-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Janner, Grit Sommer, Michael Groessl, Christa E Flück, Premature Adrenarche in Girls Characterized By Enhanced 17,20-Lyase and 17β-Hydroxysteroid Dehydrogenase Activities, The Journal of Clinical Endocrinology & Metabolism, Volume 105, Issue 12, December 2020, Pages e4439–e4451, https://doi.org/10.1210/clinem/dgaa598
Close - Share Icon Share
Abstract
Girls with premature adrenarche (PA) may have a higher risk of developing polycystic ovary syndrome (PCOS) and metabolic syndrome. The biological purpose of adrenarche is unknown and the role of novel biosynthetic pathways remains unclear.
To compare the urinary steroid metabolome and enzyme activities of girls with PA to age-matched control girls and to published steroid values of girls with normal adrenarche and of women with PCOS and their newborn daughters.
Prospective observational study from 2009 to 2014.
Academic pediatric endocrinology referral center.
Twenty-three girls with PA and 22 healthy, age-matched girls.
Steroid metabolites in 24-hour urine samples, including 4 progesterones, 5 corticosterones, aldosterone, 13 androgens, 2 estrogens, 14 glucocorticoids, and enzyme activities represented by metabolite ratios.
Girls with PA had a higher body mass index (mean standard deviation scores 0.9 vs -0.3, P = 0.013). Androgen excretion was higher in PA girls than in control girls (median 3257 nmol/24 hours vs 1627 nmol/24 hours, P < 0.001), in particular metabolites from alternate androgen pathways. The amount of progesterone, corticosterone, aldosterone, estrogen, and cortisol metabolites were similar between groups. Activities of 17β-hydroxysteroid-dehydrogenase and of 17,20-lyase were higher in girls with PA. Activities of 3β-hydroxysteroid-dehydrogenase, 21-hydroxylase, and 5α-reductase activity were not different between groups, in contrast to published results on girls with normal adrenarche or PCOS females.
Metabolites and enzymes involved in alternate androgen pathways appear to be markers of PA. Prospective studies should assess whether steroid production in PA also differs from adrenarche at normal timing and persists into adulthood.
Adrenarche was first described by F. Albright in 1947 and refers to the onset of production of adrenal androgens, namely dehydroepiandrosterone (DHEA), DHEA sulfate, and androstenedione, as a result of the maturation of the zona reticularis (1). Pubarche refers to its clinical signs, as the appearance of pubic and axillary hair, seborrhea, and body odor. Premature adrenarche (PA) is originally defined as the appearance of pubic hair before the age of 8 years in girls and 9 years in boys, but several studies have now shown that adrenarche is a gradual process that starts at preschool age with the appearance of focal islands of zona reticularis in the adrenal cortex and with traces of measurable adrenal C19 steroids as early as 3 years of age (2–6).
In the adrenal zona reticularis, cholesterol is converted to pregnenolone by the cholesterol side-chain cleavage enzyme (CYP11A1), and pregnenolone is further converted to 17-hydroxypregnenolone and DHEA due to the high activity of P450c17 17,20-lyase (CYP17A1), while the 3β-hydroxysteroid dehydrogenase 2 (3βHSD/HSD3B2) activity is relatively low. An important fraction of DHEA is further sulphated by hydroxysteroid sulfotransferase (SULT2A) to the weakly active androgen DHEA sulfate (2). In addition to this classic pathway of adrenal androgen production, an alternative pathway has first been described in the tammar wallaby (7) and later in humans (3), leading from 17-hydroxyprogesterone directly to dihydrotestosterone without passing through testosterone. In this alternative pathway, 17-hydroxyprogesterone is converted to 17α-hydroxyallopregnanolone by 5α- and 3α-reduction, and 17α-hydroxyallopregnanolone is further converted to the 19C androgen androsterone by 17,20-lyase, then androsterone is reduced to androstanediol by 17β-hydroxysteroid dehydrogenase 3 (17βHSD/HSD17B), finally yielding dihydrotestosterone by 17βHSD6 (HSD17B6) (8) (Fig. 1). A short-cut from androstenedione to androstanedione may also lead to this alternative pathway. Androstenedione and testosterone are largely converted by 11β-hydroxylase (encoded by CYP11B1) to 11-hydroxyandrostenedione and 11-hydroxytestosterone, respectively, in the adrenals. 11-hydroxy-testosterone is further converted to 11-hydroxydihydrotestosterone by 5α-reductase. These three 11-hydroxy-androgens are further reduced to 11-keto-androstenedione, 11-keto-testosterone, and 11-keto-dihydrotestosterone by adrenal 11β-hydroxysteroid dehydrogenase (11βHSD/HSD11B). This indicates that 11 oxygenated androgens, namely 11-keto-androstenedione, are the predominant circulating bioactive androgen during adrenarche and PA (9, 10) (Fig. 1).
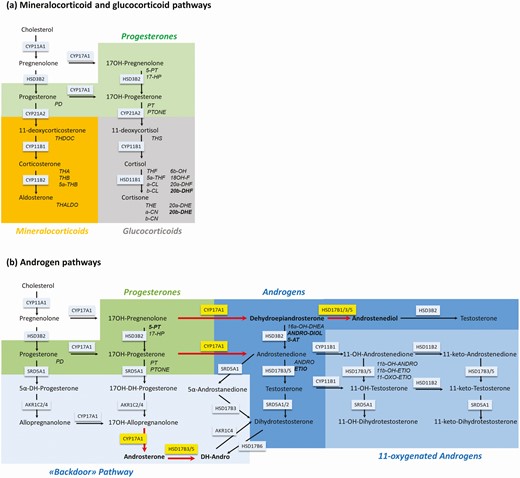
Schematic representation of the steroid biosynthesis pathways. Steroids are colored according to their classes. Normal script refers to steroids primarily found in serum and capitalized italic script refers to the corresponding urinary metabolites. Enzymes involved in the pathway are represented in boxes. For metabolite abbreviations see Table 1. (a) Schematic representation of the mineralocorticoid and glucocorticoid pathway. Green background depicts progesterones, orange background depicts mineralocorticoids, and grey background depicts glucocorticoids. (b) Schematic representation of the androgen pathway. Enzymes with higher activity in girls with premature adrenarche (PA) than in control girls are shown in yellow boxes with red arrows indicating the direction of the reaction. Hormones and their metabolites, which were higher in urine of girls with PA than in control girls are highlighted in bold. Green background depicts progesterones and blue background depicts androgens. Androgen metabolites of the classical pathway are shaded in dark blue, androgen metabolites of the backdoor pathway are shaded in light blue, and 11-oxygenated androgens are shaded in medium blue.
The impact of PA on endocrine function and general health is still matter of debate. While an early study from Spain reported functional ovarian hyperandrogenism in pubertal girls with a history of PA (11), studies that are more recent did not observe this (1). The relationship between PA and body mass index (BMI), metabolic syndrome, and cardiovascular risk is also controversial. Early obesity could trigger PA (12), and obesity at presentation of PA seems to be associated with insulin resistance and metabolic syndrome (13). But several other studies could not find any difference in the components of the metabolic syndrome between PA and controls (14, 15).
To establish the diagnosis of PA, clinicians must first rule out other causes of excessive androgen production like late-onset congenital adrenal hyperplasia (CAH), androgen producing tumors, and central precocious puberty. While the diagnosis of an androgen producing tumor and central precocious puberty are straightforward on a clinical and biochemical basis, the distinction between PA and late-onset CAH can be challenging.
Steroid profiling by mass spectrometric methods is a powerful tool for both routine diagnostic applications and for new discoveries. Multiple steroids can be measured simultaneously from different matrices facilitating the characterization of complex steroid disorders (16, 17). The detection of the androgen secretion pattern in serum during adrenarche and PA has yielded conflicting results, probably due to methodological issues (4, 5, 9). A 24-hour urinary steroid analysis by gas chromatography mass spectrometry (GC-MS) is a noninvasive, validated method to measure the urinary steroid metabolome in children (6, 18). However, the urinary steroid metabolome of children with PA has not been studied yet.
Therefore, we conducted a prospective study to assess the urinary steroid metabolome of girls with PA compared with age-matched control girls. We then compared the urinary steroid pattern of our girls with PA to the pattern of girls with a normal-timed adrenarche from a German study (6), and to the pattern of women with PCOS and their 1–3 year old daughters from a US study (19).
Participants and Methods
Study design and participants
This was a prospective study including 23 girls with PA (mean age 7 years) and 22 healthy, age-matched girls. The study was approved by the Ethics Board of Canton, Bern, Switzerland (BASEC ID PB 216_02175). The legal representatives of all participants provided written, informed consent.
Our outpatient clinic recruited the girls with PA, and a pediatric general practice recruited healthy girls that served as the comparison group. The inclusion criteria for PA were Tanner stage II pubic hair before the age of 8 and the absence of breast development. We excluded girls who had known disorders of steroid enzymes; adrenocortical tumors; chronic diseases; endocrine diseases such as hypogonadism, differences of sex development, diabetes mellitus, or thyroid dysfunction; or received medications affecting adrenal function.
Clinical measurements
All participants underwent physical examination. Standing height was measured with an Ulmer stadiometer (Busse design, Ulm, Germany) to the nearest 0.1 cm and body weight was measured to the nearest 0.1 kg using a Seca scale (Seca GmbH, Hamburg, Germany). Body mass index was calculated as weight (kg) divided by square height (m). We collected gestational age and birth weight from medical records. Standard deviation scores (SDS) were calculated for birth weight, height, weight, and BMI by the learning management system (LMS) method using the references from Swiss girls. Pubertal staging was assessed by the Tanner method.
Sample collection and biochemical measurements
We instructed participants and their parents to collect girls’ urine over a period of 24 hours. Samples were stored at >-20°C before assessing the steroid profile with an in-house method of GC-MS (18). We measured 39 steroid metabolites comprising progesterones, corticosterones, aldosterone, androgens, estrogens, and glucocorticoids in the urine samples. Table 1 provides an overview of the measured steroid metabolites and the abbreviations used in this study. In brief, the method comprises a pre-extraction on a Sep-Pak C18 column, an enzymatic hydrolysis following extraction on a Sep-Pak C18 cartridge, and derivatization and purification on a Lipidex 5000 column. A gas chromatograph 7890A from Agilent Technologies (La Jolla, California) coupled to a mass selective detector, Hewlett-Packard (Palo Alto, California, USA) 5975C, providing selected ion monitoring (SIM) was used.
List of steroid metabolites analyzed in the study, including abbreviation, trivial name, and systemic name
| Class . | Abbreviation . | Trivial Name . | Systematic Name . |
|---|---|---|---|
| Progesterone metabolites | 17-HP | 17-Hydroxypregnanolone | 3α,17α-dihydroxy-5-pregnen-20-one |
| PD | Pregnanediol | 5β-pregnane-3α,20α-diol | |
| PT | Pregnanetriol | 5β-pregnane-3α,17α,20α-triol | |
| PTONE | Pregnanetriolone | 3α,17α,20α-trihydroxy-5β-pregnan-11-one | |
| Corticosterone metabolites | THDOC | Tetrahydrodeoxycorticosterone | 3α,21-dihydroxy-5β-pregnan-20-one |
| THA | Tetrahydro-11-dehydrocorticosterone | 3α,21-dihydroxy-5β-pregnane-11,20-dione | |
| THB | Tetrahydrocorticosterone | 3α,11β,21-trihydroxy-5β-pregnan-20-one | |
| 5a-THB | 5α-Tetrahydrocorticosterone | 3α,11β,21-trihydroxy-5α-pregnan-20-one | |
| Aldosterone metabolites | THALDO | Tetrahydroaldosterone | 11β,18-epoxy-3α,18,21-trihydroxy-5β-pregnan-20-one |
| Androgen metabolites | ANDRO | Androsterone | 3α-hydroxy-5α-androstan-17-one |
| ETIO | Etiocholanolone | 3α-Hydroxy-5β-androstan-17-one | |
| DH-ANDRO | Androstanediol, Dihydroandrosterone | 5α-androstane-3α,17β-diol | |
| 11-OXO-ETIO | 11-oxo-Etiocholanolone | 3α-hydroxy-5β-androstane-11,17-dione | |
| 11b-OH-ANDRO | 11β-Hydroxyandrosterone | 3α,11β-dihydroxy-5α-androstan-17-one | |
| 11b-OH-ETIO | 11β-Hydroxyetiocholanolone | 3α,11β-dihydroxy-5β-androstan-17-one | |
| DHEA | Dehydroepiandrosterone | 3β-hydroxy-5-androsten-17-one | |
| ANDRO-DIOL | Androstenediol | 5-androstene-3β,17β-diol | |
| 16a-OH-DHEA | 16α-Hydroxy- Dehydroepiandrosterone | 3β,16α-dihydroxy-5-androsten-17-one | |
| 5-AT | Androstenetriol | 5-androstene-3β,16α,17β-triol | |
| 5-PT | Pregnenetriol | 5-pregnene-3β,17α,20α-triol | |
| TST | Testosterone | 17β-hydroxy-4-androsten-3-one | |
| DH-TST | 5α-Dihydrotestosterone | 17β-hydroxy-5α-androstan-3-one | |
| Estrogen metabolites | ESTRIOL | Estriol | 1,3,5 (10)-estratriene-3,16α,17β-triol |
| ESTRADIOL | 17β-Estradiol | 1,3,5 (10)-estratriene-3,17β-diol | |
| 11-Deoxycortisol metabolites | THS | Tetrahydro-11-deoxycortisol | 3α,17,21-trihydroxy-5β-pregnan-20-one |
| Cortisol metabolites | E | Cortisone | 17,21-dihydroxy-4-pregnene-3,11,20-trione |
| THE | Tetrahydrocortisone | 3α,17,21-trihydroxy-5β-pregnan-11,20-dione | |
| b-CN | β-Cortolone | 3α,17α,20β,21-tetrahydroxy-5β-pregnane-11-one | |
| 20a-DHE | 20α-Dihydrocortisone | 17α,20α,21-trihydroxy-4-pregnene-3,11-dione | |
| 20b-DHE | 20β-Dihydrocortisone | 17α,20β,21-trihydroxy-4-pregnene-3,11-dione | |
| F | Cortisol | 11β,17,21-trihydroxy-4-pregnene-3,20-dione | |
| THF | Tetrahydrocortisol | 3α,11β,17,21-tetrahydroxy-5β-pregnan-20-one | |
| 5a-THF | 5α-Tetrahydrocortisol | 3α,11β,17,21-tetrahydroxy-5α-pregnan-20-one | |
| a-CL | α-Cortol | 5β-pregnane-3α,11β,17α,20α,21-pentol | |
| b-CL | β-Cortol | 5β-pregnane-3α,11β,17α,20β,21-pentol | |
| 20a-DHF | 20α-Dihydrocortisol | 11β,17,20α,21-tetrahydroxy-4-pregnen-3-one | |
| 6b-OH-F | 6β-Hydroxycortisol | 6β,11β,17α,21-tetrahydroxy-4-pregnene-3,20-dione | |
| 18-OH-F | 18-Hydroxycortisol | 11,17,18,21-tetrahydroxy-4-pregnene-3,20-dione | |
| a-CN | α-Cortolone | 3α,17α,20α,21-tetrahydroxy-5β-pregnane-11-one |
| Class . | Abbreviation . | Trivial Name . | Systematic Name . |
|---|---|---|---|
| Progesterone metabolites | 17-HP | 17-Hydroxypregnanolone | 3α,17α-dihydroxy-5-pregnen-20-one |
| PD | Pregnanediol | 5β-pregnane-3α,20α-diol | |
| PT | Pregnanetriol | 5β-pregnane-3α,17α,20α-triol | |
| PTONE | Pregnanetriolone | 3α,17α,20α-trihydroxy-5β-pregnan-11-one | |
| Corticosterone metabolites | THDOC | Tetrahydrodeoxycorticosterone | 3α,21-dihydroxy-5β-pregnan-20-one |
| THA | Tetrahydro-11-dehydrocorticosterone | 3α,21-dihydroxy-5β-pregnane-11,20-dione | |
| THB | Tetrahydrocorticosterone | 3α,11β,21-trihydroxy-5β-pregnan-20-one | |
| 5a-THB | 5α-Tetrahydrocorticosterone | 3α,11β,21-trihydroxy-5α-pregnan-20-one | |
| Aldosterone metabolites | THALDO | Tetrahydroaldosterone | 11β,18-epoxy-3α,18,21-trihydroxy-5β-pregnan-20-one |
| Androgen metabolites | ANDRO | Androsterone | 3α-hydroxy-5α-androstan-17-one |
| ETIO | Etiocholanolone | 3α-Hydroxy-5β-androstan-17-one | |
| DH-ANDRO | Androstanediol, Dihydroandrosterone | 5α-androstane-3α,17β-diol | |
| 11-OXO-ETIO | 11-oxo-Etiocholanolone | 3α-hydroxy-5β-androstane-11,17-dione | |
| 11b-OH-ANDRO | 11β-Hydroxyandrosterone | 3α,11β-dihydroxy-5α-androstan-17-one | |
| 11b-OH-ETIO | 11β-Hydroxyetiocholanolone | 3α,11β-dihydroxy-5β-androstan-17-one | |
| DHEA | Dehydroepiandrosterone | 3β-hydroxy-5-androsten-17-one | |
| ANDRO-DIOL | Androstenediol | 5-androstene-3β,17β-diol | |
| 16a-OH-DHEA | 16α-Hydroxy- Dehydroepiandrosterone | 3β,16α-dihydroxy-5-androsten-17-one | |
| 5-AT | Androstenetriol | 5-androstene-3β,16α,17β-triol | |
| 5-PT | Pregnenetriol | 5-pregnene-3β,17α,20α-triol | |
| TST | Testosterone | 17β-hydroxy-4-androsten-3-one | |
| DH-TST | 5α-Dihydrotestosterone | 17β-hydroxy-5α-androstan-3-one | |
| Estrogen metabolites | ESTRIOL | Estriol | 1,3,5 (10)-estratriene-3,16α,17β-triol |
| ESTRADIOL | 17β-Estradiol | 1,3,5 (10)-estratriene-3,17β-diol | |
| 11-Deoxycortisol metabolites | THS | Tetrahydro-11-deoxycortisol | 3α,17,21-trihydroxy-5β-pregnan-20-one |
| Cortisol metabolites | E | Cortisone | 17,21-dihydroxy-4-pregnene-3,11,20-trione |
| THE | Tetrahydrocortisone | 3α,17,21-trihydroxy-5β-pregnan-11,20-dione | |
| b-CN | β-Cortolone | 3α,17α,20β,21-tetrahydroxy-5β-pregnane-11-one | |
| 20a-DHE | 20α-Dihydrocortisone | 17α,20α,21-trihydroxy-4-pregnene-3,11-dione | |
| 20b-DHE | 20β-Dihydrocortisone | 17α,20β,21-trihydroxy-4-pregnene-3,11-dione | |
| F | Cortisol | 11β,17,21-trihydroxy-4-pregnene-3,20-dione | |
| THF | Tetrahydrocortisol | 3α,11β,17,21-tetrahydroxy-5β-pregnan-20-one | |
| 5a-THF | 5α-Tetrahydrocortisol | 3α,11β,17,21-tetrahydroxy-5α-pregnan-20-one | |
| a-CL | α-Cortol | 5β-pregnane-3α,11β,17α,20α,21-pentol | |
| b-CL | β-Cortol | 5β-pregnane-3α,11β,17α,20β,21-pentol | |
| 20a-DHF | 20α-Dihydrocortisol | 11β,17,20α,21-tetrahydroxy-4-pregnen-3-one | |
| 6b-OH-F | 6β-Hydroxycortisol | 6β,11β,17α,21-tetrahydroxy-4-pregnene-3,20-dione | |
| 18-OH-F | 18-Hydroxycortisol | 11,17,18,21-tetrahydroxy-4-pregnene-3,20-dione | |
| a-CN | α-Cortolone | 3α,17α,20α,21-tetrahydroxy-5β-pregnane-11-one |
List of steroid metabolites analyzed in the study, including abbreviation, trivial name, and systemic name
| Class . | Abbreviation . | Trivial Name . | Systematic Name . |
|---|---|---|---|
| Progesterone metabolites | 17-HP | 17-Hydroxypregnanolone | 3α,17α-dihydroxy-5-pregnen-20-one |
| PD | Pregnanediol | 5β-pregnane-3α,20α-diol | |
| PT | Pregnanetriol | 5β-pregnane-3α,17α,20α-triol | |
| PTONE | Pregnanetriolone | 3α,17α,20α-trihydroxy-5β-pregnan-11-one | |
| Corticosterone metabolites | THDOC | Tetrahydrodeoxycorticosterone | 3α,21-dihydroxy-5β-pregnan-20-one |
| THA | Tetrahydro-11-dehydrocorticosterone | 3α,21-dihydroxy-5β-pregnane-11,20-dione | |
| THB | Tetrahydrocorticosterone | 3α,11β,21-trihydroxy-5β-pregnan-20-one | |
| 5a-THB | 5α-Tetrahydrocorticosterone | 3α,11β,21-trihydroxy-5α-pregnan-20-one | |
| Aldosterone metabolites | THALDO | Tetrahydroaldosterone | 11β,18-epoxy-3α,18,21-trihydroxy-5β-pregnan-20-one |
| Androgen metabolites | ANDRO | Androsterone | 3α-hydroxy-5α-androstan-17-one |
| ETIO | Etiocholanolone | 3α-Hydroxy-5β-androstan-17-one | |
| DH-ANDRO | Androstanediol, Dihydroandrosterone | 5α-androstane-3α,17β-diol | |
| 11-OXO-ETIO | 11-oxo-Etiocholanolone | 3α-hydroxy-5β-androstane-11,17-dione | |
| 11b-OH-ANDRO | 11β-Hydroxyandrosterone | 3α,11β-dihydroxy-5α-androstan-17-one | |
| 11b-OH-ETIO | 11β-Hydroxyetiocholanolone | 3α,11β-dihydroxy-5β-androstan-17-one | |
| DHEA | Dehydroepiandrosterone | 3β-hydroxy-5-androsten-17-one | |
| ANDRO-DIOL | Androstenediol | 5-androstene-3β,17β-diol | |
| 16a-OH-DHEA | 16α-Hydroxy- Dehydroepiandrosterone | 3β,16α-dihydroxy-5-androsten-17-one | |
| 5-AT | Androstenetriol | 5-androstene-3β,16α,17β-triol | |
| 5-PT | Pregnenetriol | 5-pregnene-3β,17α,20α-triol | |
| TST | Testosterone | 17β-hydroxy-4-androsten-3-one | |
| DH-TST | 5α-Dihydrotestosterone | 17β-hydroxy-5α-androstan-3-one | |
| Estrogen metabolites | ESTRIOL | Estriol | 1,3,5 (10)-estratriene-3,16α,17β-triol |
| ESTRADIOL | 17β-Estradiol | 1,3,5 (10)-estratriene-3,17β-diol | |
| 11-Deoxycortisol metabolites | THS | Tetrahydro-11-deoxycortisol | 3α,17,21-trihydroxy-5β-pregnan-20-one |
| Cortisol metabolites | E | Cortisone | 17,21-dihydroxy-4-pregnene-3,11,20-trione |
| THE | Tetrahydrocortisone | 3α,17,21-trihydroxy-5β-pregnan-11,20-dione | |
| b-CN | β-Cortolone | 3α,17α,20β,21-tetrahydroxy-5β-pregnane-11-one | |
| 20a-DHE | 20α-Dihydrocortisone | 17α,20α,21-trihydroxy-4-pregnene-3,11-dione | |
| 20b-DHE | 20β-Dihydrocortisone | 17α,20β,21-trihydroxy-4-pregnene-3,11-dione | |
| F | Cortisol | 11β,17,21-trihydroxy-4-pregnene-3,20-dione | |
| THF | Tetrahydrocortisol | 3α,11β,17,21-tetrahydroxy-5β-pregnan-20-one | |
| 5a-THF | 5α-Tetrahydrocortisol | 3α,11β,17,21-tetrahydroxy-5α-pregnan-20-one | |
| a-CL | α-Cortol | 5β-pregnane-3α,11β,17α,20α,21-pentol | |
| b-CL | β-Cortol | 5β-pregnane-3α,11β,17α,20β,21-pentol | |
| 20a-DHF | 20α-Dihydrocortisol | 11β,17,20α,21-tetrahydroxy-4-pregnen-3-one | |
| 6b-OH-F | 6β-Hydroxycortisol | 6β,11β,17α,21-tetrahydroxy-4-pregnene-3,20-dione | |
| 18-OH-F | 18-Hydroxycortisol | 11,17,18,21-tetrahydroxy-4-pregnene-3,20-dione | |
| a-CN | α-Cortolone | 3α,17α,20α,21-tetrahydroxy-5β-pregnane-11-one |
| Class . | Abbreviation . | Trivial Name . | Systematic Name . |
|---|---|---|---|
| Progesterone metabolites | 17-HP | 17-Hydroxypregnanolone | 3α,17α-dihydroxy-5-pregnen-20-one |
| PD | Pregnanediol | 5β-pregnane-3α,20α-diol | |
| PT | Pregnanetriol | 5β-pregnane-3α,17α,20α-triol | |
| PTONE | Pregnanetriolone | 3α,17α,20α-trihydroxy-5β-pregnan-11-one | |
| Corticosterone metabolites | THDOC | Tetrahydrodeoxycorticosterone | 3α,21-dihydroxy-5β-pregnan-20-one |
| THA | Tetrahydro-11-dehydrocorticosterone | 3α,21-dihydroxy-5β-pregnane-11,20-dione | |
| THB | Tetrahydrocorticosterone | 3α,11β,21-trihydroxy-5β-pregnan-20-one | |
| 5a-THB | 5α-Tetrahydrocorticosterone | 3α,11β,21-trihydroxy-5α-pregnan-20-one | |
| Aldosterone metabolites | THALDO | Tetrahydroaldosterone | 11β,18-epoxy-3α,18,21-trihydroxy-5β-pregnan-20-one |
| Androgen metabolites | ANDRO | Androsterone | 3α-hydroxy-5α-androstan-17-one |
| ETIO | Etiocholanolone | 3α-Hydroxy-5β-androstan-17-one | |
| DH-ANDRO | Androstanediol, Dihydroandrosterone | 5α-androstane-3α,17β-diol | |
| 11-OXO-ETIO | 11-oxo-Etiocholanolone | 3α-hydroxy-5β-androstane-11,17-dione | |
| 11b-OH-ANDRO | 11β-Hydroxyandrosterone | 3α,11β-dihydroxy-5α-androstan-17-one | |
| 11b-OH-ETIO | 11β-Hydroxyetiocholanolone | 3α,11β-dihydroxy-5β-androstan-17-one | |
| DHEA | Dehydroepiandrosterone | 3β-hydroxy-5-androsten-17-one | |
| ANDRO-DIOL | Androstenediol | 5-androstene-3β,17β-diol | |
| 16a-OH-DHEA | 16α-Hydroxy- Dehydroepiandrosterone | 3β,16α-dihydroxy-5-androsten-17-one | |
| 5-AT | Androstenetriol | 5-androstene-3β,16α,17β-triol | |
| 5-PT | Pregnenetriol | 5-pregnene-3β,17α,20α-triol | |
| TST | Testosterone | 17β-hydroxy-4-androsten-3-one | |
| DH-TST | 5α-Dihydrotestosterone | 17β-hydroxy-5α-androstan-3-one | |
| Estrogen metabolites | ESTRIOL | Estriol | 1,3,5 (10)-estratriene-3,16α,17β-triol |
| ESTRADIOL | 17β-Estradiol | 1,3,5 (10)-estratriene-3,17β-diol | |
| 11-Deoxycortisol metabolites | THS | Tetrahydro-11-deoxycortisol | 3α,17,21-trihydroxy-5β-pregnan-20-one |
| Cortisol metabolites | E | Cortisone | 17,21-dihydroxy-4-pregnene-3,11,20-trione |
| THE | Tetrahydrocortisone | 3α,17,21-trihydroxy-5β-pregnan-11,20-dione | |
| b-CN | β-Cortolone | 3α,17α,20β,21-tetrahydroxy-5β-pregnane-11-one | |
| 20a-DHE | 20α-Dihydrocortisone | 17α,20α,21-trihydroxy-4-pregnene-3,11-dione | |
| 20b-DHE | 20β-Dihydrocortisone | 17α,20β,21-trihydroxy-4-pregnene-3,11-dione | |
| F | Cortisol | 11β,17,21-trihydroxy-4-pregnene-3,20-dione | |
| THF | Tetrahydrocortisol | 3α,11β,17,21-tetrahydroxy-5β-pregnan-20-one | |
| 5a-THF | 5α-Tetrahydrocortisol | 3α,11β,17,21-tetrahydroxy-5α-pregnan-20-one | |
| a-CL | α-Cortol | 5β-pregnane-3α,11β,17α,20α,21-pentol | |
| b-CL | β-Cortol | 5β-pregnane-3α,11β,17α,20β,21-pentol | |
| 20a-DHF | 20α-Dihydrocortisol | 11β,17,20α,21-tetrahydroxy-4-pregnen-3-one | |
| 6b-OH-F | 6β-Hydroxycortisol | 6β,11β,17α,21-tetrahydroxy-4-pregnene-3,20-dione | |
| 18-OH-F | 18-Hydroxycortisol | 11,17,18,21-tetrahydroxy-4-pregnene-3,20-dione | |
| a-CN | α-Cortolone | 3α,17α,20α,21-tetrahydroxy-5β-pregnane-11-one |
Statistical analysis
We described clinical characteristics of girls with PA and control girls with mean and SD, and compared them with a student’s t-test. We compared metabolites and metabolite ratios between girls with PA and control girls using Mann-Whitney U tests and accounted for multiple testing using Bonferroni correction. We used the statistical softwares Stata (Version 15, Stata Corporation, Austin, Texas) for all analyses and RStudio (Version 1.1.383, Boston, Massachusetts) for creating the boxplots.
Results
Baseline characteristics are shown in Table 2. Girls with PA were heavier and had a higher BMI than age-matched control girls. There was no difference in height, gestational age, birth weight, or blood pressure between the groups.
| Clinical Characteristics . | PA . | CON . | P-valuea . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | N = 23 . | N = 22 . | . | ||||||
| . | Mean . | SD . | Range . | Mean . | SD . | Range . | . | ||
| . | . | . | min . | max . | . | . | min . | max . | . |
| Age at consultation (years) | 6.7 | 1.1 | 3.9 | 8.4 | 6.5 | 1.1 | 4.3 | 8.5 | 5.161 |
| Weight (kg) | 26.7 | 4.5 | 19.9 | 38.0 | 21.5 | 3.4 | 14.1 | 30.4 | 0.003 |
| Weight (SDS) | 1.2 | 1.0 | -0.3 | 3.1 | -0.1 | 0.9 | -1.4 | 2.0 | 0.008 |
| Height (kg) | 124.1 | 7.8 | 109.1 | 139.2 | 120.2 | 7.2 | 101.4 | 134.0 | 0.454 |
| Height (SDS) | 1.0 | 1.1 | -1.6 | 3.6 | 0.2 | 1.0 | -1.5 | 2.0 | 0.523 |
| BMI (kg/m2) | 17.3 | 2.1 | 14.2 | 22.4 | 15.0 | 1.3 | 13.7 | 18.8 | 0.004 |
| BMI (SDS) | 0.9 | 1.0 | -0.9 | 2.8 | -0.3 | 0.8 | -1.3 | 1.7 | 0.006 |
| Gestational age (weeks) | 39 | 2 | 33 | 41 | 40 | 1 | 37 | 42 | 3.934 |
| Birth weight (g) | 3100 | 735 | 1625 | 5040 | 3300 | 496 | 2270 | 3900 | 4.626 |
| Birth weight (SDS) | -0.4 | 1.3 | -2.5 | 3.7 | -0.4 | 1.1 | -2.6 | 2.0 | 8.106 |
| Clinical Characteristics . | PA . | CON . | P-valuea . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | N = 23 . | N = 22 . | . | ||||||
| . | Mean . | SD . | Range . | Mean . | SD . | Range . | . | ||
| . | . | . | min . | max . | . | . | min . | max . | . |
| Age at consultation (years) | 6.7 | 1.1 | 3.9 | 8.4 | 6.5 | 1.1 | 4.3 | 8.5 | 5.161 |
| Weight (kg) | 26.7 | 4.5 | 19.9 | 38.0 | 21.5 | 3.4 | 14.1 | 30.4 | 0.003 |
| Weight (SDS) | 1.2 | 1.0 | -0.3 | 3.1 | -0.1 | 0.9 | -1.4 | 2.0 | 0.008 |
| Height (kg) | 124.1 | 7.8 | 109.1 | 139.2 | 120.2 | 7.2 | 101.4 | 134.0 | 0.454 |
| Height (SDS) | 1.0 | 1.1 | -1.6 | 3.6 | 0.2 | 1.0 | -1.5 | 2.0 | 0.523 |
| BMI (kg/m2) | 17.3 | 2.1 | 14.2 | 22.4 | 15.0 | 1.3 | 13.7 | 18.8 | 0.004 |
| BMI (SDS) | 0.9 | 1.0 | -0.9 | 2.8 | -0.3 | 0.8 | -1.3 | 1.7 | 0.006 |
| Gestational age (weeks) | 39 | 2 | 33 | 41 | 40 | 1 | 37 | 42 | 3.934 |
| Birth weight (g) | 3100 | 735 | 1625 | 5040 | 3300 | 496 | 2270 | 3900 | 4.626 |
| Birth weight (SDS) | -0.4 | 1.3 | -2.5 | 3.7 | -0.4 | 1.1 | -2.6 | 2.0 | 8.106 |
Data are mean, SD, and range for each characteristic of girls with PA and control girls.
Abbreviations: BMI, body mass index; CON, controls; max, maximum; min, minimum; N, number; PA, premature adrenarche; SD, standard deviation; SDS, standard deviation score.
aP-values derived from Student’s t- test to test for differences between girls with PA and control girls. All comparisons account for multiple testing using Bonferroni correction. Data in bold are significantly different between groups (P < 0.05).
| Clinical Characteristics . | PA . | CON . | P-valuea . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | N = 23 . | N = 22 . | . | ||||||
| . | Mean . | SD . | Range . | Mean . | SD . | Range . | . | ||
| . | . | . | min . | max . | . | . | min . | max . | . |
| Age at consultation (years) | 6.7 | 1.1 | 3.9 | 8.4 | 6.5 | 1.1 | 4.3 | 8.5 | 5.161 |
| Weight (kg) | 26.7 | 4.5 | 19.9 | 38.0 | 21.5 | 3.4 | 14.1 | 30.4 | 0.003 |
| Weight (SDS) | 1.2 | 1.0 | -0.3 | 3.1 | -0.1 | 0.9 | -1.4 | 2.0 | 0.008 |
| Height (kg) | 124.1 | 7.8 | 109.1 | 139.2 | 120.2 | 7.2 | 101.4 | 134.0 | 0.454 |
| Height (SDS) | 1.0 | 1.1 | -1.6 | 3.6 | 0.2 | 1.0 | -1.5 | 2.0 | 0.523 |
| BMI (kg/m2) | 17.3 | 2.1 | 14.2 | 22.4 | 15.0 | 1.3 | 13.7 | 18.8 | 0.004 |
| BMI (SDS) | 0.9 | 1.0 | -0.9 | 2.8 | -0.3 | 0.8 | -1.3 | 1.7 | 0.006 |
| Gestational age (weeks) | 39 | 2 | 33 | 41 | 40 | 1 | 37 | 42 | 3.934 |
| Birth weight (g) | 3100 | 735 | 1625 | 5040 | 3300 | 496 | 2270 | 3900 | 4.626 |
| Birth weight (SDS) | -0.4 | 1.3 | -2.5 | 3.7 | -0.4 | 1.1 | -2.6 | 2.0 | 8.106 |
| Clinical Characteristics . | PA . | CON . | P-valuea . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | N = 23 . | N = 22 . | . | ||||||
| . | Mean . | SD . | Range . | Mean . | SD . | Range . | . | ||
| . | . | . | min . | max . | . | . | min . | max . | . |
| Age at consultation (years) | 6.7 | 1.1 | 3.9 | 8.4 | 6.5 | 1.1 | 4.3 | 8.5 | 5.161 |
| Weight (kg) | 26.7 | 4.5 | 19.9 | 38.0 | 21.5 | 3.4 | 14.1 | 30.4 | 0.003 |
| Weight (SDS) | 1.2 | 1.0 | -0.3 | 3.1 | -0.1 | 0.9 | -1.4 | 2.0 | 0.008 |
| Height (kg) | 124.1 | 7.8 | 109.1 | 139.2 | 120.2 | 7.2 | 101.4 | 134.0 | 0.454 |
| Height (SDS) | 1.0 | 1.1 | -1.6 | 3.6 | 0.2 | 1.0 | -1.5 | 2.0 | 0.523 |
| BMI (kg/m2) | 17.3 | 2.1 | 14.2 | 22.4 | 15.0 | 1.3 | 13.7 | 18.8 | 0.004 |
| BMI (SDS) | 0.9 | 1.0 | -0.9 | 2.8 | -0.3 | 0.8 | -1.3 | 1.7 | 0.006 |
| Gestational age (weeks) | 39 | 2 | 33 | 41 | 40 | 1 | 37 | 42 | 3.934 |
| Birth weight (g) | 3100 | 735 | 1625 | 5040 | 3300 | 496 | 2270 | 3900 | 4.626 |
| Birth weight (SDS) | -0.4 | 1.3 | -2.5 | 3.7 | -0.4 | 1.1 | -2.6 | 2.0 | 8.106 |
Data are mean, SD, and range for each characteristic of girls with PA and control girls.
Abbreviations: BMI, body mass index; CON, controls; max, maximum; min, minimum; N, number; PA, premature adrenarche; SD, standard deviation; SDS, standard deviation score.
aP-values derived from Student’s t- test to test for differences between girls with PA and control girls. All comparisons account for multiple testing using Bonferroni correction. Data in bold are significantly different between groups (P < 0.05).
Comparison of 24-hour urine steroid metabolite excretion between girls with PA and controls is summarized in Table 3 and depicted in Fig. 2. Girls with PA nearly doubled the controls in total androgen metabolite excretion (Table 3 and Fig. 2A). We found differences in median 24-hour steroid metabolite excretion in the PA group compared with control girls for androsterone (ANDRO), etiochonanolone (ETIO), dihydroandrosterone (DH-ANDRO), DHEA, androstenediol (ANDRO-DIOL), androstenetriol (5-AT), and pregnenetriol (5-PT). Some of the metabolites with higher excretion came from the alternative pathway, specifically ANDRO, 5-AT, and DH-ANDRO (ANDRO-DIOL) (Fig. 1). From the total androgen metabolite excretion of 3257 nmol/24 hours in PA (1627 nmol/24 hours in controls), ANDRO accounted for 34% (controls 17%), and 5-AT for 3% in PA (controls 1%). We could not find any difference in total cortisol metabolite excretion and most of the single cortisol metabolites between PA and controls except for higher amounts of 20β-dihydrocortisone in PA girls (79 nmol vs 42 nmol, P = 0.024).
| Urinary Steroid Metabolites (nmol/24 h) . | PA . | CON . | P-valuea . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | N = 23 . | N = 22 . | . | ||||||||
| . | Percentiles . | Percentiles . | . | ||||||||
| . | P5 . | P25 . | P50 . | P75 . | P95 . | P5 . | P25 . | P50 . | P75 . | P95 . | . |
| Progesterone metabolites (sum) | 238 | 460 | 544 | 1015 | 2793 | 151 | 273 | 344 | 507 | 797 | 0.065 |
| 17-HP | 23 | 36 | 60 | 90 | 212 | 12 | 23 | 35 | 51 | 68 | 0.264 |
| PD | 64 | 108 | 151 | 226 | 895 | 31 | 60 | 86 | 127 | 232 | 0.214 |
| PT | 118 | 240 | 354 | 685 | 1144 | 72 | 147 | 196 | 277 | 422 | 0.103 |
| PTONE | 4 | 8 | 15 | 25 | 44 | 7 | 9 | 17 | 32 | 80 | 1.000 |
| Corticosterone metabolites (sum) | 333 | 579 | 713 | 1322 | 1890 | 248 | 331 | 524 | 860 | 1249 | 0.453 |
| THDOC | 5 | 6 | 8 | 13 | 17 | 2 | 4 | 7 | 10 | 14 | 1.000 |
| THA | 119 | 152 | 214 | 324 | 578 | 60 | 97 | 139 | 188 | 290 | 0.161 |
| THB | 50 | 84 | 117 | 172 | 274 | 47 | 63 | 83 | 121 | 195 | 1.000 |
| 5a-THB | 0.002 | 0.006 | 0.008 | 0.009 | 0.014 | 0.002 | 0.004 | 0.006 | 0.009 | 0.017 | 1.000 |
| Aldosterone metabolites | |||||||||||
| THALDO | 11 | 17 | 37 | 54 | 83 | 10 | 19 | 26 | 31 | 46 | 1.000 |
| Androgene metabolites (sum) | 1426 | 2186 | 3257 | 4884 | 20931 | 650 | 1157 | 1627 | 2098 | 2779 | 0.001 |
| ANDRO | 187 | 739 | 1106 | 1668 | 5944 | 61 | 183 | 278 | 577 | 931 | 0.001 |
| ETIO | 130 | 272 | 539 | 934 | 1757 | 60 | 76 | 179 | 241 | 350 | 0.004 |
| DH-ANDRO | 10 | 17 | 25 | 47 | 106 | 3 | 8 | 12 | 18 | 26 | 0.019 |
| 11-OXO-ETIO | 59 | 137 | 279 | 485 | 636 | 58 | 127 | 286 | 500 | 642 | 1.000 |
| 11b-OH-ANDRO | 279 | 340 | 513 | 622 | 2600 | 151 | 231 | 325 | 397 | 568 | 0.082 |
| 11b-OH-ETIO | 29 | 40 | 140 | 283 | 801 | 18 | 80 | 143 | 223 | 340 | 1.000 |
| DHEA | 6 | 16 | 38 | 88 | 538 | 3 | 5 | 8 | 19 | 37 | 0.005 |
| ANDRO-DIOL | 22 | 32 | 46 | 71 | 234 | 8 | 11 | 20 | 32 | 100 | 0.013 |
| 16a-OH-DHEA | 29 | 60 | 137 | 191 | 4986 | 4 | 19 | 39 | 92 | 171 | 0.056 |
| 5-AT | 41 | 80 | 110 | 161 | 981 | 9 | 16 | 24 | 47 | 188 | 0.001 |
| 5-PT | 5 | 15 | 90 | 175 | 1101 | 2 | 4 | 13 | 22 | 92 | 0.047 |
| TST | 4 | 7 | 15 | 24 | 34 | 1 | 3 | 5 | 12 | 22 | 0.150 |
| DH-TST | 11 | 18 | 35 | 49 | 93 | 7 | 10 | 21 | 26 | 44 | 0.347 |
| Estrogen metabolites (sum) | 1 | 2 | 5 | 9 | 23 | 1 | 2 | 4 | 6 | 9 | 1.000 |
| ESTRIOL | 0.6 | 1 | 3 | 5 | 9 | 0.6 | 1 | 2 | 4 | 8 | 1.000 |
| ESTRADIOL | 0.5 | 0.8 | 1 | 3 | 7 | 0.2 | 0.6 | 0.9 | 2 | 3 | 1.000 |
| 11-Deoxycortisol metabolites | |||||||||||
| THS | 43 | 66 | 114 | 150 | 280 | 56 | 67 | 94 | 133 | 155 | 1.000 |
| Cortisol metabolites (sum) | 7103 | 9007 | 12536 | 17772 | 30629 | 6393 | 7568 | 8452 | 10868 | 13404 | 0.076 |
| E | 128 | 176 | 247 | 358 | 501 | 44 | 107 | 150 | 258 | 303 | 0.264 |
| THE | 1938 | 3254 | 4115 | 5254 | 9944 | 1471 | 2367 | 2751 | 3777 | 4625 | 0.200 |
| b-CN | 400 | 578 | 919 | 1130 | 2044 | 395 | 534 | 702 | 898 | 993 | 1.000 |
| 20a-DHE | 11 | 19 | 30 | 46 | 87 | 10 | 12 | 19 | 26 | 54 | 0.625 |
| 20b-DHE | 40 | 45 | 79 | 96 | 152 | 22 | 27 | 42 | 52 | 74 | 0.027 |
| F | 51 | 82 | 131 | 218 | 265 | 39 | 61 | 102 | 145 | 180 | 1.000 |
| THF | 472 | 820 | 1112 | 1693 | 2531 | 484 | 708 | 812 | 926 | 1355 | 0.753 |
| 5a-THF | 497 | 1024 | 1830 | 2664 | 4078 | 772 | 957 | 1343 | 1601 | 2369 | 1.000 |
| a-CL | 117 | 160 | 258 | 339 | 494 | 100 | 136 | 178 | 204 | 341 | 1.000 |
| b-CL | 141 | 329 | 409 | 660 | 997 | 140 | 257 | 339 | 603 | 754 | 1.000 |
| 20a-DHF | 28 | 33 | 47 | 62 | 146 | 18 | 22 | 31 | 53 | 120 | 0.708 |
| 6b-OH-F | 62 | 94 | 137 | 235 | 275 | 32 | 65 | 117 | 204 | 295 | 1.000 |
| 18-OH-F | 59 | 136 | 231 | 346 | 724 | 33 | 111 | 200 | 274 | 404 | 1.000 |
| a-CN | 603 | 906 | 1174 | 1969 | 2849 | 591 | 691 | 836 | 1001 | 1297 | 0.214 |
| Urinary Steroid Metabolites (nmol/24 h) . | PA . | CON . | P-valuea . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | N = 23 . | N = 22 . | . | ||||||||
| . | Percentiles . | Percentiles . | . | ||||||||
| . | P5 . | P25 . | P50 . | P75 . | P95 . | P5 . | P25 . | P50 . | P75 . | P95 . | . |
| Progesterone metabolites (sum) | 238 | 460 | 544 | 1015 | 2793 | 151 | 273 | 344 | 507 | 797 | 0.065 |
| 17-HP | 23 | 36 | 60 | 90 | 212 | 12 | 23 | 35 | 51 | 68 | 0.264 |
| PD | 64 | 108 | 151 | 226 | 895 | 31 | 60 | 86 | 127 | 232 | 0.214 |
| PT | 118 | 240 | 354 | 685 | 1144 | 72 | 147 | 196 | 277 | 422 | 0.103 |
| PTONE | 4 | 8 | 15 | 25 | 44 | 7 | 9 | 17 | 32 | 80 | 1.000 |
| Corticosterone metabolites (sum) | 333 | 579 | 713 | 1322 | 1890 | 248 | 331 | 524 | 860 | 1249 | 0.453 |
| THDOC | 5 | 6 | 8 | 13 | 17 | 2 | 4 | 7 | 10 | 14 | 1.000 |
| THA | 119 | 152 | 214 | 324 | 578 | 60 | 97 | 139 | 188 | 290 | 0.161 |
| THB | 50 | 84 | 117 | 172 | 274 | 47 | 63 | 83 | 121 | 195 | 1.000 |
| 5a-THB | 0.002 | 0.006 | 0.008 | 0.009 | 0.014 | 0.002 | 0.004 | 0.006 | 0.009 | 0.017 | 1.000 |
| Aldosterone metabolites | |||||||||||
| THALDO | 11 | 17 | 37 | 54 | 83 | 10 | 19 | 26 | 31 | 46 | 1.000 |
| Androgene metabolites (sum) | 1426 | 2186 | 3257 | 4884 | 20931 | 650 | 1157 | 1627 | 2098 | 2779 | 0.001 |
| ANDRO | 187 | 739 | 1106 | 1668 | 5944 | 61 | 183 | 278 | 577 | 931 | 0.001 |
| ETIO | 130 | 272 | 539 | 934 | 1757 | 60 | 76 | 179 | 241 | 350 | 0.004 |
| DH-ANDRO | 10 | 17 | 25 | 47 | 106 | 3 | 8 | 12 | 18 | 26 | 0.019 |
| 11-OXO-ETIO | 59 | 137 | 279 | 485 | 636 | 58 | 127 | 286 | 500 | 642 | 1.000 |
| 11b-OH-ANDRO | 279 | 340 | 513 | 622 | 2600 | 151 | 231 | 325 | 397 | 568 | 0.082 |
| 11b-OH-ETIO | 29 | 40 | 140 | 283 | 801 | 18 | 80 | 143 | 223 | 340 | 1.000 |
| DHEA | 6 | 16 | 38 | 88 | 538 | 3 | 5 | 8 | 19 | 37 | 0.005 |
| ANDRO-DIOL | 22 | 32 | 46 | 71 | 234 | 8 | 11 | 20 | 32 | 100 | 0.013 |
| 16a-OH-DHEA | 29 | 60 | 137 | 191 | 4986 | 4 | 19 | 39 | 92 | 171 | 0.056 |
| 5-AT | 41 | 80 | 110 | 161 | 981 | 9 | 16 | 24 | 47 | 188 | 0.001 |
| 5-PT | 5 | 15 | 90 | 175 | 1101 | 2 | 4 | 13 | 22 | 92 | 0.047 |
| TST | 4 | 7 | 15 | 24 | 34 | 1 | 3 | 5 | 12 | 22 | 0.150 |
| DH-TST | 11 | 18 | 35 | 49 | 93 | 7 | 10 | 21 | 26 | 44 | 0.347 |
| Estrogen metabolites (sum) | 1 | 2 | 5 | 9 | 23 | 1 | 2 | 4 | 6 | 9 | 1.000 |
| ESTRIOL | 0.6 | 1 | 3 | 5 | 9 | 0.6 | 1 | 2 | 4 | 8 | 1.000 |
| ESTRADIOL | 0.5 | 0.8 | 1 | 3 | 7 | 0.2 | 0.6 | 0.9 | 2 | 3 | 1.000 |
| 11-Deoxycortisol metabolites | |||||||||||
| THS | 43 | 66 | 114 | 150 | 280 | 56 | 67 | 94 | 133 | 155 | 1.000 |
| Cortisol metabolites (sum) | 7103 | 9007 | 12536 | 17772 | 30629 | 6393 | 7568 | 8452 | 10868 | 13404 | 0.076 |
| E | 128 | 176 | 247 | 358 | 501 | 44 | 107 | 150 | 258 | 303 | 0.264 |
| THE | 1938 | 3254 | 4115 | 5254 | 9944 | 1471 | 2367 | 2751 | 3777 | 4625 | 0.200 |
| b-CN | 400 | 578 | 919 | 1130 | 2044 | 395 | 534 | 702 | 898 | 993 | 1.000 |
| 20a-DHE | 11 | 19 | 30 | 46 | 87 | 10 | 12 | 19 | 26 | 54 | 0.625 |
| 20b-DHE | 40 | 45 | 79 | 96 | 152 | 22 | 27 | 42 | 52 | 74 | 0.027 |
| F | 51 | 82 | 131 | 218 | 265 | 39 | 61 | 102 | 145 | 180 | 1.000 |
| THF | 472 | 820 | 1112 | 1693 | 2531 | 484 | 708 | 812 | 926 | 1355 | 0.753 |
| 5a-THF | 497 | 1024 | 1830 | 2664 | 4078 | 772 | 957 | 1343 | 1601 | 2369 | 1.000 |
| a-CL | 117 | 160 | 258 | 339 | 494 | 100 | 136 | 178 | 204 | 341 | 1.000 |
| b-CL | 141 | 329 | 409 | 660 | 997 | 140 | 257 | 339 | 603 | 754 | 1.000 |
| 20a-DHF | 28 | 33 | 47 | 62 | 146 | 18 | 22 | 31 | 53 | 120 | 0.708 |
| 6b-OH-F | 62 | 94 | 137 | 235 | 275 | 32 | 65 | 117 | 204 | 295 | 1.000 |
| 18-OH-F | 59 | 136 | 231 | 346 | 724 | 33 | 111 | 200 | 274 | 404 | 1.000 |
| a-CN | 603 | 906 | 1174 | 1969 | 2849 | 591 | 691 | 836 | 1001 | 1297 | 0.214 |
Data are the median (P50) and 5th, 25th, 75th, and 95th percentiles for urinary steroid metabolites in girls with PA and control girls collected over 24 hours.
Abbreviations: CON, controls; h, hours N, number; PA, premature adrenarche. For metabolite abbreviations see Table 1.
aP-values derived from Mann-Whitney-U tests to test for differences between girls with PA and control girls. All comparisons account for multiple testing using Bonferroni correction. Data in bold are significantly different between groups (P < 0.05).
| Urinary Steroid Metabolites (nmol/24 h) . | PA . | CON . | P-valuea . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | N = 23 . | N = 22 . | . | ||||||||
| . | Percentiles . | Percentiles . | . | ||||||||
| . | P5 . | P25 . | P50 . | P75 . | P95 . | P5 . | P25 . | P50 . | P75 . | P95 . | . |
| Progesterone metabolites (sum) | 238 | 460 | 544 | 1015 | 2793 | 151 | 273 | 344 | 507 | 797 | 0.065 |
| 17-HP | 23 | 36 | 60 | 90 | 212 | 12 | 23 | 35 | 51 | 68 | 0.264 |
| PD | 64 | 108 | 151 | 226 | 895 | 31 | 60 | 86 | 127 | 232 | 0.214 |
| PT | 118 | 240 | 354 | 685 | 1144 | 72 | 147 | 196 | 277 | 422 | 0.103 |
| PTONE | 4 | 8 | 15 | 25 | 44 | 7 | 9 | 17 | 32 | 80 | 1.000 |
| Corticosterone metabolites (sum) | 333 | 579 | 713 | 1322 | 1890 | 248 | 331 | 524 | 860 | 1249 | 0.453 |
| THDOC | 5 | 6 | 8 | 13 | 17 | 2 | 4 | 7 | 10 | 14 | 1.000 |
| THA | 119 | 152 | 214 | 324 | 578 | 60 | 97 | 139 | 188 | 290 | 0.161 |
| THB | 50 | 84 | 117 | 172 | 274 | 47 | 63 | 83 | 121 | 195 | 1.000 |
| 5a-THB | 0.002 | 0.006 | 0.008 | 0.009 | 0.014 | 0.002 | 0.004 | 0.006 | 0.009 | 0.017 | 1.000 |
| Aldosterone metabolites | |||||||||||
| THALDO | 11 | 17 | 37 | 54 | 83 | 10 | 19 | 26 | 31 | 46 | 1.000 |
| Androgene metabolites (sum) | 1426 | 2186 | 3257 | 4884 | 20931 | 650 | 1157 | 1627 | 2098 | 2779 | 0.001 |
| ANDRO | 187 | 739 | 1106 | 1668 | 5944 | 61 | 183 | 278 | 577 | 931 | 0.001 |
| ETIO | 130 | 272 | 539 | 934 | 1757 | 60 | 76 | 179 | 241 | 350 | 0.004 |
| DH-ANDRO | 10 | 17 | 25 | 47 | 106 | 3 | 8 | 12 | 18 | 26 | 0.019 |
| 11-OXO-ETIO | 59 | 137 | 279 | 485 | 636 | 58 | 127 | 286 | 500 | 642 | 1.000 |
| 11b-OH-ANDRO | 279 | 340 | 513 | 622 | 2600 | 151 | 231 | 325 | 397 | 568 | 0.082 |
| 11b-OH-ETIO | 29 | 40 | 140 | 283 | 801 | 18 | 80 | 143 | 223 | 340 | 1.000 |
| DHEA | 6 | 16 | 38 | 88 | 538 | 3 | 5 | 8 | 19 | 37 | 0.005 |
| ANDRO-DIOL | 22 | 32 | 46 | 71 | 234 | 8 | 11 | 20 | 32 | 100 | 0.013 |
| 16a-OH-DHEA | 29 | 60 | 137 | 191 | 4986 | 4 | 19 | 39 | 92 | 171 | 0.056 |
| 5-AT | 41 | 80 | 110 | 161 | 981 | 9 | 16 | 24 | 47 | 188 | 0.001 |
| 5-PT | 5 | 15 | 90 | 175 | 1101 | 2 | 4 | 13 | 22 | 92 | 0.047 |
| TST | 4 | 7 | 15 | 24 | 34 | 1 | 3 | 5 | 12 | 22 | 0.150 |
| DH-TST | 11 | 18 | 35 | 49 | 93 | 7 | 10 | 21 | 26 | 44 | 0.347 |
| Estrogen metabolites (sum) | 1 | 2 | 5 | 9 | 23 | 1 | 2 | 4 | 6 | 9 | 1.000 |
| ESTRIOL | 0.6 | 1 | 3 | 5 | 9 | 0.6 | 1 | 2 | 4 | 8 | 1.000 |
| ESTRADIOL | 0.5 | 0.8 | 1 | 3 | 7 | 0.2 | 0.6 | 0.9 | 2 | 3 | 1.000 |
| 11-Deoxycortisol metabolites | |||||||||||
| THS | 43 | 66 | 114 | 150 | 280 | 56 | 67 | 94 | 133 | 155 | 1.000 |
| Cortisol metabolites (sum) | 7103 | 9007 | 12536 | 17772 | 30629 | 6393 | 7568 | 8452 | 10868 | 13404 | 0.076 |
| E | 128 | 176 | 247 | 358 | 501 | 44 | 107 | 150 | 258 | 303 | 0.264 |
| THE | 1938 | 3254 | 4115 | 5254 | 9944 | 1471 | 2367 | 2751 | 3777 | 4625 | 0.200 |
| b-CN | 400 | 578 | 919 | 1130 | 2044 | 395 | 534 | 702 | 898 | 993 | 1.000 |
| 20a-DHE | 11 | 19 | 30 | 46 | 87 | 10 | 12 | 19 | 26 | 54 | 0.625 |
| 20b-DHE | 40 | 45 | 79 | 96 | 152 | 22 | 27 | 42 | 52 | 74 | 0.027 |
| F | 51 | 82 | 131 | 218 | 265 | 39 | 61 | 102 | 145 | 180 | 1.000 |
| THF | 472 | 820 | 1112 | 1693 | 2531 | 484 | 708 | 812 | 926 | 1355 | 0.753 |
| 5a-THF | 497 | 1024 | 1830 | 2664 | 4078 | 772 | 957 | 1343 | 1601 | 2369 | 1.000 |
| a-CL | 117 | 160 | 258 | 339 | 494 | 100 | 136 | 178 | 204 | 341 | 1.000 |
| b-CL | 141 | 329 | 409 | 660 | 997 | 140 | 257 | 339 | 603 | 754 | 1.000 |
| 20a-DHF | 28 | 33 | 47 | 62 | 146 | 18 | 22 | 31 | 53 | 120 | 0.708 |
| 6b-OH-F | 62 | 94 | 137 | 235 | 275 | 32 | 65 | 117 | 204 | 295 | 1.000 |
| 18-OH-F | 59 | 136 | 231 | 346 | 724 | 33 | 111 | 200 | 274 | 404 | 1.000 |
| a-CN | 603 | 906 | 1174 | 1969 | 2849 | 591 | 691 | 836 | 1001 | 1297 | 0.214 |
| Urinary Steroid Metabolites (nmol/24 h) . | PA . | CON . | P-valuea . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | N = 23 . | N = 22 . | . | ||||||||
| . | Percentiles . | Percentiles . | . | ||||||||
| . | P5 . | P25 . | P50 . | P75 . | P95 . | P5 . | P25 . | P50 . | P75 . | P95 . | . |
| Progesterone metabolites (sum) | 238 | 460 | 544 | 1015 | 2793 | 151 | 273 | 344 | 507 | 797 | 0.065 |
| 17-HP | 23 | 36 | 60 | 90 | 212 | 12 | 23 | 35 | 51 | 68 | 0.264 |
| PD | 64 | 108 | 151 | 226 | 895 | 31 | 60 | 86 | 127 | 232 | 0.214 |
| PT | 118 | 240 | 354 | 685 | 1144 | 72 | 147 | 196 | 277 | 422 | 0.103 |
| PTONE | 4 | 8 | 15 | 25 | 44 | 7 | 9 | 17 | 32 | 80 | 1.000 |
| Corticosterone metabolites (sum) | 333 | 579 | 713 | 1322 | 1890 | 248 | 331 | 524 | 860 | 1249 | 0.453 |
| THDOC | 5 | 6 | 8 | 13 | 17 | 2 | 4 | 7 | 10 | 14 | 1.000 |
| THA | 119 | 152 | 214 | 324 | 578 | 60 | 97 | 139 | 188 | 290 | 0.161 |
| THB | 50 | 84 | 117 | 172 | 274 | 47 | 63 | 83 | 121 | 195 | 1.000 |
| 5a-THB | 0.002 | 0.006 | 0.008 | 0.009 | 0.014 | 0.002 | 0.004 | 0.006 | 0.009 | 0.017 | 1.000 |
| Aldosterone metabolites | |||||||||||
| THALDO | 11 | 17 | 37 | 54 | 83 | 10 | 19 | 26 | 31 | 46 | 1.000 |
| Androgene metabolites (sum) | 1426 | 2186 | 3257 | 4884 | 20931 | 650 | 1157 | 1627 | 2098 | 2779 | 0.001 |
| ANDRO | 187 | 739 | 1106 | 1668 | 5944 | 61 | 183 | 278 | 577 | 931 | 0.001 |
| ETIO | 130 | 272 | 539 | 934 | 1757 | 60 | 76 | 179 | 241 | 350 | 0.004 |
| DH-ANDRO | 10 | 17 | 25 | 47 | 106 | 3 | 8 | 12 | 18 | 26 | 0.019 |
| 11-OXO-ETIO | 59 | 137 | 279 | 485 | 636 | 58 | 127 | 286 | 500 | 642 | 1.000 |
| 11b-OH-ANDRO | 279 | 340 | 513 | 622 | 2600 | 151 | 231 | 325 | 397 | 568 | 0.082 |
| 11b-OH-ETIO | 29 | 40 | 140 | 283 | 801 | 18 | 80 | 143 | 223 | 340 | 1.000 |
| DHEA | 6 | 16 | 38 | 88 | 538 | 3 | 5 | 8 | 19 | 37 | 0.005 |
| ANDRO-DIOL | 22 | 32 | 46 | 71 | 234 | 8 | 11 | 20 | 32 | 100 | 0.013 |
| 16a-OH-DHEA | 29 | 60 | 137 | 191 | 4986 | 4 | 19 | 39 | 92 | 171 | 0.056 |
| 5-AT | 41 | 80 | 110 | 161 | 981 | 9 | 16 | 24 | 47 | 188 | 0.001 |
| 5-PT | 5 | 15 | 90 | 175 | 1101 | 2 | 4 | 13 | 22 | 92 | 0.047 |
| TST | 4 | 7 | 15 | 24 | 34 | 1 | 3 | 5 | 12 | 22 | 0.150 |
| DH-TST | 11 | 18 | 35 | 49 | 93 | 7 | 10 | 21 | 26 | 44 | 0.347 |
| Estrogen metabolites (sum) | 1 | 2 | 5 | 9 | 23 | 1 | 2 | 4 | 6 | 9 | 1.000 |
| ESTRIOL | 0.6 | 1 | 3 | 5 | 9 | 0.6 | 1 | 2 | 4 | 8 | 1.000 |
| ESTRADIOL | 0.5 | 0.8 | 1 | 3 | 7 | 0.2 | 0.6 | 0.9 | 2 | 3 | 1.000 |
| 11-Deoxycortisol metabolites | |||||||||||
| THS | 43 | 66 | 114 | 150 | 280 | 56 | 67 | 94 | 133 | 155 | 1.000 |
| Cortisol metabolites (sum) | 7103 | 9007 | 12536 | 17772 | 30629 | 6393 | 7568 | 8452 | 10868 | 13404 | 0.076 |
| E | 128 | 176 | 247 | 358 | 501 | 44 | 107 | 150 | 258 | 303 | 0.264 |
| THE | 1938 | 3254 | 4115 | 5254 | 9944 | 1471 | 2367 | 2751 | 3777 | 4625 | 0.200 |
| b-CN | 400 | 578 | 919 | 1130 | 2044 | 395 | 534 | 702 | 898 | 993 | 1.000 |
| 20a-DHE | 11 | 19 | 30 | 46 | 87 | 10 | 12 | 19 | 26 | 54 | 0.625 |
| 20b-DHE | 40 | 45 | 79 | 96 | 152 | 22 | 27 | 42 | 52 | 74 | 0.027 |
| F | 51 | 82 | 131 | 218 | 265 | 39 | 61 | 102 | 145 | 180 | 1.000 |
| THF | 472 | 820 | 1112 | 1693 | 2531 | 484 | 708 | 812 | 926 | 1355 | 0.753 |
| 5a-THF | 497 | 1024 | 1830 | 2664 | 4078 | 772 | 957 | 1343 | 1601 | 2369 | 1.000 |
| a-CL | 117 | 160 | 258 | 339 | 494 | 100 | 136 | 178 | 204 | 341 | 1.000 |
| b-CL | 141 | 329 | 409 | 660 | 997 | 140 | 257 | 339 | 603 | 754 | 1.000 |
| 20a-DHF | 28 | 33 | 47 | 62 | 146 | 18 | 22 | 31 | 53 | 120 | 0.708 |
| 6b-OH-F | 62 | 94 | 137 | 235 | 275 | 32 | 65 | 117 | 204 | 295 | 1.000 |
| 18-OH-F | 59 | 136 | 231 | 346 | 724 | 33 | 111 | 200 | 274 | 404 | 1.000 |
| a-CN | 603 | 906 | 1174 | 1969 | 2849 | 591 | 691 | 836 | 1001 | 1297 | 0.214 |
Data are the median (P50) and 5th, 25th, 75th, and 95th percentiles for urinary steroid metabolites in girls with PA and control girls collected over 24 hours.
Abbreviations: CON, controls; h, hours N, number; PA, premature adrenarche. For metabolite abbreviations see Table 1.
aP-values derived from Mann-Whitney-U tests to test for differences between girls with PA and control girls. All comparisons account for multiple testing using Bonferroni correction. Data in bold are significantly different between groups (P < 0.05).
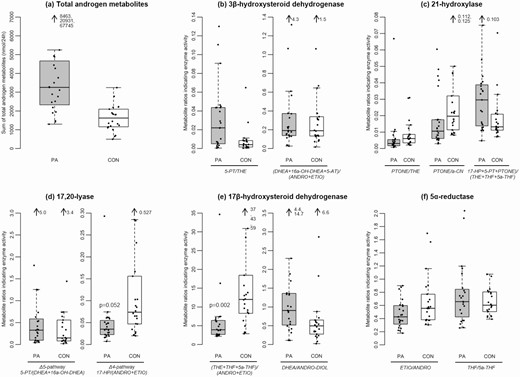
Differences in androgen excretion and steroid enzyme activities calculated from substrate to product conversion ratios between girls with premature adrenarche (PA) and control girls. Boxplots show the range of total androgen excretion and metabolite ratios for PA girls and controls. Ratios are given as substrate over product conversion by specific enzymes, thus a lower ratio reflects a higher enzyme activity. Grey boxes represent PA girls (PA), white boxes represent control girls (CON), black dots are individual values of patients or controls. Each box covers the distribution of the data from the first quartile to the third quartile, and the horizontal line inside the box represents the median. The whiskers extend to 1.5x interquartile range below the first quartile and 1.5x interquartile range above the third quartile, respectively. (a) Total androgen metabolite secretion (nmol/24 hours) amounts of ANDRO, ETIO, DH-ANDRO, 11-OXO-ETIO, 11b-OH-ANDRO, 11b-OH-ETIO, DHEA, ANDRO-DIOL, 16a-OH-DHEA, 5-AT, 5-PT, TST, and 5a-TST. (b) 3β-hydroxysteroid dehydrogenase activity, calculated from 5-PT/THE and (DHEA + 16a-OH-DHEA + 5-AT)/(ANDRO + ETIO). (c) 21-hydroxylase activity, calculated from PTONE/THE, PTONE/a-CN, and 17-HP + 5-PT + PTONE)/(THE + THF + 5a-THF). (d) 17,20-lyase Δ5-pathway activity calculated from 5-PT/(DHEA + 16a-OH-DHEA) and 17,20-lyase Δ4-pathway calculated from 17-HP/(ANDRO + ETIO). (e) 17β-hydroxysteroid dehydrogenase activity calculated from (THE + THF + 5a-THF)/(ANDRO + ETIO) and DHEA/ANDRO-DIOL. (f) 5α-reductase activity calculated from ETIO/ANDRO and THF/5a-THF. Mann-Whitney U tests for differences between PA girls and control girls detected higher activities of 17,20-lyase (CYP17A1) Δ4-pathway (P = 0.052) and of 17β-hydroxysteroid dehydrogenase (HSD17B), as indicated by the excretion of C19 metabolites (P = 0.002) in PA girls. Abbreviations: For metabolite abbreviations see Table 1.
Steroid enzyme activities were assessed by metabolite ratios, as published for diagnosing various forms of congenital adrenal hyperplasia (18) and for characterizing adrenarche and PCOS (6, 19). Ratios are given as substrate over product conversion by specific enzymes, thus a lower ratio reflects a higher enzyme activity. The results are shown in Table 4. Compared to controls, PA girls showed similar enzyme activities for 3βHSD and 21-hydroxylase (Fig. 2B and 2C). These enzyme activities decrease with age during normal adrenarche (6). However, in contrast to control girls, girls with PA had higher 17,20-lyase activity through the ∆ 4 pathway, likely leading to the backdoor pathway and higher 17βHSD activity within the backdoor pathway, as indicated by the urinary excretion of C19 metabolites (Fig. 2D and 2E). Higher activities of these enzymes were not observed during normal adrenarche over time (6). Ratios for 5α-reductase activity were similar in girls with PA and control girls of our study (Fig. 2F) and also did not change age-dependently with adrenarche in the literature (6), while women with PCOS and their daughters had higher 5α-reductase activity compared with controls (19).
Steroid hormone enzyme activities represented by steroid hormone metabolite ratios in girls with PA and control girls
| Urinary Steroid Metabolite Ratios Indicating Enzyme Activities . | Cases . | Controls . | P-valuea . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | N = 23 . | N = 22 . | . | ||||||||
| . | Percentiles . | Percentiles . | . | ||||||||
| . | P5 . | P25 . | P50 . | P75 . | P95 . | P5 . | P25 . | P50 . | P75 . | P95 . | . |
| 21-hydroxylase | |||||||||||
| PTONE/THE | 0.001 | 0.002 | 0.003 | 0.006 | 0.012 | 0.002 | 0.003 | 0.006 | 0.009 | 0.031 | 0.437 |
| PTONE/a-CN | 0.004 | 0.006 | 0.011 | 0.018 | 0.048 | 0.009 | 0.011 | 0.020 | 0.032 | 0.112 | 0.320 |
| (17-HP + 5-PT + PTONE)/ (THE + THF + 5a-THF) | 0.011 | 0.013 | 0.030 | 0.040 | 0.075 | 0.007 | 0.011 | 0.013 | 0.021 | 0.039 | 0.386 |
| 3β-hydroxysteroid dehydrogenase | |||||||||||
| 5-PT/THE | 0.001 | 0.005 | 0.022 | 0.044 | 0.111 | 0.001 | 0.002 | 0.004 | 0.008 | 0.028 | 0.154 |
| (DHEA + 16a-OH-DHEA + 5-AT)/ (ANDRO + ETIO) | 0.035 | 0.132 | 0.192 | 0.375 | 1.319 | 0.073 | 0.134 | 0.190 | 0.340 | 1.062 | 1.000 |
| 11β-hydroxylase | |||||||||||
| THS/THE | 0.010 | 0.017 | 0.021 | 0.033 | 0.060 | 0.020 | 0.025 | 0.031 | 0.041 | 0.053 | 0.386 |
| CYP17 global (17α-hydroxylase and 17,20-lyase) | |||||||||||
| PD/(ANDRO + ETIO) | 0.035 | 0.066 | 0.111 | 0.163 | 0.230 | 0.035 | 0.109 | 0.201 | 0.452 | 1.02 | 0.341 |
| 17α-hydroxylase global | |||||||||||
| (THA + THB + 5αTHB)/THE | 0.095 | 0.142 | 0.169 | 0.215 | 0.337 | 0.062 | 0.111 | 0.157 | 0.256 | 0.431 | 1.000 |
| 17α-hydroxylase Δ4-pathway | |||||||||||
| PD/17-HP | 1.30 | 2.28 | 2.80 | 3.75 | 6.50 | 0.554 | 1.73 | 2.56 | 4.40 | 6.14 | 1.000 |
| 17,20-lyase global | |||||||||||
| THE/(ANDRO + ETIO) | 1.29 | 1.63 | 2.40 | 3.97 | 10.98 | 1.67 | 4.69 | 7.02 | 10.11 | 26.34 | 0.011 |
| 17,20-lyase Δ5-pathway | |||||||||||
| 5-PT/(DHEA + 16a-OH-DHEA) | 0.053 | 0.09 | 0.329 | 0.629 | 1.81 | 0.044 | 0.081 | 0.151 | 0.559 | 1.44 | 1.000 |
| 17,20-lyase Δ4-pathway | |||||||||||
| 17-HP/(ANDRO + ETIO) | 0.016 | 0.023 | 0.035 | 0.059 | 0.074 | 0.022 | 0.047 | 0.074 | 0.156 | 0.285 | 0.052 |
| CYP17 global Δ4- vs. Δ5-pathway | |||||||||||
| (DHEA + 116a-OH-DHEA + ANDRO-DIOL)/ 11b-OH-ANDRO | 0.085 | 0.262 | 0.473 | 0.848 | 1.92 | 0.098 | 0.139 | 0.253 | 0.491 | 0.890 | 0.663 |
| 17β-hydroxysteroid dehydrogenase | |||||||||||
| (THE + THF + 5a-THF)/(ANDRO + ETIO) | 2.42 | 2.67 | 3.94 | 6.62 | 16.31 | 3.25 | 8.31 | 12.02 | 18.44 | 43.02 | 0.002 |
| DHEA/ANDRO-DIOL | 0.113 | 0.491 | 0.907 | 1.37 | 4.37 | 0.083 | 0.278 | 0.497 | 0.652 | 2.86 | 0.464 |
| 5α-reductase | |||||||||||
| ETIO/ANDRO | 0.216 | 0.291 | 0.427 | 0.608 | 0.878 | 0.317 | 0.382 | 0.557 | 0.771 | 1.57 | 1.000 |
| THF/5a-THF | 0.265 | 0.371 | 0.658 | 0.866 | 1.93 | 0.424 | 0.506 | 0.605 | 0.806 | 1.07 | 1.000 |
| Aromatase (CYP19A1) | |||||||||||
| TST/ESTRADIOL | 1.61 | 2.93 | 9.31 | 23.87 | 40.49 | 0.910 | 3.13 | 5.93 | 11.77 | 26.16 | 1.000 |
| 11β-hydroxysteroid dehydrogenase type 2 | |||||||||||
| (F + E)/(THE + THF + 5a-THF) | 0.022 | 0.041 | 0.047 | 0.071 | 0.094 | 0.021 | 0.034 | 0.048 | 0.067 | 0.118 | 1.000 |
| 11β-hydroxysteroid dehydrogenase type 1 | |||||||||||
| THE/(THF + 5a-THF) | 0.857 | 1.07 | 1.50 | 1.91 | 3.29 | 0.899 | 1.09 | 1.37 | 1.65 | 2.21 | 1.000 |
| 20α-hydroxysteroid dehydrogenase | |||||||||||
| (THE + THF + 5a-THF)/(a-CL + a-CN) | 2.97 | 4.30 | 4.76 | 6.47 | 8.52 | 3.29 | 4.24 | 5.18 | 5.66 | 6.80 | 1.000 |
| 20β-hydroxysteroid dehydrogenase | |||||||||||
| (THE + THF + 5a-THF)/(b-CL + b-CN) | 3.79 | 4.65 | 5.60 | 6.69 | 9.75 | 3.03 | 4.16 | 4.78 | 5.67 | 8.26 | 1.000 |
| 20α- vs. 20β-hydroxysteroid dehydrogenase | |||||||||||
| (a-CL + a-CN)/(b-CL + b-CN) | 0.671 | 0.815 | 1.05 | 1.58 | 2.07 | 0.543 | 0.862 | 1.03 | 1.17 | 1.38 | 1.000 |
| 3α-hydroxysteroid dehydrogenase | |||||||||||
| (THF + 5a-THF)/20a-DHF | 23.35 | 31.10 | 61.27 | 78.86 | 132.42 | 32.87 | 46.06 | 56.37 | 97.56 | 123.26 | 1.000 |
| Urinary Steroid Metabolite Ratios Indicating Enzyme Activities . | Cases . | Controls . | P-valuea . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | N = 23 . | N = 22 . | . | ||||||||
| . | Percentiles . | Percentiles . | . | ||||||||
| . | P5 . | P25 . | P50 . | P75 . | P95 . | P5 . | P25 . | P50 . | P75 . | P95 . | . |
| 21-hydroxylase | |||||||||||
| PTONE/THE | 0.001 | 0.002 | 0.003 | 0.006 | 0.012 | 0.002 | 0.003 | 0.006 | 0.009 | 0.031 | 0.437 |
| PTONE/a-CN | 0.004 | 0.006 | 0.011 | 0.018 | 0.048 | 0.009 | 0.011 | 0.020 | 0.032 | 0.112 | 0.320 |
| (17-HP + 5-PT + PTONE)/ (THE + THF + 5a-THF) | 0.011 | 0.013 | 0.030 | 0.040 | 0.075 | 0.007 | 0.011 | 0.013 | 0.021 | 0.039 | 0.386 |
| 3β-hydroxysteroid dehydrogenase | |||||||||||
| 5-PT/THE | 0.001 | 0.005 | 0.022 | 0.044 | 0.111 | 0.001 | 0.002 | 0.004 | 0.008 | 0.028 | 0.154 |
| (DHEA + 16a-OH-DHEA + 5-AT)/ (ANDRO + ETIO) | 0.035 | 0.132 | 0.192 | 0.375 | 1.319 | 0.073 | 0.134 | 0.190 | 0.340 | 1.062 | 1.000 |
| 11β-hydroxylase | |||||||||||
| THS/THE | 0.010 | 0.017 | 0.021 | 0.033 | 0.060 | 0.020 | 0.025 | 0.031 | 0.041 | 0.053 | 0.386 |
| CYP17 global (17α-hydroxylase and 17,20-lyase) | |||||||||||
| PD/(ANDRO + ETIO) | 0.035 | 0.066 | 0.111 | 0.163 | 0.230 | 0.035 | 0.109 | 0.201 | 0.452 | 1.02 | 0.341 |
| 17α-hydroxylase global | |||||||||||
| (THA + THB + 5αTHB)/THE | 0.095 | 0.142 | 0.169 | 0.215 | 0.337 | 0.062 | 0.111 | 0.157 | 0.256 | 0.431 | 1.000 |
| 17α-hydroxylase Δ4-pathway | |||||||||||
| PD/17-HP | 1.30 | 2.28 | 2.80 | 3.75 | 6.50 | 0.554 | 1.73 | 2.56 | 4.40 | 6.14 | 1.000 |
| 17,20-lyase global | |||||||||||
| THE/(ANDRO + ETIO) | 1.29 | 1.63 | 2.40 | 3.97 | 10.98 | 1.67 | 4.69 | 7.02 | 10.11 | 26.34 | 0.011 |
| 17,20-lyase Δ5-pathway | |||||||||||
| 5-PT/(DHEA + 16a-OH-DHEA) | 0.053 | 0.09 | 0.329 | 0.629 | 1.81 | 0.044 | 0.081 | 0.151 | 0.559 | 1.44 | 1.000 |
| 17,20-lyase Δ4-pathway | |||||||||||
| 17-HP/(ANDRO + ETIO) | 0.016 | 0.023 | 0.035 | 0.059 | 0.074 | 0.022 | 0.047 | 0.074 | 0.156 | 0.285 | 0.052 |
| CYP17 global Δ4- vs. Δ5-pathway | |||||||||||
| (DHEA + 116a-OH-DHEA + ANDRO-DIOL)/ 11b-OH-ANDRO | 0.085 | 0.262 | 0.473 | 0.848 | 1.92 | 0.098 | 0.139 | 0.253 | 0.491 | 0.890 | 0.663 |
| 17β-hydroxysteroid dehydrogenase | |||||||||||
| (THE + THF + 5a-THF)/(ANDRO + ETIO) | 2.42 | 2.67 | 3.94 | 6.62 | 16.31 | 3.25 | 8.31 | 12.02 | 18.44 | 43.02 | 0.002 |
| DHEA/ANDRO-DIOL | 0.113 | 0.491 | 0.907 | 1.37 | 4.37 | 0.083 | 0.278 | 0.497 | 0.652 | 2.86 | 0.464 |
| 5α-reductase | |||||||||||
| ETIO/ANDRO | 0.216 | 0.291 | 0.427 | 0.608 | 0.878 | 0.317 | 0.382 | 0.557 | 0.771 | 1.57 | 1.000 |
| THF/5a-THF | 0.265 | 0.371 | 0.658 | 0.866 | 1.93 | 0.424 | 0.506 | 0.605 | 0.806 | 1.07 | 1.000 |
| Aromatase (CYP19A1) | |||||||||||
| TST/ESTRADIOL | 1.61 | 2.93 | 9.31 | 23.87 | 40.49 | 0.910 | 3.13 | 5.93 | 11.77 | 26.16 | 1.000 |
| 11β-hydroxysteroid dehydrogenase type 2 | |||||||||||
| (F + E)/(THE + THF + 5a-THF) | 0.022 | 0.041 | 0.047 | 0.071 | 0.094 | 0.021 | 0.034 | 0.048 | 0.067 | 0.118 | 1.000 |
| 11β-hydroxysteroid dehydrogenase type 1 | |||||||||||
| THE/(THF + 5a-THF) | 0.857 | 1.07 | 1.50 | 1.91 | 3.29 | 0.899 | 1.09 | 1.37 | 1.65 | 2.21 | 1.000 |
| 20α-hydroxysteroid dehydrogenase | |||||||||||
| (THE + THF + 5a-THF)/(a-CL + a-CN) | 2.97 | 4.30 | 4.76 | 6.47 | 8.52 | 3.29 | 4.24 | 5.18 | 5.66 | 6.80 | 1.000 |
| 20β-hydroxysteroid dehydrogenase | |||||||||||
| (THE + THF + 5a-THF)/(b-CL + b-CN) | 3.79 | 4.65 | 5.60 | 6.69 | 9.75 | 3.03 | 4.16 | 4.78 | 5.67 | 8.26 | 1.000 |
| 20α- vs. 20β-hydroxysteroid dehydrogenase | |||||||||||
| (a-CL + a-CN)/(b-CL + b-CN) | 0.671 | 0.815 | 1.05 | 1.58 | 2.07 | 0.543 | 0.862 | 1.03 | 1.17 | 1.38 | 1.000 |
| 3α-hydroxysteroid dehydrogenase | |||||||||||
| (THF + 5a-THF)/20a-DHF | 23.35 | 31.10 | 61.27 | 78.86 | 132.42 | 32.87 | 46.06 | 56.37 | 97.56 | 123.26 | 1.000 |
Data are the median (P50) and 5th, 25th, 75th, and 95th percentiles for urinary steroid metabolites in girls with PA and control girls collected over 24 hours.
Abbreviations: N, number. For metabolite abbreviations see Table 1.
aP-values derived from Mann-Whitney U tests to test for differences between girls with PA and control girls. All comparisons account for multiple testing using Bonferroni correction. Data in bold are significantly different between groups (P < 0.05).
Steroid hormone enzyme activities represented by steroid hormone metabolite ratios in girls with PA and control girls
| Urinary Steroid Metabolite Ratios Indicating Enzyme Activities . | Cases . | Controls . | P-valuea . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | N = 23 . | N = 22 . | . | ||||||||
| . | Percentiles . | Percentiles . | . | ||||||||
| . | P5 . | P25 . | P50 . | P75 . | P95 . | P5 . | P25 . | P50 . | P75 . | P95 . | . |
| 21-hydroxylase | |||||||||||
| PTONE/THE | 0.001 | 0.002 | 0.003 | 0.006 | 0.012 | 0.002 | 0.003 | 0.006 | 0.009 | 0.031 | 0.437 |
| PTONE/a-CN | 0.004 | 0.006 | 0.011 | 0.018 | 0.048 | 0.009 | 0.011 | 0.020 | 0.032 | 0.112 | 0.320 |
| (17-HP + 5-PT + PTONE)/ (THE + THF + 5a-THF) | 0.011 | 0.013 | 0.030 | 0.040 | 0.075 | 0.007 | 0.011 | 0.013 | 0.021 | 0.039 | 0.386 |
| 3β-hydroxysteroid dehydrogenase | |||||||||||
| 5-PT/THE | 0.001 | 0.005 | 0.022 | 0.044 | 0.111 | 0.001 | 0.002 | 0.004 | 0.008 | 0.028 | 0.154 |
| (DHEA + 16a-OH-DHEA + 5-AT)/ (ANDRO + ETIO) | 0.035 | 0.132 | 0.192 | 0.375 | 1.319 | 0.073 | 0.134 | 0.190 | 0.340 | 1.062 | 1.000 |
| 11β-hydroxylase | |||||||||||
| THS/THE | 0.010 | 0.017 | 0.021 | 0.033 | 0.060 | 0.020 | 0.025 | 0.031 | 0.041 | 0.053 | 0.386 |
| CYP17 global (17α-hydroxylase and 17,20-lyase) | |||||||||||
| PD/(ANDRO + ETIO) | 0.035 | 0.066 | 0.111 | 0.163 | 0.230 | 0.035 | 0.109 | 0.201 | 0.452 | 1.02 | 0.341 |
| 17α-hydroxylase global | |||||||||||
| (THA + THB + 5αTHB)/THE | 0.095 | 0.142 | 0.169 | 0.215 | 0.337 | 0.062 | 0.111 | 0.157 | 0.256 | 0.431 | 1.000 |
| 17α-hydroxylase Δ4-pathway | |||||||||||
| PD/17-HP | 1.30 | 2.28 | 2.80 | 3.75 | 6.50 | 0.554 | 1.73 | 2.56 | 4.40 | 6.14 | 1.000 |
| 17,20-lyase global | |||||||||||
| THE/(ANDRO + ETIO) | 1.29 | 1.63 | 2.40 | 3.97 | 10.98 | 1.67 | 4.69 | 7.02 | 10.11 | 26.34 | 0.011 |
| 17,20-lyase Δ5-pathway | |||||||||||
| 5-PT/(DHEA + 16a-OH-DHEA) | 0.053 | 0.09 | 0.329 | 0.629 | 1.81 | 0.044 | 0.081 | 0.151 | 0.559 | 1.44 | 1.000 |
| 17,20-lyase Δ4-pathway | |||||||||||
| 17-HP/(ANDRO + ETIO) | 0.016 | 0.023 | 0.035 | 0.059 | 0.074 | 0.022 | 0.047 | 0.074 | 0.156 | 0.285 | 0.052 |
| CYP17 global Δ4- vs. Δ5-pathway | |||||||||||
| (DHEA + 116a-OH-DHEA + ANDRO-DIOL)/ 11b-OH-ANDRO | 0.085 | 0.262 | 0.473 | 0.848 | 1.92 | 0.098 | 0.139 | 0.253 | 0.491 | 0.890 | 0.663 |
| 17β-hydroxysteroid dehydrogenase | |||||||||||
| (THE + THF + 5a-THF)/(ANDRO + ETIO) | 2.42 | 2.67 | 3.94 | 6.62 | 16.31 | 3.25 | 8.31 | 12.02 | 18.44 | 43.02 | 0.002 |
| DHEA/ANDRO-DIOL | 0.113 | 0.491 | 0.907 | 1.37 | 4.37 | 0.083 | 0.278 | 0.497 | 0.652 | 2.86 | 0.464 |
| 5α-reductase | |||||||||||
| ETIO/ANDRO | 0.216 | 0.291 | 0.427 | 0.608 | 0.878 | 0.317 | 0.382 | 0.557 | 0.771 | 1.57 | 1.000 |
| THF/5a-THF | 0.265 | 0.371 | 0.658 | 0.866 | 1.93 | 0.424 | 0.506 | 0.605 | 0.806 | 1.07 | 1.000 |
| Aromatase (CYP19A1) | |||||||||||
| TST/ESTRADIOL | 1.61 | 2.93 | 9.31 | 23.87 | 40.49 | 0.910 | 3.13 | 5.93 | 11.77 | 26.16 | 1.000 |
| 11β-hydroxysteroid dehydrogenase type 2 | |||||||||||
| (F + E)/(THE + THF + 5a-THF) | 0.022 | 0.041 | 0.047 | 0.071 | 0.094 | 0.021 | 0.034 | 0.048 | 0.067 | 0.118 | 1.000 |
| 11β-hydroxysteroid dehydrogenase type 1 | |||||||||||
| THE/(THF + 5a-THF) | 0.857 | 1.07 | 1.50 | 1.91 | 3.29 | 0.899 | 1.09 | 1.37 | 1.65 | 2.21 | 1.000 |
| 20α-hydroxysteroid dehydrogenase | |||||||||||
| (THE + THF + 5a-THF)/(a-CL + a-CN) | 2.97 | 4.30 | 4.76 | 6.47 | 8.52 | 3.29 | 4.24 | 5.18 | 5.66 | 6.80 | 1.000 |
| 20β-hydroxysteroid dehydrogenase | |||||||||||
| (THE + THF + 5a-THF)/(b-CL + b-CN) | 3.79 | 4.65 | 5.60 | 6.69 | 9.75 | 3.03 | 4.16 | 4.78 | 5.67 | 8.26 | 1.000 |
| 20α- vs. 20β-hydroxysteroid dehydrogenase | |||||||||||
| (a-CL + a-CN)/(b-CL + b-CN) | 0.671 | 0.815 | 1.05 | 1.58 | 2.07 | 0.543 | 0.862 | 1.03 | 1.17 | 1.38 | 1.000 |
| 3α-hydroxysteroid dehydrogenase | |||||||||||
| (THF + 5a-THF)/20a-DHF | 23.35 | 31.10 | 61.27 | 78.86 | 132.42 | 32.87 | 46.06 | 56.37 | 97.56 | 123.26 | 1.000 |
| Urinary Steroid Metabolite Ratios Indicating Enzyme Activities . | Cases . | Controls . | P-valuea . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | N = 23 . | N = 22 . | . | ||||||||
| . | Percentiles . | Percentiles . | . | ||||||||
| . | P5 . | P25 . | P50 . | P75 . | P95 . | P5 . | P25 . | P50 . | P75 . | P95 . | . |
| 21-hydroxylase | |||||||||||
| PTONE/THE | 0.001 | 0.002 | 0.003 | 0.006 | 0.012 | 0.002 | 0.003 | 0.006 | 0.009 | 0.031 | 0.437 |
| PTONE/a-CN | 0.004 | 0.006 | 0.011 | 0.018 | 0.048 | 0.009 | 0.011 | 0.020 | 0.032 | 0.112 | 0.320 |
| (17-HP + 5-PT + PTONE)/ (THE + THF + 5a-THF) | 0.011 | 0.013 | 0.030 | 0.040 | 0.075 | 0.007 | 0.011 | 0.013 | 0.021 | 0.039 | 0.386 |
| 3β-hydroxysteroid dehydrogenase | |||||||||||
| 5-PT/THE | 0.001 | 0.005 | 0.022 | 0.044 | 0.111 | 0.001 | 0.002 | 0.004 | 0.008 | 0.028 | 0.154 |
| (DHEA + 16a-OH-DHEA + 5-AT)/ (ANDRO + ETIO) | 0.035 | 0.132 | 0.192 | 0.375 | 1.319 | 0.073 | 0.134 | 0.190 | 0.340 | 1.062 | 1.000 |
| 11β-hydroxylase | |||||||||||
| THS/THE | 0.010 | 0.017 | 0.021 | 0.033 | 0.060 | 0.020 | 0.025 | 0.031 | 0.041 | 0.053 | 0.386 |
| CYP17 global (17α-hydroxylase and 17,20-lyase) | |||||||||||
| PD/(ANDRO + ETIO) | 0.035 | 0.066 | 0.111 | 0.163 | 0.230 | 0.035 | 0.109 | 0.201 | 0.452 | 1.02 | 0.341 |
| 17α-hydroxylase global | |||||||||||
| (THA + THB + 5αTHB)/THE | 0.095 | 0.142 | 0.169 | 0.215 | 0.337 | 0.062 | 0.111 | 0.157 | 0.256 | 0.431 | 1.000 |
| 17α-hydroxylase Δ4-pathway | |||||||||||
| PD/17-HP | 1.30 | 2.28 | 2.80 | 3.75 | 6.50 | 0.554 | 1.73 | 2.56 | 4.40 | 6.14 | 1.000 |
| 17,20-lyase global | |||||||||||
| THE/(ANDRO + ETIO) | 1.29 | 1.63 | 2.40 | 3.97 | 10.98 | 1.67 | 4.69 | 7.02 | 10.11 | 26.34 | 0.011 |
| 17,20-lyase Δ5-pathway | |||||||||||
| 5-PT/(DHEA + 16a-OH-DHEA) | 0.053 | 0.09 | 0.329 | 0.629 | 1.81 | 0.044 | 0.081 | 0.151 | 0.559 | 1.44 | 1.000 |
| 17,20-lyase Δ4-pathway | |||||||||||
| 17-HP/(ANDRO + ETIO) | 0.016 | 0.023 | 0.035 | 0.059 | 0.074 | 0.022 | 0.047 | 0.074 | 0.156 | 0.285 | 0.052 |
| CYP17 global Δ4- vs. Δ5-pathway | |||||||||||
| (DHEA + 116a-OH-DHEA + ANDRO-DIOL)/ 11b-OH-ANDRO | 0.085 | 0.262 | 0.473 | 0.848 | 1.92 | 0.098 | 0.139 | 0.253 | 0.491 | 0.890 | 0.663 |
| 17β-hydroxysteroid dehydrogenase | |||||||||||
| (THE + THF + 5a-THF)/(ANDRO + ETIO) | 2.42 | 2.67 | 3.94 | 6.62 | 16.31 | 3.25 | 8.31 | 12.02 | 18.44 | 43.02 | 0.002 |
| DHEA/ANDRO-DIOL | 0.113 | 0.491 | 0.907 | 1.37 | 4.37 | 0.083 | 0.278 | 0.497 | 0.652 | 2.86 | 0.464 |
| 5α-reductase | |||||||||||
| ETIO/ANDRO | 0.216 | 0.291 | 0.427 | 0.608 | 0.878 | 0.317 | 0.382 | 0.557 | 0.771 | 1.57 | 1.000 |
| THF/5a-THF | 0.265 | 0.371 | 0.658 | 0.866 | 1.93 | 0.424 | 0.506 | 0.605 | 0.806 | 1.07 | 1.000 |
| Aromatase (CYP19A1) | |||||||||||
| TST/ESTRADIOL | 1.61 | 2.93 | 9.31 | 23.87 | 40.49 | 0.910 | 3.13 | 5.93 | 11.77 | 26.16 | 1.000 |
| 11β-hydroxysteroid dehydrogenase type 2 | |||||||||||
| (F + E)/(THE + THF + 5a-THF) | 0.022 | 0.041 | 0.047 | 0.071 | 0.094 | 0.021 | 0.034 | 0.048 | 0.067 | 0.118 | 1.000 |
| 11β-hydroxysteroid dehydrogenase type 1 | |||||||||||
| THE/(THF + 5a-THF) | 0.857 | 1.07 | 1.50 | 1.91 | 3.29 | 0.899 | 1.09 | 1.37 | 1.65 | 2.21 | 1.000 |
| 20α-hydroxysteroid dehydrogenase | |||||||||||
| (THE + THF + 5a-THF)/(a-CL + a-CN) | 2.97 | 4.30 | 4.76 | 6.47 | 8.52 | 3.29 | 4.24 | 5.18 | 5.66 | 6.80 | 1.000 |
| 20β-hydroxysteroid dehydrogenase | |||||||||||
| (THE + THF + 5a-THF)/(b-CL + b-CN) | 3.79 | 4.65 | 5.60 | 6.69 | 9.75 | 3.03 | 4.16 | 4.78 | 5.67 | 8.26 | 1.000 |
| 20α- vs. 20β-hydroxysteroid dehydrogenase | |||||||||||
| (a-CL + a-CN)/(b-CL + b-CN) | 0.671 | 0.815 | 1.05 | 1.58 | 2.07 | 0.543 | 0.862 | 1.03 | 1.17 | 1.38 | 1.000 |
| 3α-hydroxysteroid dehydrogenase | |||||||||||
| (THF + 5a-THF)/20a-DHF | 23.35 | 31.10 | 61.27 | 78.86 | 132.42 | 32.87 | 46.06 | 56.37 | 97.56 | 123.26 | 1.000 |
Data are the median (P50) and 5th, 25th, 75th, and 95th percentiles for urinary steroid metabolites in girls with PA and control girls collected over 24 hours.
Abbreviations: N, number. For metabolite abbreviations see Table 1.
aP-values derived from Mann-Whitney U tests to test for differences between girls with PA and control girls. All comparisons account for multiple testing using Bonferroni correction. Data in bold are significantly different between groups (P < 0.05).
Discussion
This prospective study found that total urinary androgen excretion in girls with PA was higher than in age-matched controls. Metabolites from the alternative pathway such as ANDRO and 5-AT were higher in girls with PA, but not the measured 11-hydroxy-metabolites. Our results indicate that this pattern of urinary metabolome is due to higher 17,20 lyase activity, which seems to result from higher metabolic flux through alternate pathways (Fig. 1). Similarly, we found higher 17βHSD activity promoting conversion in the backdoor pathway in PA girls compared with controls. By contrast, we found no difference between PA and age-matched girls in 3βHSD and 21-hydroxylase activity, which decreased gradually, and age-dependent in German girls with normal adrenarche (6). These results indicate that PA might not only be a variant of normal adrenarche occurring at an earlier time point, but that it also has its own metabolic profile likely due to underlying alterations affecting androgen production in comparison to normal development.
To our knowledge, only 1 study assessed the urinary steroid profile of 400 healthy children aged 3–18 years (6). This study from Germany showed that urinary adrenal androgen secretion in children increased significantly after age 7–8 years at adrenarche, when DHEA and its 16α-hydroxylated downstream metabolites, as well as ANDRO-DIOL increase by 2- to 4-fold in girls. Likewise the sum of C19 androgens, including ETIO and ANDRO increases by age 7–8 years. These changes seemed to be due to age-dependent changes in steroidogenic enzyme activities, including a decrease in 3βHSD and 21-hydroxylase, while activities of 17βHSD and 5α-reductase seemed unchanged (6). In our study, girls with PA showed a very similar pattern of high androgens compared with German girls during normal adrenarche. But in contrast to the German girls with normal adrenarche, our girls had similar 3βHSD and 21-hydroxylase activities but higher 17,20-lyase and 17βHSD activities compared with age-matched control girls. These enzymes are essential for androgen production through the classic and the alternate pathway (Fig. 1). These findings suggest that PA is not only a timing problem of normal adrenarche and that it involves changes of androgen production.
High activities of 17,20-lyase and 17βHSD activities calculated from metabolite ratios of alternate androgen production pathways hint at more profound alterations of the developmental regulation underlying premature versus normal adrenarche targeting these enzymes. To test this hypothesis, further prospective studies should assess the steroid metabolome of normal adrenarche and PA longitudinally, and compare data with respect to developmental events beyond puberty of girls with PA to age-matched controls, but also in relation to bone age and pubertal stages. Such a prospective longitudinal study should expand its focus to deep profiling of phenotype, transcriptome, metabolome, and complex analysis of all data in a combinatory fashion to better understand the underlying cause or pathomechanism of PA.
The 11-oxygenated C19 steroid pathway plays an important role in the human androgen metabolism (20). Plasma steroid profiling revealed that adrenal-derived steroid sulfates, testosterone (TST), and 11-oxyandrogens increased with adrenarche. Among the 11-oxyandrogens, the increase of 11-keto-testosterone was higher in PA compared with controls (9). In our study, the 11β-hydroxy-metabolites as precursors of the 11-ketoandrogens were not higher. However, we did not directly measure the 11-ketoandrogenes and might therefore have missed this effect.
Earlier studies searching for causes of PA found elevated 17,20-lyase activity (21), increased IGF-1 (22), and anti-Müllerian hormone (23). Plasma steroid profiling showed evidence of increased 17,20-lyase activity and increased body weight in girls with PA (21), 2 findings that our study showed as well.
Specific monogenic steroid hormone biosynthesis defects are rare in children with PA except for late-onset, nonclassical CAH due to 21-hydroxylase deficiency, which is recommended to be considered in the diagnostic workup of PA (24, 25). In a study looking for the causes and patterns of androgen excess in 140 prepubertal and 59 postpubertal children, a diagnosis of PA was found in 61.4% of the prepuberal group, while 4.3% had CAH. Fourty percent of postpubertal girls had a PCOS diagnosis, and 14% had a CAH diagnosis (24). Isolated elevated plasma DHEA sulfate was the most common finding with PA among the studied laboratory parameters (DHEA sulfate, androstenedione, and testosterone). Exclusion of 21-hydroxylase CAH is possible by measuring basal serum 17-hydroxyprogesterone, which distinguished PA from CAH at a threshold of 6 nmol/L (2 ng/ml), with high sensitivity and specificity (26). Whether the more specific metabolite 21-deoxycortisol will replace 17α-hydroxyprogesterone as a serum marker steroid of CAH is currently being discussed (27). A urinary steroid profile measured by GC-MS provides a more comprehensive picture of overall steroid metabolism and therefore allows the diagnosis of 21-hydroxylase and other forms of CAH with a high sensitivity and specificity (17).Thus, CAH in the PA girls of our study was excluded.
In very rare cases PA can be caused by apparent cortisone reductase deficiency due to enzyme deficiency of 11-hydroxysteroid dehydrogenase type 1 or by cofactor variants in 3’-phosphoadenosine 5’-phosphosulfate synthase 2 (PAPSS2), leading to DHEA sulfotransferase deficiency (20). Similarly, glucocorticoid resistance and androgen receptor hypersensitivity due to a CAG repeat polymorphism have been reported (20). But these extremely rare monogenic disorders eventually manifest with additional characteristics, at least with follow-up and by family history, and are therefore not included in the regular workup of PA.
The question whether PA girls are at risk to develop functional ovarian hyperandrogenism or PCOS later in adolescence and adulthood is controversial and not solved (1, 11). Using the same urinary steroid profiling method, we recently studied the steroid metabolome in young PCOS women and compared them to age-matched controls (28). Polycystic ovary syndrome women had higher total androgen metabolites in their urine than controls, similar to girls with PA, compared with controls, as observed by this study. The urinary steroid signature of women with PCOS showed a considerable overlap with the profile of PA, specifically for DHEA and its metabolites, ANDRO, DH-ANDRO, and ETIO. The comparison revealed also some interesting differences. For example, women with PCOS showed higher TST, 5α-dihydrotestosterone (DH-TST) secretion, tetrahydrocortisone (THE), tetrahydrocortisol (THF), and 11β-hydroxy-metabolites, and lower estriol (ESTRIOL) and pregnanediol (PD) compared with controls, while we did not observe these differences in girls with PA (28). Higher age and the contribution of activated ovarian steroidogenesis to the urinary metabolome in PCOS women may explain the different patterns between PCOS women and girls with PA. Enzyme ratio calculations showed higher global 17,20-lyase activity and within the ∆-4 pathway, specifically in both young PCOS women and in girls with PA. But unlike girls with PA, PCOS women had similar 17βHSD activity as controls (28). Conversely, PCOS women had higher 11β-hydroxylase and P450 oxidoreductase activities than controls, while our PA girls had similar activities of these enzymes as controls. Our results indicate that there is considerable overlap between the androgen secretion pattern in girls with PA and PCOS women, distinguishing them from healthy females. But these results don’t answer the question whether there is a common underlying cause, which would allow us to predict that PA girls are at risk for developing functional ovarian hyperandrogenism or PCOS.
This is the first prospective study measuring the urinary steroid metabolome in girls with PA compared with age-matched controls. There are some limitations of the study. The number of participants was relatively low and, therefore, we did not include covariables such as age and BMI in the statistical analyses. However, we controlled for age using age-matched controls. Body mass index was higher in girls with PA (0.9 SDS) than in controls (-0.3), but both groups were normal weight. Considering the large magnitude of observed differences in most metabolic parameters, the study has high statistical power to detect differences between groups and it shows robust results. We did not measure 11-keto-androgens and, thus, we may have missed their contribution to the urinary steroid signature of PA. At the beginning of this study, their relevance was still unknown. Finally, this study only assesses one time point in the development of adrenarche. It will be important to assess the metabolome (and other characteristics) of normal adrenarche and PA longitudinally beyond puberty in future studies. There is some evidence that androgens and anthropometric parameters normalize in some PA girls after puberty (29).
In conclusion, the urinary steroid secretion pattern of PA suggests higher activities of 17,20-lyase in the Δ4 pathway and 17βHSD when calculated from metabolites of the alternate androgen pathway and compared with age-matched controls. These enzymes play an important role in alternate pathways of androgen production. The pattern of elevated metabolites and activities of enzymes of alternate androgen pathways may hint towards a PA diagnosis.
Both PCOS women and girls with PA had high 17,20-lyase activity. Future longitudinal studies investigating the metabolome of PA and controls in puberty and adulthood should assess whether PA girls also have a different metabolic pattern compared with girls at timing of normal adrenarche over time and whether PA girls eventually develop functional ovarian hyperandrogenism or PCOS later in life.
Acknowledgments
We thank all the girls and families for participating in the study.
Financial Support: This work was supported by the Swiss National Science Foundation (SNF 320030-146127) to CEF.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
Author notes
The authors contributed in equal parts to the study.



