH 0.560 uIU/mL, FT4 1.52 ng/dL, T3 68 ng/dL). Treatment with prednisone and methimazole was continued with no change in dose. After an additional week, thyroid function tests were within the normal range (TSH 0.953 uIU/mL, FT4 1.22 ng/dL, T3 63 ng/dL). The same treatment was continued for 7 more days and a further improvement was observed (TSH 1.45 uIU/mL and FT4 0.95 ng/dL). At that point, therapy was continued with prednisone 40 mg/d and methimazole tapered down to 5 mg/d.
Follow-up and monitoring
Given the relatively common occurrence of thyroid disorders among patients taking amiodarone, it is advisable to check thyroid function before and at 3-month intervals during treatment (48).
The same monitoring should continue for at least 1 year after amiodarone withdrawal because the drug has a half-life of about 100 days and can accumulate in the adipose tissue and muscles. It has been observed that 7% of patients developed type 2 AIT as long as 7 to 16 months after amiodarone withdrawal (65). Whether the initial presentation was of hypothyroidism or thyrotoxicosis, patients should be monitored closely. When patients with type 2 are treated with prednisolone, weekly changes in FT3/TT3 are expected, with normalization after an average of 8 days, whereas patients with type 1 on methimazole require an average of 4 weeks to achieve a normal FT3 (66). After glucocorticoid discontinuation, 16% of type 2 patients can be expected to be permanently hypothyroid (36).
Patients with amiodarone-associated thyroid disorder can have alterations in the efficacy of their anticoagulant therapy. The warfarin effect on vitamin K-dependent clotting factors can increase with thyrotoxicosis thereby increasing the risk of bleeding (67). Furthermore, amiodarone itself is an inhibitor of a number of cytochrome P450 enzymes, potentially affecting not only warfarin but also rivaroxaban and apixaban pharmacokinetics (20, 31, 68–71). Because a reduction in warfarin clearance may lead to a sudden and pronounced increase in prothrombin time and international normalized ratio (20, 68), appropriate adjustment in the anticoagulant therapy dosage and careful monitoring should be performed.
Patient discussion summary
The presentation of AIT in our patient worsened his underlying cardiac condition. A life-threatening situation marked by frequent bouts of ventricular fibrillation and tachyarrhythmias indicated the need of extracorporeal membrane oxygenation and management in an intensive care unit. The background characteristics of his medical history (long-term amiodarone treatment, no previous thyroid disorders), together with the absence of thyroid gland pathologic features on US, oriented his physicians toward the diagnosis of type 2 AIT. Although prednisone treatment alone was immediately initiated, therapy with both methimazole and prednisone could have been considered from the outset. The patient presented as an emergency, and the rapid normalization of thyroid hormone levels could have played an important role in his outcome. We have no diagnostic test that can accurately differentiate between type 2 and mixed type1/type2 AIT and when in doubt in a critical situation, it may be more effective to treat the patient for both type 1 and type 2 disease. In the face of persistent elevation in thyroid hormone levels despite a 1-week course of prednisone, methimazole was added to the therapy and improvements in thyroid hormonal values and in his general condition were observed.
The dose of methimazole generally recommended in type 1 AIT varies from 30 to 40 mg/d. In our patient, a lower dose of 10 mg was chosen in accordance with the only mildly elevated values of FT4. In type 2 AIT, although a salutary effect of prednisone is anticipated within the first few weeks, in some cases a longer treatment period is required to see an improvement in thyroid hormone levels. Thus, in our patient, given an absence of a history of thyroid disease and a normal thyroid US (abnormalities of which would relate more to type 2 AIT) a type 2 AIT with a delayed effect of prednisone could not be excluded. Had the patient been in a more stable clinical state, methimazole initiation could be questioned and more time could have been allowed to elapse to achieve a beneficial effect of prednisone. However, in a patient deemed to be in critical condition, as often may be the case in clinical practice, treatment with both methimazole and prednisone can be beneficial when a mixed type 1 and type 2 AIT is suspected. In our patient, a rapid normalization of thyroid hormones levels was thought to be crucial. Amiodarone treatment was not suspended because it was deemed essential for his cardiac condition; moreover, his thyroid hormone levels had started to normalize under treatment. In the future, the option of a thyroidectomy may be discussed with the patient, but in the meantime, the patient will be closely followed with indicated adjustments in methimazole and prednisone therapy.
Summary
Patients taking amiodarone should be carefully monitored because amiodarone-induced thyroid disorders may present at any time during amiodarone treatment or after amiodarone discontinuation. Both forms, hypothyroidism and thyrotoxicosis, require proper diagnosis to allow effective treatment. Hypothyroidism is treated with levothyroxine, whereas treatment of AIT is based on whether it is a type 1 or type 2, with methimazole used for type 1, and prednisone used for type 2. However, when the presentation is a mixed type 1/type 2 or difficult to distinguish, use of both methimazole and prednisone is suggested. Thyroidectomy can be considered with consultation and management by a multidisciplinary team. Diagnosis and management of amiodarone-induced thyroid disorders can be challenging, and patients require continued careful monitoring and follow-up during and after amiodarone suspension.
Abbreviations
- 99mTcO4–
99m pertechnetate
- AIH
amiodarone-induced hypothyroidism
- AIT
amiodarone-induced thyrotoxicosis
- ETA
European Thyroid Association
- FT3
free T3
- FT4
free T4
- LVEF
left ventricular ejection fraction
- MIBI
99mTcO4 2-methoxy-isobutyl-isonitrile
- RAI
radioiodine
- TT3
total triiodothyronine
- TPO
antithyroperoxidase
- US
ultrasound
Acknowledgments
Financial Support: This work was supported by The Catherine L. Heron and Albert C. Schneider Vanguard Charitable thyroid fellowship.
Additional Information
Disclosure summary: The authors have nothing to declare. None of the authors have a conflict of interest.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.



