-
PDF
- Split View
-
Views
-
Cite
Cite
Daniela Gorduza, Ingrid Plotton, Laurent Remontet, Claire-Lise Gay, Meriem El Jani, Alaa Cheikhelard, Thomas Blanc, Alaa El Ghoneimi, Marc-David Leclair, Pascal Roy, Fabrice Pirot, Yanis Mimouni, Segolene Gaillard,, Pierre Chatelain, Yves Morel, Behrouz Kassai, Pierre Mouriquand, Preoperative Topical Estrogen Treatment vs Placebo in 244 Children With Midshaft and Posterior Hypospadias: Results of a Prospective, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial, The Journal of Clinical Endocrinology & Metabolism, Volume 105, Issue 7, July 2020, Pages 2422–2429, https://doi.org/10.1210/clinem/dgaa231
Close - Share Icon Share
Abstract
Urethral fistula and dehiscence are common after hypospadias surgery. Preoperative androgens have been considered to reduce these complications although this consideration is not evidence-based. Dermatologists have reported the benefits of topical estrogens on skin healing. We investigated whether the preoperative use of topical promestriene could reduce healing complications in hypospadias surgery. Our primary objective was to demonstrate a reduction of healing complications with promestriene vs placebo. Impact on reoperations and other complications, clinical tolerance, bone growth, and biological systemic effects of the treatment were also considered.
We conducted a prospective, randomized, placebo-controlled, double-blind, parallel group trial between 2011 and 2015 in 4 French centers. One-stage transverse preputial island flap urethroplasty (onlay urethroplasty) was selected for severe hypospadias. Promestriene or placebo was applied on the penis for 2 months prior to surgery. The primary outcome was the presence of postoperative urethral fistula or dehiscence in the first year postsurgery. For safety reasons, hormonal and anatomical screenings were performed.
Out of 241 patients who received surgery, 122 patients were randomized to receive placebo, and 119 patients received promestriene. The primary outcome was unavailable for 11 patients. Healing complications were assessed at 16.4% (19/116) in the placebo vs 14.9% (17/114) in the promestriene arm, and the odds ratio adjusted on center was 0.93 (95% confidence interval 0.45-1.94), P = 0.86.
Although we observed an overall lower risk of complications compared to previous publications, postsurgery complications were not different between promestriene and placebo, because of a lack of power of the study or the inefficacy of promestriene.
Healing complications mostly represented by urethral fistula and dehiscence are common after hypospadias surgery. They usually occur during the first few months following surgery and are more frequent in mid-shaft and proximal hypospadias (1). Although classifications of hypospadias are often reported as related to the position of the urethral meatus, the true landmark for defining the severity of hypospadias is the level of division of the corpus spongiosum and the subsequent hypoplasia of the tissues forming the ventral aspect of the penis, which is responsible of the ventral curvature (2). The more proximal this division sits, the more severe the hypospadias is. Beside the severity of hypospadias, the technique of urethroplasty used and the experience of the surgeon seem to be relevant for the outcome of this surgery (3,4). Preoperative androgen treatment to increase the size of the penis and improve the vascularity of penile tissues has been used for decades and reported by some to be beneficial for the outcome (5,6). Studies of the endocrinology of skin healing by dermatologists demonstrated that androgens were detrimental to the healing process whereas estrogens had much more favorable effects (7-9). Estrogen receptors alpha and beta seem to play a role in the healing process. Both receptors have been identified and are expressed in neonatal foreskin keratinocytes (10,11).These findings triggered the present study, which hypothesized that a topical estrogen treatment could improve the outcome of hypospadias surgery in children receiving the same type of urethroplasty. Our main objective was to demonstrate a reduction of healing postsurgery complications in the estrogen arm versus placebo. Impact on reoperations and other complications, clinical tolerance, bone growth, and biological systemic effects of the treatment were also reported.
Patients and Methods
Trial design
This study was a multicenter randomized, placebo-controlled, double blind, parallel group trial.
The study was approved by the French ethic committee (Comité de Protection des Personnes CPP Sud Est II) and the French competent authority (Agence National de sécurité du médicament et des produits de santé). It was registered under EudraCT number 2010-023686-22, and its methodology fulfilled the national regulations (Commission Nationale de l’Informatique et des Libertés). The study was also conducted in accordance with the French legislation, the Good Clinical Practice, and the Declaration of Helsinki.
Participants
The eligible patients were those with a hypospadias associated with a midshaft or more proximal division of the corpus spongiosum for which a transverse island flap urethroplasty (onlay urethroplasty) was the selected technique of urethral repair (12). Parents were duly informed of the research protocol, and surgery was planned between 9 and 36 months of age by a surgeon with more than 5 year experience in hypospadias surgery. Patients who received androgen stimulation (systemic androgen or human chorionic gonadotrophin [HCG]) less than 6 months prior to surgery, those who were previously circumcised, and those with a known risk of tumors were excluded.
Parents/legal guardian of eligible participants were informed orally and with a written document provided before inclusion. After signature of the consent by the parents or legal guardian, eligible participants were included in the study.
A data and safety monitoring board (DSMB) was constituted to review safety and conduct of the study.
Outcomes
The primary outcome was the proportion of patients with postoperative urethral fistula and dehiscence of the reconstructed urethra during the first year following surgery.
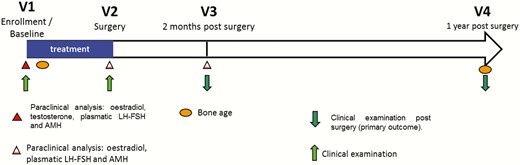
Two secondary outcomes were assessed: (i) in terms of efficiency, the total number and type of postsurgical complications and the total number of reoperations whatever the cause and (ii) and in terms of safety, the plasma concentrations of estradiol, testosterone, luteinizing hormone (LH)/follicle-stimulating hormone (FSH), and anti-Müllerian hormone; bone age (centrally reviewed by an independent endocrinologist, using Maturos® software); and the clinical tolerance of the treatment and its possible adverse reactions.
Sample size
The estimation of the sample size was based on data of a previous cohort of hypospadias patients for which a 26% healing complication rate was found (2). With an alpha risk of 0.05, a power of 80%, 485 patients were required to demonstrate a difference in the proportion of children with complications of 11%, using a 2-tailed test and accounting for 15% loss for follow-up and interim analysis (13).
Randomization and study treatment
Participants were randomized in 2 arms, in a 1:1 ratio: promestriene 1% or placebo cream. Randomization sequences were generated by the department of biostatistics of the Hospices Civils de Lyon, using R software and a method of blocks (block size of 4). It was stratified according to the hormonal treatment received prior to the study (treated or not by HCG or long-acting testosterone) and according to each participating center. Randomization was centralized by phone call to the coordination center, which provided a treatment number. The coordination center, investigators, nurses, and pharmacist were blinded to the treatment. Immediately after randomization, participants received study treatment, as tubes of cream, for 2 months prior to surgery, with the last day of study treatment being the day before surgery. Adherence to treatment was evaluated by weighting tubes before and after use. A study diary was given and a systematic interview of parents was also performed during the hospital stay (Fig. 1).
Blinding
Promestriene 1% and placebo had the same aspect, texture, scent, and presentation. All participants and investigators were blinded to the allocation group. Surgeons (assessors of the principal outcome) were also blinded to the allocated group. The Anti-Poison Centre of the Hospices Civils de Lyon maintained the allocation list to process to the unblinding 24/7 and to guide investigators.
Statistical methods
The main characteristics of the patients were described per treatment arm. Quantitative data were reported by their means and standard deviations. Qualitative data were reported as numbers and percentages.
The treatment effect was measured by comparing the rate of patients presenting postoperative urethral fistula and dehiscence within 1 year post-surgery in the treatment arm versus placebo. The main analysis used a logistic regression model taking into account a center effect. The placebo group being coded 0, and the promestriene group was coded 1; the treatment effect was assessed using a bilateral Wald test and an alpha risk of 5%. The main analysis was performed as intent to treat (ITT). The ITT analysis concerned all randomized patients. The ITT population was also used for safety analysis.
A secondary per-protocol analysis was planned in patients having received the onlay urethroplasty.
An interim analysis was performed and submitted to the DSMB after enrollment of 100 patients to ensure that the study treatment had no deleterious effects. Consequently, the values used for the inferiority test at the final and interim analysis were defined to preserve a global alpha risk of 2.5% (1.25% spent at each step) (13). The interim analysis did not have any consequence on the power of the final analysis on efficacy endpoint.
Secondary efficacy analysis compared the total number of complications in both arms after 1 year of treatment including reoperation for fistula or dehiscence not related to stenosis.
Safety analysis compared the plasma levels of estradiol, testosterone, LH, and FSH, and bone age before and after treatment in both arms.
No particular procedure was used to account for missing data.
Results
Recruitment
The flow diagram of the study is presented in Fig. 2. Among 370 eligible subjects, 105 (28%) parents refused the participation of their child to the study. From May 26, 2011 to December 29, 2015, 244 patients were enrolled in the study, in 4 French centers (Lyon, Paris Necker, Nantes, and Paris Robert-Debré).
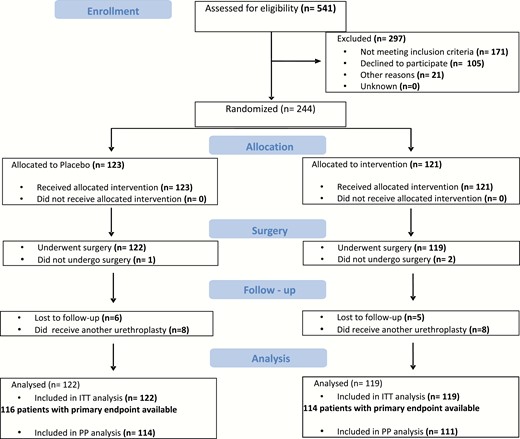
Four hundred and eighty five patients were initially planned to be enrolled over a 3-year and 6-month period. The recruitment rate was lower than expected, and the study was extended by 1 more year. It was eventually stopped after 4 years and 6 months.
Three children could not receive surgery and subsequently dropped out from the main analysis (2 in the promestriene arm, 1 in the placebo arm). Out of 241 patients who received surgery, 122 were included in the placebo arm and 119 in the promestriene arm as originally assigned. All 241 subjects were enrolled in the ITT analysis. Two hundred and twenty-five patients who received an onlay urethroplasty were considered for the per-protocol analysis.
Baseline data
Baseline demographics and clinical characteristics of the patients are presented in Table 1. Mean age at surgery was 17.5 (±5.1) months. The description of the hypospadias was similar between the 2 arms. None of the subjects presented with any gynecomastia. Six (4.9%) and 4 (3.3%) patients presented midline anomalies and 5 (4.1%) and 1 (1%) had other associated malformations in the placebo and the promestriene arms, respectively.
| . | Placebo N = 122 . | Promestriene N = 119 . |
|---|---|---|
| Age, months, mean (±SD) | 14.9 (±5.1) | 15.1 (±4.8) |
| Age at surgery, months, mean (±SD) | 17.4 (±5.2) | 17.5 (±5.0) |
| Weight, kg, mean (±SD) | 10.5 (±1.9) | 10.3 (±2.0) |
| Height, cm, mean (±SD) | 77.4 (±5.6) | 77.9 (±6.6) |
| Penile length, cm, mean (±SD) | 4.0 (±0.6) | 3.9 (±0.5) |
| Diameter, cm, mean (±SD) | 1.48 (±0.18) | 1.46 (±0.26) |
| Ventral aspect (Hypoplasia), n (%) | 104 (85) | 109 (92) |
| Meatal position, n (%) Distal Mid shaft Proximal | 44 (36) 59 (48) 19 (16) | 50 (42) 49 (41) 20 (17) |
| Division of corpus spongiosum, n (%) Distal Mid shaft Proximal | 2 (1) 74 (61) 46 (38) | 3 (2) 78 (66) 38 (32) |
| Curvature, n (%) No curvature Mild curvature Severe curvature | 6 (5) 74 (61) 42 (34) | 5 (4) 79 (66) 35 (30) |
| Preputial aspect, n (%) Complete Uncomplete | 0 (0) 122 (100) | 1 (1) 118 (99) |
| Previous treatment with androgen, n (%) | 13 (11) | 14 (12) |
| Previous treatment with HCG, n( %) | 9 (7) | 7 (6) |
| . | Placebo N = 122 . | Promestriene N = 119 . |
|---|---|---|
| Age, months, mean (±SD) | 14.9 (±5.1) | 15.1 (±4.8) |
| Age at surgery, months, mean (±SD) | 17.4 (±5.2) | 17.5 (±5.0) |
| Weight, kg, mean (±SD) | 10.5 (±1.9) | 10.3 (±2.0) |
| Height, cm, mean (±SD) | 77.4 (±5.6) | 77.9 (±6.6) |
| Penile length, cm, mean (±SD) | 4.0 (±0.6) | 3.9 (±0.5) |
| Diameter, cm, mean (±SD) | 1.48 (±0.18) | 1.46 (±0.26) |
| Ventral aspect (Hypoplasia), n (%) | 104 (85) | 109 (92) |
| Meatal position, n (%) Distal Mid shaft Proximal | 44 (36) 59 (48) 19 (16) | 50 (42) 49 (41) 20 (17) |
| Division of corpus spongiosum, n (%) Distal Mid shaft Proximal | 2 (1) 74 (61) 46 (38) | 3 (2) 78 (66) 38 (32) |
| Curvature, n (%) No curvature Mild curvature Severe curvature | 6 (5) 74 (61) 42 (34) | 5 (4) 79 (66) 35 (30) |
| Preputial aspect, n (%) Complete Uncomplete | 0 (0) 122 (100) | 1 (1) 118 (99) |
| Previous treatment with androgen, n (%) | 13 (11) | 14 (12) |
| Previous treatment with HCG, n( %) | 9 (7) | 7 (6) |
| . | Placebo N = 122 . | Promestriene N = 119 . |
|---|---|---|
| Age, months, mean (±SD) | 14.9 (±5.1) | 15.1 (±4.8) |
| Age at surgery, months, mean (±SD) | 17.4 (±5.2) | 17.5 (±5.0) |
| Weight, kg, mean (±SD) | 10.5 (±1.9) | 10.3 (±2.0) |
| Height, cm, mean (±SD) | 77.4 (±5.6) | 77.9 (±6.6) |
| Penile length, cm, mean (±SD) | 4.0 (±0.6) | 3.9 (±0.5) |
| Diameter, cm, mean (±SD) | 1.48 (±0.18) | 1.46 (±0.26) |
| Ventral aspect (Hypoplasia), n (%) | 104 (85) | 109 (92) |
| Meatal position, n (%) Distal Mid shaft Proximal | 44 (36) 59 (48) 19 (16) | 50 (42) 49 (41) 20 (17) |
| Division of corpus spongiosum, n (%) Distal Mid shaft Proximal | 2 (1) 74 (61) 46 (38) | 3 (2) 78 (66) 38 (32) |
| Curvature, n (%) No curvature Mild curvature Severe curvature | 6 (5) 74 (61) 42 (34) | 5 (4) 79 (66) 35 (30) |
| Preputial aspect, n (%) Complete Uncomplete | 0 (0) 122 (100) | 1 (1) 118 (99) |
| Previous treatment with androgen, n (%) | 13 (11) | 14 (12) |
| Previous treatment with HCG, n( %) | 9 (7) | 7 (6) |
| . | Placebo N = 122 . | Promestriene N = 119 . |
|---|---|---|
| Age, months, mean (±SD) | 14.9 (±5.1) | 15.1 (±4.8) |
| Age at surgery, months, mean (±SD) | 17.4 (±5.2) | 17.5 (±5.0) |
| Weight, kg, mean (±SD) | 10.5 (±1.9) | 10.3 (±2.0) |
| Height, cm, mean (±SD) | 77.4 (±5.6) | 77.9 (±6.6) |
| Penile length, cm, mean (±SD) | 4.0 (±0.6) | 3.9 (±0.5) |
| Diameter, cm, mean (±SD) | 1.48 (±0.18) | 1.46 (±0.26) |
| Ventral aspect (Hypoplasia), n (%) | 104 (85) | 109 (92) |
| Meatal position, n (%) Distal Mid shaft Proximal | 44 (36) 59 (48) 19 (16) | 50 (42) 49 (41) 20 (17) |
| Division of corpus spongiosum, n (%) Distal Mid shaft Proximal | 2 (1) 74 (61) 46 (38) | 3 (2) 78 (66) 38 (32) |
| Curvature, n (%) No curvature Mild curvature Severe curvature | 6 (5) 74 (61) 42 (34) | 5 (4) 79 (66) 35 (30) |
| Preputial aspect, n (%) Complete Uncomplete | 0 (0) 122 (100) | 1 (1) 118 (99) |
| Previous treatment with androgen, n (%) | 13 (11) | 14 (12) |
| Previous treatment with HCG, n( %) | 9 (7) | 7 (6) |
The onlay urethroplasty was confirmed and used in 225 patients: 114 (93%) and 111 (93%) patients in the placebo and promestriene arms, respectively.
Efficacy outcomes
Of 241 patients, the primary endpoint was not available for 11 patients. Results are presented in Table 2.
Number of patients presenting postsurgery complications and distribution of complications
| . | Placebo n (%) N = 122 . | Promestriene n (%) N = 119 . |
|---|---|---|
| Missing data | 6 (4.9) | 5 (4.2) |
| Patients presenting at 1 complication | 19 (16.4) | 17 (14.9) |
| Patients presenting no complication | 95 (77.9) | 96 (80.7) |
| Patients presenting 1 complication | 19 (15.6) | 17 (14.3) |
| Patients presenting 2 complications | 6 (4.9) | 4 (3.4) |
| Patients presenting 3 complications | 1 (0.8) | 2 (1.7) |
| Patients presenting 4 complications | 1 (0.8 ) | 0 (0.0) |
| Number of patients presenting at least 1 complication (onlay surgery only)a | 15 (13.9) | 15 (14.0) |
| . | Placebo n (%) N = 122 . | Promestriene n (%) N = 119 . |
|---|---|---|
| Missing data | 6 (4.9) | 5 (4.2) |
| Patients presenting at 1 complication | 19 (16.4) | 17 (14.9) |
| Patients presenting no complication | 95 (77.9) | 96 (80.7) |
| Patients presenting 1 complication | 19 (15.6) | 17 (14.3) |
| Patients presenting 2 complications | 6 (4.9) | 4 (3.4) |
| Patients presenting 3 complications | 1 (0.8) | 2 (1.7) |
| Patients presenting 4 complications | 1 (0.8 ) | 0 (0.0) |
| Number of patients presenting at least 1 complication (onlay surgery only)a | 15 (13.9) | 15 (14.0) |
aPlacebo: n = 114; promestriene: n = 111.
Number of patients presenting postsurgery complications and distribution of complications
| . | Placebo n (%) N = 122 . | Promestriene n (%) N = 119 . |
|---|---|---|
| Missing data | 6 (4.9) | 5 (4.2) |
| Patients presenting at 1 complication | 19 (16.4) | 17 (14.9) |
| Patients presenting no complication | 95 (77.9) | 96 (80.7) |
| Patients presenting 1 complication | 19 (15.6) | 17 (14.3) |
| Patients presenting 2 complications | 6 (4.9) | 4 (3.4) |
| Patients presenting 3 complications | 1 (0.8) | 2 (1.7) |
| Patients presenting 4 complications | 1 (0.8 ) | 0 (0.0) |
| Number of patients presenting at least 1 complication (onlay surgery only)a | 15 (13.9) | 15 (14.0) |
| . | Placebo n (%) N = 122 . | Promestriene n (%) N = 119 . |
|---|---|---|
| Missing data | 6 (4.9) | 5 (4.2) |
| Patients presenting at 1 complication | 19 (16.4) | 17 (14.9) |
| Patients presenting no complication | 95 (77.9) | 96 (80.7) |
| Patients presenting 1 complication | 19 (15.6) | 17 (14.3) |
| Patients presenting 2 complications | 6 (4.9) | 4 (3.4) |
| Patients presenting 3 complications | 1 (0.8) | 2 (1.7) |
| Patients presenting 4 complications | 1 (0.8 ) | 0 (0.0) |
| Number of patients presenting at least 1 complication (onlay surgery only)a | 15 (13.9) | 15 (14.0) |
aPlacebo: n = 114; promestriene: n = 111.
Thirty-six out of 230 patients presented at least 1 postoperative urethral fistula or dehiscence of the reconstructed urethra during the first year following surgery: 19 (16.4%) and 17 (14.9%) patients in the placebo and promestriene arms, respectively (odds ratio [OR] = 0.89, [95% confidence interval (CI) 0.44-1.82], P = 0.76). After adjusting on the centers, the OR was 0.93 (95% CI 0.45-1.94), P = 0.86.
A sensitivity analysis was performed taking into account the 11 subjects for whom the primary endpoint was missing. The analysis considered that complications were attributed to subjects receiving promestriene versus no complication for subjects in the placebo group. The OR was 1.31 (95% CI 0.65-2.63), P = 0.44.
In the 215 patients who underwent an onlay urethroplasty and for whom the primary endpoint was available, the number of patients presenting at least 1 postoperative urethral fistula or dehiscence of the reconstructed urethra during the first year following surgery was 15 (13.9%) and 15 (14.0%) patients in the placebo and Promestriene arms, respectively (OR = 1.20 [95% CI 0.53-2.73], P = 0.65 after adjusting on the centers).
Out of the 241 ITT population, 50 patients presented a total of 69 postsurgical complications of any type. Distribution of the complications per subject is presented in Table 2.
Safety outcomes
Hormonal safety screenings were measured in the first 169 patients and subsequently stopped after recommendation from the DSMB.
Results of hormonal screenings before and after treatment are presented in Table 3. After treatment, mean values of LH (P = 0.71), FSH (P = 0.27), anti-Müllerian hormone (P = 0.18), and estradiol (P = 0.72) were not different between the 2 arms.
| . | . | Before Treatment At Baseline . | After Treatment At Surgery . | ||
|---|---|---|---|---|---|
| Hormonal safety . | . | Placebo N = 85 . | Promestriene N = 84 . | Placebo N = 85 . | Promestriene N = 84 . |
| LH (UI/L) | Mean (+/- SD) | 0.07 (±0.07) | 0.07 (±0.06) | 0.46 (±0.40) | 0.49 (±0.44) |
| FSH (UI/L) | Mean (+/- SD) | 0.33 (±0.27) | 0.34 (±0.31) | 0.61 (±0.40) | 0.53 (±0.41) |
| AMH (pmol/L) | Mean (±SD) | 1689 (±771) | 1703 (±758) | 1362 (±691) | 1511 (±717) |
| Estradiol (pmol/L) | Mean (±SD) | 1.33 (±8.17) | 2.63 (±8.48) | 1.91 (±9.20) | 2.40 (±8.09) |
| Bone age safety | Placebo N = 70 | Promestriene N = 67 | Placebo N = 70 | Promestriene N = 67 | |
| Estimated bone age (months) | Mean (±SD) | 14.8 (±8.65) | 12.8 (±9.6) | 31.0 (±8.9) | 30.1 (±10.6) |
| Effective age (months) | Mean (±SD) | 15.7(±5.2) | 15.8 (±5.1) | 30.4 (±5.3) | 30.7 (±5.7) |
| . | . | Before Treatment At Baseline . | After Treatment At Surgery . | ||
|---|---|---|---|---|---|
| Hormonal safety . | . | Placebo N = 85 . | Promestriene N = 84 . | Placebo N = 85 . | Promestriene N = 84 . |
| LH (UI/L) | Mean (+/- SD) | 0.07 (±0.07) | 0.07 (±0.06) | 0.46 (±0.40) | 0.49 (±0.44) |
| FSH (UI/L) | Mean (+/- SD) | 0.33 (±0.27) | 0.34 (±0.31) | 0.61 (±0.40) | 0.53 (±0.41) |
| AMH (pmol/L) | Mean (±SD) | 1689 (±771) | 1703 (±758) | 1362 (±691) | 1511 (±717) |
| Estradiol (pmol/L) | Mean (±SD) | 1.33 (±8.17) | 2.63 (±8.48) | 1.91 (±9.20) | 2.40 (±8.09) |
| Bone age safety | Placebo N = 70 | Promestriene N = 67 | Placebo N = 70 | Promestriene N = 67 | |
| Estimated bone age (months) | Mean (±SD) | 14.8 (±8.65) | 12.8 (±9.6) | 31.0 (±8.9) | 30.1 (±10.6) |
| Effective age (months) | Mean (±SD) | 15.7(±5.2) | 15.8 (±5.1) | 30.4 (±5.3) | 30.7 (±5.7) |
| . | . | Before Treatment At Baseline . | After Treatment At Surgery . | ||
|---|---|---|---|---|---|
| Hormonal safety . | . | Placebo N = 85 . | Promestriene N = 84 . | Placebo N = 85 . | Promestriene N = 84 . |
| LH (UI/L) | Mean (+/- SD) | 0.07 (±0.07) | 0.07 (±0.06) | 0.46 (±0.40) | 0.49 (±0.44) |
| FSH (UI/L) | Mean (+/- SD) | 0.33 (±0.27) | 0.34 (±0.31) | 0.61 (±0.40) | 0.53 (±0.41) |
| AMH (pmol/L) | Mean (±SD) | 1689 (±771) | 1703 (±758) | 1362 (±691) | 1511 (±717) |
| Estradiol (pmol/L) | Mean (±SD) | 1.33 (±8.17) | 2.63 (±8.48) | 1.91 (±9.20) | 2.40 (±8.09) |
| Bone age safety | Placebo N = 70 | Promestriene N = 67 | Placebo N = 70 | Promestriene N = 67 | |
| Estimated bone age (months) | Mean (±SD) | 14.8 (±8.65) | 12.8 (±9.6) | 31.0 (±8.9) | 30.1 (±10.6) |
| Effective age (months) | Mean (±SD) | 15.7(±5.2) | 15.8 (±5.1) | 30.4 (±5.3) | 30.7 (±5.7) |
| . | . | Before Treatment At Baseline . | After Treatment At Surgery . | ||
|---|---|---|---|---|---|
| Hormonal safety . | . | Placebo N = 85 . | Promestriene N = 84 . | Placebo N = 85 . | Promestriene N = 84 . |
| LH (UI/L) | Mean (+/- SD) | 0.07 (±0.07) | 0.07 (±0.06) | 0.46 (±0.40) | 0.49 (±0.44) |
| FSH (UI/L) | Mean (+/- SD) | 0.33 (±0.27) | 0.34 (±0.31) | 0.61 (±0.40) | 0.53 (±0.41) |
| AMH (pmol/L) | Mean (±SD) | 1689 (±771) | 1703 (±758) | 1362 (±691) | 1511 (±717) |
| Estradiol (pmol/L) | Mean (±SD) | 1.33 (±8.17) | 2.63 (±8.48) | 1.91 (±9.20) | 2.40 (±8.09) |
| Bone age safety | Placebo N = 70 | Promestriene N = 67 | Placebo N = 70 | Promestriene N = 67 | |
| Estimated bone age (months) | Mean (±SD) | 14.8 (±8.65) | 12.8 (±9.6) | 31.0 (±8.9) | 30.1 (±10.6) |
| Effective age (months) | Mean (±SD) | 15.7(±5.2) | 15.8 (±5.1) | 30.4 (±5.3) | 30.7 (±5.7) |
Bone age assessments were also stopped after DSMB recommendation. Data were available for 137 patients at inclusion and at the end of their participation (Table 3). Twelve months postsurgery, the mean difference (bone age minus effective age) was not statistically different between arms: 0.61 (±7.4) in the placebo versus −0.58 (±8.3) in the promestriene arm.
At the end of the treatment, 1 (0.8%) and 2 (1.7%) patients presented with a gynecomastia in the placebo and promestriene arms, respectively.
Forty-one serious adverse events were notified to the pharmacovigilance center: 24 and 17 in the placebo and promestriene arms, respectively. One expected event, erythema, was considered as related to promestriene. It resolved without sequelae.
Discussion
To our knowledge, this is the first randomized, double-blind, placebo-controlled trial to evaluate the efficacy of an estrogen therapy in the surgery of hypospadias. In the ITT population, there were fewer patients presenting with a healing complication in the promestriene vs the placebo arm (14.9% vs 16.4%, respectively), but this difference was not statistically significant (P = 0.86) nor clinically relevant. Furthermore, it should be noted that this difference is in favor of the placebo arm in the per-protocol population but again with no statistical significant (P = 0.65). Thus, the study did not demonstrate that promestriene was better than placebo in reducing healing complications in postsurgery hypospadias.
This study probably suffered from a lack of power. Indeed, it was calibrated to detect an 11% decrease (ie, 26% to 15%) in the number of patients presenting a healing complication. That choice was guided by previous quantifications of complications in clinical cohorts of hypospadias and lead to a number of subjects required of 485 (2). However, only 244 patients were included, the drop of patients from 485 to 244 was explained by the unexpected reduction of patients’ flow in the 4 centers. The trial was stopped early after DSMB recommendation and because the trial completion would take 4.7 more years. Moreover, the choice of 26% for the placebo arm is probably a choice that overestimated the real rate; this was confirmed by the observation of a rate of healing complications of 13.3% in Lyon during the period of the study in 113 hypospadias patients who did not enter the study but were operated using an onlay urethroplasty (note that in Lyon we observed 9.8% in the promestriene arm and 10.3% in the placebo arm in the present study).
In our cohort of 241 patients, the overall healing complication rate dropped significantly (15.7%) compared to previous studies: 45% of complications (including 6% of fistula) in a series of 374 onlay urethroplasties (14), 31% of complications (17% of fistula) for Wiener et al who compared the results after an onlay vs a tubularized incised plate urethroplasty for proximal hypospadias (15), 22.5% complications in a series of 421 onlay urethroplasties (16), 20% fistula for Wallis et al (17), 25% fistula in 40 patients reported by Braga et al (18). The incidence of fistula and dehiscence after onlay procedure in our initial study (2) was 15% and 11.3%, respectively, which is comparable to several other publications.
It is striking that, in our cohort, the postsurgery complication rate dropped compared to previous studies. There is no clear explanation. The simple massage of the penis for 2 months preceding surgery (with placebo or active treatment) is unlikely to bring a positive effect, and patients not included in the protocol who underwent the same operation during the same period had almost the same outcome. So the massage is probably not the answer to this drop.
Overall, in the present study, promestriene was well tolerated with no increase in gynecomastia and no hormonological or radiological safety concerns. An increase in the LH values for both groups between pretreatment and posttreatment assessments were observed without explanation for this elevation.
Preoperative testosterone is commonly used to increase the size of the genital tubercle and the blood supply of its tissues to facilitate surgery and, possibly, to improve the healing outcome. Systemic testosterone, dihydrotestosterone ointment, and HCG have been used prior to surgery with variable protocols. A previous study of 126 onlay urethroplasties performed in our institution showed that preoperative testosterone administered less than 3 months prior to surgery significantly increased the healing complication rate compared to those who did not receive any hormonal stimulation or received it more than 3 months prior to surgery (57% vs 21.7%) (19). Published articles on this subject are equivocal and protocols too heterogeneous to establish comparisons. The effect of preoperative androgen stimulation on operative outcomes remains discussed between those who found improved outcomes (5,20-22) and those who reported negative effects (19,23). Meta-analyses are contradictory, highlighting the lack of consensus on this subject (24,25). The role of sex hormones on wound healing has been studied mainly because of the difference in healing observed between males and females. Dermatologists demonstrated that androgens slow down the healing process (8,26-29). Conversely, estrogens were reported to be beneficial by promoting the healing process (11,30-32). Their action depends of the type of hormone administered, the route of administration, the dose, the experimental model, and the animal model (9). Systemic administration of estrogens leads to breast development and may increase the risk of breast cancer, although this is not documented in the pediatric population. New molecules with topic administration may reduce these general effects (33). In Paiva et al’s study, 24 hypospadias patients received topical estrogen (0.01%) on the penis 30 days prior to surgery. Development of pubic hair in 14% of cases and darker genital skin in 50% were reported (6).
In our study, promestriene (17 beta-methoxy-3-propoxyestra-1,3,5 (10)-triene) was selected because of nonsignificant systemic effects (34,35) and significant local estrogenic effects (34). This product has a 24-h half-life, and less than 1% of the product goes into the blood circulation. In an ex vivo experiment on promestriene absorption into and through the foreskin, less than 2% of the initial dose were absorbed within 24 h. Steroid penetration into both epidermis and dermis was quantified and was found more than 6-fold higher in epidermis than in dermis where the healing process occurs (36). This study confirmed the safety of use of promestriene with no particular secondary effects detected.
Conclusion
The present study did not demonstrate any effect of promestriene versus placebo in reducing the healing complications postsurgery of the hypospadias. We did not detect any safety concerns related to the use of promestriene. The drop of complications using the same surgical technique between our initial cohort and the present series remains unexplained.
Acknowledgments
The study was coordinated by the Clinical Investigation Centre of Lyon: Hanane Gheit, Malika Hadid, Elhame Kahale, and Meriem El Jani (CIC Lyon). Patrick Chevarier (ClinInfo SA) was responsible for the data management. The Biostatistics Unit of the Hospices Civils de Lyon performed the statistical analysis. The hormonal analysis were performed by the “ Laboratoire d’Hormonologie d’Endocrinologie Moléculaire et des Maladies Rares” (HCL). The authors would like to thank the assistants/study nurses/clinical investigation centers’ staff from the different sites who greatly contributed to the project: Catherine Hoffer, Marie-Claire Kien, Corinne Spagnol, Anne Siaudeau, Kathy De-Boissieu-Du-Tiret (site nurses and assistant, Lyon), Sophie Pfister, Saphia Faked (CIC Necker), Marion Boivin (CIC Nantes), and Lila Benadjaoud (CIC Robert Debré). They also thank the central pharmacy (Christine PIVOT, HCL) for preparing the double-blind study treatment, the study monitors for their subsequent quality control (DRCi Lyon), and the DSMB members for their expertise and recommendations throughout the project: Silvy Laporte (Saint Etienne), Claire Bouvattier (Bicêtre), and Piet Hoebeke (Leuven).
Financial Support: This study was funded by a grant from the French government: Programme Hospitalier de Recherche Clinique (PHRC) national 2010. The study treatment was provided by former Theramex now Teva Pharmaceutical Industries Ltd.
Clinical Trial Information: This study was registered in the clinicaltrials.gov website under number NCT01370798.
Authors Contributions: DG (article author, study investigator, patient recruitment); PM (main project coordinator, co-author, patient recruitment); LR and PR (statistical analysis); AC, TBC, AEG, and MDL, (patient recruitment); CLG, PC (endocrinology evaluations); IP and YM (hormonal evaluations); FP (investigational product management); and ME-J, YM, SG, and BK (study management, methodology, interpretation of results). All authors read and approved the final manuscript.
Additional Information
Disclosure Summary: The authors declare that they have no competing interests.
Data Availability: Data can be shared following regulatory and confidentiality requirements to other researchers on request after thorough review of the objective.
References



