-
PDF
- Split View
-
Views
-
Cite
Cite
Alessandro Brancatella, Debora Ricci, Nicola Viola, Daniele Sgrò, Ferruccio Santini, Francesco Latrofa, Subacute Thyroiditis After Sars-COV-2 Infection, The Journal of Clinical Endocrinology & Metabolism, Volume 105, Issue 7, July 2020, Pages 2367–2370, https://doi.org/10.1210/clinem/dgaa276
Close - Share Icon Share
Abstract
Subacute thyroiditis (SAT) is a thyroid disease of viral or postviral origin. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that began in Wuhan, China, has spread rapidly worldwide and Italy has been severely affected by this outbreak.
The objective of this work is to report the first case of SAT related to SARS-CoV-2 infection.
We describe the clinical, laboratory, and imaging features of an 18-year-old woman who came to our attention for fever, neck pain radiated to the jaw, and palpitations occurring 15 days after a SARS-CoV-2–positive oropharyngeal swab. Coronavirus disease 2019 (COVID-19) had been mild and the patient had completely recovered in a few days.
At physical examination the patient presented with a slightly increased heart rate and a painful and enlarged thyroid on palpation. At laboratory exams free thyroxine and free triiodothyronine were high, thyrotropin undetectable, and inflammatory markers and white blood cell count elevated. Bilateral and diffuse hypoechoic areas were detected at neck ultrasound. One month earlier, thyroid function and imaging both were normal. We diagnosed SAT and the patient started prednisone. Neck pain and fever recovered within 2 days and the remaining symptoms within 1 week. Thyroid function and inflammatory markers normalized in 40 days.
We report the first case of SAT after a SARS-CoV-2 infection. We alert clinicians to additional and unreported clinical manifestations associated with COVID-19.
Subacute thyroiditis (SAT) is a self-limited inflammatory thyroid disease characterized by neck pain, general symptoms, and thyroid dysfunction (1, 2). SAT is usually preceded by an upper respiratory tract infection. Direct and indirect evidence support a viral or postviral origin of this disease, and many viruses have been reported as potentially causative agents (3).
Since January 2020, coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in an emerging respiratory infection with a pandemical diffusion (4). Here, we report the first case of SAT in a patient affected by SARS-CoV-2.
Material and Methods
Case presentation
On February 28, 2020, an 18-year-old woman underwent an oropharyngeal swab for SARS-CoV-2 on the evidence that his father, who lived with her, had been hospitalized 2 days earlier because of COVID-19. Her swab turned out to be positive. In the next few days, the woman developed mild upper respiratory symptoms (rhinorrhea and cough). She was left untreated and recovered completely in 4 days (Fig. 1). Two additional swabs for SARS-CoV-2 (March 13 and 14) both turned out to be negative.
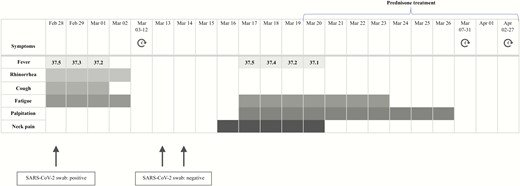
Symptoms and maximum body temperatures referred to by the patient.
On March 17, the patient presented with sudden fever (37.5 °C), fatigue, palpitations, and anterior neck pain radiating to the jaw (Fig. 1). Because of worsening of the neck pain, on March 19 the patient was referred to us. For the previous finding of isolated hyperthyrotropinemia, on February 21 she had undergone thyroid evaluation: Thyroid function was normal, antibodies to thyroglobulin (TgAb) and to thyroperoxidase (TPOAb) negative, and thyroid ultrasound unremarkable (Table 1).
| Measure . | Reference range . | February 21 . | March 19 . | April 1 . | April 27 . |
|---|---|---|---|---|---|
| FT4, nmol/L | 11-23 | 15.4 | 27.2 | 21.7 | 16.2 |
| FT3, pmol/L | 4.6-8.4 | 5.5 | 8.7 | 7.5 | 5.3 |
| TSH, mIU/L | 0.5-4.1 | 2.1 | < 0.04 | 0.2 | 2.9 |
| TgAb, IU/mL | < 30 | < 30 | 120.2 | ||
| TPOAb, IU/mL | < 10 | < 10 | < 10 | ||
| TRAb, IU/mL | < 1.5 | < 1.5 | |||
| Tg, µg/L | 5.6 | ||||
| White cell count, per L | 3800-11 000 | 11 200 | 6900 | 6600 | |
| ESR, mm/h | 0-13 | 90 | 28 | 2 | |
| CRP, mg/L | < 2 | 6.9 | 1.2 | 0.9 |
| Measure . | Reference range . | February 21 . | March 19 . | April 1 . | April 27 . |
|---|---|---|---|---|---|
| FT4, nmol/L | 11-23 | 15.4 | 27.2 | 21.7 | 16.2 |
| FT3, pmol/L | 4.6-8.4 | 5.5 | 8.7 | 7.5 | 5.3 |
| TSH, mIU/L | 0.5-4.1 | 2.1 | < 0.04 | 0.2 | 2.9 |
| TgAb, IU/mL | < 30 | < 30 | 120.2 | ||
| TPOAb, IU/mL | < 10 | < 10 | < 10 | ||
| TRAb, IU/mL | < 1.5 | < 1.5 | |||
| Tg, µg/L | 5.6 | ||||
| White cell count, per L | 3800-11 000 | 11 200 | 6900 | 6600 | |
| ESR, mm/h | 0-13 | 90 | 28 | 2 | |
| CRP, mg/L | < 2 | 6.9 | 1.2 | 0.9 |
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FT3, free triiodothyronine; FT4, free thyroxine; Tg, thyroglobulin; TgAb, thyroglobulin antibodies; TPOAb, thyroperoxidase antibodies; TRAb, TSH receptor antibodies; TSH, thyrotropin.
| Measure . | Reference range . | February 21 . | March 19 . | April 1 . | April 27 . |
|---|---|---|---|---|---|
| FT4, nmol/L | 11-23 | 15.4 | 27.2 | 21.7 | 16.2 |
| FT3, pmol/L | 4.6-8.4 | 5.5 | 8.7 | 7.5 | 5.3 |
| TSH, mIU/L | 0.5-4.1 | 2.1 | < 0.04 | 0.2 | 2.9 |
| TgAb, IU/mL | < 30 | < 30 | 120.2 | ||
| TPOAb, IU/mL | < 10 | < 10 | < 10 | ||
| TRAb, IU/mL | < 1.5 | < 1.5 | |||
| Tg, µg/L | 5.6 | ||||
| White cell count, per L | 3800-11 000 | 11 200 | 6900 | 6600 | |
| ESR, mm/h | 0-13 | 90 | 28 | 2 | |
| CRP, mg/L | < 2 | 6.9 | 1.2 | 0.9 |
| Measure . | Reference range . | February 21 . | March 19 . | April 1 . | April 27 . |
|---|---|---|---|---|---|
| FT4, nmol/L | 11-23 | 15.4 | 27.2 | 21.7 | 16.2 |
| FT3, pmol/L | 4.6-8.4 | 5.5 | 8.7 | 7.5 | 5.3 |
| TSH, mIU/L | 0.5-4.1 | 2.1 | < 0.04 | 0.2 | 2.9 |
| TgAb, IU/mL | < 30 | < 30 | 120.2 | ||
| TPOAb, IU/mL | < 10 | < 10 | < 10 | ||
| TRAb, IU/mL | < 1.5 | < 1.5 | |||
| Tg, µg/L | 5.6 | ||||
| White cell count, per L | 3800-11 000 | 11 200 | 6900 | 6600 | |
| ESR, mm/h | 0-13 | 90 | 28 | 2 | |
| CRP, mg/L | < 2 | 6.9 | 1.2 | 0.9 |
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FT3, free triiodothyronine; FT4, free thyroxine; Tg, thyroglobulin; TgAb, thyroglobulin antibodies; TPOAb, thyroperoxidase antibodies; TRAb, TSH receptor antibodies; TSH, thyrotropin.
Results
On March 19, at physical examination, the patient’s heart rate was 90 beats per minute and on palpation the thyroid gland was markedly painful and slightly tender and enlarged. The patient did not refer to any upper respiratory symptom. At laboratory exams, free thyroxine (FT4) and free triiodothyronine (FT3) were both mildly elevated, thyrotropin (TSH) undetectable, thyroglobulin (Tg) detectable at low level with positive TgAb. TPOAb and antibodies to the TSH receptor were negative. Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white cell blood count were high (Table 1). Thyroid ultrasound showed multiple, diffuse hypoechoic areas (Fig. 2). Thus, SAT was diagnosed and March 20 the patient started prednisone (25 mg/d). Neck pain and fever disappeared within 2 days and the remaining symptoms within 1 week (Fig. 1). On April 1, FT4 concentration had declined to the upper normal range, TSH had risen to almost normal level, ESR was slightly elevated, and white cell blood count and CRP were normal (Table 1). Steroid was progressively tapered and at the last evaluation (April 27), while taking prednisone 16 mg/day, the patient was asymptomatic. Thyroid functional tests and inflammatory markers were normal (Fig. 1 and Table 1).
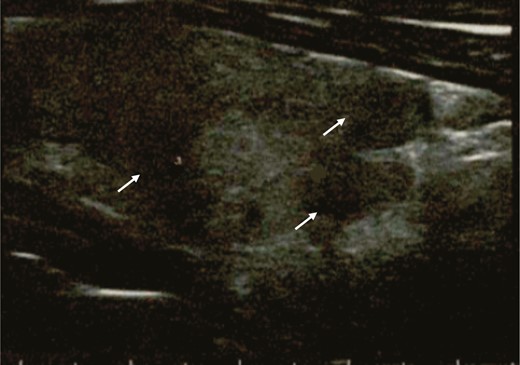
Thyroid ultrasound performed during the thyrotoxic phase, showing multiple hypoechoic areas (arrows).
Discussion
SAT is a self-limited, inflammatory disorder characterized by neck pain and general symptoms, often associated with thyroid dysfunction (1, 2). Clinically, neck pain, frequently radiating to the upper neck, is the presenting symptom of SAT. On palpation, the thyroid is painful, tender, and enlarged. Systemic symptoms, including fever, fatigue, myalgia, and anorexia are common (1, 2). Thyroid dysfunction usually follows a triphasic course (thyrotoxicosis, hypothyroidism, and euthyroidism) that usually lasts 3 months. Symptomatic thyrotoxicosis occurs in the majority of patients, whereas clinical hypothyroidism is uncommon.
Although the diagnosis of SAT is usually based on clinical grounds, laboratory tests and neck imaging are useful (1, 2, 5). A marked elevation of inflammatory markers (ESR and CRP) is common. In the acute phase, most individuals present with overt thyrotoxicosis (high FT4 levels and low to undetectable TSH levels) (1). As a consequence of the destructive process, the FT3/FT4 ratio is usually low and Tg elevated (1, 2). TSH receptor antibodies are generally absent, whereas TPOAb are positive in some and TgAb in a larger number of patients (6-8). During the acute phase, radionuclide thyroid scanning typically shows reduced or absent tracer uptake, whereas thyroid ultrasound shows bilateral hypoechoic areas associated with low to absent vascularization (5).
Thyroid evaluation performed 1 month earlier in the patient herein reported had ruled out thyroid diseases. She then presented with clinical, biochemical, and imaging stigmata of SAT. Of note, as previously reported, SAT was associated with the de novo appearance of TgAb (6, 8). Because of positive TgAb, Tg levels were inappropriately normal (9). Although the benefit of glucocorticoid is debated (10), in our experience the administration of prednisone during the acute phase is associated with a faster resolution of symptoms and a lower risk of recurrence (11). Indeed, within 2 weeks of treatment, we obtained a complete recovery of symptoms and a remarkable improvement both in thyroid function and inflammatory markers.
The etiology and the pathogenesis of SAT have not been completely understood, but it is common opinion that the disease is due to a viral infection or to a postviral inflammatory reaction in genetically predisposed individuals (1, 3). Some human leukocyte antigen (HLA) haplotypes (mainly HLA-Bw35, but also HLA-B67, HLA-B15/62, and HLA-Drw8) have been reported to predispose to SAT (12, 13). SAT is usually preceded by an upper respiratory infection and usually presents in association with characteristic symptoms of viral disease. In addition, several cases of the disease have been reported during viral outbreaks (2, 3). Some authors have reported a higher incidence of SAT in summer, during the outbreaks of echovirus and coxsackievirus (14, 15). Many other viruses, including mumps, adenovirus, orthomyxovirus, Epstein-Barr virus, hepatitis E, HIV, cytomegalovirus, dengue fever, and rubella have also been related to SAT (3, 16-18). At autopsy, thyroid injury was demonstrated in patients with SARS-CoV infection during the 2002 outbreak (19). However, coronavirus infection has never been associated with clinical SAT.
In conclusion, to our knowledge, this is the first case of SAT related to SARS-CoV-2. Because of the chronological association, SARS-CoV-2 may be considered accountable for the onset of SAT. COVID-19, originating from Wuhan, is spreading around the world as a pandemic disease (4). Since initial detection of the virus, more than 3 million cases of COVID-19 have been confirmed worldwide and Italy has been severely affected since February 2020 (20).
We therefore believe physicians should be alerted about the possibility of this additional clinical manifestation related to SARS-CoV-2 infection.
Abbreviations
- COVID-19
coronavirus disease 2019
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- FT3
free triiodothyronine
- FT4
free thyroxine
- HLA
human leukocyte antigen
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SAT
subacute thyroiditis
- Tg
thyroglobulin
- TgAb
thyroglobulin antibodies
- TPOAb
thyroperoxidase antibodies
- TSH
thyrotropin
Acknowledgments
We thank the patient, whose written informed consent was obtained and who graciously agreed to collaborate with this study. We also thank Ms Laura Macrì for her technical help in drawing the figure.
Financial Support: This work was supported by “Fondi di Ateneo 2018,” University of Pisa (to F.L.).
Author Contributions: Discussion of study results: A.B., D.R., N.V., D.S., F.S., and F.L.; writing of the manuscript: A.B., F.S., and F.L.
Additional Information
Disclosure Summary: The authors declare no potential conflicts of interest.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References



