-
PDF
- Split View
-
Views
-
Cite
Cite
Mabel Toribio, Tomas G Neilan, Magid Awadalla, Lauren A Stone, Adam Rokicki, Corinne Rivard, Connor P Mulligan, Diana Cagliero, Lindsay T Fourman, Takara L Stanley, Jennifer E Ho, Virginia A Triant, Tricia H Burdo, Michael D Nelson, Lidia S Szczepaniak, Markella V Zanni, Intramyocardial Triglycerides Among Women With vs Without HIV: Hormonal Correlates and Functional Consequences, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 12, December 2019, Pages 6090–6100, https://doi.org/10.1210/jc.2019-01096
Close - Share Icon Share
Abstract
Women with HIV (WHIV) on anti-retroviral therapy (ART) are living longer but facing heightened vulnerability to heart failure.
We investigated metabolic/hormonal/immune parameters relating to diastolic dysfunction—a precursor to heart failure—among WHIV without known cardiovascular disease (CVD).
Nineteen ART-treated WHIV and 11 non-HIV-infected women without known CVD enrolled and successfully completed relevant study procedures [cardiac magnetic resonance spectroscopy (MRS) and cardiac MRI]. Groups were matched on age and body mass index. Primary outcome measures included intramyocardial triglyceride content (cardiac MRS) and diastolic function (cardiac MRI). Relationships between intramyocardial triglyceride content and clinical parameters were also assessed.
Among WHIV (vs non-HIV-infected women), intramyocardial triglyceride content was threefold higher [1.2 (0.4, 3.1) vs 0.4 (0.1, 0.5)%, P = 0.01], and diastolic function was reduced (left atrial passive ejection fraction: 27.2 ± 9.6 vs 35.9 ± 6.4%, P = 0.007). There was a strong inverse relationship between intramyocardial triglyceride content and diastolic function (ρ = −0.62, P = 0.004). Among the whole group, intramyocardial triglyceride content did not relate to chronologic age but did increase across the reproductive aging spectrum (P = 0.02). HIV status and reproductive aging status remained independent predictors of intramyocardial triglyceride content after adjusting for relevant cardiometabolic parameters (overall model R2 = 0.56, P = 0.003; HIV status P = 0.01, reproductive aging status P = 0.02).
For asymptomatic WHIV, increased intramyocardial triglyceride content is associated with diastolic dysfunction. Moreover, relationships between intramyocardial triglyceride accumulation and women’s reproductive aging are noted.
Anti-retroviral therapy (ART)–treated women with HIV (WHIV) are living longer but facing heightened vulnerability to heart failure (1). WHIV have a fourfold increased risk for heart failure (2) and, importantly, are particularly susceptible to developing heart failure with preserved ejection fraction (2), a heart failure subtype intransigent to medical therapy (3). Once heart failure develops among WHIV, clinical outcomes are poor (2). Indeed, compared with non-HIV-infected women with heart failure, WHIV with heart failure exhibit increased rates of heart failure hospitalization, cardiovascular disease (CVD) mortality, and all-cause mortality (2). Diastolic dysfunction—a potentially reversible condition characterized by left ventricular stiffness/impaired relaxation and elevated cardiac-filling pressures—antecedes clinical heart failure (4). A better understanding of mechanisms underlying the development of diastolic dysfunction among WHIV may help shape strategies to preserve diastolic function and prevent progression to heart failure.
In the current study, we applied advanced, noninvasive cardiovascular imaging techniques [cardiac magnetic resonance spectroscopy (MRS)/cardiac MRI] to probe metabolic/hormonal mechanisms of HIV-associated diastolic dysfunction specifically among women. Cardiac MRS/MRI studies, to date, exploring correlates of HIV-associated diastolic dysfunction have been conducted among predominantly male cohorts (5–8). With an a priori focus on women’s biology, we prospectively recruited ART-treated WHIV and matched non-HIV-infected women without known CVD to test the following hypothesis: ART-treated WHIV, who have a known diathesis to metabolic dysregulation and ectopic fat deposition (9–11), would evidence increased intramyocardial triglyceride content in association with reduced diastolic function. Furthermore, we sought within our all-women cohort to characterize sex-specific hormonal pathways relevant to heightened intramyocardial triglyceride content. Based on general-population studies linking estrogen depletion to ectopic fat deposition (12, 13), we postulated a potential relationship between reproductive aging and intramyocardial triglyceride content. We reasoned that the identification of hormonal factors predictive of intramyocardial triglyceride content in our cohort might highlight therapeutic targets for heart-failure prevention strategies tailored to WHIV.
Methods
Study design and participants
ART-treated WHIV, aged 40 to 75 years without known CVD or diabetes, and non-HIV-infected women group matched on age and body mass index (BMI) were prospectively recruited from the Greater Boston Area between August 2016 and November 2017. Women were excluded from the study for stable/unstable angina, severe renal dysfunction, select systemic infections, cancer, contraindication to MRI/MRS, or use of potentially confounding medications (e.g., immunosuppressants, lipid-lowering medications, or medications that block the renin-angiotensin-aldosterone pathway). Informed consent was obtained from all participants. Findings on myocardial fibrosis and diastolic strain rate (derived from cardiac MRI), among women in our full cohort, have been previously published (14), but findings on intramyocardial triglyceride content and correlates among the subset of women who also underwent cardiac MRS are explored here. Evaluable imaging data for this analysis (including cardiac MRS and MRI data) were available for 30 women—19 WHIV and 11 non-HIV-infected controls. An additional five WHIV who signed consent did not complete the study (one participant was ineligible as a result of diabetes, two participants withdrew consent after screening, one participant was lost to follow-up after screening, and one participant was unable to complete successfully both cardiac MRS and MRI). Eight non-HIV-infected women who signed consent did not complete the study (four participants withdrew consent after screening, and four participants were unable to complete successfully both cardiac MRS and MRI). The study received approval from the Partners Institutional Review Board and was registered on clinicaltrials.gov (NCT02874703).
Study procedures
Clinical research study visits
Participants underwent history attentive to demographics, cardiometabolic risk factors, HIV-specific parameters (time since HIV diagnosis, ART regimen, time since ART initiation, nadir CD4+ T cell count), and reproductive/menstrual health. Physical examination was performed, including anthropometric measurements, such as height, weight, and waist-to-hip ratio. BMI was calculated. Blood samples were drawn for assessment of metabolic, immune, and hormonal parameters described below.
Metabolic, immune, and hormonal phenotyping
Creatinine, HbA1c, and lipid levels were measured using standard techniques. For WHIV, ultrasensitive RT-PCR was used to determine viral load (Cobas Ampliprep/Cobas TaqMan HIV-1 test version 2.0; Roche Diagnostics, Rotkreuz, Switzerland). Non-HIV-infected women underwent confirmatory HIV antibody testing by ELISA (Abbott, Abbott Park, IL). Anti-Müllerian hormone (AMH) levels were quantified by ELISA using a standard 1:2 dilution for women over the age of 40 (Ansh Laboratories, Webster, TX). Ten-year atherosclerotic CVD (ASCVD) risk score was calculated using a standard calculator (15).
Delineation of reproductive aging groups
Data on menstrual history and AMH levels were synthesized to classify women along a continuum of reproductive aging subgroups in a manner comparable with that previously published by our group (16). Women with at least one menstrual cycle within the past year and with detectable AMH levels were classified as premenopausal (Group 1). Women with either 1 year of amenorrhea and detectable AMH levels or with at least one menstrual cycle within the past year and with undetectable AMH levels were classified as having reduced ovarian reserve (Group 2). Women with 1 year of amenorrhea and undetectable AMH levels were classified as postmenopausal (Group 3). Women who reported a history of hysterectomy without oophorectomy (n = 3) were excluded from the classification system. Notably, none of the participants reported use of hormonal therapies, which would be expected to cause amenorrhea or oligomenorrhea, e.g., progesterone-eluting intrauterine devices.
Cardiac MRS
Cardiac proton spectroscopy (1H-MRS) studies were performed on a 3T system (Tim Trio; Siemens, Erlangen, Germany) using an 18-channel, phased-array torso coil, as previously described (17, 18). Cardiac MRS was performed before cardiac MRI. For the MRS, ECG electrodes were used to track physiological motion, and respiratory motion was controlled with a navigator, Prospective Acquisition Correction Sequence. A sampling volume for spectroscopy (voxel) was positioned in the ventricular septum on reference cardiac images, as shown in Fig. 1A. Cardiac spectroscopy data were collected with the following parameters: repetition time = 3:4 seconds, depending on respiratory rate length, and echo time = 35 ms, 1024 data points over a 1000-kHz spectral width. Cardiac triglyceride and water signal intensities were evaluated by the line-fit procedure using the external program NUTS from Acorn NMR (Livermore, CA). The final water and fat signal intensities were adjusted for spin-spin (T2) relaxation (19). Intramyocardial triglyceride content was expressed as a percentage of myocardial water content. A typical cardiac spectrum is presented in Fig. 1B.
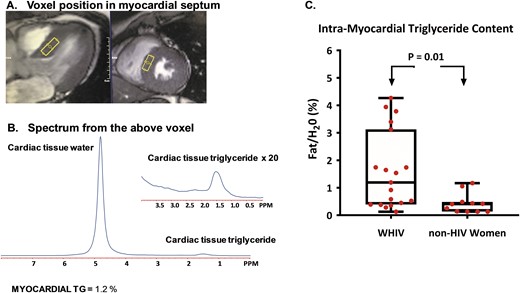
Cardiac MRS and intramyocardial triglyceride content in women with and without HIV. (A) Intramyocardial triglyceride content was measured for each participant by placing voxels (rectangle) over the intraventricular septum, as shown in representative (right) sagittal and (left) axial cardiac cine images. The intraventricular septum was chosen as the site of measurement because it contains myocardial tissue without overlying epicardial adipose tissue, which would affect the assessment of intramyocardial triglyceride content. (B) An example of a cardiac proton spectrum is shown. The spectrum includes a myocardial water signal (the larger peak) and myocardial triglyceride signal (the smaller peak). The intramyocardial triglyceride for this example spectrum shown is 1.2% and was calculated as a percent of the myocardial water signal. (C) Intramyocardial triglyceride content is displayed as median (interquartile range) for each subgroup, with whiskers representing minimum and maximum values, respectively. Intramyocardial triglyceride content was significantly higher among WHIV compared with non-HIV-infected women (P = 0.01). PPM, parts per million; TG, triglyceride
Cardiac MRI
Cardiac MRI was performed on a second 3.0-T MRI system (Skyra; Siemens Healthcare, Erlangen, Germany). Cardiac MRI images were acquired with ECG gating and breath holding, with the participant in a supine position, as previously described (20, 21). The basic MRI protocol consisted of cine steady-state free precession imaging for quantification of left ventricular mass and function, consistent with previous practice (20, 21). After imaging, data transfer was achieved through application of a cloud-based network (Teamplay; Siemens Healthcare). A commercial software package (Medis® Suite MR; Medis Medical Imaging Systems, Leiden, Netherlands) was used to perform offline analysis of left atrial passive ejection fraction, using methods previously described and validated (22, 23). In brief, left atrial volumes were measured at (i) the beginning of left ventricular diastole (defined as the frame immediately before the opening of the mitral leaflets [volumes at maximum left atrial size (VOLmax)]), (ii) the end of passive left ventricular filling [defined as the frame immediately before left atrial contraction (VOLbac)], and (iii) the end of left atrial contraction (VOLmin). To calculate left atrial volumes and derive left atrial passive ejection fraction, we measured atrial length (from the midpoint of the mitral annulus plane) and border (excluding atrial appendage and pulmonary veins) in the two- and four-chamber views. We then applied the biplane area–length method: left atrial volume = (four-chamber area) × (two-chamber area) × 0.85/atrial length. The left atrial passive ejection fraction was calculated as (VOLmax − VOLbac)/VOLmax × 100, consistent with previous methodology (22, 23), as shown in Fig. 2A.
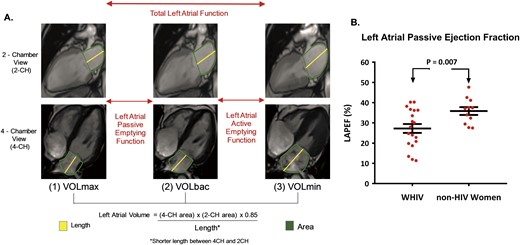
Cardiac MRI and diastolic function in women with and without HIV. (A) Diastolic function was assessed through measurement of the left atrial passive ejection fraction (LAPEF) on cardiac MRI. Left atrial passive ejection fraction was determined as the difference between VOLmax and VOLbac. (B) Diastolic function (quantified by left atrial passive ejection fraction, %) is displayed as mean ± SEM for each group. Diastolic function was significantly reduced among WHIV compared with non-HIV-infected women (P = 0.007). CH, chamber.
Statistical analysis
Participant characteristics, as well as primary outcomes of interest (intramyocardial triglyceride content and diastolic function) were compared among WHIV vs non-HIV-infected women. The relationship between intramyocardial triglyceride content and diastolic function was assessed in the whole group and in each subgroup. Additional factors relating to intramyocardial triglyceride content were assessed in the whole group and in each subgroup. Normality of the data was determined using a Shapiro-Wilk test. Normally distributed variables are reported as means ± SD (SD), and non-normally distributed data are reported as median [interquartile range (IQR)]. For a between-group comparison of individual parameters, Student t test, Wilcoxon rank sum test, or χ2 test was used as appropriate. Intramyocardial triglyceride content was compared across reproductive aging groups using a Kruskal-Wallis test. Bivariate analyses were performed using a Spearman correlation coefficient if at least one variable was non-normally distributed. Multivariable regression modeling was performed to determine whether intramyocardial triglyceride content remained related to diastolic function among WHIV controlling for relevant variables. Additionally, multivariable regression modeling was performed among WHIV to assess whether detectable viral load related to intramyocardial triglyceride content controlling for other relevant parameters. Finally, multivariable regression modeling was performed in the whole group to assess the relationship between HIV status and intramyocardial triglyceride content controlling for other relevant factors, including reproductive aging status. JMP Pro software (version 11.0; SAS Institute, Cary, NC) was used for statistical analyses, with P < 0.05 statistically significant.
Results
Participant characteristics
By design, groups of WHIV vs non-HIV-infected women were similar in age (52 ± 4 vs 53 ± 6 years, P = 0.84) and BMI (32 ± 7 vs 32 ± 8 kg/m2, P = 0.83; Table 1). There were no significant between-group differences in race or ethnicity. Also comparable between groups were levels of total cholesterol (P = 0.91) and low-density lipoprotein–cholesterol (LDL-C; P = 0.76). With respect to reproductive health parameters, there were no significant differences between groups in median years since last menstrual period or in levels of AMH. No woman in either group reported taking estrogen-containing hormone-replacement therapy. Between-group distributions across reproductive aging categories did not differ significantly (P = 0.67). Among WHIV, the median CD4+ count was 872 (574, 1220) cells/mm3, and the median viral load was undetectable (Table 1).
| . | WHIV (n = 19) . | Non-HIV-Infected Women (n = 11) . | P Value . |
|---|---|---|---|
| Demographic and traditional CVD-risk parameters | |||
| Age, y | 52 ± 4 | 53 ± 6 | 0.84 |
| Race, % | 0.62 | ||
| White | 42 (8/19) | 64 (7/11) | |
| Black/African American | 37 (7/19) | 27 (3/11) | |
| Othera | 21 (4/19) | 9 (1/11) | |
| Ethnicity, Hispanic, % | 11 (2/19) | 18 (2/11) | 0.56 |
| Current hypertension, % | 26 (5/19) | 36 (4/11) | 0.57 |
| Current anti-hypertensive, % | 16 (3/19) | 9 (1/11) | 0.59 |
| Current smoking, % | 53 (10/19) | 27 (3/11) | 0.17 |
| Current cocaine use, % | 0 (0/19) | 0 (0/11) | — |
| Total cholesterol, mg/dL | 200 ± 42 | 201 ± 26 | 0.91 |
| LDL-C, mg/dL | 114 ± 34 | 110 ± 27 | 0.76 |
| HDL-C, mg/dL | 59 ± 14 | 65 ± 23 | 0.47 |
| Triglycerides, mg/dL | 107 (91, 167) | 92 (53, 145) | 0.21 |
| 10-Year ASCVD risk score, % | 3.6 (1.3, 4.9), 3.8 ± 3.2 | 1.6 (1.3, 3.7), 3.3 ± 3.5 | 0.49 |
| HbA1c, % | 5.6 ± 0.4 | 5.5 ± 0.2 | 0.54 |
| BMI, kg/m2 | 32 ± 7 | 32 ± 8 | 0.83 |
| WHR | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.17 |
| Current HCV, % | 11 (2/19) | 0 (0/11) | 0.17 |
| HIV-specific parameters | |||
| Time since HIV diagnosis, y | 19 ± 8 | — | — |
| Total duration ART, y | 17 (8, 22) | — | — |
| NRTI use, % | 84 (16/19) | — | — |
| NNRTI use, % | 32 (6/19) | — | — |
| PI use, % | 42 (8/19) | — | — |
| INSTI use, % | 63 (12/19) | — | — |
| CCR5 antagonist use, % | 5 (1/19) | — | — |
| CD4+ T cell count, cells/mm3 | 872 (574, 1220) | — | — |
| CD4+/CD8+ T cell count | 1.1 ± 0.5 | ||
| Nadir CD4+ T cell count, cells/mm3 | 170 ± 155 | — | — |
| HIV viral load, copies/mLb | 19 (19, 19) | — | — |
| Hormonal status | |||
| Time since LMP, y | 6 (2, 10) | 5 (0, 11) | 0.52 |
| AMH levels, pg/mLc | 7 (7, 7) | 7 (7, 7) | 1.0 |
| Current hormone therapy, % | 0 (0/19) | 0 (0/11) | — |
| . | WHIV (n = 19) . | Non-HIV-Infected Women (n = 11) . | P Value . |
|---|---|---|---|
| Demographic and traditional CVD-risk parameters | |||
| Age, y | 52 ± 4 | 53 ± 6 | 0.84 |
| Race, % | 0.62 | ||
| White | 42 (8/19) | 64 (7/11) | |
| Black/African American | 37 (7/19) | 27 (3/11) | |
| Othera | 21 (4/19) | 9 (1/11) | |
| Ethnicity, Hispanic, % | 11 (2/19) | 18 (2/11) | 0.56 |
| Current hypertension, % | 26 (5/19) | 36 (4/11) | 0.57 |
| Current anti-hypertensive, % | 16 (3/19) | 9 (1/11) | 0.59 |
| Current smoking, % | 53 (10/19) | 27 (3/11) | 0.17 |
| Current cocaine use, % | 0 (0/19) | 0 (0/11) | — |
| Total cholesterol, mg/dL | 200 ± 42 | 201 ± 26 | 0.91 |
| LDL-C, mg/dL | 114 ± 34 | 110 ± 27 | 0.76 |
| HDL-C, mg/dL | 59 ± 14 | 65 ± 23 | 0.47 |
| Triglycerides, mg/dL | 107 (91, 167) | 92 (53, 145) | 0.21 |
| 10-Year ASCVD risk score, % | 3.6 (1.3, 4.9), 3.8 ± 3.2 | 1.6 (1.3, 3.7), 3.3 ± 3.5 | 0.49 |
| HbA1c, % | 5.6 ± 0.4 | 5.5 ± 0.2 | 0.54 |
| BMI, kg/m2 | 32 ± 7 | 32 ± 8 | 0.83 |
| WHR | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.17 |
| Current HCV, % | 11 (2/19) | 0 (0/11) | 0.17 |
| HIV-specific parameters | |||
| Time since HIV diagnosis, y | 19 ± 8 | — | — |
| Total duration ART, y | 17 (8, 22) | — | — |
| NRTI use, % | 84 (16/19) | — | — |
| NNRTI use, % | 32 (6/19) | — | — |
| PI use, % | 42 (8/19) | — | — |
| INSTI use, % | 63 (12/19) | — | — |
| CCR5 antagonist use, % | 5 (1/19) | — | — |
| CD4+ T cell count, cells/mm3 | 872 (574, 1220) | — | — |
| CD4+/CD8+ T cell count | 1.1 ± 0.5 | ||
| Nadir CD4+ T cell count, cells/mm3 | 170 ± 155 | — | — |
| HIV viral load, copies/mLb | 19 (19, 19) | — | — |
| Hormonal status | |||
| Time since LMP, y | 6 (2, 10) | 5 (0, 11) | 0.52 |
| AMH levels, pg/mLc | 7 (7, 7) | 7 (7, 7) | 1.0 |
| Current hormone therapy, % | 0 (0/19) | 0 (0/11) | — |
There were no significant differences in baseline characteristics among WHIV and non-HIV-infected women. Normally distributed variables are presented as means ±SD; non-normally distributed data are presented as median (IQR). P values were determined by Student two-tailed t test, Wilcoxon rank sum test, and χ2 test for normally distributed, non-normally distributed, and categorical variables, respectively.
Abbreviations: CCR5, C-C chemokine receptor type 5; HCV, hepatitis C virus; HDL-C, high-density lipoprotein–cholesterol; INSTI, integrase inhibitor; LDL-C, low-density lipoprotein–cholesterol; LMP, last menstrual period; NNRTI, non-nucleoside reverse transcription inhibitor; NRTI, nucleoside reverse transcription inhibitor; PI, protease inhibitor; WHR, waist-to-hip ratio.
Category includes “American Indian/Alaskan native,” “more than one race,” and “other.”
The lower limit of detection for viral load using applied assay was 20 copies/mL; a value of 19 copies per milliliter was imputed for those women with viral load levels below the limit of detection.
The lower limit of detection for AMH using applied assay was 8 pg/mL; a value of 7 pg/mL was imputed for those women with an AMH level below the level of detection.
| . | WHIV (n = 19) . | Non-HIV-Infected Women (n = 11) . | P Value . |
|---|---|---|---|
| Demographic and traditional CVD-risk parameters | |||
| Age, y | 52 ± 4 | 53 ± 6 | 0.84 |
| Race, % | 0.62 | ||
| White | 42 (8/19) | 64 (7/11) | |
| Black/African American | 37 (7/19) | 27 (3/11) | |
| Othera | 21 (4/19) | 9 (1/11) | |
| Ethnicity, Hispanic, % | 11 (2/19) | 18 (2/11) | 0.56 |
| Current hypertension, % | 26 (5/19) | 36 (4/11) | 0.57 |
| Current anti-hypertensive, % | 16 (3/19) | 9 (1/11) | 0.59 |
| Current smoking, % | 53 (10/19) | 27 (3/11) | 0.17 |
| Current cocaine use, % | 0 (0/19) | 0 (0/11) | — |
| Total cholesterol, mg/dL | 200 ± 42 | 201 ± 26 | 0.91 |
| LDL-C, mg/dL | 114 ± 34 | 110 ± 27 | 0.76 |
| HDL-C, mg/dL | 59 ± 14 | 65 ± 23 | 0.47 |
| Triglycerides, mg/dL | 107 (91, 167) | 92 (53, 145) | 0.21 |
| 10-Year ASCVD risk score, % | 3.6 (1.3, 4.9), 3.8 ± 3.2 | 1.6 (1.3, 3.7), 3.3 ± 3.5 | 0.49 |
| HbA1c, % | 5.6 ± 0.4 | 5.5 ± 0.2 | 0.54 |
| BMI, kg/m2 | 32 ± 7 | 32 ± 8 | 0.83 |
| WHR | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.17 |
| Current HCV, % | 11 (2/19) | 0 (0/11) | 0.17 |
| HIV-specific parameters | |||
| Time since HIV diagnosis, y | 19 ± 8 | — | — |
| Total duration ART, y | 17 (8, 22) | — | — |
| NRTI use, % | 84 (16/19) | — | — |
| NNRTI use, % | 32 (6/19) | — | — |
| PI use, % | 42 (8/19) | — | — |
| INSTI use, % | 63 (12/19) | — | — |
| CCR5 antagonist use, % | 5 (1/19) | — | — |
| CD4+ T cell count, cells/mm3 | 872 (574, 1220) | — | — |
| CD4+/CD8+ T cell count | 1.1 ± 0.5 | ||
| Nadir CD4+ T cell count, cells/mm3 | 170 ± 155 | — | — |
| HIV viral load, copies/mLb | 19 (19, 19) | — | — |
| Hormonal status | |||
| Time since LMP, y | 6 (2, 10) | 5 (0, 11) | 0.52 |
| AMH levels, pg/mLc | 7 (7, 7) | 7 (7, 7) | 1.0 |
| Current hormone therapy, % | 0 (0/19) | 0 (0/11) | — |
| . | WHIV (n = 19) . | Non-HIV-Infected Women (n = 11) . | P Value . |
|---|---|---|---|
| Demographic and traditional CVD-risk parameters | |||
| Age, y | 52 ± 4 | 53 ± 6 | 0.84 |
| Race, % | 0.62 | ||
| White | 42 (8/19) | 64 (7/11) | |
| Black/African American | 37 (7/19) | 27 (3/11) | |
| Othera | 21 (4/19) | 9 (1/11) | |
| Ethnicity, Hispanic, % | 11 (2/19) | 18 (2/11) | 0.56 |
| Current hypertension, % | 26 (5/19) | 36 (4/11) | 0.57 |
| Current anti-hypertensive, % | 16 (3/19) | 9 (1/11) | 0.59 |
| Current smoking, % | 53 (10/19) | 27 (3/11) | 0.17 |
| Current cocaine use, % | 0 (0/19) | 0 (0/11) | — |
| Total cholesterol, mg/dL | 200 ± 42 | 201 ± 26 | 0.91 |
| LDL-C, mg/dL | 114 ± 34 | 110 ± 27 | 0.76 |
| HDL-C, mg/dL | 59 ± 14 | 65 ± 23 | 0.47 |
| Triglycerides, mg/dL | 107 (91, 167) | 92 (53, 145) | 0.21 |
| 10-Year ASCVD risk score, % | 3.6 (1.3, 4.9), 3.8 ± 3.2 | 1.6 (1.3, 3.7), 3.3 ± 3.5 | 0.49 |
| HbA1c, % | 5.6 ± 0.4 | 5.5 ± 0.2 | 0.54 |
| BMI, kg/m2 | 32 ± 7 | 32 ± 8 | 0.83 |
| WHR | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.17 |
| Current HCV, % | 11 (2/19) | 0 (0/11) | 0.17 |
| HIV-specific parameters | |||
| Time since HIV diagnosis, y | 19 ± 8 | — | — |
| Total duration ART, y | 17 (8, 22) | — | — |
| NRTI use, % | 84 (16/19) | — | — |
| NNRTI use, % | 32 (6/19) | — | — |
| PI use, % | 42 (8/19) | — | — |
| INSTI use, % | 63 (12/19) | — | — |
| CCR5 antagonist use, % | 5 (1/19) | — | — |
| CD4+ T cell count, cells/mm3 | 872 (574, 1220) | — | — |
| CD4+/CD8+ T cell count | 1.1 ± 0.5 | ||
| Nadir CD4+ T cell count, cells/mm3 | 170 ± 155 | — | — |
| HIV viral load, copies/mLb | 19 (19, 19) | — | — |
| Hormonal status | |||
| Time since LMP, y | 6 (2, 10) | 5 (0, 11) | 0.52 |
| AMH levels, pg/mLc | 7 (7, 7) | 7 (7, 7) | 1.0 |
| Current hormone therapy, % | 0 (0/19) | 0 (0/11) | — |
There were no significant differences in baseline characteristics among WHIV and non-HIV-infected women. Normally distributed variables are presented as means ±SD; non-normally distributed data are presented as median (IQR). P values were determined by Student two-tailed t test, Wilcoxon rank sum test, and χ2 test for normally distributed, non-normally distributed, and categorical variables, respectively.
Abbreviations: CCR5, C-C chemokine receptor type 5; HCV, hepatitis C virus; HDL-C, high-density lipoprotein–cholesterol; INSTI, integrase inhibitor; LDL-C, low-density lipoprotein–cholesterol; LMP, last menstrual period; NNRTI, non-nucleoside reverse transcription inhibitor; NRTI, nucleoside reverse transcription inhibitor; PI, protease inhibitor; WHR, waist-to-hip ratio.
Category includes “American Indian/Alaskan native,” “more than one race,” and “other.”
The lower limit of detection for viral load using applied assay was 20 copies/mL; a value of 19 copies per milliliter was imputed for those women with viral load levels below the limit of detection.
The lower limit of detection for AMH using applied assay was 8 pg/mL; a value of 7 pg/mL was imputed for those women with an AMH level below the level of detection.
Intramyocardial triglyceride content and diastolic function
Intramyocardial triglyceride content was threefold higher among WHIV vs non-HIV-infected women [1.2 (0.4, 3.1) vs 0.4 (0.1, 0.5)%, P = 0.01; Fig. 1C]. In addition, diastolic function (as quantified by left atrial passive ejection fraction) was reduced among the WHIV compared with the non-HIV-infected women (27.2 ± 9.6 vs 35.9 ± 6.4%, P = 0.007; Fig. 2B). Other conventional MRI measures, including left ventricular end-diastolic volume, left ventricular mass index, left ventricular ejection fraction, and left atrial maximum volume, did not differ significantly between groups (Table 2).
| . | WHIV (n = 19) . | Non-HIV-Infected Women (n = 11) . | P Value . |
|---|---|---|---|
| LVEDV, mL | 131.9 ± 22.6 | 140.3 ± 25.0 | 0.37 |
| LVMI, g/m2 | 47.3 (43.8, 56.0) | 44.1 (39.9, 47.9) | 0.08 |
| LVEF, % | 57.9 ± 4.2 | 61.1 ± 4.8 | 0.08 |
| LA max vol, mL/m2 | 67.7 ± 13.8 | 71.8 ± 19.2 | 0.54 |
| LAAEF, % | 39.0 ± 7.0 | 37.5 ± 5.5 | 0.52 |
| LAPEF, % | 27.2 ± 9.6 | 35.9 ± 6.4 | 0.007 |
| . | WHIV (n = 19) . | Non-HIV-Infected Women (n = 11) . | P Value . |
|---|---|---|---|
| LVEDV, mL | 131.9 ± 22.6 | 140.3 ± 25.0 | 0.37 |
| LVMI, g/m2 | 47.3 (43.8, 56.0) | 44.1 (39.9, 47.9) | 0.08 |
| LVEF, % | 57.9 ± 4.2 | 61.1 ± 4.8 | 0.08 |
| LA max vol, mL/m2 | 67.7 ± 13.8 | 71.8 ± 19.2 | 0.54 |
| LAAEF, % | 39.0 ± 7.0 | 37.5 ± 5.5 | 0.52 |
| LAPEF, % | 27.2 ± 9.6 | 35.9 ± 6.4 | 0.007 |
Normally distributed variables are presented as means ± SD; non-normally distributed data are presented as median (IQR). P values were determined by Student two-tailed t test for normally distributed variables and Wilcoxon rank sum test for non-normally distributed variables. P value <0.05 is shown in boldface.
Abbreviations: LAAEF, left atrial active ejection fraction; LA max vol, left atrial maximum volume; LAPEF, left atrial passive ejection fraction; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
| . | WHIV (n = 19) . | Non-HIV-Infected Women (n = 11) . | P Value . |
|---|---|---|---|
| LVEDV, mL | 131.9 ± 22.6 | 140.3 ± 25.0 | 0.37 |
| LVMI, g/m2 | 47.3 (43.8, 56.0) | 44.1 (39.9, 47.9) | 0.08 |
| LVEF, % | 57.9 ± 4.2 | 61.1 ± 4.8 | 0.08 |
| LA max vol, mL/m2 | 67.7 ± 13.8 | 71.8 ± 19.2 | 0.54 |
| LAAEF, % | 39.0 ± 7.0 | 37.5 ± 5.5 | 0.52 |
| LAPEF, % | 27.2 ± 9.6 | 35.9 ± 6.4 | 0.007 |
| . | WHIV (n = 19) . | Non-HIV-Infected Women (n = 11) . | P Value . |
|---|---|---|---|
| LVEDV, mL | 131.9 ± 22.6 | 140.3 ± 25.0 | 0.37 |
| LVMI, g/m2 | 47.3 (43.8, 56.0) | 44.1 (39.9, 47.9) | 0.08 |
| LVEF, % | 57.9 ± 4.2 | 61.1 ± 4.8 | 0.08 |
| LA max vol, mL/m2 | 67.7 ± 13.8 | 71.8 ± 19.2 | 0.54 |
| LAAEF, % | 39.0 ± 7.0 | 37.5 ± 5.5 | 0.52 |
| LAPEF, % | 27.2 ± 9.6 | 35.9 ± 6.4 | 0.007 |
Normally distributed variables are presented as means ± SD; non-normally distributed data are presented as median (IQR). P values were determined by Student two-tailed t test for normally distributed variables and Wilcoxon rank sum test for non-normally distributed variables. P value <0.05 is shown in boldface.
Abbreviations: LAAEF, left atrial active ejection fraction; LA max vol, left atrial maximum volume; LAPEF, left atrial passive ejection fraction; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
Among all participants, there was an inverse relationship between intramyocardial triglyceride content and diastolic function (ρ = −0.57, P = 0.001). This inverse relationship was also significant within the subgroup of WHIV (ρ = −0.62, P = 0.004). Among the WHIV, intramyocardial triglyceride content independently predicted diastolic function even after controlling for traditional cardiometabolic risk factors encompassed in the 10-year ASCVD risk score (which includes age, sex, race, blood pressure, anti-hypertensive medication therapy, lipid levels, diabetes, and cigarette smoking; overall model R2 = 0.34, P = 0.04; intramyocardial triglyceride content, P = 0.01).
Predictors of intramyocardial triglyceride content
Among the whole group, intramyocardial triglyceride content did not relate to chronologic age (ρ = 0.27, P = 0.14) but did increase across the reproductive aging spectrum: 0.1 (0.1, 1.3)% among premenopausal women, 0.4 (0.3, 1.2)% among women with reduced ovarian reserve, and 1.1 (0.5, 3.3)% among postmenopausal women (P = 0.02 for Kruskal-Wallis test comparing groups; Table 3). Among WHIV, on bivariate analysis, intramyocardial triglyceride content related to HbA1c (ρ = 0.51, P = 0.03) but did not relate to age, 10-year ASCVD risk score, BMI, or HIV-specific parameters (Table 3). However, multivariable modeling among WHIV revealed that detectable (vs undetectable) viral load and the CD4+/CD8+ T cell ratio remained significantly associated with intramyocardial triglyceride content after adjusting for HbA1c and race (overall model R2 = 0.78, P = 0.02; detectable viral load, P = 0.01; CD4+/CD8+ T cell ratio, P = 0.04). Among non-HIV-infected women, intramyocardial triglyceride content related to select lipid parameters (e.g., triglyceride levels, ρ = 0.68, P = 0.02; LDL-C level, ρ = 0.67, P = 0.03) but did not relate to age, 10-year ASCVD risk score, or BMI (Table 3). Among the whole group, in multivariable modeling, HIV status and reproductive aging status remained independent predictors of intramyocardial triglyceride content even after adjusting for HbA1c and non–high-density lipoprotein–cholesterol (HDL-C; overall model R2 = 0.56, P = 0.003; HIV status, P = 0.01; reproductive aging status, P = 0.02; Table 4).
| Demographic and Traditional CVD Risk Parameters . | WHIV . | Non-HIV-Infected Women . | Whole Group . | |||
|---|---|---|---|---|---|---|
| Spearman ρ . | P Value . | Spearman ρ . | P Value . | Spearman ρ . | P Value . | |
| Age, y | 0.26 | 0.29 | 0.25 | 0.45 | 0.27 | 0.14 |
| Total cholesterol, mg/dL | 0.10 | 0.69 | 0.58 | 0.06 | 0.18 | 0.34 |
| LDL-C, mg/dL | 0.005 | 0.98 | 0.67 | 0.03 | 0.16 | 0.40 |
| HDL-C, mg/dL | 0.40 | 0.09 | −0.45 | 0.16 | 0.08 | 0.69 |
| Triglycerides, mg/dL | 0.02 | 0.92 | 0.68 | 0.02 | 0.35 | 0.06 |
| 10-Year ASCVD risk score, % | 0.23 | 0.35 | −0.18 | 0.59 | 0.20 | 0.29 |
| HbA1c, % | 0.51 | 0.03 | −0.04 | 0.91 | 0.33 | 0.08 |
| BMI, kg/m2 | −0.01 | 0.97 | 0.24 | 0.51 | 0.04 | 0.82 |
| WHR | 0.07 | 0.79 | −0.05 | 0.89 | 0.11 | 0.56 |
| Time since HIV diagnosis, years | −0.26 | 0.27 | — | — | — | — |
| Total duration ART, years | −0.04 | 0.88 | — | — | — | — |
| CD4+ T cell count, cells/mm3 | 0.12 | 0.62 | — | — | — | — |
| HIV viral load, copies/mL | 0.28 | 0.26 | — | — | — | — |
| Intramyocardial lipid content and reproductive aging status (whole group) | ||||||
| Reproductive aging groups | Intramyocardial lipid content, % | P value | ||||
| Group 1 | 0.1 (0.1, 1.3) | 0.02 | ||||
| Group 2 | 0.4 (0.3, 1.2) | |||||
| Group 3 | 1.1 (0.5, 3.3) | |||||
| Demographic and Traditional CVD Risk Parameters . | WHIV . | Non-HIV-Infected Women . | Whole Group . | |||
|---|---|---|---|---|---|---|
| Spearman ρ . | P Value . | Spearman ρ . | P Value . | Spearman ρ . | P Value . | |
| Age, y | 0.26 | 0.29 | 0.25 | 0.45 | 0.27 | 0.14 |
| Total cholesterol, mg/dL | 0.10 | 0.69 | 0.58 | 0.06 | 0.18 | 0.34 |
| LDL-C, mg/dL | 0.005 | 0.98 | 0.67 | 0.03 | 0.16 | 0.40 |
| HDL-C, mg/dL | 0.40 | 0.09 | −0.45 | 0.16 | 0.08 | 0.69 |
| Triglycerides, mg/dL | 0.02 | 0.92 | 0.68 | 0.02 | 0.35 | 0.06 |
| 10-Year ASCVD risk score, % | 0.23 | 0.35 | −0.18 | 0.59 | 0.20 | 0.29 |
| HbA1c, % | 0.51 | 0.03 | −0.04 | 0.91 | 0.33 | 0.08 |
| BMI, kg/m2 | −0.01 | 0.97 | 0.24 | 0.51 | 0.04 | 0.82 |
| WHR | 0.07 | 0.79 | −0.05 | 0.89 | 0.11 | 0.56 |
| Time since HIV diagnosis, years | −0.26 | 0.27 | — | — | — | — |
| Total duration ART, years | −0.04 | 0.88 | — | — | — | — |
| CD4+ T cell count, cells/mm3 | 0.12 | 0.62 | — | — | — | — |
| HIV viral load, copies/mL | 0.28 | 0.26 | — | — | — | — |
| Intramyocardial lipid content and reproductive aging status (whole group) | ||||||
| Reproductive aging groups | Intramyocardial lipid content, % | P value | ||||
| Group 1 | 0.1 (0.1, 1.3) | 0.02 | ||||
| Group 2 | 0.4 (0.3, 1.2) | |||||
| Group 3 | 1.1 (0.5, 3.3) | |||||
Spearman ρ is shown for association between intramyocardial triglyceride content (non-normally distributed) and each baseline characteristic within all participants, WHIV, and non-HIV-infected women, respectively.
As shown above, intramyocardial triglyceride content (shown as median IQR) differed significantly across three reproductive aging groups (P = 0.02). P values <0.05 are shown in boldface.
| Demographic and Traditional CVD Risk Parameters . | WHIV . | Non-HIV-Infected Women . | Whole Group . | |||
|---|---|---|---|---|---|---|
| Spearman ρ . | P Value . | Spearman ρ . | P Value . | Spearman ρ . | P Value . | |
| Age, y | 0.26 | 0.29 | 0.25 | 0.45 | 0.27 | 0.14 |
| Total cholesterol, mg/dL | 0.10 | 0.69 | 0.58 | 0.06 | 0.18 | 0.34 |
| LDL-C, mg/dL | 0.005 | 0.98 | 0.67 | 0.03 | 0.16 | 0.40 |
| HDL-C, mg/dL | 0.40 | 0.09 | −0.45 | 0.16 | 0.08 | 0.69 |
| Triglycerides, mg/dL | 0.02 | 0.92 | 0.68 | 0.02 | 0.35 | 0.06 |
| 10-Year ASCVD risk score, % | 0.23 | 0.35 | −0.18 | 0.59 | 0.20 | 0.29 |
| HbA1c, % | 0.51 | 0.03 | −0.04 | 0.91 | 0.33 | 0.08 |
| BMI, kg/m2 | −0.01 | 0.97 | 0.24 | 0.51 | 0.04 | 0.82 |
| WHR | 0.07 | 0.79 | −0.05 | 0.89 | 0.11 | 0.56 |
| Time since HIV diagnosis, years | −0.26 | 0.27 | — | — | — | — |
| Total duration ART, years | −0.04 | 0.88 | — | — | — | — |
| CD4+ T cell count, cells/mm3 | 0.12 | 0.62 | — | — | — | — |
| HIV viral load, copies/mL | 0.28 | 0.26 | — | — | — | — |
| Intramyocardial lipid content and reproductive aging status (whole group) | ||||||
| Reproductive aging groups | Intramyocardial lipid content, % | P value | ||||
| Group 1 | 0.1 (0.1, 1.3) | 0.02 | ||||
| Group 2 | 0.4 (0.3, 1.2) | |||||
| Group 3 | 1.1 (0.5, 3.3) | |||||
| Demographic and Traditional CVD Risk Parameters . | WHIV . | Non-HIV-Infected Women . | Whole Group . | |||
|---|---|---|---|---|---|---|
| Spearman ρ . | P Value . | Spearman ρ . | P Value . | Spearman ρ . | P Value . | |
| Age, y | 0.26 | 0.29 | 0.25 | 0.45 | 0.27 | 0.14 |
| Total cholesterol, mg/dL | 0.10 | 0.69 | 0.58 | 0.06 | 0.18 | 0.34 |
| LDL-C, mg/dL | 0.005 | 0.98 | 0.67 | 0.03 | 0.16 | 0.40 |
| HDL-C, mg/dL | 0.40 | 0.09 | −0.45 | 0.16 | 0.08 | 0.69 |
| Triglycerides, mg/dL | 0.02 | 0.92 | 0.68 | 0.02 | 0.35 | 0.06 |
| 10-Year ASCVD risk score, % | 0.23 | 0.35 | −0.18 | 0.59 | 0.20 | 0.29 |
| HbA1c, % | 0.51 | 0.03 | −0.04 | 0.91 | 0.33 | 0.08 |
| BMI, kg/m2 | −0.01 | 0.97 | 0.24 | 0.51 | 0.04 | 0.82 |
| WHR | 0.07 | 0.79 | −0.05 | 0.89 | 0.11 | 0.56 |
| Time since HIV diagnosis, years | −0.26 | 0.27 | — | — | — | — |
| Total duration ART, years | −0.04 | 0.88 | — | — | — | — |
| CD4+ T cell count, cells/mm3 | 0.12 | 0.62 | — | — | — | — |
| HIV viral load, copies/mL | 0.28 | 0.26 | — | — | — | — |
| Intramyocardial lipid content and reproductive aging status (whole group) | ||||||
| Reproductive aging groups | Intramyocardial lipid content, % | P value | ||||
| Group 1 | 0.1 (0.1, 1.3) | 0.02 | ||||
| Group 2 | 0.4 (0.3, 1.2) | |||||
| Group 3 | 1.1 (0.5, 3.3) | |||||
Spearman ρ is shown for association between intramyocardial triglyceride content (non-normally distributed) and each baseline characteristic within all participants, WHIV, and non-HIV-infected women, respectively.
As shown above, intramyocardial triglyceride content (shown as median IQR) differed significantly across three reproductive aging groups (P = 0.02). P values <0.05 are shown in boldface.
| Covariate . | β Estimate . | β SE . | P Value . |
|---|---|---|---|
| HIV status (positive) | 0.21 | 0.07 | 0.01 |
| Reproductive aging category | 0.02 | ||
| Postmenopausal (Group 3) | Reference | ||
| Reduced ovarian reserve (Group 2) | −0.02 | 0.12 | |
| Premenopausal (Group 1) | −0.28 | 0.13 | |
| Non-HDL-C, mg/dL | 0.001 | 0.002 | 0.60 |
| HbA1c, % | 0.29 | 0.23 | 0.23 |
| Covariate . | β Estimate . | β SE . | P Value . |
|---|---|---|---|
| HIV status (positive) | 0.21 | 0.07 | 0.01 |
| Reproductive aging category | 0.02 | ||
| Postmenopausal (Group 3) | Reference | ||
| Reduced ovarian reserve (Group 2) | −0.02 | 0.12 | |
| Premenopausal (Group 1) | −0.28 | 0.13 | |
| Non-HDL-C, mg/dL | 0.001 | 0.002 | 0.60 |
| HbA1c, % | 0.29 | 0.23 | 0.23 |
Overall model R2 = 0.56, P = 0.003. Multivariable modeling was performed using log intramyocardial triglyceride content as the dependent variable. Covariates added to this model included HIV+ status, reproductive aging status, non-HDL-C, and HbA1c. For reproductive aging status, the postmenopausal Group 3 represents the reference group; β estimates are provided for the reduced ovarian reserve Group 2 and the premenopausal Group 1. Reproductive aging status was entered as a nominal variable. For each covariate, the β estimate and β estimate SE are shown. As shown above, HIV+ status and reproductive aging status independently predicted intramyocardial triglyceride content, controlling for non-HDL-C and HbA1c. P values <0.05 are shown in boldface.
| Covariate . | β Estimate . | β SE . | P Value . |
|---|---|---|---|
| HIV status (positive) | 0.21 | 0.07 | 0.01 |
| Reproductive aging category | 0.02 | ||
| Postmenopausal (Group 3) | Reference | ||
| Reduced ovarian reserve (Group 2) | −0.02 | 0.12 | |
| Premenopausal (Group 1) | −0.28 | 0.13 | |
| Non-HDL-C, mg/dL | 0.001 | 0.002 | 0.60 |
| HbA1c, % | 0.29 | 0.23 | 0.23 |
| Covariate . | β Estimate . | β SE . | P Value . |
|---|---|---|---|
| HIV status (positive) | 0.21 | 0.07 | 0.01 |
| Reproductive aging category | 0.02 | ||
| Postmenopausal (Group 3) | Reference | ||
| Reduced ovarian reserve (Group 2) | −0.02 | 0.12 | |
| Premenopausal (Group 1) | −0.28 | 0.13 | |
| Non-HDL-C, mg/dL | 0.001 | 0.002 | 0.60 |
| HbA1c, % | 0.29 | 0.23 | 0.23 |
Overall model R2 = 0.56, P = 0.003. Multivariable modeling was performed using log intramyocardial triglyceride content as the dependent variable. Covariates added to this model included HIV+ status, reproductive aging status, non-HDL-C, and HbA1c. For reproductive aging status, the postmenopausal Group 3 represents the reference group; β estimates are provided for the reduced ovarian reserve Group 2 and the premenopausal Group 1. Reproductive aging status was entered as a nominal variable. For each covariate, the β estimate and β estimate SE are shown. As shown above, HIV+ status and reproductive aging status independently predicted intramyocardial triglyceride content, controlling for non-HDL-C and HbA1c. P values <0.05 are shown in boldface.
Discussion
Our study revealed substantial subclinical cardiac pathology among ART-treated WHIV without known CVD or diabetes. Compared with matched, non-HIV-infected women, WHIV evidenced a threefold increase in intramyocardial triglyceride content, as well as reduced diastolic function. Moreover, there was a strong, inverse correlation between elevated intramyocardial triglyceride content and lower diastolic function, both within the whole group and specifically among WHIV. Furthermore, among WHIV, intramyocardial triglyceride content independently predicted lower diastolic function, even after controlling for traditional cardiometabolic risk factors. Within the whole group, intramyocardial triglyceride content did not relate to chronologic age or traditional cardiometabolic risk factors but did increase along a reproductive aging continuum. Additionally, differential parameters associated with intramyocardial triglyceride content within each subgroup: HbA1c among WHIV and levels of both circulating triglycerides and LDL-C among non-HIV-infected women. Within the whole group, HIV status and reproductive aging status independently predicted intramyocardial triglyceride content in a multivariable model controlling for HbA1c and non-HDL-C. Overall, our work highlights key pathways related to diastolic dysfunction risk among ART-treated WHIV and suggests that strategies to reduce intramyocardial triglyceride accumulation may be investigated for potential to prevent heart failure and/or improve heart failure outcomes in this population. Moreover, our observation that among women, intramyocardial triglyceride content increases across a reproductive aging continuum suggests a need to consider reduced ovarian reserve as a potential risk factor for the development of diastolic dysfunction and heart failure.
In this study, we used advanced cardiac MRS techniques (17) to demonstrate, among asymptomatic, ART-treated WHIV (vs matched, non-HIV-infected women), increased intramyocardial triglyceride content. Cardiac MRS represents the gold-standard noninvasive imaging test for assessment of intracellular triglycerides, correlating closely with histopathological quantification (24). Typically, human myocardial cells store miniscule amounts of fat (25). In our cohort of women without known CVD, the percentage of intramyocardial triglycerides was 0.4 among non-HIV-infected women vs 1.2 among WHIV—a threefold relative increase for WHIV. Furthermore, compared with non-HIV-infected women, WHIV demonstrated a substantial relative impairment in diastolic function on cardiac MRI. Among WHIV, intramyocardial triglyceride content related strongly and independently to this subclinical functional cardiac pathology. Thus, among ART-treated WHIV, ectopic fat accumulation in myocardial cells (an atypical fat-storage depot) appears to be pathologic, adversely influencing diastolic function. This finding dovetails with literature relating intramyocardial triglyceride content to diastolic dysfunction among patients with other disease processes, including obesity and diabetes (18, 26–29).
Our primary findings on correlates of diastolic dysfunction among ART-treated WHIV are supported by and build on insights derived from prior cross-sectional, cardiac MRS/MRI-based studies conducted among predominantly male cohorts (5–8). Taken together, these studies highlighted subclinical cardiac steatosis and diastolic dysfunction as problems among ART-treated people with HIV (PHIV) and established relationships between intramyocardial fat accumulation and cardiac dysfunction (5–8). Our study adds an a priori focus on women’s biology in a cohort of aging women (≥40 years), with and without HIV, who do not have known CVD or diabetes and who are not on medications that might confound assessment of intramyocardial triglyceride content (e.g., statins). Importantly, previous work has suggested that insights on mechanisms of HIV-associated CVD gleaned from all-male or predominantly male cohorts do not consistently generalize to women (30–34). Through the current study, we confirm that among WHIV, as among men with HIV, cardiac steatosis is closely related to diastolic dysfunction.
Interested in the impact of women’s biology on HIV-associated myocardial pathology, we prospectively collected data enabling us to examine relationships between reproductive aging and intramyocardial triglyceride content. Among women, postmenopausal status represents a known risk factor for the development of ASCVD and/or heart failure (35); however, the determination of the menopause status among WHIV can be challenging. The most frequently cited reproductive aging classification schemes [Stages of Reproductive Aging Workshop criteria (36) and revised Stages of Reproductive Aging Workshop 10 (37) criteria]—reliant on menstrual history, as well as follicle-stimulating hormone and estradiol timed to menstrual cycle day 3—were validated among healthy women and are not recommended for application among women with chronic disease or menstrual irregularity. Cognizant of this challenge, we collected data on menstrual/reproductive history and on circulating levels of AMH, with a goal of situating participants along a reproductive aging spectrum. AMH, secreted by ovarian granulosa cells, reflects a woman’s ovarian reserve, with levels dropping to undetectable a few years before menopause (38, 39). For our study, data on menstrual history and AMH were synthesized to classify women along the following continuum: premenopausal → reduced ovarian reserve → postmenopausal (see Methods). Of interest, in our study of women with and without HIV, intramyocardial triglyceride content did not vary by chronologic age but did increase across this reproductive aging continuum. Reduced endogenous estrogen production has been previously shown to predispose to visceral adiposity (12) and liver fat (13); however, our study establishes a relationship between women’s reproductive aging and intramyocardial triglyceride content. Our observation may help to explain why early menopause represents a risk factor for the development of heart failure among women in the general population (40).
In our study of age/BMI-matched women, with vs without HIV, reproductive aging classification did not vary significantly by HIV status, and in multivariable modeling, both HIV status and reproductive aging status independently predicted intramyocardial triglyceride content even after controlling for other relevant parameters. Of note, a landmark analysis, led by Scherzer et al. (41) and based out of the large US Women’s Interagency HIV Study, suggests that WHIV, with access to ART, tend to have lower AMH levels than women without HIV (41). Taken together, findings from our study and the Scherzer et al. study (41) suggest that aging ART-treated WHIV may be at a particularly high risk for the development for cardiac steatosis and associated diastolic dysfunction.
Key questions remain as to pathways, independent of reproductive aging, predisposing ART-treated WHIV to increased intramyocardial triglyceride accumulation. A previous study by Thiara et al. (7) showed a relationship between duration of ART and intramyocardial triglyceride content among PHIV. This observation is bolstered by biologic plausibility, given that select anti-retroviral therapeutics induce subcutaneous fat dysfunction and attendant ectopic fat deposition (10). Relationships between ART (type, duration) and intramyocardial triglyceride content could not be discerned in our study, primarily because of our relatively small sample size of WHIV. Thiara et al. (7) also showed a relationship between circulating triglyceride levels and intramyocardial triglyceride content. We identified such a relationship in our whole group and in our non-HIV-infected subgroup but not among our WHIV. Among our group of WHIV, we did note a direct relationship between HbA1c level and intramyocardial triglyceride content. Furthermore, in our overall group, HIV status related to intramyocardial triglyceride content even after controlling for HbA1c and non-HDL-C. This finding implies that additional biologic differences and/or differential environmental risk-factor exposures between women with/without HIV may contribute to intramyocardial triglyceride accumulation. Additionally, among WHIV, detectable viremia and the CD4+/CD8+ T cell ratio related to intramyocardial triglyceride content, controlling for other relevant clinical parameters. Future investigations focused on unearthing mechanisms of increased intramyocardial triglyceride accumulation among WHIV may usefully assess HIV-specific processes, including HIV proteins, such as viral protein R (42, 43); HIV-mediated downregulation/inactivation of the endoribonuclease (microRNA processing enzyme) Dicer (44, 45); HIV-associated immune activation, persistent despite ART (46); and HIV-associated relative growth hormone deficiency (47), all of which may engender metabolic dysregulation and/or ectopic fat deposition (48). Moreover, new-start ART studies are needed to investigate differences in the effects of various first-line regimens on intramyocardial triglyceride accumulation.
BMI and the waist-to-hip ratio were comparable among women with and without HIV in our study, and neither anthropometric parameter related to intramyocardial triglyceride content in the whole group or either subgroup. Notably, however, the average BMI in both groups was 32 kg/m2 corresponding with a “moderately obese” designation. In the general population, obesity is known to promote increased intramyocardial triglyceride accumulation (26), invoking an “overflow” model, whereby the triglyceride storage capacity of subcutaneous fat becomes saturated, prompting the accumulation of excess triglycerides in ectopic fat depots (viscera, liver, heart) (49). Several large HIV cohort studies have documented that ART initiation appears to engender weight gain, more so in women than in men (50, 51). Given that ART is now typically initiated immediately upon HIV diagnosis (most often before the development of AIDS-related cachexia), post-ART weight gain may not purely reflect a “return to health” but rather, a potentially maladaptive metabolic response. Indeed, studies have shown that a striking proportion of ART-treated PHIV is overweight or obese (50, 52). As the epidemics of HIV and obesity continue to intersect globally (53), it will be important to determine whether ART-treated PHIV, and particularly WHIV, deposit excess fat ectopically at lower BMI thresholds and to identify the precise mechanisms underlying this predilection.
Our study identifies increased intramyocardial triglyceride accumulation and associated diastolic dysfunction as concerns for aging, ART-treated WHIV. Furthermore, through this study, we discerned a relationship between reproductive aging status and intramyocardial triglyceride content in women. Study limitations include a relatively small sample size and a cross-sectional study design, which preclude conclusions on causality. Specifically, our study design does not enable us to assess differential effects of unchecked HIV infection vs ART (type, duration) on intramyocardial fat accumulation among ART-treated WHIV. Moreover, all participants in this study were recruited from the Greater Boston Area—the generalizability of our findings to WHIV in other regions remains unknown. These limitations notwithstanding, our study assesses intramyocardial triglyceride accumulation and diastolic dysfunction among women with and without HIV, factoring in considerations pertaining to women’s reproductive health. Our study synthesizes hormonal data with MRS/MRI-derived surrogates of cardiovascular pathology. We show among WHIV (vs non-HIV-infected women) increased intramyocardial triglyceride content relating significantly to reduced diastolic dysfunction. Diastolic dysfunction is known to progress toward heart failure with preserved ejection fraction, a highly morbid disease for which no effective therapies have been identified (3). Our work suggests that additional HIV-CVD research-testing strategies to forestall myocardial steatosis and preserve diastolic dysfunction, particularly among aging WHIV, may advance heart-failure prevention efforts. Based on evolving knowledge on parameters predisposing to myocardial steatosis in this population, such strategies may target HIV-specific parameters, sex-specific parameters, or both.
Acknowledgments
We thank the participants in this study and the nursing staff of the Massachusetts General Hospital Clinical Research Center. We also thank Jacob Calkins, Mary Foley, and Larry White from the Martinos Center for Biomedical Imaging.
Financial Support: This project was funded through a Collaborative Feasibility award from the National Institutes of Health (NIH)/Harvard Center for AIDS Research (Grant P30AI060354 to M.V.Z. and T.G.N.). This project was also supported by NIH Grants M01RR01066, 1UL1RR025758, and 8UL1TR000170 to the Harvard Clinical and Translational Science Center. Support was also received from NIH P30DK040561 and the Nutrition and Obesity Research Center at Harvard. M.T. received support from NIH 5KL2TR001100-05. T.G.N. received support from the A. Curt Greer, Pamela Kohlberg, and Kohlberg Foundation; American Heart Association Fellow to Faculty Award 12FTF12060588, NIH 1R01HL130539, NIH 1R01HL137562, and NIH K24HL113128-06. L.T.F. received support from NIH T32DK007028 and NIH/National Heart, Lung, and Blood Institute 5T32HL076136. V.A.T. received support from NIH R01HL132786. J.E.H. received support from NIH R01HL140224. M.V.Z. received support from NIH 1R01HL137562.
Clinical Trial Information: ClinicalTrials.gov no. NCT02874703 (registered 22 August 2016).
Author Contributions: M.T. was involved in study recruitment/performance, data acquisition, data analysis/interpretation, and manuscript writing. T.G.N. was involved in study concept/design, data acquisition, data analysis/interpretation, and manuscript writing. M.A. was involved in data acquisition, data analysis/interpretation, and manuscript writing. L.A.S. was involved in study recruitment/performance, data analysis/interpretation, and manuscript writing. A.R., C.R., and C.P.M. were involved in study recruitment/performance. D.C., L.T.F., T.L.S., J.E.H., V.A.T., and T.H.B. were involved in data analysis/interpretation. M.D.N. and L.S.S. were involved in data acquisition and data analysis/interpretation. M.V.Z. was involved in study concept/design, study recruitment/performance, data acquisition, data analysis/interpretation, and manuscript writing. All authors contributed to critical revision of the manuscript.
Additional Information
Disclosure Summary: T.G.N. has participated in a Scientific Advisory Board Meeting for Bristol-Myers Squibb and has served as a consultant for Aprea Therapeutics, Parexel, and Intrinsic Imaging, all for work unrelated to this project. T.L.S. has received investigator-initiated research funding from Novo Nordisk and Kowa Pharmaceuticals. J.E.H. has received investigator-initiated research funding from Gilead and research supplies from EcoNugenics. M.V.Z. has received investigator-initiated research funding from Gilead. All disclosures are unrelated to this manuscript. The remaining authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Abbreviations:
- AMH
anti-Müllerian hormone
- ART
anti-retroviral therapy
- ASCVD
atherosclerotic cardiovascular disease
- BMI
body mass index
- CVD
cardiovascular disease
- HDL-C
high-density lipoprotein–cholesterol
- IQR
interquartile range
- LDL-C
low-density lipoprotein–cholesterol
- MRS
magnetic resonance spectroscopy
- PHIV
people with HIV
- VOLbac
left atrial size before atrial contraction
- VOLmax
volume at maximum left atrial size
- VOLmin
left atrial size at end of contraction
- WHIV
women with HIV
References and Notes
Author notes
M.T. and T.G.N. contributed equally to this study.



