-
PDF
- Split View
-
Views
-
Cite
Cite
Matthew D Ettleson, Antonio C Bianco, Individualized Therapy for Hypothyroidism: Is T4 Enough for Everyone?, The Journal of Clinical Endocrinology & Metabolism, Volume 105, Issue 9, September 2020, Pages e3090–e3104, https://doi.org/10.1210/clinem/dgaa430
Close - Share Icon Share
Abstract
It is well recognized that some hypothyroid patients on levothyroxine (LT4) remain symptomatic, but why patients are susceptible to this condition, why symptoms persist, and what is the role of combination therapy with LT4 and liothyronine (LT3), are questions that remain unclear. Here we explore evidence of abnormal thyroid hormone (TH) metabolism in LT4-treated patients, and offer a rationale for why some patients perceive LT4 therapy as a failure.
This review is based on a collection of primary and review literature gathered from a PubMed search of “hypothyroidism,” “levothyroxine,” “liothyronine,” and “desiccated thyroid extract,” among other keywords. PubMed searches were supplemented by Google Scholar and the authors’ prior knowledge of the subject.
In most LT4-treated patients, normalization of serum thyrotropin levels results in decreased serum T3/T4 ratio, with relatively lower serum T3 levels; in at least 15% of the cases, serum T3 levels are below normal. These changes can lead to a reduction in TH action, which would explain the slower rate of metabolism and elevated serum cholesterol levels. A small percentage of patients might also experience persistent symptoms of hypothyroidism, with impaired cognition and tiredness. We propose that such patients carry a key clinical factor, for example, specific genetic and/or immunologic makeup, that is well compensated while the thyroid function is normal but might become apparent when compounded with relatively lower serum T3 levels.
After excluding other explanations, physicians should openly discuss and consider therapy with LT4 and LT3 with those hypothyroid patients who have persistent symptoms or metabolic abnormalities despite normalization of serum thyrotropin level. New clinical trials focused on symptomatic patients, genetic makeup, and comorbidities, with the statistical power to identify differences between monotherapy and combination therapy, are needed.
I was diagnosed with hypothyroidism 15 years ago. Finding a treatment that was effective for me was difficult as the four doctors I visited just wouldn’t listen to me. There was an established treatment: levothyroxine. Do it and we don’t want to talk about it. After doing my own research, I distinctly remember an endocrinologist telling me, “You let me worry about all that.” I finally met a doctor who would listen to me and saw more than just my TSH [thyrotropin] level. He recommended a mixture of T4 and T3. After two weeks, my ten-year clinical depression disappeared, I lost 30 pounds with minimal effort, and I felt great with no side effects. I have considered it a success from that time forward. For me, there is currently no other approach to getting T4 and T3. Before, they said, “Just take levothyroxine!” but this did not work for me. On combination therapy, I’m doing better.
This account of a hypothyroid woman illustrates a problem faced by physicians on an almost daily basis: how to manage hypothyroid patients who remain symptomatic despite having a normal serum thyrotropin level. Hypothyroidism is a common diagnosis, and thyroid hormone (TH) replacement is the primary form of treatment (1, 2). Before the development of the thyrotropin assay, hypothyroidism was diagnosed via signs, symptoms, and metabolic parameters and treated with desiccated thyroid extract (DTE), which contains both thyroxine (T4) and triiodothyronine (T3). DTE was the mainstay of treatment until the early 1970s despite inconsistent TH content (3-7). The introduction of the thyrotropin assay into clinical practice led to the recognition that many patients were being overtreated. Although levothyroxine (LT4) became commercially available in the 1950s, its use in the treatment of hypothyroidism, along with L-triiodothyronine (LT3), was uncommon, as DTE remained the dominant form of treatment (8, 9). The discovery of peripheral conversion of T4 to T3 via deiodination opened the door for LT4 alone to be used in the treatment of hypothyroidism. It became the recommended treatment for hypothyroidism in all current professional guidelines, and rose to be a top-prescribed medication in the United States and other countries (10-13). However, many studies have revealed limitations of serum thyrotropin as a tool to adjust the dose of LT4, which have invariably led to relatively lower serum T3 levels. Although the majority of patients do have a satisfactory response on LT4 therapy, some continue to experience hypothyroid symptoms, have metabolic abnormalities, or are otherwise dissatisfied with their treatment despite normalized thyrotropin levels (14-20). Exact population estimates are sparse, but according to one general health scoring method, 34% of those on LT4 with normal thyrotropin values met case criteria for psychological distress, while 26% of the control population met the same threshold (15). It is conceivable that the relatively lower serum levels of T3 is compounded by one or more factors unique to the individual patient, a second hit that exhausts their capacity to compensate. In this article we present a case for the inclusion of LT3 in an individualized approach for treatment of hypothyroidism for our patient listed earlier and so many others. Although there is concern about long-term use of LT3, a series of trials following almost 1000 patients for up to 1 year shows that, similarly to LT4, combination therapy restores euthyroidism while maintaining a normal serum thyrotropin without additional adverse drug reactions (21).
The purpose of this narrative review is to present relevant, up-to-date scientific literature on the treatment of hypothyroidism and TH metabolism. To that end, a PubMed review of the literature was conducted using search terms including: “hypothyroidism,” “thyroid stimulating hormone,” “thyroxine,” “triiodothyronine,” “levothyroxine,” “liothyronine,” “combination therapy,” “desiccated thyroid extract,” and “deiodinase.” The search was supplemented using Google Scholar using the same keywords. Specific searches using these keywords associated with particular biomarkers (eg, “cholesterol” and “cognitive function”) were undertaken. References lists from relevant review articles were also reviewed.
Serum Thyrotropin: An Extraordinary Tool to Diagnose Hypothyroidism
The link between myxedema and the thyroid was forged in observations of animals and patients following thyroidectomy (22). In 1883, Kocher noted that patients undergoing total thyroidectomy exhibited similar symptoms to what at the time was considered cretinism. Subsequently, it became clear that several separate conditions described at the time—cretinism, congenital and acquired myxedema, and “cachexia strumpriva”—were due to “arrest of the function of the thyroid gland” (22). Continued research in the thyroid field led to a series of seminal discoveries, and today we know that the thyroid gland secretes a mixture of T4 and T3 at a roughly 11:1 ratio (23). Whereas the thyroid is the only source of T4, most T3 in the circulation is produced outside of the thyroid gland. Hence, it is understandable that the hypothalamus-pituitary-thyroid (HPT) feedback mechanism that controls thyroid activity evolved to be sensitive to circulating levels of T4 (24). Mechanistically, this is made possible through the type 2 deiodinase (D2) that is expressed and transforms circulating T4 into T3 in tanycytes, ependymal cells in the median eminence, and thyrotrophs, pituitary cells that produce thyrotropin (25). D2 is uniquely regulated in the hypothalamus, making it a faithful indicator of circulating levels of T4 to the structures expressing thyrotropin-releasing factor (TRH) and thyrotropin (26). This mechanism evolved to preserve TH signaling in the face of iodine deficiency; environmental levels of iodine served as a powerful evolutionary pressure that shaped the HPT axis into defending against iodine deficiency and low serum T4, which promptly elevates serum thyrotropin (24). Thyrotropin in turn stimulates thyroid growth and iodine uptake, and also increases the fractional secretion of T3 (27). During the initial phases of hypothyroidism, even minimal reductions in circulating levels of T4 are sufficient to accelerate thyrotropin secretion, elevating its levels in the circulation. This frequently occurs while plasma T3 is stable, preserved by a number of thyroidal and extrathyroidal homeostatic mechanisms that increase the relative T3 production (28). The integrated transduction of the circulating levels of T4 and T3 and the coordinated actions of D2 within the HPT axis explain the remarkable value of obtaining serum thyrotropin levels for the diagnosis of subclinical or clinical hypothyroidism.
Although the basic function of the HPT axis was progressively understood through the 20th century, during the 1960s there was no method available clinically to determine a patient’s thyroid status on the basis of thyrotropin levels. Utiger developed the first sensitive thyrotropin immunoassay that could reliably identify individuals with hypothyroidism exhibiting thyrotropin levels ranging between 20 and 180 mμg/mL (29). Detection of serum thyrotropin levels was immediately recognized as superior to measurements of serum cholesterol and basal metabolic rate. As Utiger states, “The TSH radioimmunoassay should greatly improve and simplify the diagnosis and treatment of many thyroid disorders” (30). Within a few years, serum thyrotropin measurement became and remains the standard biochemical measure of thyroid status for the diagnosis of hypothyroidism (31, 32).
Some limitations have been identified in the universal application of serum thyrotropin alone to determine thyroid status (33). Large cohort studies in the United States and other countries have demonstrated variability in the mean and upper and lower bounds of thyrotropin of patients of different ages, sexes, and races (1, 34, 35). In general, thyrotropin appears to be increased in older people, women, and non-Hispanic individuals. These findings have led some to suggest age- and race-specific cutoffs for thyrotropin, although these cutoffs have not been widely adopted in clinical practice (36, 37). Evidence to support a higher thyrotropin cutoff for elderly patients is somewhat more compelling, with data suggesting either less mortality with slightly elevated thyrotropin and increased mortality with lower normal thyrotropin levels (38, 39). In addition to specific patient characteristics, a number of medications and supplements have been shown to affect thyrotropin levels, notably metformin, glucocorticoids, and biotin, which can interfere with the assay itself (40-42).
Thyroid Hormone Replacement in Hypothyroidism
From the late 19th century until the 1970s, DTE was the treatment of choice for patients with hypothyroidism. The dosing of DTE was adjusted based on clinical assessment and many times supported by the basal metabolic rate (BMR); later, serum protein-bound iodine (PBI) levels were also used (43-45). DTE remained the standard of care even after synthesis of LT4 was streamlined and the existence of T3 was discovered in the 1950s (46-48). At that time, T4 and T3 were considered unrelated hormones produced by the thyroid gland; both could, independently, reproduce the pleomorphic actions of thyroid secretion. Yet, significant differences were seen when either was used to treat hypothyroidism (8, 9). LT4 was a slow-acting molecule with prolonged effects, and required doses that would result in disproportionally high PBI. The metabolic contribution provided by T3 was thought to be lacking in LT4-treated patients. Conversely, LT3 actions were rapid but brief, with brisk but short-lived effects on patients. In contrast to therapy with LT4, PBI levels in patients on full LT3 replacement doses were very low. The fact that both LT4 and LT3 were independently effective in restoring euthyroidism but neither normalized PBI pointed to a dissociation between the patient’s thyroid status and circulating TH levels (49). Thus, given these inconsistencies, the concept that TH replacement had to be made with a mixture of LT4 and LT3 was conceived (8, 9).
However, within a few years a turning point occurred when Braverman and colleagues discovered the peripheral conversion of T4 to T3 in athyreotic patients, providing a solid physiological rationale for treatment with LT4 alone, with deiodination restoring T3 levels (6). The use of DTE and LT3 fell dramatically out of favor, and LT4 monotherapy became the predominant treatment of hypothyroidism (7). Therapy with LT4 is largely effective, safe, relatively inexpensive, and far and away the most common treatment of hypothyroidism (10, 11, 50). Two recent large population studies have shown that, provided hypothyroid patients achieve sustained normal serum thyrotropin levels, mortality is comparable to the background population (51, 52).
The Use of Serum Thyrotropin Led to a Significant Reduction in Thyroid Hormone Replacement Dose
Unsurprisingly, following its success to diagnose hypothyroidism, serum thyrotropin quickly became the gold standard to adjust doses of LT4. Although the first-generation thyrotropin assays could not detect subnormal levels, later versions of the assay detected very low levels of thyrotropin, distinguishing between hyperthyroid and normal individuals. When applied to patients on TH replacement, it became clear that most patients on TH replacement who were managed on a clinical basis were mildly thyrotoxic and had low/suppressed thyrotropin levels (50). The belief that high serum T4 levels were required in the treatment of athyreotic patients to make up for the absence of T3 led to standard recommendations of TH replacement at daily doses equivalent to 200 to 400 μg of LT4 (53). These recommendations were based on prior treatment with DTE and early studies observing changes in BMR, PBI, and cholesterol levels in hypothyroid patients treated with LT4 (54, 55). The assessment provided by serum thyrotropin, as compared to BMR and PBI, led to a reduction in LT4 replacement dosing. In some cases, 75% reductions were needed to adequately normalize serum thyrotropin (53, 56, 57). By the early 1980s, it was widely recognized that LT4 doses in the 100- to 200-μg range (or less) were needed to normalize thyrotropin levels (58). For most patients, this transition was satisfactory. However, with the reduction in LT4 based on an objective thyrotropin level and less on clinical symptomatology, some patients had recurrence of symptoms similar to those experienced prior to treatment.
Levothyroxine Monotherapy Normalizes Serum Thyrotropin but Creates a Low Serum Triiodothyronine/Thyroxine Ratio
As the thyrotropin assay became universally accepted as the primary tool to manage hypothyroid patients, and with the realization that T3 is the biologically active TH, a valid question was whether therapy with LT4 restored serum T3 levels to the normal range. Unexpectedly, this became a controversial point (59). Early studies show that serum concentration of T3 in 44 LT4-treated treated patients was slightly less than that in control participants (53); however, the same group found later that “...TSH levels of 19 patients on LT4 returned to normal when serum T3 levels were not significantly different from those of controls” (60). Multiple subsequent cross-sectional studies involving 20 to 135 patients at a time found either low (61, (62-68) or normal (69-71) serum T3 in LT4-treated patients. A key study compared serum T3 in 50 patients before and after surgical thyroidectomy and concluded that treatment with LT4 that normalizes serum thyrotropin also restores serum T3 to presurgical levels (72). However, a similarly designed study of 135 patients concluded the opposite (73). Although most studies agree that serum T4 levels are relatively higher in LT4-treated patients, the reasons for the inconsistency around serum T3 are not clear, but are likely to include (i) small numbers of patient samples, (ii) variability associated with measuring low serum T3 levels by immunoassays (72), and (iii) changes in laboratory assays and practices over the duration of the study, all of which would decrease the ability to accurately measure serum T3 levels.
Two cross-sectional studies involving a large number of patients point to serum T3 being lower in LT4-treated patients. The first study compared serum T3 levels in 1811 LT4-treated athyreotic patients who had normal serum thyrotropin levels with 3875 euthyroid controls. Serum T4 was higher and serum T3 was lower for every serum thyrotropin quintile analyzed. In addition, among the LT4-treated patients, 15.2% had serum T3 below the normal reference range (67). The second study used cross-sectional data from the US National Health and Nutrition Examination Survey (NHANES) (2001-2012) to match, based on sex, age, ethnic background, and serum thyrotropin, 469 participants taking LT4 with the same number of participants not taking LT4 (74). Participants using LT4 had higher serum total and free T4 and about 10% lower serum total and free T3 in relation to matched controls. Thus, the American Thyroid Association guidelines on hypothyroidism recognized that LT4-treated hypothyroid patients with normal serum thyrotropin values may have serum T3 levels that are at the lower end of the reference range, or even below the reference range. They also indicate that more studies are needed to clarify its clinical significance (32).
The mechanistic basis of how therapy with LT4 normalizes serum thyrotropin, but results in reduced serum T3/T4 ratio, lies in 2 facts: (i) D2 exhibits a “suicidal behavior”: It is ubiquitinated and degraded after it interacts with T4 and catalyzes the deiodination reaction; thus, lower serum T4 levels accelerate the activity of the D2 pathway by prolonging D2’s half-life. This increases the extrathyroidal fractional production of T3 (75); (ii) in contrast to the rest of the body, D2 in the hypothalamus is more stable; it converts T4 to T3 without undergoing much ubiquitination (76). This critical difference in D2’s behavior sets the stage for 3 events that occur simultaneously when a hypothyroid patient is treated with LT4 monotherapy: (i) peripheral D2 converts T4 to T3, which equilibrates with the systemic circulation, (ii) hypothalamus-pituitary D2 converts T4 to T3 locally, suppressing TRH/thyrotropin secretion, and (iii) D2 activity in both systems is progressively lost as the serum T4 levels return to normal. However, owing to insufficient D2 ubiquitination in the hypothalamus (76), D2 activity is lost predominantly in the periphery when compared to the HPT axis, leading to relatively greater T3 production in the HPT when compared to the periphery. Hence, serum thyrotropin is normalized at higher serum T4 levels that are insufficient to normalize serum T3. Clinically, it is noted that the dose of LT4 supplementation required to normalize circulating T3 may lead to thyrotropin suppression, but this is not seen in all cases (72, 73, 77).
Patients on Levothyroxine Therapy With Normal Thyrotropin Are Not Necessarily Euthyroid
As physicians were getting used to managing hypothyroid patients with LT4 and serum thyrotropin, Ridgway and colleagues analyzed thyroid function tests and several TH-dependent biomarkers in a group of 10 hypothyroid patients during treatment with escalating doses of LT3 in 4-week intervals and after transition to LT4 (68). To overcome the known issue that serum T3 levels spike shortly after ingestion of LT3 tablets and then decline, the integrated levels of serum T3 were calculated to accurately report T3 exposure. This was based on multiple measurements taken throughout the 24-hour period after LT3 ingestion (Fig. 1A). Treatment with 10 to 25 μg/day of LT3 did not normalize integrated serum T3 levels; only 50-μg/day LT3 brought serum T3 to the reference range, although still below the median level observed in euthyroid individuals. The analysis of the TH biomarkers revealed that (i) cardiac function and (ii) serum creatinine phosphokinase (CPK) activity, which reflect TH action in the skeletal muscle, were normalized starting with 20-μg/day LT3, but that (iii) BMR was normalized only with 50-μg/day LT3; and (iv) serum cholesterol levels and (v) serum thyrotropin levels decreased with LT3, but neither normalized on any LT3 dose. On switching therapy to LT4, serum thyrotropin levels dropped further, near normalization, and cardiac function and CPK activity remained normal. However, integrated serum T3 also dropped back to the lower limit of the reference range, and the BMR slowed down to subnormal values; serum cholesterol remained elevated (Fig. 1A and 1B).
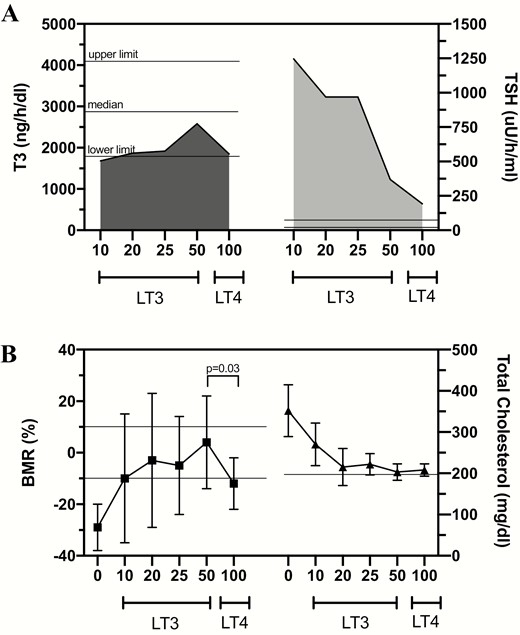
A, Integrated mean 24-hour serum triiodothyronine (T3) and thyrotropin (TSH) profiles following the indicated L-triiodothyronine (LT3) doses and levothyroxine (LT4) therapy. LT4 doses varied between 100 and 150 μg/day. The 24-hour serum T3 profiles were similar after LT3 20 or 25 μg/day or LT4 100 to 150 μg/day but 24-hour serum thyrotropin profiles were significantly less on LT4 than either LT3 20 or 25 μg/day (P < .001). Furthermore, the 24-hour serum T3 profile on LT3 50 μg/day was significantly higher (P < .01) than on LT4, yet the 24-hour serum thyrotropin profile was significantly lower on LT4 than LT3 50 μg/day (P ~ .05). Statistics were performed by paired t test of the 24-hour integrated hormone values. B, On LT3 10 μg/day the serum cholesterol was significantly reduced (352 to 270 mg/dL; P < .05). After the LT3 dose was increased to 20 μg/day, mean basal metabolic rate (BMR) (–3%) and serum cholesterol (215 mg/dL) were significantly different from baseline values (P < .001). On LT3 25 and 50 μg/day, and after 6 weeks of LT4 therapy, mean BMR and serum cholesterol were similar to and not significantly different from those values obtained while on LT3 20 μg/day. However, switching from LT3 50 μg/day to LT4 resulted in a significant drop in BMR (P < .03). Reproduced from (68).
It is not clear why during replacement therapy with TH some systems are normalized even as serum T3 levels are subnormal, for example, cardiovascular, at the same time that other systems remain hypothyroid, for example, metabolism. It is conceivable that tissues that express D2 are better equipped to endure TH deficiency. Indeed, the human heart and skeletal muscle express D2 but at a very low activity level (78, 79). In addition, TH receptor (TR)-α predominates in skeletal and cardiac muscles, whereas TR-β is found in the pituitary, liver, adipose tissue, and other metabolic tissues (80). The biomarkers in tissues with TR-α (CPK and cardiac function) normalized with low doses of LT3 and LT4, but those on TR-β tissues (BMR, cholesterol) appear to require higher levels of T3 to be normalized, and did not normalize on LT4 therapy. Although the Ridgway study was completed more than 40 years ago with a small number of patients, its findings help explain why many patients on LT4 monotherapy remain partially hypothyroid despite a normalized thyrotropin, although only some have perceived symptoms.
Subnormal energy expenditure
Metabolic studies show that LT4-treated patients with normal serum thyrotropin exhibit slower BMR (68), weigh about 10 pounds more, and report a reduced level of physical activity (74). In one study, many LT4-treated patients with normalized thyrotropin levels had a BMR about 10% slower than that of normal controls (18). This was also seen in groups of women on LT4 therapy with normal and suppressed thyrotropin levels, and healthy controls (77). The suppressed thyrotropin group had a mean thyrotropin of 0.14 μU/ml and a BMR that was similar to healthy controls. However, the LT4 group, which had an average thyrotropin of 2.1 μU/ml, had a BMR significantly slower than the healthy controls and the thyrotropin-suppressed group. A retrospective analysis of 649 obese women (body mass index [BMI] > 30 kg/m2) and normal serum thyrotropin revealed that those with primary hypothyroidism taking LT4 therapy (n = 85) exhibited a BMR 6% slower (28.59 ± 3.26 vs 29.91 ± 3.59 kcal/kg fat-free mass/day), including when adjusted for age, BMI, body composition, and level of physical activity, compared to the control group (81). In a study comparing LT4-treated patients with thyrotropin levels in the high and low end of the normal range, no significant differences in BMI, BMR, body composition, or dietary intake were identified (82). However, significantly positive correlations between free T3 levels and BMI and resting energy expenditure were found, suggesting that serum T3, rather than thyrotropin, may be a better marker of energy expenditure in LT4-treated patients.
Abnormal lipid metabolism
TRs in the liver are mostly occupied with T3 derived from the plasma (83); consequently, hepatic TH signaling is very sensitive to circulating T3 levels (84). Hence, it makes sense that cholesterol metabolism is affected when serum T3 is low, as in LT4-treated patients (85). This was tested in a preclinical LT4-treated thyroidectomized rat model that, similarly to patients, exhibits normal serum thyrotropin with a higher serum T4/T3 ratio and reduction in serum T3 levels. These animals exhibited reduced T3 signaling in the liver, along with elevated serum cholesterol levels (26). Subsequent studies revealed that patients on LT4 therapy exhibited an increase of 15 mg/dL in total cholesterol levels and 6 mg/dl in serum low-density lipoprotein levels, despite normalization of serum thyrotropin (86, 87). This was expanded and confirmed in a meta-analysis of 65 studies that compared 1878 LT4-treated patients to 14 493 healthy controls (20). In addition, NHANES data revealed that individuals on LT4 are 54% more likely to be on statins as compared to controls matched for age, sex, and ethnic background (74). In some of these studies, normalization of serum cholesterol was achieved by increasing the dose of LT4 (73, 87, 88), a change that also normalizes serum T3 but lowers serum thyrotropin below the normal reference range. This issue requires further study, because clinical trial data have not found significant differences in lipid levels between those on LT4 vs combination therapy (89).
Dissatisfaction and lower scores of quality of life
Most practicing clinicians likely have firsthand experience with hypothyroid patients on treatment with a normal thyrotropin and persistent symptoms, similar to the patient’s story retold in the introduction. Patients on LT4 with thyrotropin values in the normal range exhibit reduced psychological well-being compared to age- and sex-matched controls (15). Others examined quality of life and thyrotropin levels before and up to 6 months after initiation of LT4 therapy for Hashimoto thyroiditis (17). Median thyrotropin dropped from 8 to 3 mIU/L, and ThyPRO scores (validated questionnaire for thyroid symptoms) improved in several domains, but notably no improvement was seen in hypothyroid symptoms, cognitive complaints, and impaired sex life. This issue is addressed directly in recent guidelines on hypothyroidism, which acknowledge that a minority of patients with normal thyrotropin levels may perceive poor health status (32).
In an effort to further characterize those patients dissatisfied with their current hypothyroid treatment, a large-scale survey was developed to assess patients’ self-perceptions about hypothyroidism, with sobering findings (14). Overall, the median response for satisfaction with treatment was 5 out of 10, and almost all participants believed that new treatments for hypothyroidism are needed for better care. Notably, those patients on DTE were more satisfied than those on either LT4 monotherapy or combination therapy. Interpretation of these survey results are complicated by the likely association between hypothyroidism and other chronic diseases, including depression and anxiety disorders, although subgroup analyses were conducted in an effort to minimize confounding effects of reported comorbidities (90, 91).
It cannot be overstated that symptoms present in patients treated with LT4 should not be automatically presumed to be due to dysfunctional TH metabolism. Anecdotally, a growing number of patients are placed on LT4 without a solid diagnosis of hypothyroidism. In a unique study of patients on LT4 in which no clear diagnosis of hypothyroidism could be found, more than 60% remained euthyroid off LT4 over the study period (92). Thus, investigating the existence of thyroid disease in a patient on LT4 with persistent symptoms should be part of the clinical approach. In addition, patients who for many years have done well on LT4 but only recently have become symptomatic should be carefully evaluated for other conditions. Other causes of residual symptoms, such as suboptimal LT4 treatment, somatoform disorders, menopausal syndrome, and the coexistence of chronic diseases that may present with similar symptoms, should be evaluated for and treated, if found. Stronger candidates for combination therapy are those patients in whom alternative explanations are explored and eliminated, and persistent symptoms, which can be traced back to when the patient became hypothyroid, remain despite optimal TH therapy.
One-two punch: Why do some patients on levothyroxine seem to suffer more from persistent symptoms and metabolic abnormalities?
Most patients on LT4 with normal serum thyrotropin exhibit a decreased serum T3/T4 ratio. Given that T3 is the metabolically active TH, it is conceivable that the metabolic abnormalities detected in these patients (slower BMR and increased serum cholesterol) are the result of relatively lower serum T3 levels. BMR and serum cholesterol both are normalized by increasing the replacement dose of LT4 to the point that serum thyrotropin is suppressed (73, 77, 88). However, in contrast to the changes in metabolism, the vast majority of patients on LT4 with normal serum thyrotropin are asymptomatic. They successfully compensate for potential LT4 shortcomings and remain under the care of an internist, rarely visiting an endocrinologist to manage their thyroid disease. Why then do only a small fraction of LT4-treated patients have persistent symptoms? Given that hypothyroidism is so prevalent, we propose a “2-hit” hypothesis in which the underlying relatively low serum T3 levels is compounded by 1 or more factors, specific to the individual patient, exhausting his or her ability to compensate, for example, a condition that would further compromise conversion of T4 to T3, or make the patient more sensitive to minor changes in organ dysfunction. This would then intensify the development of perceived symptoms such as cognitive dysfunction, fatigue, and management of body weight.
A genetic rationale
In LT4-treated patients, most (> 80%) circulating T3 is derived via the D2 pathway (93). Thus, it is logical to place emphasis on D2 when investigating possible explanations for impaired T4 to T3 conversion. Depending on ethnic background, individuals may carry a commonly inherited DIO2polymorphism (rs225014) that results in an amino acid substitution (Thr92Ala) in D2, initially described in association with obesity and insulin resistance (94). This change in amino acid in D2 occurs where its susceptibility to ubiquitination and proteasomal degradation is regulated, modifying its subcellular distribution and reducing its enzymatic velocity by about 20% (95). In rodents, and possibly in humans, most T3 in the brain is produced via the D2 pathway (96). This makes the central nervous system (CNS) particularly susceptible to reduced D2 activity. The impact of the Thr92Ala-DIO2 polymorphism was tested in a mouse model carrying the Thr92-to-Ala substitution in D2. In these animals, distinct brain areas exhibited signs of localized hypothyroidism, which was associated with stress of the endoplasmic reticulum and the appropriate unfolded protein response. These mice exhibited normal growth and reproduction, but refrained from physical activity, slept 4 times more, and required additional time to memorize objects and social interactions. Enhancing T3 signaling in the brain with administration of LT3 improved cognition, whereas restoring proteostasis with the chemical chaperone 4-phenylbutyrate eliminated the mouse phenotype. In contrast, primary hypothyroidism intensified the Ala92-Dio2 phenotype, with only partial response to LT4 therapy (95).
The molecular mechanisms associated with the Thr92Ala-DIO2 polymorphism were also studied in humans. Using microarray analyses of 19 postmortem human temporal pole samples, Thr92Ala-DIO2 carriers were found to exhibit a transcriptional fingerprint that included sets of genes involved in CNS diseases, mitochondrial dysfunction, inflammation, oxidative stress, and apoptosis. Some of these changes were associated with the ectopic presence of Ala92-D2 in the Golgi apparatus, where its presence and/or ensuing oxidative stress disrupted basic cellular functions and increased preapoptosis. These findings are reminiscent of disease mechanisms observed in neurodegenerative disorders such as Huntington disease, and could contribute to the unresolved neurocognitive symptoms of affected carriers (97). In fact, to determine whether Thr92Ala-DIO2 was associated with incident Alzheimer disease (AD), community-based cohorts from Chicago and northeastern Illinois and religious clergymen from across the United States—3054 African Americans (AAs) and 9304 European Americans (EAs)—were evaluated for the incidence of AD. AAs with Thr92Ala-D2 had 1.3 times greater odds of developing AD. A secondary analysis of a representative sample of the US population revealed that AAs carriers of Thr92Ala-DIO2 showed a trend toward increased odds of dementia and 1.35 times greater odds of developing cognitive impairment not demented. Meta-analysis showed that AAs with Thr92Ala-DIO2 had 1.3 times increased odds of developing AD/dementia, whereas in EAs, no association was found between Thr92Ala-DIO2 and AD, dementia, or cognitive impairment not demented. It is conceivable that Thr92Ala-DIO2 might represent one factor contributing to racial discrepancies in incident AD (98).
Not demented/AD carriers of the Thr92Ala-DIO2 polymorphism are reportedly asymptomatic when surveyed through quality of life or thyroid-specific questionnaires (99). A phenotype has been reported only in connection with diagnosis and treatment for hypothyroidism (100). In contrast with the Ala92-Dio2 mice, carriers of the Thr92Ala-DIO2 polymorphism have the potential to adapt to and correct defects through lifelong learning and training, a phenomenon seen in animals (101) and individuals with neurodevelopmental disorders (102). However, it is conceivable that, once carriers of the Thr92Ala-DIO2 polymorphism become hypothyroid and are treated with LT4, these adaptive mechanisms are exhausted, bringing out a phenotype that is similar to the Ala92-Dio2 mice. The approximately 10% lower serum T3 levels observed in adequately LT4-treated hypothyroid patients could be the key element that tips the balance toward cognitive dysfunction.
However, association studies of single-nucleotide polymorphisms in humans are in general less powerful because they explain only a small number of phenotypic variations among individuals (103). Epigenetic interference, the existence of alleles with additive effects or synergistic interactions, includes some of the genetic elements that could explain this (103). Thus, it is understandable that data on Thr92Ala-DIO2 obtained across the world may be conflicting. One study from the United Kingdom demonstrated that the presence of Thr92Ala-DIO2 in the study population was associated with an enhanced response to combination therapy measured by psychological well-being scoring (100). Whereas the UK study did not observe an association between Thr92Ala-DIO2 and serum TH levels, a subsequent study in Italy found that LT4-treated hypothyroid carriers of the DIO2 polymorphism have lower serum T3 levels (104). In contrast, no impact of Thr92Ala-DIO2 was found on the response to LT4 monotherapy in a study from the Netherlands (99). The interference of other genetic polymorphism has been reported as well. A randomized crossover trial with a group of autoimmune hypothyroid patients adequately treated with LT4 showed that those with a combination of DIO2 and MCT10 (rs17606253) polymorphisms preferred combination therapy over LT4 alone (105). This could be quite relevant given that MCT10 is a transmembrane TH transporter that may affect how much T3 accumulates in the brain. Notably, there are dozens of proteins involved in the control of TH signaling, including other membrane transporters, deiodinases, TRs, and their coregulators. This highlights the potential for isolated or combined gene polymorphisms to interfere in the success of LT4 therapy.
In contrast to the D2 pathway, the D1 pathway accounts for less than 20% of the circulating T3 in LT4-treated patients. Thus, although less significant, a defect in the D1 pathway could also compromise T4 activation to T3 in LT4-treated patients. Recently, 2 families have been reported in which mutations in DIO1 compromise its catalytic activity—Asn94Lys-DIO1 and Met201Ile-DIO1—and result in a phenotype of elevated serum reverse T3 levels (106). Whereas these mutations would go unnoticed in individuals with a normal thyroid, LT4-treated carriers are likely to exhibit reduced serum T3 levels given that D1 does not participate in the feedback regulation of thyrotropin and TRH.
An immunological rationale
Cognitive dysfunction is a primary symptom of hypothyroid patients. Several areas may be affected, including general intelligence, attention/concentration, memory, perceptual function, language, psychomotor function, and executive function. Treatment with LT4 is usually effective in restoring these functions (107). However, it is not clear why a number of patients on LT4 have persistent cognitive symptoms. For example, a group of LT4-treated individuals with normal serum thyrotropin exhibited reductions in health status, psychological function, working memory, and motor learning compared to euthyroid controls (71). In another group of hypothyroid patients on LT4 with normal thyrotropin levels, there were lower scores on several neurocognitive and well-being scoring methodologies compared to reference values (108), but scores did not correlate with thyrotropin or antibody levels.
The rare association between encephalopathy associated with autoimmune thyroiditis, known as Hashimoto encephalopathy (109), points to the fact that thyroid autoimmunity might play an underlying role. Indeed, patients with Hashimoto thyroiditis have been shown to have higher titers of autoantibodies against structures of the CNS compared to control individuals (110), and this might have functional consequences. For example, using 99mTc ethyl cysteine dimer and single-photon emission computed tomography, a significant difference in brain perfusion was detected in a group of 41 euthyroid patients with Hashimoto thyroiditis with normal neurological investigation as compared to a matched control group (111). In a study using magnetic resonance spectroscopy, individuals with autoimmune thyroiditis on LT4 with normal thyrotropin levels were shown to have abnormal cerebral metabolite ratios, which can lead to a reduction of neuronal activity, while having no typical magnetic resonance imaging abnormalities (112). Although no definitive differences in magnetic resonance imaging were detected in a study between long-term LT4-treated patients with autoimmune thyroiditis and healthy participants, a correlation between thyroid autoantibodies and activity in mood-related brain regions was identified (113). Altogether, these findings strengthen the possibility of cerebral involvement in autoimmune thyroiditis in individual cases, with cerebral hypoperfusion suggesting cerebral vasculitis as the most likely pathogenetic model (111). Unfortunately, considering these elements in assessing effectiveness of therapy with LT4 in clinical practice is not always feasible because it requires the use of advanced diagnostic tools. It is hoped these assessments will be considered in future clinical trials comparing LT4 monotherapy and combination therapy.
Other low-grade autoimmune processes that do not target the CNS per se can also lead to cognitive dysfunction and nonspecific symptoms that resemble those of hypothyroidism. For example, vitamin B12 deficiency can cause numbness or tingling in the extremities, balance problems, difficulty thinking and reasoning (cognitive difficulties), or memory loss, weakness, and fatigue, even before anemia develops (114). In fact, B12 deficiency is prevalent among patients with thyroid autoimmunity through the association with atrophic gastritis and pernicious anemia, both conditions leading to impaired B12 absorption. Atrophic gastritis has been found in 35% to 40% of patients with thyroid autoimmunity and pernicious anemia in 16% (115).
Definitive Clinical Trial Data With Patients With Persistent Symptoms Are Lacking
Despite evidence that LT4 monotherapy that normalizes serum thyrotropin leads to low circulating T3 levels, the connection between low serum T3 and persistent hypothyroid symptoms is still elusive. First, not all clinical trials measure serum T3. Second, the analysis of serum T3 in patients taking LT3 requires multiple sampling (68). Third, there is relatively high variability of the T3 assays as discussed above. Fourth, serum T3 does not necessarily reflect tissue T3 content because of the activity of D2 and type 3 deiodinase (D3); this is particularly relevant in the brain, where D2 and D3 are highly expressed.
In the aforementioned NHANES study matching individuals by thyrotropin on and off LT4 therapy, despite a relatively lower T3 level in the LT4 group, multivariable analysis did not reveal an association between the T3/T4 ratio and any of the assessed clinical parameters other than age and sex (74). Conversely, a longitudinal study of patients with thyroid cancer on LT4 therapy demonstrated an inverse association between hypothyroid symptoms and free T3 levels that was present at thyrotropin levels within and below the normal range (116). Symptom relief was associated with free T3 levels in the upper half of the normal range. Clinical trials have been undertaken comparing LT4 and combination therapy to determine whether one mode of treatment is more efficacious than the other, and, by extension, whether the elevated serum T3 levels associated with combination therapy correlated with improved hypothyroid signs and symptoms (117).
Prior to evaluating the data available from clinical trials, it is important to note that it is difficult to perform a robust comparative study between therapies for hypothyroidism because of the nonspecific nature of both biomarkers and symptoms of hypothyroidism. It can be difficult to pinpoint whether improvement or decline in any of these aspects is due to a change in systemic thyroid status, a comorbid condition, or some other unknown confounder. A major challenge that any study examining hypothyroid patients with persistent symptoms must overcome is the placebo effect inherent in providing a new therapy to an already dissatisfied individual. Trials involving large numbers of participants tend to be the most resistant to bias (118). As more health care data are readily available electronically, large databases containing thousands of patients have been used to answer clinical questions, although the use of “big data” has its own limitations (119).
To date, the general consensus among professional societies is that there is insufficient evidence that combination therapy is superior to LT4 monotherapy, although the British Thyroid Association and the European Thyroid Association state that combination therapy could be considered as “experimental therapy” if the patient is persistently symptomatic (50). A meta-analysis of combination therapy vs LT4 therapy trials concluded there was no significant difference in symptoms or adverse effects between the 2 treatments (89). Indeed, LT4 and combination therapy have been repeatedly shown to be equivalent as to primary outcomes (efficacy and adverse events) in the general hypothyroid population (120-123). However, when considered among other factors such as safety index, cost, and convenience, professional guidelines have argued that LT4 should remain the preferred treatment for patients with hypothyroidism.
However, a criticism of the available trials is lack of statistical power to address the issue of residual symptoms while on LT4, because many studies have not enrolled sufficient numbers of patients with this complaint. Several clinical trials have included subpopulations of patients who have symptoms on LT4 at baseline, but the small number of patients enrolled did not enable those trials to demonstrate clear benefit in this population (124-127). When aggregated and analyzed together, no significant differences between LT4 and combination therapy emerged (89). Only one trial addressed the issue of genetic polymorphism, albeit the largest trial on combination therapy so far (100). Therefore, it is well recognized that the available trials have limitations (117, 128-131), and it is expected that future trials consider the recruitment of a sufficient number of individuals who exhibit residual symptoms on LT4 (130). Patients should as much as possible be screened for genetic (ideally obtain genome-wide association study data), autoimmune and other interfering variables. An issue to be considered is the variability in responses to the quality of life or thyroid-specific symptoms questionnaires. Patient preference could be brought to the forefront as an outcome, given its simplicity to obtain.
Universal Therapy vs an Individualized Approach
The role of genomic medicine has expanded into clinical practice, giving rise to the concept of “personalized medicine,” which has entered into all medical specialties, from the bedside to large professional guidelines (132). There are several examples within the guidelines of treatment of common chronic diseases, such as hemoglobin A1c (HbA1c) targets in diabetes mellitus and statin use for hyperlipidemia, that have attempted to balance individualized treatment with standardization of care (133). Most hypothyroid guidelines are in support of the standardized approach with thyrotropin-targeted LT4 therapy, but some guidelines more recently have introduced the concept of individualized therapy (50).
A prominent example of goal-directed therapy in chronic disease is the management of diabetes, with evidenced-based targets of HbA1c, blood pressure, and low-density lipoprotein cholesterol (134, 135). Targeted goals of therapy have been codified in standards of care for diabetes because of well-documented reductions in microvascular and macrovascular complications of diabetes associated with specific HbA1c, cholesterol, and blood pressure cutoffs (136). Significant metabolic improvements among patients with diabetes have been seen since the standardization of evidence-based guidelines for the treatment of diabetes (136, 137). The standard of care has evolved to include not only new scientific findings and classes of medical therapies, but also specific language to foster a more “individualized” approach to the management of diabetes, with different glycemic targets for different populations of patients (138, 139). Notably, the public health ramifications of the transition from uniform to individualized targets of HbA1c have been explored with mixed results. On one side of the coin, a cost-effectiveness analysis demonstrated the reduced costs and improved quality of life of the individualized approach, but on the other side, individualized goals may exacerbate existing disparities in treatment of diabetes between patients of different socioeconomic groups (140, 141).
The public health implications of liberalizing recommendations for the treatment of hypothyroidism to more readily prescribe LT3 and lessen the emphasis on a specific thyrotropin target range are not clear. Based on the recent tendencies among endocrinologists to more readily prescribe combination therapy, presumably due to increased deference given to patient preferences, LT3 prescriptions would likely increase if guidelines were liberalized (142, 143). Although the concerns with the use of LT3 are being dissipated with new data (144, 145), we agree that more compelling, large-scale trials demonstrating the long-term safety and efficacy of combination therapy are needed (130). However, we also acknowledge the known deficiencies in current hypothyroid management strategies. Large observational studies across multiple countries have demonstrated that an alarming number of patients on LT4 monotherapy were not within the therapeutic range at the time of testing (146, 147), in addition to the previously discussed subpopulation of patients with normal thyrotropin levels who have persistent hypothyroid symptoms and remain dissatisfied with therapy. At this time, we suggest physicians treat each hypothyroid patient individually after considering both biochemical function and symptomology, recognizing that normalization of thyrotropin level might not be equivalent to euthyroidism, in particular those with a likely “second hit” contributing to their clinical presentation of hypothyroidism.
Combination Therapy Should Be Used Judiciously
Most professional guidelines support an individualized approach to the treatment of hypothyroidism, which may include combination therapy in patients with persistent symptoms on LT4. However, these groups have cautioned against using LT3 in certain populations that may be at increased risk of adverse reactions, that is, in the pediatric population, in pregnant women, and in older adults, because the effects of the rapid serum T3 fluctuation have not been studied. In particular, although large studies examining the safety of combination therapy in older individuals are not available, caution should be exercised based on the detrimental cardiovascular and bone metabolism effects of subclinical hyperthyroidism in older patients (148, 149). Although specific guidance on the dosing and monitoring of combination therapy is outside the scope of this review, we generally refer to the European Thyroid Association guidelines, which suggest initiating a 3-month trial of LT4 and LT3 in about a 15:1 ratio dosed by weight, with LT3 divided into 2 daily doses (150). Notwithstanding, a critically important factor to consider when planning for a combination regimen is maintaining serum thyrotropin within normal range. For the clinical follow-up, in addition to monitoring symptoms and clinical signs, including heart rate and weight, specific attention should be paid to cardiovascular and bone health, including lipid levels and bone density, in patients treated longer term. In addition to professional guidelines, clinicians considering a trial of combination therapy may find a review of suggested guidance for the use of combination therapy helpful (21, 131).
Conclusion
The development of the thyrotropin assay and the recognition of peripheral conversion of T4 to T3 led to sweeping changes in treatment of hypothyroidism, including significant TH dose reduction and transition away from DTE and to LT4 monotherapy. These changes led some patients, like our patient introduced at the beginning of this article, to complain of persistent symptoms and metabolic abnormalities despite normal thyrotropin levels. Although the great majority of LT4-treated patients are well despite a relatively lower serum T3/T4 ratio, we propose that some patients likely have a compounding condition, a second hit that increases the likelihood of developing symptoms. Professional guidelines support the use of LT3 in combination with LT4 for those patients who have been properly screened and unambiguously not benefited from LT4. As long as serum thyrotropin stays within normal range, replacing a small fraction of the LT4 dose by LT3 once or twice a day has not been associated with adverse drug reactions (21). We acknowledge the importance of close clinical and laboratory follow-up in any patient being treated with LT3, and, as such, reliability of patient follow-up should be considered before initiation. For the benefit of the large number of patients with hypothyroidism, clinical trials robustly designed and powered appropriately to identify potential benefits of combination therapy in those who respond poorly to LT4 are needed. Only until these data are available can professional guidance be given on the use of combination therapy in patients with persistent symptoms.
Abbreviations
- AA
African American
- AD
Alzheimer disease
- BMI
body mass index
- BMR
basal metabolic rate
- CNS
central nervous system
- CPK
creatinine phosphokinase
- D2
type 2 deiodinase
- D3
type 3 deiodinase
- DTE
desiccated thyroid extract
- EA
European American
- HbA1c
hemoglobin A1c
- HPT
hypothalamus-pituitary-thyroid
- LT3
L-triiodothyronine
- LT4
levothyroxine
- NHANES
National Health and Nutrition Examination Survey
- PBI
protein-bound iodine
- T3
triiodothyronine
- T4
thyroxine
- TH
thyroid hormone
- TR
thyroid hormone receptor
- TRH
thyrotropin-releasing factor
Acknowledgments
Financial Support: This work was supported NIH DK58538, DK65055, DK77148.
Additional Information
Disclosure Summary: A.B. is a consultant for Allergan Inc and Synthonics Inc. M.D.E. has nothing to disclose.
Data Availability: Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References



