-
PDF
- Split View
-
Views
-
Cite
Cite
Anna Aulinas, Franziska Plessow, Elisa Asanza, Lisseth Silva, Dean A Marengi, WuQiang Fan, Parisa Abedi, Joseph Verbalis, Nicholas A Tritos, Lisa Nachtigall, Alexander T Faje, Karen K Miller, Elizabeth A Lawson, Low Plasma Oxytocin Levels and Increased Psychopathology in Hypopituitary Men With Diabetes Insipidus, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 8, August 2019, Pages 3181–3191, https://doi.org/10.1210/jc.2018-02608
Close - Share Icon Share
Abstract
Oxytocin (OT) and vasopressin share anatomical pathways of synthesis and secretion, and patients with central diabetes insipidus (CDI) presumably are at risk for OT deficiency. However, an OT-deficient state in hypopituitary patients has not been established.
We hypothesized that men with CDI compared to patients with similar anterior pituitary deficiencies (APD) but no CDI and healthy controls (HC) of similar age and body mass index, would have lower plasma OT levels, associated with increased psychopathology.
Cross-sectional.
Clinical research center.
Sixty-two men (20 CDI, 20 APD, 22 HC), age 18 to 60 years.
Frequent sampling of blood every 5 minutes for OT over 1 hour and validated questionnaires to assess psychopathology.
Pooled plasma OT levels; depressive, anxiety, and alexithymia symptoms; and quality of life.
The mean 1-hour pool of fasting OT levels was lower in CDI compared with APD and HC (P = 0.02 and P = 0.009, respectively), with no differences between APD and HC (P = 0.78). Symptoms of depression, anxiety, and alexithymia were more pronounced in CDI than in HC (P = 0.001, P = 0.004, and P = 0.02, respectively). Although CDI and APD reported worse physical health compared with HC (P = 0.001 and P = 0.005) with no differences between APD and CDI, only CDI reported worse mental health compared with HC (P = 0.009).
We have demonstrated low plasma OT levels and increased psychopathology in hypopituitary men with CDI, suggestive of a possible OT-deficient state. Larger studies of both sexes are required to confirm these findings and clinically characterize hypopituitary patients with OT deficiency.
Anterior and/or posterior pituitary hormone deficiencies can result from congenital anomalies, tumor, infiltrative or inflammatory disease, traumatic brain injury, or surgery involving the hypothalamic pituitary axes (1). The prevalence of hypopituitarism, probably underestimated, has been reported to be ∼45/100,000 individuals (2). Anterior pituitary insufficiencies can be diagnosed with standard testing and treated with appropriate hormone replacements, resulting in known clinical benefits (1, 3–5). Similarly, central diabetes insipidus (CDI), a deficiency of the posterior pituitary hormone vasopressin (AVP), can be identified based on clinical symptoms and laboratory test results and successfully replaced with 1-desamino-8-d-arginine vasopressin (desmopressin) (6). However, whether a deficiency in the posterior pituitary hormone oxytocin (OT) is also present in hypopituitary patients is an important question that has not been adequately studied.
Similar to AVP, OT is a nine-amino acid peptide hormone produced in the supraoptic and paraventricular nuclei of the hypothalamus that is secreted throughout the brain and enters the peripheral circulation through the posterior pituitary gland. OT mediates a range of physiologic processes and has important neuropsychiatric effects, including antidepressant, anxiolytic, and socioemotional functioning properties (7, 8). OT-based therapeutic agents are under active investigation for the treatment of neuropsychiatric disorders, including autism, depression, and anxiety disorders (9–11), and OT replacement is feasible in hypopituitary patients if a deficient state is established.
Despite the shared anatomical pathways of the AVP and OT systems, such that patients with CDI are presumably at a high risk of OT deficiency, an OT deficiency syndrome has yet to be established in hypopituitary patients with CDI. In animal models of acquired CDI due to neurohypophysectomy or pituitary stalk compression, plasma OT levels are undetectable (12, 13), and a reduction in AVP and OT mRNA expression at the hypothalamic supraoptic nucleus and paraventricular nucleus was observed after pituitary stalk compression in rats (14). Only a few studies with divergent results have examined OT levels in patients with hypopituitarism (15–17). The data focused primarily on patients with craniopharyngioma (CP), without specifically examining those with CDI. The results were mostly from salivary OT levels (the clinical significance of which is unknown), rather than OT levels in the peripheral blood that directly receives OT from the posterior pituitary (15–17). Moreover, most studies have measured OT at only a single time point. Similar to other hypothalamic pituitary hormones, the levels at a single basal OT measurement might overlap in patients with normal vs insufficient hormone stores. OT is a pulsatile hormone with a short half-life in plasma on the order of minutes (18–20). The identification of OT deficiency might, therefore, require a more sophisticated approach (e.g., frequent sampling for an integrated measure of OT secretion to leverage the pulsatile secretory dynamics of OT or the use of provocative testing). Although insulin-induced hypoglycemia has been shown to stimulate OT secretion (21–23), its clinical use might be limited in these patients because of the risks associated with cardiovascular comorbidities and structural brain damage and an attenuated response in patients with obesity (24).
Hypopituitarism is associated with increased psychopathology and impaired quality of life, with increased incapacitation and an increased number of sick days (25–28). A recent study reported a greater prevalence of psychiatric conditions requiring medical therapy in patients with childhood-onset CP (29). These patients also had a greater rate of CDI (79% vs 43%) compared with adult-onset CP (29). Although neuropsychological dysfunction in hypopituitary patients is likely multifactorial, including the effects of cranial irradiation, surgery, visual disturbances, and other pituitary hormone deficiencies, insufficient OT production could be a factor in a subset of patients. Along these lines, a child with CP was reported to have demonstrated improvements in socioemotional functioning beginning within the first 24 hours of low-dose intranasal OT (30). Also, in a small subset of patients with CP and anterior hypothalamic damage, a single dose of intranasal OT resulted in improved ability to identify others’ emotions (31).
In the present pilot study intended to uncover an OT deficient state in hypopituitary patients, we focused on men to avoid cyclic fluctuations in estradiol and progesterone, which are known to affect the levels of OT. We aimed to identify reduced OT levels in men with CDI using an integrated measure of pooled plasma OT (which captures basal levels and OT pulses) collected every 5 minutes (given the short half-life of OT) over 1 hour under standard conditions (fasted morning with minimal social stimulation). We hypothesized that in men with CDI compared with patients with similar anterior pituitary deficiencies (APD) but no CDI and healthy male controls (HC) of similar age and body mass index (BMI), the OT levels would be lower and associated with increased psychopathology (i.e., depressive, anxiety, and alexithymia symptoms) and impaired quality of life.
Methods
We performed a cross-sectional study of 62 male subjects, aged 18 to 60 years: 20 with CDI, 20 with APD only, and 22 HC of similar age and BMI. By design, we selected individuals with APD who had similar anterior pituitary deficiencies to the CDI group. Participants with CDI and APD were primarily recruited through the Neuroendocrine and Pituitary Tumor Clinical Center at Massachusetts General Hospital (Boston, MA). The HC were recruited from the surrounding community. All the participants provided written informed consent with a study physician or nurse practitioner before any procedures. The institutional review board of Partners HealthCare approved the present study.
The pituitary diagnoses were established as per routine clinical practice based on clinical symptoms, sodium levels, and the water deprivation test if needed for CDI, and a combination of basal and validated dynamic tests to assess anterior pituitary function. All the CDI participants and none of the APD participants were receiving desmopressin treatment for CDI. The CDI and APD subjects both had at least one anterior pituitary insufficiency. All subjects had been receiving stable hormone replacement for the previous 3 months. Normal total or free T4 and normal free testosterone levels, calculated from total testosterone and SHBG by the laws of mass action (32), were required for participation. Participants were excluded from the study if they were receiving human chorionic gonadotropin, which can increase estradiol levels, or if their laboratory tests showed a creatinine level >1.5 mg/dL, alanine aminotransferase or aspartate aminotransferase levels >2.5 times the upper limit of normal, or hematocrit <34%. An additional exclusion criterion for the APD participants included the presence of CDI. Additional exclusion criteria for the HC were a history of a brain or pituitary tumor, radiation involving the hypothalamus or pituitary, hypopituitarism, and treatment with testosterone.
All the subjects were admitted to the Translational and Clinical Research Center (TCRC) at Massachusetts General Hospital for two outpatient visits: a screening visit and, if eligible, a main study visit. The screening visit included medical history review, physical examination, and blood tests to assess eligibility. To assess psychopathology, all participants completed the following measures. The Beck Depression Inventory-IA (BDI) is a validated and reliable questionnaire to assess the severity of depressive symptoms during the previous week (33). Based on responses to 21 items, a total score is calculated. The total scores range from 0 to 63, with higher scores indicating a greater severity of depressive symptoms (score ≤16, mild; 17 to 29, moderate, and score ≥30, severe). The State-Trait Anxiety Inventory (34) is a well-validated, reliable instrument for assessing trait and state anxiety. We used the State-Trait Anxiety Trait Scale (STAI-T) to determine the general anxiety levels. Based on responses to 20 items, with scores ranging from 1 (“almost never”) to 4 (“almost always”), a total score is calculated. The total Trait scores range from 20 to 80, with higher scores indicating more pronounced anxiety and scores >45 indicating clinically significant anxiety symptoms (35). The Toronto Alexithymia Scale (TAS-20) (36) was used to evaluate socioemotional functioning, measuring the ability to express and identify one’s emotions. Based on responses to 20 items ranging from 1 (“strongly disagree”) to 5 (“strongly agree”), a global score is calculated. A global score of ≥61 indicates alexithymia (difficulty understanding one’s own emotions), a score of 52 to 60 possible alexithymia, and a score of ≤51 nonalexithymia. In addition, the 36-item Short-Form Survey (SF-36) was used to assess overall physical and mental health. In the SF-36, eight multi-item scales are used to calculate the overall dimensions. The overall physical health dimension is calculated using the scores obtained from physical functioning, bodily pain, role limitations due to physical health problems, and general health perception scales. The overall mental health dimension is calculated using the scores obtained from role limitations due to personal or emotional problems, general mental health, social functioning, and energy/fatigue scales (37). For each overall dimension, the scores range from 0 to 100, with higher scores indicating a more favorable health state and less disability.
For the main visit, the participants presented to the TCRC after an 8-hour fast. Medical history and medication use since the screening visit were updated and a physical examination, including BMI and waist/hip ratio (calculated by dividing the measurement of the waist iliac circumference in centimeters by the broadest hip circumference in centimeters), were performed. Hypertension was defined as systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥80 mm Hg, the use of antihypertensive medications, or self-report in the medical history (38). Dyslipidemia was defined as total cholesterol >220 mg/dL (>5.8 mmol/L), triglycerides ≥150 mg/dL (1.7 mmol/L), treatment with lipid-lowering medication, or self-report in the medical history. Type 2 diabetes mellitus was confirmed by fasting glucose ≥126 mg/dL (7 mmol/L) in two consecutive determinations, the use of antidiabetic medications, or self-report in the medical history. Desmopressin replacement was classified according to the dose used as low (dose equivalent, ≤200 µg/d orally), medium (dose equivalent, >200 to ≤600 µg/d orally), or high (dose equivalent, >600 µg/d orally) (1).
An intravenous catheter was placed, and the subjects were allowed to acclimate to their rooms for at least 30 minutes before the fasting blood sampling for blood chemical tests (basic metabolic panel, lipid profile, glucose) and hormones (OT, AVP, total testosterone, free T4, IGF-1, prolactin). This was followed by frequent sampling of blood every 5 minutes from 8:00 to 9:00 am for assessment of the pooled OT. Blood sampling was performed by experienced research nurses at the TCRC with identical procedures used between the patients and controls. Interactions and conversation were kept to a minimum (i.e., no cell phone, reading, television, radio) to avoid the effects of social stimulation on OT levels.
Biochemical analyses
Equal aliquots of plasma from each time point, available from all participants, other than one APD and two HC subjects, were combined into a 1-hour pool for an integrated measure of OT for each participant.
The basic metabolic panel, lipids, glucose, free T4, SHBG, and total testosterone were obtained using standard techniques by LabCorp (Burlington, NC). Serum and plasma for other hormones were stored at −80°C and run in a single batch. An RIA of extracted plasma OT was performed in duplicate using antibody Pitt-Ab-2. The minimum limit of detection was 0.4 pg/mL, with an intra-assay coefficient of variation (CV) of 4.2% and interassay CV of 12.4%. The antiserum displayed significant cross-reactivity with arginine vasotocin but <1% cross-reactivity with AVP, lysine vasopressin, and desmopressin (39). An RIA of plasma AVP was performed in duplicate after acetone-ether extraction. The minimal detectable AVP concentration in extracted plasma was 0.5 pg/mL. The intra-assay CV was 6.4% and the interassay CV was 8.8%. The antiserum displayed substantial cross-reactivity with lysine vasopressin but <1% cross-reactivity with OT, AVP, and desmopressin. IGF-1 was measured in serum using liquid chromatography–mass spectrometry (Quest Diagnostics, San Juan Capistrano, CA). Prolactin was measured using a competitive ELISA kit (ALPCO, Salem, NH) with a minimum detection limit of 2 ng/mL, an interassay CV of 5.3%, and an intra-assay CV of 3.7%.
Statistical analysis
Statistical analyses were performed using STATA software, version 14.2 (StataCorp LLC, College Station, TX). Quantitative data are expressed as the mean ± SEM (Gaussian distribution) or the median and interquartile range (non-Gaussian distribution) and categorical data as percentages. The data distribution was analyzed using the Shapiro-Wilk test. Logarithmic transformations were performed when necessary to normalize the distribution of a particular measure. The mean values across the three groups (HC, APD, and CDI) were compared with one-way analyses of variance, followed by the Fisher least significant difference test for pairwise comparisons if the variables analyzed were overall significantly different. The Fisher exact test was performed to compare the categorical variables across the groups. Linear correlation analyses between the OT levels and other quantitative clinical variables of interest were performed using the Spearman rank correlation. For the two-group comparisons (low vs high desmopressin dose, low vs high 1-hour pool of OT), the mean values across two groups were compared using the Wilcoxon rank-sum test. Statistical significance was defined as a bilateral P < 0.05.
Results
Clinical characteristics
The clinical characteristics of the study participants are summarized in Table 1. The mean age was 44.5 ± 1.5 years, and mean BMI was 30.3 ± 0.6 kg/m2. Per study design, age and BMI were similar across the groups. None of the participants had been currently smoking. APD participants had a greater prevalence of hypertension compared with CDI and HC groups despite the similar age and BMI; six APD and one CDI participants were taking antihypertensive medications. Both APD and CDI participants had a greater prevalence of dyslipidemia compared with HC. Five APD and three CDI participants were taking a statin. Three APD and two CDI participants had type 2 diabetes mellitus, all of whom were taking antidiabetic medications. One APD and five CDI participants were taking selective serotonin reuptake inhibitors and two CDI participants were taking anxiolytic medication (lorazepam and clonazepam). HC participants were not taking any medications, except for multivitamin supplements by two and selective serotonin reuptake inhibitors by one HC participant.
| Variable . | HC (n = 22) . | APD (n = 20) . | CDI (n = 20) . | P Value . |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, y | 42.5 ± 2.3 | 47.1 ± 2.5 | 43.9 ± 2.9 | 0.43 |
| Hypertension | 1 (4.6) | 8 (40.0) | 2 (10.0) | 0.008a,c |
| Dyslipidemia | 2 (9.1) | 15 (75.0) | 10 (50.0) | 0.0001a,b |
| Diabetes | 0 (0) | 3 (15.0) | 2 (10.0) | 0.18 |
| BMI, kg/m2 | 30.5 ± 1.1 | 31.3 ± 1.6 | 29.7 ± 1.1 | 0.63 |
| Waist/hip ratio | 0.93 ± 0.01 | 0.96 ± 0.02 | 0.95 ± 0.02 | 0.47 |
| SBP, mm Hg | 127.3 ± 2.5 | 132.1 ± 3.4 | 121.9 ± 3.3 | 0.07 |
| DBP, mm Hg | 75.6 ± 1.9 | 82.8 ± 1.9 | 75.8 ± 2.1 | 0.02a,c |
| Biochemical parameters | ||||
| Fasting glucose, mg/dL | 92.1 ± 8.4 | 96.5 ± 26.1 | 85.5 ± 12.3 | 0.07 |
| Sodium, mmol/L | 139.7 ± 2.0 | 139.1 ± 2.6 | 139.3 ± 4.2 | 0.82 |
| Total cholesterol, mg/dL | 161 ± 28 | 183 ± 38 | 181 ± 28 | 0.05a |
| LDL cholesterol, mg/dL | 98.1 ± 22.9 | 110.2 ± 29.8 | 111.9 ± 24.4 | 0.17 |
| HDL cholesterol, mg/dL | 44.7 ± 11.7 | 41.1 ± 10.8 | 41.9 ± 10.3 | 0.54 |
| Triglycerides, mg/dL | 90.9 ± 37.9 | 158.1 ± 77.6 | 149.5 ± 128.4 | 0.03a |
| Free T4, ng/dL | 1.22 ± 0.2 | 1.25 ± 0.3 | 1.37 ± 0.3 | 0.11 |
| SHBG, nmol/L | 32.8 ± 13.1 | 28.9 ± 14.3 | 34.6 ± 29.2 | 0.66 |
| Total testosterone, ng/dL | 486 ± 164 | 603 ± 332 | 590 ± 420 | 0.43 |
| Prolactin, ng/mL | 9.1 ± 0.8 | 12.0 ± 1.5 | 9.5 ± 1.4 | 0.21 |
| IGF-1, ng/mL | 167.3 ± 8.6 | 143.9 ± 17.6 | 159.8 ± 17.0 | 0.16 |
| Psychosocial evaluation | ||||
| BDI | 0.7 ± 0.7 | 2.8 ± 0.8 | 4.5 ± 0.8 | 0.005b,d |
| STAI-T | 25.2 ± 1.5 | 30.3 ± 1.6 | 32.6 ± 1.6 | 0.004b,d |
| TAS-20 total | 36.7 ± 2.5 | 42.0 ± 2.6 | 45.7 ± 2.6 | 0.05b |
| Quality of life | ||||
| SF-36 Overall physical health | 94.8 ± 2.9 | 82.3 ± 3.1 | 80.2 ± 3.0 | 0.002a,b |
| SF-36 Overall mental health | 91.2 ± 2.6 | 86.6 ± 2.8 | 79.6 ± 2.7 | 0.01b,d |
| Variable . | HC (n = 22) . | APD (n = 20) . | CDI (n = 20) . | P Value . |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, y | 42.5 ± 2.3 | 47.1 ± 2.5 | 43.9 ± 2.9 | 0.43 |
| Hypertension | 1 (4.6) | 8 (40.0) | 2 (10.0) | 0.008a,c |
| Dyslipidemia | 2 (9.1) | 15 (75.0) | 10 (50.0) | 0.0001a,b |
| Diabetes | 0 (0) | 3 (15.0) | 2 (10.0) | 0.18 |
| BMI, kg/m2 | 30.5 ± 1.1 | 31.3 ± 1.6 | 29.7 ± 1.1 | 0.63 |
| Waist/hip ratio | 0.93 ± 0.01 | 0.96 ± 0.02 | 0.95 ± 0.02 | 0.47 |
| SBP, mm Hg | 127.3 ± 2.5 | 132.1 ± 3.4 | 121.9 ± 3.3 | 0.07 |
| DBP, mm Hg | 75.6 ± 1.9 | 82.8 ± 1.9 | 75.8 ± 2.1 | 0.02a,c |
| Biochemical parameters | ||||
| Fasting glucose, mg/dL | 92.1 ± 8.4 | 96.5 ± 26.1 | 85.5 ± 12.3 | 0.07 |
| Sodium, mmol/L | 139.7 ± 2.0 | 139.1 ± 2.6 | 139.3 ± 4.2 | 0.82 |
| Total cholesterol, mg/dL | 161 ± 28 | 183 ± 38 | 181 ± 28 | 0.05a |
| LDL cholesterol, mg/dL | 98.1 ± 22.9 | 110.2 ± 29.8 | 111.9 ± 24.4 | 0.17 |
| HDL cholesterol, mg/dL | 44.7 ± 11.7 | 41.1 ± 10.8 | 41.9 ± 10.3 | 0.54 |
| Triglycerides, mg/dL | 90.9 ± 37.9 | 158.1 ± 77.6 | 149.5 ± 128.4 | 0.03a |
| Free T4, ng/dL | 1.22 ± 0.2 | 1.25 ± 0.3 | 1.37 ± 0.3 | 0.11 |
| SHBG, nmol/L | 32.8 ± 13.1 | 28.9 ± 14.3 | 34.6 ± 29.2 | 0.66 |
| Total testosterone, ng/dL | 486 ± 164 | 603 ± 332 | 590 ± 420 | 0.43 |
| Prolactin, ng/mL | 9.1 ± 0.8 | 12.0 ± 1.5 | 9.5 ± 1.4 | 0.21 |
| IGF-1, ng/mL | 167.3 ± 8.6 | 143.9 ± 17.6 | 159.8 ± 17.0 | 0.16 |
| Psychosocial evaluation | ||||
| BDI | 0.7 ± 0.7 | 2.8 ± 0.8 | 4.5 ± 0.8 | 0.005b,d |
| STAI-T | 25.2 ± 1.5 | 30.3 ± 1.6 | 32.6 ± 1.6 | 0.004b,d |
| TAS-20 total | 36.7 ± 2.5 | 42.0 ± 2.6 | 45.7 ± 2.6 | 0.05b |
| Quality of life | ||||
| SF-36 Overall physical health | 94.8 ± 2.9 | 82.3 ± 3.1 | 80.2 ± 3.0 | 0.002a,b |
| SF-36 Overall mental health | 91.2 ± 2.6 | 86.6 ± 2.8 | 79.6 ± 2.7 | 0.01b,d |
Data presented as mean ± SEM or n (%).
Abbreviations: DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
P < 0.05 HC vs APD.
P < 0.05 HC vs CDI.
P < 0.05 APD vs CDI.
P < 0.1 HC vs APD.
| Variable . | HC (n = 22) . | APD (n = 20) . | CDI (n = 20) . | P Value . |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, y | 42.5 ± 2.3 | 47.1 ± 2.5 | 43.9 ± 2.9 | 0.43 |
| Hypertension | 1 (4.6) | 8 (40.0) | 2 (10.0) | 0.008a,c |
| Dyslipidemia | 2 (9.1) | 15 (75.0) | 10 (50.0) | 0.0001a,b |
| Diabetes | 0 (0) | 3 (15.0) | 2 (10.0) | 0.18 |
| BMI, kg/m2 | 30.5 ± 1.1 | 31.3 ± 1.6 | 29.7 ± 1.1 | 0.63 |
| Waist/hip ratio | 0.93 ± 0.01 | 0.96 ± 0.02 | 0.95 ± 0.02 | 0.47 |
| SBP, mm Hg | 127.3 ± 2.5 | 132.1 ± 3.4 | 121.9 ± 3.3 | 0.07 |
| DBP, mm Hg | 75.6 ± 1.9 | 82.8 ± 1.9 | 75.8 ± 2.1 | 0.02a,c |
| Biochemical parameters | ||||
| Fasting glucose, mg/dL | 92.1 ± 8.4 | 96.5 ± 26.1 | 85.5 ± 12.3 | 0.07 |
| Sodium, mmol/L | 139.7 ± 2.0 | 139.1 ± 2.6 | 139.3 ± 4.2 | 0.82 |
| Total cholesterol, mg/dL | 161 ± 28 | 183 ± 38 | 181 ± 28 | 0.05a |
| LDL cholesterol, mg/dL | 98.1 ± 22.9 | 110.2 ± 29.8 | 111.9 ± 24.4 | 0.17 |
| HDL cholesterol, mg/dL | 44.7 ± 11.7 | 41.1 ± 10.8 | 41.9 ± 10.3 | 0.54 |
| Triglycerides, mg/dL | 90.9 ± 37.9 | 158.1 ± 77.6 | 149.5 ± 128.4 | 0.03a |
| Free T4, ng/dL | 1.22 ± 0.2 | 1.25 ± 0.3 | 1.37 ± 0.3 | 0.11 |
| SHBG, nmol/L | 32.8 ± 13.1 | 28.9 ± 14.3 | 34.6 ± 29.2 | 0.66 |
| Total testosterone, ng/dL | 486 ± 164 | 603 ± 332 | 590 ± 420 | 0.43 |
| Prolactin, ng/mL | 9.1 ± 0.8 | 12.0 ± 1.5 | 9.5 ± 1.4 | 0.21 |
| IGF-1, ng/mL | 167.3 ± 8.6 | 143.9 ± 17.6 | 159.8 ± 17.0 | 0.16 |
| Psychosocial evaluation | ||||
| BDI | 0.7 ± 0.7 | 2.8 ± 0.8 | 4.5 ± 0.8 | 0.005b,d |
| STAI-T | 25.2 ± 1.5 | 30.3 ± 1.6 | 32.6 ± 1.6 | 0.004b,d |
| TAS-20 total | 36.7 ± 2.5 | 42.0 ± 2.6 | 45.7 ± 2.6 | 0.05b |
| Quality of life | ||||
| SF-36 Overall physical health | 94.8 ± 2.9 | 82.3 ± 3.1 | 80.2 ± 3.0 | 0.002a,b |
| SF-36 Overall mental health | 91.2 ± 2.6 | 86.6 ± 2.8 | 79.6 ± 2.7 | 0.01b,d |
| Variable . | HC (n = 22) . | APD (n = 20) . | CDI (n = 20) . | P Value . |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, y | 42.5 ± 2.3 | 47.1 ± 2.5 | 43.9 ± 2.9 | 0.43 |
| Hypertension | 1 (4.6) | 8 (40.0) | 2 (10.0) | 0.008a,c |
| Dyslipidemia | 2 (9.1) | 15 (75.0) | 10 (50.0) | 0.0001a,b |
| Diabetes | 0 (0) | 3 (15.0) | 2 (10.0) | 0.18 |
| BMI, kg/m2 | 30.5 ± 1.1 | 31.3 ± 1.6 | 29.7 ± 1.1 | 0.63 |
| Waist/hip ratio | 0.93 ± 0.01 | 0.96 ± 0.02 | 0.95 ± 0.02 | 0.47 |
| SBP, mm Hg | 127.3 ± 2.5 | 132.1 ± 3.4 | 121.9 ± 3.3 | 0.07 |
| DBP, mm Hg | 75.6 ± 1.9 | 82.8 ± 1.9 | 75.8 ± 2.1 | 0.02a,c |
| Biochemical parameters | ||||
| Fasting glucose, mg/dL | 92.1 ± 8.4 | 96.5 ± 26.1 | 85.5 ± 12.3 | 0.07 |
| Sodium, mmol/L | 139.7 ± 2.0 | 139.1 ± 2.6 | 139.3 ± 4.2 | 0.82 |
| Total cholesterol, mg/dL | 161 ± 28 | 183 ± 38 | 181 ± 28 | 0.05a |
| LDL cholesterol, mg/dL | 98.1 ± 22.9 | 110.2 ± 29.8 | 111.9 ± 24.4 | 0.17 |
| HDL cholesterol, mg/dL | 44.7 ± 11.7 | 41.1 ± 10.8 | 41.9 ± 10.3 | 0.54 |
| Triglycerides, mg/dL | 90.9 ± 37.9 | 158.1 ± 77.6 | 149.5 ± 128.4 | 0.03a |
| Free T4, ng/dL | 1.22 ± 0.2 | 1.25 ± 0.3 | 1.37 ± 0.3 | 0.11 |
| SHBG, nmol/L | 32.8 ± 13.1 | 28.9 ± 14.3 | 34.6 ± 29.2 | 0.66 |
| Total testosterone, ng/dL | 486 ± 164 | 603 ± 332 | 590 ± 420 | 0.43 |
| Prolactin, ng/mL | 9.1 ± 0.8 | 12.0 ± 1.5 | 9.5 ± 1.4 | 0.21 |
| IGF-1, ng/mL | 167.3 ± 8.6 | 143.9 ± 17.6 | 159.8 ± 17.0 | 0.16 |
| Psychosocial evaluation | ||||
| BDI | 0.7 ± 0.7 | 2.8 ± 0.8 | 4.5 ± 0.8 | 0.005b,d |
| STAI-T | 25.2 ± 1.5 | 30.3 ± 1.6 | 32.6 ± 1.6 | 0.004b,d |
| TAS-20 total | 36.7 ± 2.5 | 42.0 ± 2.6 | 45.7 ± 2.6 | 0.05b |
| Quality of life | ||||
| SF-36 Overall physical health | 94.8 ± 2.9 | 82.3 ± 3.1 | 80.2 ± 3.0 | 0.002a,b |
| SF-36 Overall mental health | 91.2 ± 2.6 | 86.6 ± 2.8 | 79.6 ± 2.7 | 0.01b,d |
Data presented as mean ± SEM or n (%).
Abbreviations: DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
P < 0.05 HC vs APD.
P < 0.05 HC vs CDI.
P < 0.05 APD vs CDI.
P < 0.1 HC vs APD.
The clinical history of pituitary disease in participants with APD and CDI is summarized in Table 2. The mean age at the diagnosis of pituitary disease was 30.7 ± 2.3 years. As per the study design, the number of anterior pituitary deficiencies was similar across the groups, with a mean of 3.3 ± 0.1. The most frequent pituitary diseases were nonfunctioning pituitary adenoma in nine (23%) and CP in nine participants (23%). In addition, 30 individuals (75%) had undergone pituitary surgery, 20 (50%) had received radiotherapy, and 2 (5%) had received chemotherapy (for medulloblastoma and Langerhans cell histiocytosis). All patients with central adrenal insufficiency, hypothyroidism, and/or hypogonadism were receiving hormone replacement therapy. The mean daily hydrocortisone-equivalent dose for the patients receiving glucocorticoid replacement therapy was 16.4 ± 0.8 mg, with no differences between groups (P = 0.62). Of those with a diagnosis of GH deficiency, 77% of the APD and 71% of the CDI participants were receiving recombinant human GH replacement. The hormone parameters (free T4, total testosterone, IGF-1, and prolactin) did not differ between the groups. All CDI participants were receiving desmopressin replacement therapy; 11 (55%) were taking oral desmopressin, 7 (35%) were taking intranasal desmopressin, and 2 (10%) were taking both intranasal and oral desmopressin dose from one to three times daily. Of the 20 CDI participants, 12 (60%) were receiving a low dose, 6 (30%) a medium dose, and 2 (10%) a high dose of desmopressin.
| Variable . | APD (n = 20) . | CDI (n = 20) . | P Value . |
|---|---|---|---|
| Age at diagnosis, y | 30.7 ± 3.6 | 30.6 ± 2.8 | 0.98 |
| Number of APDs | 3.3 ± 0.2 | 3.4 ± 0.2 | 0.73 |
| Time since first APD, y, [median (IQR)] | 6.8 (4.3) | 12.3 (13.2) | 0.41 |
| Time since CDI, y, [median (IQR)] | NA | 13.3 (16.6) | 0.41 |
| Pituitary tumor | 19 (95.0) | 14 (70.0) | 0.09 |
| Pituitary disease | 0.46 | ||
| Nonfunctioning PA | 5 (25.0) | 4 (20.0) | |
| CP | 4 (20.0) | 5 (25.0) | |
| Prolactinoma | 2 (10.0) | 0 (0) | |
| Acromegaly | 2 (10.0) | 1 (5.0) | |
| Cushing syndrome | 3 (15.0 | 1 (5.0) | |
| Neurosarcoidosis | 0 (0) | 2 (10.0) | |
| Histiocytosis | 0 (0) | 1 (5.0) | |
| Chondrosarcoma | 2 (10.0) | 0 (0) | |
| Pituitary hypoplasia | 1 (5.0) | 1 (5.0) | |
| Pituitary stalk enlargement | 0 (0) | 2 (10.0) | |
| Germinoma | 0 (0) | 2 (10.0) | |
| Other | 1 (5.0) (medulloblastoma) | 1 (5.0) (Rathke cleft cyst) | |
| Macroadenoma | 16 (80.0) | 11 (55.0) | 0.55 |
| Surgery | 17 (85.0) | 13 (65.0) | 0.27 |
| Transsphenoidal surgery | 11 (55.0) | 9 (45.0) | |
| Craniotomy | 4 (20.0) | 4 (20.0) | |
| Both | 2 (10.0) | 0 (0) | |
| Radiotherapy | 15 (75.0) | 5 (35.0) | 0.03 |
| Pituitary deficiencies | 1.00 | ||
| Hypogonadism | 19 (95) | 19 (95) | |
| Adrenal insufficiency | 15 (75.0) | 16 (80.0) | |
| Central hypothyroidism | 18 (90.0) | 18 (90.0) | |
| GH deficiency | 13 (65.0) | 14 (70.0) | |
| GH deficiency not tested | 2 (10.0) | 1 (5.0) |
| Variable . | APD (n = 20) . | CDI (n = 20) . | P Value . |
|---|---|---|---|
| Age at diagnosis, y | 30.7 ± 3.6 | 30.6 ± 2.8 | 0.98 |
| Number of APDs | 3.3 ± 0.2 | 3.4 ± 0.2 | 0.73 |
| Time since first APD, y, [median (IQR)] | 6.8 (4.3) | 12.3 (13.2) | 0.41 |
| Time since CDI, y, [median (IQR)] | NA | 13.3 (16.6) | 0.41 |
| Pituitary tumor | 19 (95.0) | 14 (70.0) | 0.09 |
| Pituitary disease | 0.46 | ||
| Nonfunctioning PA | 5 (25.0) | 4 (20.0) | |
| CP | 4 (20.0) | 5 (25.0) | |
| Prolactinoma | 2 (10.0) | 0 (0) | |
| Acromegaly | 2 (10.0) | 1 (5.0) | |
| Cushing syndrome | 3 (15.0 | 1 (5.0) | |
| Neurosarcoidosis | 0 (0) | 2 (10.0) | |
| Histiocytosis | 0 (0) | 1 (5.0) | |
| Chondrosarcoma | 2 (10.0) | 0 (0) | |
| Pituitary hypoplasia | 1 (5.0) | 1 (5.0) | |
| Pituitary stalk enlargement | 0 (0) | 2 (10.0) | |
| Germinoma | 0 (0) | 2 (10.0) | |
| Other | 1 (5.0) (medulloblastoma) | 1 (5.0) (Rathke cleft cyst) | |
| Macroadenoma | 16 (80.0) | 11 (55.0) | 0.55 |
| Surgery | 17 (85.0) | 13 (65.0) | 0.27 |
| Transsphenoidal surgery | 11 (55.0) | 9 (45.0) | |
| Craniotomy | 4 (20.0) | 4 (20.0) | |
| Both | 2 (10.0) | 0 (0) | |
| Radiotherapy | 15 (75.0) | 5 (35.0) | 0.03 |
| Pituitary deficiencies | 1.00 | ||
| Hypogonadism | 19 (95) | 19 (95) | |
| Adrenal insufficiency | 15 (75.0) | 16 (80.0) | |
| Central hypothyroidism | 18 (90.0) | 18 (90.0) | |
| GH deficiency | 13 (65.0) | 14 (70.0) | |
| GH deficiency not tested | 2 (10.0) | 1 (5.0) |
Data presented as mean ± SEM or n (%).
Abbreviations: IQR, interquartile range; NA, not applicable; PA, pituitary adenoma.
| Variable . | APD (n = 20) . | CDI (n = 20) . | P Value . |
|---|---|---|---|
| Age at diagnosis, y | 30.7 ± 3.6 | 30.6 ± 2.8 | 0.98 |
| Number of APDs | 3.3 ± 0.2 | 3.4 ± 0.2 | 0.73 |
| Time since first APD, y, [median (IQR)] | 6.8 (4.3) | 12.3 (13.2) | 0.41 |
| Time since CDI, y, [median (IQR)] | NA | 13.3 (16.6) | 0.41 |
| Pituitary tumor | 19 (95.0) | 14 (70.0) | 0.09 |
| Pituitary disease | 0.46 | ||
| Nonfunctioning PA | 5 (25.0) | 4 (20.0) | |
| CP | 4 (20.0) | 5 (25.0) | |
| Prolactinoma | 2 (10.0) | 0 (0) | |
| Acromegaly | 2 (10.0) | 1 (5.0) | |
| Cushing syndrome | 3 (15.0 | 1 (5.0) | |
| Neurosarcoidosis | 0 (0) | 2 (10.0) | |
| Histiocytosis | 0 (0) | 1 (5.0) | |
| Chondrosarcoma | 2 (10.0) | 0 (0) | |
| Pituitary hypoplasia | 1 (5.0) | 1 (5.0) | |
| Pituitary stalk enlargement | 0 (0) | 2 (10.0) | |
| Germinoma | 0 (0) | 2 (10.0) | |
| Other | 1 (5.0) (medulloblastoma) | 1 (5.0) (Rathke cleft cyst) | |
| Macroadenoma | 16 (80.0) | 11 (55.0) | 0.55 |
| Surgery | 17 (85.0) | 13 (65.0) | 0.27 |
| Transsphenoidal surgery | 11 (55.0) | 9 (45.0) | |
| Craniotomy | 4 (20.0) | 4 (20.0) | |
| Both | 2 (10.0) | 0 (0) | |
| Radiotherapy | 15 (75.0) | 5 (35.0) | 0.03 |
| Pituitary deficiencies | 1.00 | ||
| Hypogonadism | 19 (95) | 19 (95) | |
| Adrenal insufficiency | 15 (75.0) | 16 (80.0) | |
| Central hypothyroidism | 18 (90.0) | 18 (90.0) | |
| GH deficiency | 13 (65.0) | 14 (70.0) | |
| GH deficiency not tested | 2 (10.0) | 1 (5.0) |
| Variable . | APD (n = 20) . | CDI (n = 20) . | P Value . |
|---|---|---|---|
| Age at diagnosis, y | 30.7 ± 3.6 | 30.6 ± 2.8 | 0.98 |
| Number of APDs | 3.3 ± 0.2 | 3.4 ± 0.2 | 0.73 |
| Time since first APD, y, [median (IQR)] | 6.8 (4.3) | 12.3 (13.2) | 0.41 |
| Time since CDI, y, [median (IQR)] | NA | 13.3 (16.6) | 0.41 |
| Pituitary tumor | 19 (95.0) | 14 (70.0) | 0.09 |
| Pituitary disease | 0.46 | ||
| Nonfunctioning PA | 5 (25.0) | 4 (20.0) | |
| CP | 4 (20.0) | 5 (25.0) | |
| Prolactinoma | 2 (10.0) | 0 (0) | |
| Acromegaly | 2 (10.0) | 1 (5.0) | |
| Cushing syndrome | 3 (15.0 | 1 (5.0) | |
| Neurosarcoidosis | 0 (0) | 2 (10.0) | |
| Histiocytosis | 0 (0) | 1 (5.0) | |
| Chondrosarcoma | 2 (10.0) | 0 (0) | |
| Pituitary hypoplasia | 1 (5.0) | 1 (5.0) | |
| Pituitary stalk enlargement | 0 (0) | 2 (10.0) | |
| Germinoma | 0 (0) | 2 (10.0) | |
| Other | 1 (5.0) (medulloblastoma) | 1 (5.0) (Rathke cleft cyst) | |
| Macroadenoma | 16 (80.0) | 11 (55.0) | 0.55 |
| Surgery | 17 (85.0) | 13 (65.0) | 0.27 |
| Transsphenoidal surgery | 11 (55.0) | 9 (45.0) | |
| Craniotomy | 4 (20.0) | 4 (20.0) | |
| Both | 2 (10.0) | 0 (0) | |
| Radiotherapy | 15 (75.0) | 5 (35.0) | 0.03 |
| Pituitary deficiencies | 1.00 | ||
| Hypogonadism | 19 (95) | 19 (95) | |
| Adrenal insufficiency | 15 (75.0) | 16 (80.0) | |
| Central hypothyroidism | 18 (90.0) | 18 (90.0) | |
| GH deficiency | 13 (65.0) | 14 (70.0) | |
| GH deficiency not tested | 2 (10.0) | 1 (5.0) |
Data presented as mean ± SEM or n (%).
Abbreviations: IQR, interquartile range; NA, not applicable; PA, pituitary adenoma.
Psychosocial evaluation and quality of life
In all the groups, mean scores for the BDI, STAI-T, and TAS-20 were in the nonclinical range for depression, anxiety, and alexithymia. No significant differences were found in the depressive, anxiety, and alexithymia scores in the APD group compared with the HC and CDI participants. In contrast, symptoms of depression, anxiety, and alexithymia were greater in the patients with CDI compared with the HC (Table 1; Fig. 1). The patients with CDI taking a medium to high dose of desmopressin (n = 8) had higher BDI and STAI-T scores than those taking a low desmopressin dose (n = 12; BDI, 7.8 ± 2.2 vs 2.25 ± 0.6, respectively; P = 0.007; STAI-T, 39.1 ± 3.7 vs 28.3 ± 1.6, respectively; P = 0.006), indicative of greater depressive and anxiety symptom severity. Regarding the quality of life, the CDI participants had lower scores for overall self-reported mental and physical health compared with the HC as assessed by the SF-36. The APD participants also reported worse physical health compared with the HC but did not differ from the HC in self-reported mental health (Fig. 2). The results were similar after excluding the three patients with CDI and systemic disease (sarcoidosis and histiocytosis).
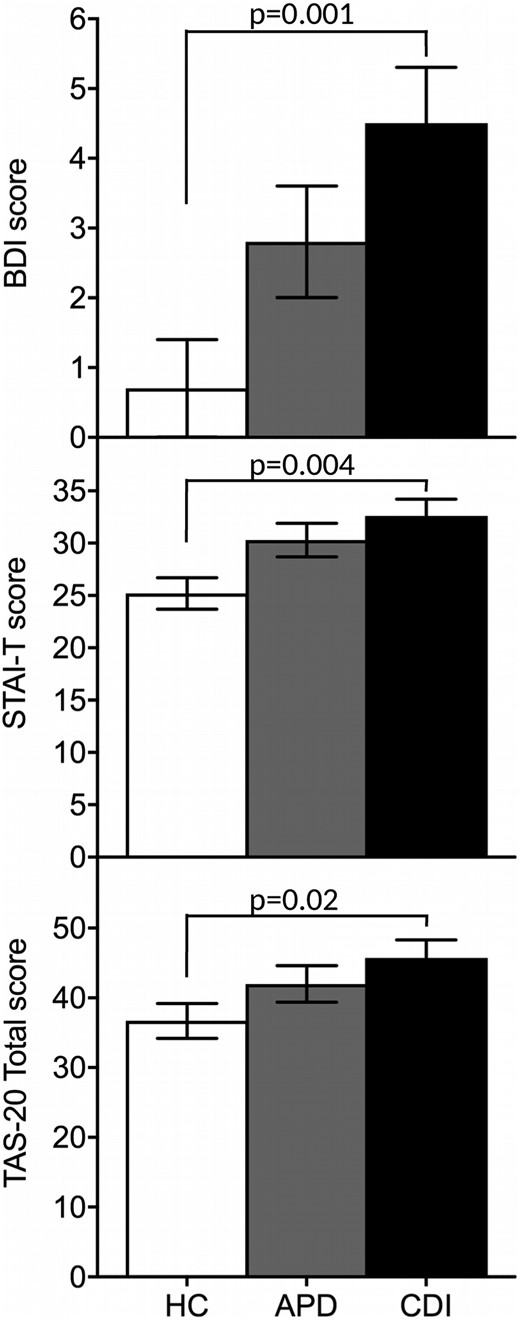
Measures of psychopathology across groups. Symptoms of depression, anxiety, and alexithymia were greater in CDI group than in HC group. Whiskers represent the SEM range.
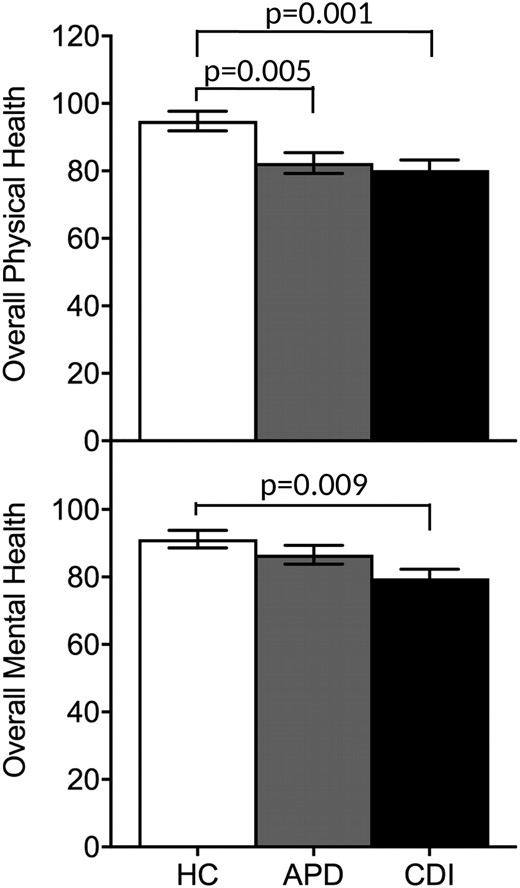
Mean mental and physical health scores assessed using the SF-36 across the study groups. The CDI and APD groups reported worse physical health compared with the HC group, and only the CDI group reported worse mental health compared with the HC group. Whiskers represent the SEM range.
Hypothalamic-posterior pituitary axis evaluation
The mean hormone levels are summarized in Table 3. The baseline fasting OT levels did not differ across the groups. However, the 1-hour pool of fasting OT levels was significantly lower in the CDI group than in the APD and HC groups (P = 0.02 and P = 0.009, respectively). In contrast, the levels were not different between the APD and HC groups (P = 0.78; Fig. 3). The CDI group had lower baseline fasting AVP levels compared with the APD and HC groups (P = 0.0002 and P = 0.005, respectively), with no differences between the APD and HC groups (P = 0.28).
| Variable . | HC (n = 22) . | APD (n = 20) . | CDI (n = 20) . | P Value . |
|---|---|---|---|---|
| Baseline OT, pg/mL | 1.06 ± 0.07 | 1.00 ± 0.07 | 1.04 ± 0.08 | 0.80 |
| 1-h Pool of OT, pg/mLa | 1.35 ± 0.06 | 1.33 ± 0.03 | 1.11 ± 0.07 | 0.02b,c |
| Baseline vasopressin, pg/mL | 0.39 ± 0.04 | 0.45 ± 0.05 | 0.23 ± 0.02 | 0.0007b,c |
| Variable . | HC (n = 22) . | APD (n = 20) . | CDI (n = 20) . | P Value . |
|---|---|---|---|---|
| Baseline OT, pg/mL | 1.06 ± 0.07 | 1.00 ± 0.07 | 1.04 ± 0.08 | 0.80 |
| 1-h Pool of OT, pg/mLa | 1.35 ± 0.06 | 1.33 ± 0.03 | 1.11 ± 0.07 | 0.02b,c |
| Baseline vasopressin, pg/mL | 0.39 ± 0.04 | 0.45 ± 0.05 | 0.23 ± 0.02 | 0.0007b,c |
One-hour pool of OT levels were not available for two HC and one APD participant.
P < 0.05 HC vs CDI.
P < 0.05 APD vs CDI.
| Variable . | HC (n = 22) . | APD (n = 20) . | CDI (n = 20) . | P Value . |
|---|---|---|---|---|
| Baseline OT, pg/mL | 1.06 ± 0.07 | 1.00 ± 0.07 | 1.04 ± 0.08 | 0.80 |
| 1-h Pool of OT, pg/mLa | 1.35 ± 0.06 | 1.33 ± 0.03 | 1.11 ± 0.07 | 0.02b,c |
| Baseline vasopressin, pg/mL | 0.39 ± 0.04 | 0.45 ± 0.05 | 0.23 ± 0.02 | 0.0007b,c |
| Variable . | HC (n = 22) . | APD (n = 20) . | CDI (n = 20) . | P Value . |
|---|---|---|---|---|
| Baseline OT, pg/mL | 1.06 ± 0.07 | 1.00 ± 0.07 | 1.04 ± 0.08 | 0.80 |
| 1-h Pool of OT, pg/mLa | 1.35 ± 0.06 | 1.33 ± 0.03 | 1.11 ± 0.07 | 0.02b,c |
| Baseline vasopressin, pg/mL | 0.39 ± 0.04 | 0.45 ± 0.05 | 0.23 ± 0.02 | 0.0007b,c |
One-hour pool of OT levels were not available for two HC and one APD participant.
P < 0.05 HC vs CDI.
P < 0.05 APD vs CDI.
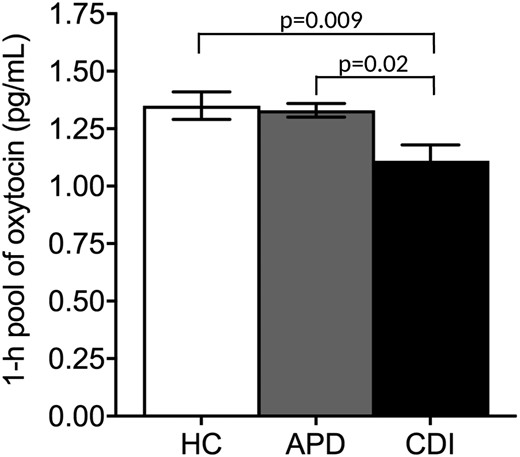
Mean 1-hour pool of fasting OT levels across groups. The 1-hour pool of fasting OT levels was lower in the CDI group compared with the APD and HC groups, and the levels between the APD and HC groups did not differ. Whiskers represent the SEM range.
Across all participants, positive correlations were found between the 1-hour pool of OT and baseline AVP (rs = 0.252; P = 0.048). The dose equivalent of desmopressin used did not correlate with the plasma OT or AVP parameters evaluated (P ≥ 0.34). The OT levels did not differ according to the time of desmopressin administration [OT levels, desmopressin before visit (n = 9) 1.01 ± 0.15 pg/mL vs desmopressin at bedtime (n = 11) 1.17 ± 0.05 pg/mL; P = 0.41].
Relationship between OT levels and clinical features in CDI
The OT levels did not differ across the etiological pituitary diagnoses (P = 0.55) or tumor size (P = 0.63). A trend for a lower 1-hour pool of OT levels was observed in the patients with CP (n = 9) compared with other pituitary diseases (n = 31; 1.07 ± 0.04 and 1.23 ± 0.07 pg/mL, respectively; P = 0.09). The 1-hour pool of OT levels did not differ significantly according to the treatment received for the pituitary disease (surgery, P = 0.85; type of surgery, P = 0.74; radiation therapy, P = 0.11). One patient with medulloblastoma (in the APD group) and another with Langerhans cell histiocytosis (in the CDI group) had previously received chemotherapy. The OT levels in these patients were close to the mean for the APD (medulloblastoma) or CDI groups (Langerhans cell histiocytosis). Six patients with CDI had persistent visual field impairment at the main study visit. The mean 1-hour pool of OT levels was lower in those with CDI with visual field impairment than in those without (0.88 ± 0.2 vs 1.2 ± 0.1 pg/mL, respectively; P = 0.02).
The median 1-hour pool of OT levels in the CDI group was 1.15 pg/mL. Pituitary disease, type of treatment received (surgery or radiotherapy, or both), number of pituitary deficiencies, and hormone replacement therapy, including desmopressin dosing, did not differ in the lower (≤1.15 pg/mL) vs higher (>1.15 pg/mL) 1-hour pool of OT levels. The OT levels were not associated with the psychosocial measures in CDI (P ≥ 0.35). No differences were found in the psychosocial measures in the lower vs higher 1-hour pool of OT groups, other than a trend toward higher anxiety scores in those with lower OT levels (35.7 ± 3.7 vs 29.5 ± 1.7, respectively; P = 0.1). We also found a trend toward a higher BMI in subjects with lower compared with higher 1-hour pool of OT levels (31.6 ± 1.9 vs 27.9 ± 0.9, respectively; P = 0.08). No significant differences in the prevalence of cardiovascular comorbidities were observed between the low 1-hour pool of OT and the high 1-hour pool of OT levels.
Discussion
To the best of our knowledge, the present study is the first to demonstrate low plasma OT levels in patients with hypopituitarism and CDI compared with well-matched patients with anterior pituitary deficiencies only and healthy individuals. We have also shown that patients with CDI, but not those with APD without CDI, had higher symptoms of depression, anxiety, and alexithymia and reported worse mental health, as assessed by the SF-36, compared with the HC. Although the psychosocial symptoms did not significantly correlate with the peripheral OT levels, these findings raise the question of whether an OT deficiency in hypopituitary patients with CDI contributes to increased psychopathology and further studies to determine causality are warranted.
One strength of our study was that we used an integrated measure of a 1-hour pool of plasma sampled every 5 minutes to capture both basal and pulsatile secretion. We have demonstrated that the basal morning OT levels were low in the CDI group compared with the APD and HC groups despite the similar age and BMI (two key factors known to affect OT levels) and, in the groups with pituitary disease, a similar number of anterior pituitary deficiencies and similar hormone replacement therapies. This is particularly striking when considering that the individuals in the APD group were more likely than were the CDI participants to have had radiotherapy (suggesting more aggressive disease) and features of metabolic syndrome (e.g., hypertension, dyslipidemia), which have previously been shown to be associated with lower OT concentrations (40, 41). Interestingly, within the CDI group, we observed a lower 1-hour pool of OT levels in those with visual field impairment, a marker of more aggressive hypothalamic pituitary disease, compared with those without visual field abnormalities.
Despite the anatomical rationale for suspecting OT deficiency in patients who are deficient in AVP, research studies assessing OT levels in CDI are lacking. Very few studies, which were limited by between-group differences in age (16, 42) and BMI (15–17), have examined OT in hypopituitarism to date. These investigations were mostly focused on patients with CP without specifically examining those with CDI. In addition, most studies measured OT in the saliva, the clinical significance of which is not clear, rather than in the circulation where OT is directly delivered via the posterior pituitary gland. Although a study of six patients with CDI showed low fasting plasma OT levels (42), a second investigation of patients with CDI (17) and two studies of patients with CP (15, 16) did not find differences in basal salivary OT levels compared with HC. In subgroup analyses, the salivary OT levels were lower in a combined group of patients with hypopituitarism with or without CDI (17) and in patients with specific types of hypothalamic damage (15, 16). We did not find a difference in a single fasting sample of plasma OT in the CDI group compared with the HC or APD groups, despite well-matched groups, suggesting, at least in some cases, the existence of residual OT synthesis and a lack of complete OT deficiency. Consistent with these data, one study of rats showed that OT neurons were more resistant to pituitary stalk damage than VP neurons after pituitary stalk compression (43). More sophisticated approaches to identify OT deficiency (e.g., integrated measures over time to capture pulsatile secretion as we performed in our study or provocative testing to unmask an insufficient response to a stimulus in the setting of some degree of residual posterior pituitary function) might be required. One previous study found that salivary OT release was blunted in response to exercise in patients with CP compared with HC; however, no differences in OT levels according to AVP status were reported (15). A study of patients with CDI (5 females and 1 male) showed a lack of an expected increase in plasma OT levels after insulin-induced hypoglycemia; however, the healthy individuals (6 males, 4 females) in their study also failed to show an increase in OT levels (42). Future research will be important to identify and validate provocative tests for OT deficiency, similar to those used in clinical practice to assess an adrenal or GH deficiency (1).
A greater prevalence of major depression and dysthymia and more self-reported social problems and overall lower quality of life have previously been documented in patients with hypopituitarism compared with HC (25, 26, 29, 44, 45). Limited data are available describing psychosocial functioning specifically in patients with hypopituitarism and CDI. Recently, patients with CDI were found to have lower empathy compared with HC (17). Furthermore, although patients with CDI and APD both had worse performance than HC in interpreting facial expressions (Reading the Mind in the Eyes Task) and the salivary OT levels did not differ between groups, the salivary OT levels were associated with performance (on a subset of easy items) in those with CDI but not those with APD (17). Evidence has shown that AVP and OT might have opposing effects on psychosocial functioning. Higher levels of AVP, in both animal models and humans, have been associated with more pronounced depressive and anxiety symptoms, social avoidance, and aggressiveness (46–48). AVP receptor antagonists, which were shown to have antidepressant and anxiolytic actions in rodents (49, 50) and improved social communication in a small study of patients with autism (51), are under investigation as a potential therapeutic option for neuropsychiatric disorders (52). From these data, one would expect a possible reduction in depressive and anxiety symptoms and improved socioemotional functioning in patients with AVP deficiency. We have demonstrated that patients with CDI have increased psychopathology, including symptoms of depression, anxiety, and alexithymia compared with HC, while the APD subjects did not significantly differ from the HC in these measures. Although the scores were suggestive of less psychopathology in those with APD than in those with CDI, these between-group differences were not statistically significant. Additionally, although physical health perception, as assessed by the SF-36, was worse in both APD and CDI groups compared with the HC group, mental health perception was worse only in the CDI group compared with the HC. Given the known effects of OT in reducing anxiety (53, 54) and depressive (8) symptoms and regulating socioemotional functioning (7, 18, 55), these data raise the possibility that OT deficiency could contribute to increased psychopathology in patients with CDI. Therefore, our results have set the foundation for future prospective studies to assess for causality and potential mechanisms, if any, involved in an OT-deficient state and the development of psychopathology in this population. Importantly, all patients were receiving stable hormone replacement therapy without differences in hormone levels between APD and CDI, and all CDI subjects were clinically and biochemically well-controlled with desmopressin. In addition, the proportion of patients with GH deficiency receiving recombinant human GH replacement did not differ across the groups, an important study attribute, because nonreplaced GH deficiency negatively affects psychopathology and quality of life (45, 56).
Interestingly, patients with CDI receiving a lower dose of desmopressin in our study had less severe symptoms of depression and anxiety compared with those patients with CDI receiving a higher dose of desmopressin. It has been reported that desmopressin, a V2R-selective agonist, does not cross the blood–brain barrier (57, 58) and does not have biological activity at the OT receptor in humans (59). We also showed that the plasma OT levels did not differ in CDI according to dose or timing (immediately before the morning study visit vs the day before) of desmopressin administration. Therefore, it is unlikely that the difference in psychopathology can be attributed to desmopressin. Thus, patients who require higher doses of desmopressin might have greater damage of AVP neurons and, therefore, be more likely to have affected OT neurons, potentially leading to the greater psychopathology in these subjects. Future studies with larger samples are necessary to determine the causality of this finding.
We did not demonstrate any association between the OT parameters and psychosocial measures in the CDI group, other than a trend toward greater anxiety symptoms in patients with lower 1-hour pool of OT levels (less than the median). The lack of association could have resulted from the small sample size, because the primary objective of the present study was to demonstrate differences in OT levels across groups, not to identify correlations. It is also possible that the pulsatile secretory characteristics (i.e., pulse mass, pulse height) or altered OT dynamics (i.e., blunted response to stimuli) rather than average OT levels would be more useful to elucidate the association between OT and psychopathology. In previous studies, serum OT pulse characteristics were more strongly associated with socioemotional functioning than were the mean OT levels in healthy individuals (18), and a lower salivary OT increase after exercise was associated with higher state anxiety in patients with CP (15).
Our study had limitations but also had several strengths. Given the rarity of CDI, our sample size was relatively small, which limited our ability to detect correlations between OT levels and clinical endpoints. Furthermore, causality cannot be inferred from a cross-sectional study alone. A key strength of our study was the comparison of CDI participants with two control groups of similar age and BMI and a similar number of anterior pituitary deficiencies to those in the APD group, minimizing differences in confounding factors for the OT levels. Although plasma levels of OT do not always mirror central OT activity, the OT levels in the peripheral blood represent a logical biomarker for posterior pituitary OT release and have previously been shown to correlate with levels of anxiety, depression, and socioemotional functioning (7, 8, 18, 53, 54). Blood was drawn under standard conditions at the same time of day with the participants in the fasting state and with minimal social interaction to minimize any external OT stimulation. We used an integrated measure of basal OT over 1 hour to capture basal and pulsatile secretion. The method for measurement of OT in human samples is controversial (60), and improved commercially available, standardized methodology will be important for clinical translation in the future. In our study, OT was measured after extraction using a well-validated extracted RIA with negligible cross-reactivity with AVP and desmopressin to avoid any interference in the measurement of OT, a critical consideration because all CDI subjects were taking desmopressin, and with lower detection limits than other methods, an important characteristic when measuring levels in individuals with a suspected deficiency.
In conclusion, we have demonstrated low plasma OT levels and increased psychopathology in hypopituitary men with CDI, suggestive of a possible OT-deficient state. Larger studies of both sexes are required to confirm these findings and further clinically characterize hypopituitary patients with OT deficiency. Development and validation of improved and standardized methods for measuring OT levels in human samples and OT stimulation tests will be valuable steps to enable reliable diagnostic testing of OT deficiency. Studies examining the safety and efficacy of OT replacement will also be important before OT can be clinically prescribed to this population.
Acknowledgments
We thank the Massachusetts General Hospital Neuroendocrine and Pituitary Tumor Clinical Center staff, the Translational and Clinical Research Center staff, and study participants for their assistance in successfully completing the present study.
FinancialSupport: The present study was supported by grants 1UL1TR002541-01, 1UL1TR001102-01, and 8UL1TR000170-05, Harvard Clinical and Translational Science Center, from the National Center for Advancing Translational Science and a Clinical Research Grant from Catalan Society of Endocrinology. A.A. was supported by a grant from Fundación Alfonso Martín Escudero. W.F. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (grant T32DK007028). The funding sources had no role in the design of the study, data analysis, or writing of the manuscript.
Disclosure Summary: E.A.L. has a financial interest in OXT Therapeutics, a company developing an intranasal oxytocin and long-acting analogs of oxytocin to treat obesity and metabolic disease. E.A.L.’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. The remaining authors have nothing to disclose.
Abbreviations:
- APD
anterior pituitary deficiency
- AVP
vasopressin
- BDI
Beck Depression Inventory-IA
- BMI
body mass index
- CDI
central diabetes insipidus
- CP
craniopharyngioma
- CV
coefficient of variation
- HC
healthy control
- IQR
interquartile range
- OT
oxytocin
- SF-36
36-item short-form survey
- STAI-T
State-Trait Anxiety Trait Scale
- TAS-20
Toronto Alexithymia Scale
- TCRC
Translational and Clinical Research Center



