-
PDF
- Split View
-
Views
-
Cite
Cite
Bas C J Majoor, Socrates E Papapoulos, P D Sander Dijkstra, Marta Fiocco, Neveen A T Hamdy, Natasha M Appelman-Dijkstra, Denosumab in Patients With Fibrous Dysplasia Previously Treated With Bisphosphonates, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 12, December 2019, Pages 6069–6078, https://doi.org/10.1210/jc.2018-02543
Close - Share Icon Share
Abstract
Fibrous dysplasia/McCune-Albright syndrome (FD/MAS) is a rare bone disorder commonly treated with bisphosphonates, but clinical and biochemical responses may be incomplete.
To evaluate the efficacy and tolerability of the receptor activator of nuclear factor-κB ligand inhibitor denosumab in the treatment of patients with FD/MAS refractory to bisphosphonate therapy.
Case series.
Academic center of expertise for rare bone diseases.
Data were collected from 12 consecutive patients with FD/MAS with persistent pain and increased biochemical markers of bone turnover (BTMs) after long-term treatment with bisphosphonates (median, 8.8 years) and were treated with subcutaneous denosumab 60 mg at 3- or 6-month intervals with a follow-up for at least 12 months.
Sustained reduction of BTMs and bone pain.
A 60 mg dose of denosumab once every 3 months, but not once every 6 months, induced a sustained reduction of BTMs. After a median treatment period of 15.5 months (range, 12 to 19) serum alkaline phosphatase activity and propeptide of type 1 procollagen levels were respectively reduced from 212 ± 39.4 IU/L to 79 ± 6.0 IU/L (P = 0.004) and from 346.2 ± 111.1 ng/mL to 55.7 ± 16.6 ng/mL (P = 0.023) and normalized in 70% and 75% of patients, respectively. Although not quantitavely measured, 10 patients reported a reduction in bone pain of whom 6 reported complete elimination of pain. Treatment with denosumab was well tolerated.
Our results indicate that 60 mg of denosumab every 3 months is a promising, well-tolerated treatment of most patients with FD/MAS refractory to bisphosphonate therapy. These results together with those of previously published case reports provide the necessary background for the design of a larger, controlled study.
Fibrous dysplasia/McCune-Albright syndrome (FD/MAS) is a rare disorder characterized by replacement of bone by highly vascularized fibrous tissue in one (monostotic) or more skeletal sites (polyostotic) that may be associated with skeletal morbidity (1, 2). The disorder is caused by postzygotic, somatic activating mutations of GNAS leading to overproduction of cAMP and abnormal cellular responses, such as increased production of the bone resorbing factors IL-6 and receptor activator of nuclear factor-κB ligand (RANKL) (3–5). Extraskeletal distribution of the GNAS mutation may be associated with manifestations in other tissues, particularly endocrine glands, such as endocrinopathies and café-au-lait skin patches in MAS and therefore the disease is referred to as FD/MAS (6).
Although there is as yet no approved medical treatment of FD/MAS, several studies have reported clinical and biochemical improvement in patients treated with different bisphosphonate regimens (7–14). However, response to bisphosphonates may be inadequate in decreasing pain symptoms, particularly in patients with polyostotic disease and high skeletal burden (12, 13). Recent case reports suggest successful response to treatment of patients with FD/MAS with the RANKL inhibitor, denosumab (15–18). In this case series we report the clinical and biochemical outcomes of treatment of 12 patients with bisphosphonate-refractory FD/MAS treated consecutively with denosumab.
Patients and Methods
Patients
Patients eligible for treatment with denosumab were adults with FD/MAS attending the outpatient clinic of the Center for Bone Quality of the Leiden University Medical Center who fulfilled the following criteria. First, patients had to have been treated previously with bisphosphonates with incomplete clinical and biochemical responses as defined by persistence of pain and/or failure to normalize serum values of total serum alkaline phosphatase (ALP) activity (in the absence of liver disease), and/or of serum amino-terminal propeptide of type 1 procollagen (P1NP). Patients had to be aged ≥18 years, had never been treated with denosumab, and to have no contraindications for the use of this agent (e.g., hypocalcemia, vitamin D deficiency, pregnancy, and lactation). Impending tooth extraction or localization of the disease in the proximal femur were not exclusion criteria.
In our center, management of patients with FD/MAS is conducted following an in-house standard care trajectory that includes collection of data about type and extent of disease, extent of skeletal involvement, screening for endocrinopathies, history of previous medical or surgical treatment, and evaluation of laboratory and clinical parameters of disease activity at predefined time intervals. Patients were seen on their regular three-month outpatient clinic visits during which nonfasting morning blood samples were collected for evaluation of bone and mineral metabolism and data on change in pain complaints were registered, although pain was not quantitively measured. All patients received calcium/D3 supplements as required. Patients with persistent hypophosphatemia resulting from renal phosphate wasting were treated with active vitamin D metabolites. Oral informed consent was obtained from all patients for the off-label use of denosumab (Prolia®, Amgen) and written approval for the retrospective analysis and reporting of the collected data were obtained from the medical ethics committee of the Leiden University Medical Center.
Methods
All patients included in the study had been treated previously with bisphosphonates, primarily olpadronate [(3-dimethylamino-1-hydroxypropylidene)-1,1 bisphosphonate], a nitrogen-containing bisphosphonate that is 5 to 10 times more potent than pamidronate that has been used in our center for the treatment of patients with Paget disease of bone, malignancies, and rare bone diseases (12, 19–26). The diagnosis of fibrous dysplasia was established based on clinical and radiological evaluation, with additional histological and genetic evaluation where required. The extent of bone involvement was determined by 99m-technetium bone scans using the validated skeletal bone score (SBS) (27). Serum calcium adjusted for albumin binding, phosphate, creatinine and γ-glutamyltransferase were measured by semiautomated techniques. Serum ALP activity was measured using a fully automated P800 modulator system (Roche BV, Woerden, Holland). PTH and 25-OH vitamin D (25-OHD) were measured using the Immulite 2500 assay (Siemens Diagnostics, Breda, Netherlands) and the LIAISON® 25-OH Vitamin D TOTAL assay (DiaSorin S.A./N.V., Brussels, Belgium), respectively. P1NP and the C-terminal telopeptide of type 1 collagen (CTX) were determined by the E-170 system (Roche BV). C-terminal fibroblast growth factor (FGF)-23 was measured in plasma by ELISA (Immutopics, San Clemente, CA). Normal ranges: ALP 40 to 98 IU/L, P1NP in premenopausal women and men <59 ng/mL, CTX <573 pg/mL, FGF-23 <125 pg/mL, PTH 0.7 to 8.0 pmol/L, 25-OHD 50 to 250 nmol/L. Maximal renal tubular phosphate reabsorption in relation to glomerular filtration rate was assessed in some patients from a nomogram (normal range, 0.8 to 1.42 mmol/L) (28).
Outcomes of treatment
The primary outcomes of treatment were the stable reduction of biochemical markers of bone turnover and the decrease or disappearance of FD-related skeletal pain. Patients were followed until the last included patient had completed 12 months of treatment with denosumab (end of observation period).
Biochemical response to treatment was evaluated with percentage change of serum levels of ALP and P1NP and normalization rates were calculated (upper limit of normal ALP 98 IU/L, P1NP 59 ng/mL). Possible attributive factors on biochemical response nine months after start of treatment with denosumab, including type of FD and dosage scheme, were evaluated in a linear mixed model with a follow-up of nine months, as patients with six-month doses were switched to three-month doses after nine months of inadequate response. Pain response was assessed at each visit and response was categorized as increased, unchanged, improved. or disappeared. Potential adverse effects of denosumab treatment were registered at each visit; radiographs were only obtained during treatment when clinically indicated.
Statistical analysis
Statistical analysis was performed using SPSS for Windows, version 23.0 (SPSS Inc., Chicago, IL). Unless otherwise stated, results are presented as median (range) or as a percentage in case of categorical data. A linear mixed model was used to assess the subtypes of FD and dose intervals as attributive factors to the response to treatment with denosumab over time. A paired sample t-test was used to compare bone turnover markers (BTMs) before starting denosumab and at the end of the observation period. Spearman correlation was used to evaluate the relation between pretreatment values and treatment outcome.
Results
Seven patients had polyostotic FD, four had MAS, and one patient had severe monostotic craniofacial disease (Table 1). Endocrine functions were stable for years in the four patients with MAS: two patients had precocious puberty and were not receiving any hormonal treatment at adult age; two had growth hormone excess and were receiving octreotide and had normal values of serum IGF1, one of whom also had hyperthyroidism and was receiving methimazole titration therapy and had normal serum TSH. All women included in the study were premenopausal.
| Patient No. . | Sex/Age . | FD Type . | SBS . | FGF-23, pg/mL . | ALP, IU/L . | P1NP, ng/mL . | CTX, pg/mL . | Dmab Duration, mo . | Duration BP Use, y . | Months Between Stop BP and Start Dmab, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/41 | PFD | 0.3 | 116 | 75 | 73 | 241 | 22 | 2.5 | 0.8 |
| 2 | F/35 | PFD | 0.6 | 71 | 94 | 80 | 114 | 18 | 4.7 | 0.3 |
| 3 | F/68 | PFD | 5.6 | 159 | 198 | 864 | 403 | 15 | 0.7 | 0 |
| 4 | M/28 | PFD | 7.8 | 115 | 275 | 273 | 443 | 14 | 5.0 | 99.2 |
| 5 | F/28 | MFD | 13.8 | 202 | 135 | 109 | 166 | 18 | 8.6 | 34.4 |
| 6 | F/36 | PFD | 16.7 | 133 | 183 | 290 | 347 | 17 | 1.7 | 0.4 |
| 7 | F/33 | PFD | 25.0 | 160 | 538 | 1235 | 600 | 30 | 11.8 | 6.4 |
| 8 | M/52 | PFD | 25.0 | 133 | 330 | 354 | 548 | 15 | 9.5 | 4.2 |
| 9 | M/33 | MAS | 31.3 | 173 | 144 | 124 | 604 | 16 | 11.8 | 103.5 |
| 10 | F/46 | MAS | 38.7 | 162 | 203 | 208 | 148 | 13 | 18.5 | 4.2 |
| 11 | F/41 | MAS | 44.0 | 161 | 161 | 198 | 333 | 15 | 22.1 | 4.1 |
| 12 | F/50 | MAS | 64.7 | 273 | 503 | 812 | 999 | 23 | 8.9 | 8.8 |
| Patient No. . | Sex/Age . | FD Type . | SBS . | FGF-23, pg/mL . | ALP, IU/L . | P1NP, ng/mL . | CTX, pg/mL . | Dmab Duration, mo . | Duration BP Use, y . | Months Between Stop BP and Start Dmab, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/41 | PFD | 0.3 | 116 | 75 | 73 | 241 | 22 | 2.5 | 0.8 |
| 2 | F/35 | PFD | 0.6 | 71 | 94 | 80 | 114 | 18 | 4.7 | 0.3 |
| 3 | F/68 | PFD | 5.6 | 159 | 198 | 864 | 403 | 15 | 0.7 | 0 |
| 4 | M/28 | PFD | 7.8 | 115 | 275 | 273 | 443 | 14 | 5.0 | 99.2 |
| 5 | F/28 | MFD | 13.8 | 202 | 135 | 109 | 166 | 18 | 8.6 | 34.4 |
| 6 | F/36 | PFD | 16.7 | 133 | 183 | 290 | 347 | 17 | 1.7 | 0.4 |
| 7 | F/33 | PFD | 25.0 | 160 | 538 | 1235 | 600 | 30 | 11.8 | 6.4 |
| 8 | M/52 | PFD | 25.0 | 133 | 330 | 354 | 548 | 15 | 9.5 | 4.2 |
| 9 | M/33 | MAS | 31.3 | 173 | 144 | 124 | 604 | 16 | 11.8 | 103.5 |
| 10 | F/46 | MAS | 38.7 | 162 | 203 | 208 | 148 | 13 | 18.5 | 4.2 |
| 11 | F/41 | MAS | 44.0 | 161 | 161 | 198 | 333 | 15 | 22.1 | 4.1 |
| 12 | F/50 | MAS | 64.7 | 273 | 503 | 812 | 999 | 23 | 8.9 | 8.8 |
Reference ranges: FGF-23 0–125 pg/mL, ALP 40–98 IU/L, P1NP <59 ng/mL, CTX <573 pg/mL.
Abbreviations: BP, bisphosphonate; Dmab, denosumab MFD, monostotic FD; PFD, polyostotic.
| Patient No. . | Sex/Age . | FD Type . | SBS . | FGF-23, pg/mL . | ALP, IU/L . | P1NP, ng/mL . | CTX, pg/mL . | Dmab Duration, mo . | Duration BP Use, y . | Months Between Stop BP and Start Dmab, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/41 | PFD | 0.3 | 116 | 75 | 73 | 241 | 22 | 2.5 | 0.8 |
| 2 | F/35 | PFD | 0.6 | 71 | 94 | 80 | 114 | 18 | 4.7 | 0.3 |
| 3 | F/68 | PFD | 5.6 | 159 | 198 | 864 | 403 | 15 | 0.7 | 0 |
| 4 | M/28 | PFD | 7.8 | 115 | 275 | 273 | 443 | 14 | 5.0 | 99.2 |
| 5 | F/28 | MFD | 13.8 | 202 | 135 | 109 | 166 | 18 | 8.6 | 34.4 |
| 6 | F/36 | PFD | 16.7 | 133 | 183 | 290 | 347 | 17 | 1.7 | 0.4 |
| 7 | F/33 | PFD | 25.0 | 160 | 538 | 1235 | 600 | 30 | 11.8 | 6.4 |
| 8 | M/52 | PFD | 25.0 | 133 | 330 | 354 | 548 | 15 | 9.5 | 4.2 |
| 9 | M/33 | MAS | 31.3 | 173 | 144 | 124 | 604 | 16 | 11.8 | 103.5 |
| 10 | F/46 | MAS | 38.7 | 162 | 203 | 208 | 148 | 13 | 18.5 | 4.2 |
| 11 | F/41 | MAS | 44.0 | 161 | 161 | 198 | 333 | 15 | 22.1 | 4.1 |
| 12 | F/50 | MAS | 64.7 | 273 | 503 | 812 | 999 | 23 | 8.9 | 8.8 |
| Patient No. . | Sex/Age . | FD Type . | SBS . | FGF-23, pg/mL . | ALP, IU/L . | P1NP, ng/mL . | CTX, pg/mL . | Dmab Duration, mo . | Duration BP Use, y . | Months Between Stop BP and Start Dmab, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/41 | PFD | 0.3 | 116 | 75 | 73 | 241 | 22 | 2.5 | 0.8 |
| 2 | F/35 | PFD | 0.6 | 71 | 94 | 80 | 114 | 18 | 4.7 | 0.3 |
| 3 | F/68 | PFD | 5.6 | 159 | 198 | 864 | 403 | 15 | 0.7 | 0 |
| 4 | M/28 | PFD | 7.8 | 115 | 275 | 273 | 443 | 14 | 5.0 | 99.2 |
| 5 | F/28 | MFD | 13.8 | 202 | 135 | 109 | 166 | 18 | 8.6 | 34.4 |
| 6 | F/36 | PFD | 16.7 | 133 | 183 | 290 | 347 | 17 | 1.7 | 0.4 |
| 7 | F/33 | PFD | 25.0 | 160 | 538 | 1235 | 600 | 30 | 11.8 | 6.4 |
| 8 | M/52 | PFD | 25.0 | 133 | 330 | 354 | 548 | 15 | 9.5 | 4.2 |
| 9 | M/33 | MAS | 31.3 | 173 | 144 | 124 | 604 | 16 | 11.8 | 103.5 |
| 10 | F/46 | MAS | 38.7 | 162 | 203 | 208 | 148 | 13 | 18.5 | 4.2 |
| 11 | F/41 | MAS | 44.0 | 161 | 161 | 198 | 333 | 15 | 22.1 | 4.1 |
| 12 | F/50 | MAS | 64.7 | 273 | 503 | 812 | 999 | 23 | 8.9 | 8.8 |
Reference ranges: FGF-23 0–125 pg/mL, ALP 40–98 IU/L, P1NP <59 ng/mL, CTX <573 pg/mL.
Abbreviations: BP, bisphosphonate; Dmab, denosumab MFD, monostotic FD; PFD, polyostotic.
All patients had been treated previously with bisphosphonates, predominantly olpadronate, for a median period of 8.8 years (range, 0.7 to 22.1). Other bisphosphonates used were pamidronate, zoledronate, alendronate, and risedronate (Table 2). Median SBS was 20.8 (0.3 to 64.7). Previous long-term treatment with bisphosphonates had led to temporary normalization of serum ALP activity or serum P1NP values in three patients, and temporary normalization of serum CTX levels in all patients (12). Bisphosphonate treatment had also resulted in intermittent responses of pain complaints in all patients, but in none of the patients did this treatment result in disappearance of pain at any point during follow-up.
| Patient No. . | Duration BP Use, y . | Time Between Stop BP and Start Denosumab, mo . | Cumulative Dose of BP . |
|---|---|---|---|
| 1 | 2.5 | 0.8 | Pamidronate 122 mg IV, olpadronate 80 mg IV and 255.00 mg oral |
| 2 | 4.7 | 0.3 | Zoledronate 4 mg IV and olpadronate 169.500 mg oral |
| 3 | 0.7 | 0 | Alendronate 3.600 mg oral and olpadronate 60 mg IV |
| 4 | 5.0 | 99.2 | Pamidronate 42 mg IV |
| 5 | 8.6 | 34.4 | Olpadronate 120 mg IV and 166.500 mg oral |
| 6 | 1.7 | 0.4 | Olpadronate 68 mg IV and 146.000 mg oral |
| 7 | 11.8 | 6.4 | Olpadronate 124 mg IV and 219.000 mg oral and zoledronate 10 mg IV |
| 8 | 9.5 | 4.2 | Alendronate 900 mg oral, risedronate 900 mg oral, zoledronate 28 mg IV, and olpadronate 24 mg IV |
| 9 | 11.8 | 103.5 | Olpadronate 20 mg IV and 328.500 mg oral |
| 10 | 18.5 | 4.2 | Alendronate 105.00 mg oral, risedronate 4.200 mg oral, zoledronate 4 mg IV, pamidronate 20 mg IV, olpadronate 116 mg IV and 629.750 mg oral |
| 11 | 22.1 | 4.1 | Olpadronate 764 mg IV and 144.000 mg oral |
| 12 | 8.9 | 8.8 | Olpadronate 720 mg IV and 365.000 mg oral |
| Patient No. . | Duration BP Use, y . | Time Between Stop BP and Start Denosumab, mo . | Cumulative Dose of BP . |
|---|---|---|---|
| 1 | 2.5 | 0.8 | Pamidronate 122 mg IV, olpadronate 80 mg IV and 255.00 mg oral |
| 2 | 4.7 | 0.3 | Zoledronate 4 mg IV and olpadronate 169.500 mg oral |
| 3 | 0.7 | 0 | Alendronate 3.600 mg oral and olpadronate 60 mg IV |
| 4 | 5.0 | 99.2 | Pamidronate 42 mg IV |
| 5 | 8.6 | 34.4 | Olpadronate 120 mg IV and 166.500 mg oral |
| 6 | 1.7 | 0.4 | Olpadronate 68 mg IV and 146.000 mg oral |
| 7 | 11.8 | 6.4 | Olpadronate 124 mg IV and 219.000 mg oral and zoledronate 10 mg IV |
| 8 | 9.5 | 4.2 | Alendronate 900 mg oral, risedronate 900 mg oral, zoledronate 28 mg IV, and olpadronate 24 mg IV |
| 9 | 11.8 | 103.5 | Olpadronate 20 mg IV and 328.500 mg oral |
| 10 | 18.5 | 4.2 | Alendronate 105.00 mg oral, risedronate 4.200 mg oral, zoledronate 4 mg IV, pamidronate 20 mg IV, olpadronate 116 mg IV and 629.750 mg oral |
| 11 | 22.1 | 4.1 | Olpadronate 764 mg IV and 144.000 mg oral |
| 12 | 8.9 | 8.8 | Olpadronate 720 mg IV and 365.000 mg oral |
Data on treatment with bisphosphonates prior to start of denosuman injections.
Olpadronate was given orally (range, 50–200 mg daily) or IV (range, 4–8 mg for 3–5 consecutive d at 3-mo to 6-mo intervals). Risendronate was given orally (range, 10–30 mg daily), alendronate was given orally (70 mg weekly), pamidronate was given IV (30 mg for 3 consecutive d in 3-mo cycles), Zoledronate was given IV (6–12 mo cycles of single infusions with 4 mg).
Abbreviation: BP, bisphosphonate.
| Patient No. . | Duration BP Use, y . | Time Between Stop BP and Start Denosumab, mo . | Cumulative Dose of BP . |
|---|---|---|---|
| 1 | 2.5 | 0.8 | Pamidronate 122 mg IV, olpadronate 80 mg IV and 255.00 mg oral |
| 2 | 4.7 | 0.3 | Zoledronate 4 mg IV and olpadronate 169.500 mg oral |
| 3 | 0.7 | 0 | Alendronate 3.600 mg oral and olpadronate 60 mg IV |
| 4 | 5.0 | 99.2 | Pamidronate 42 mg IV |
| 5 | 8.6 | 34.4 | Olpadronate 120 mg IV and 166.500 mg oral |
| 6 | 1.7 | 0.4 | Olpadronate 68 mg IV and 146.000 mg oral |
| 7 | 11.8 | 6.4 | Olpadronate 124 mg IV and 219.000 mg oral and zoledronate 10 mg IV |
| 8 | 9.5 | 4.2 | Alendronate 900 mg oral, risedronate 900 mg oral, zoledronate 28 mg IV, and olpadronate 24 mg IV |
| 9 | 11.8 | 103.5 | Olpadronate 20 mg IV and 328.500 mg oral |
| 10 | 18.5 | 4.2 | Alendronate 105.00 mg oral, risedronate 4.200 mg oral, zoledronate 4 mg IV, pamidronate 20 mg IV, olpadronate 116 mg IV and 629.750 mg oral |
| 11 | 22.1 | 4.1 | Olpadronate 764 mg IV and 144.000 mg oral |
| 12 | 8.9 | 8.8 | Olpadronate 720 mg IV and 365.000 mg oral |
| Patient No. . | Duration BP Use, y . | Time Between Stop BP and Start Denosumab, mo . | Cumulative Dose of BP . |
|---|---|---|---|
| 1 | 2.5 | 0.8 | Pamidronate 122 mg IV, olpadronate 80 mg IV and 255.00 mg oral |
| 2 | 4.7 | 0.3 | Zoledronate 4 mg IV and olpadronate 169.500 mg oral |
| 3 | 0.7 | 0 | Alendronate 3.600 mg oral and olpadronate 60 mg IV |
| 4 | 5.0 | 99.2 | Pamidronate 42 mg IV |
| 5 | 8.6 | 34.4 | Olpadronate 120 mg IV and 166.500 mg oral |
| 6 | 1.7 | 0.4 | Olpadronate 68 mg IV and 146.000 mg oral |
| 7 | 11.8 | 6.4 | Olpadronate 124 mg IV and 219.000 mg oral and zoledronate 10 mg IV |
| 8 | 9.5 | 4.2 | Alendronate 900 mg oral, risedronate 900 mg oral, zoledronate 28 mg IV, and olpadronate 24 mg IV |
| 9 | 11.8 | 103.5 | Olpadronate 20 mg IV and 328.500 mg oral |
| 10 | 18.5 | 4.2 | Alendronate 105.00 mg oral, risedronate 4.200 mg oral, zoledronate 4 mg IV, pamidronate 20 mg IV, olpadronate 116 mg IV and 629.750 mg oral |
| 11 | 22.1 | 4.1 | Olpadronate 764 mg IV and 144.000 mg oral |
| 12 | 8.9 | 8.8 | Olpadronate 720 mg IV and 365.000 mg oral |
Data on treatment with bisphosphonates prior to start of denosuman injections.
Olpadronate was given orally (range, 50–200 mg daily) or IV (range, 4–8 mg for 3–5 consecutive d at 3-mo to 6-mo intervals). Risendronate was given orally (range, 10–30 mg daily), alendronate was given orally (70 mg weekly), pamidronate was given IV (30 mg for 3 consecutive d in 3-mo cycles), Zoledronate was given IV (6–12 mo cycles of single infusions with 4 mg).
Abbreviation: BP, bisphosphonate.
Patients were treated for at least one year with denosumab with a median follow-up of 15.5 months (range, 12 to 19 months). Median time between the last bisphosphonate treatment and first denosumab injection was 4.0 months (range, 0 to 103 months).
At the start of denosumab treatment, all 12 patients reported having pain at the site of their bone lesions. Prior to start of treatment with denosumab, 8 patients used a variety of analgesics, including paracetamol (n = 8), nonsteroidal anti-inflammatory drugs (NSAIDs; n = 4), morphine derivatives (e.g., oxycodone; n = 5), and 1 patient used pregabalin. The 4 patients who did not use any analgesics did however have pain, but previous attempts to relieve the pain with analgesics were unsuccessful and they had stopped taking them. Serum values of biochemical markers of bone turnover varied markedly between patients at the time of starting treatment with denosumab (Table 1). Serum ALP activity was increased in 10 of 12 patients (median, 191 IU/L; range, 75 to 538 IU/L), serum P1NP in 12 of 12 patients (median, 241 ng/mL; range, 73 to 1235 ng/mL), and serum CTX in 3 of 12 patients (median, 375 pg/mL; range, 114 to 999 pg/mL). However, blood was collected in the nonfasting state, which can affect measured values of CTX, so that serum CTX levels should be interpreted with caution in this case series (28). Serum calcium, adjusted for albumin, phosphate, creatinine and 25-OHD vitamin D concentrations, and plasma PTH levels were within their respective normal laboratory reference ranges in all studied patients. In contrast, serum FGF-23 values were increased in 9 of the 12 patients (Table 1).
Biochemical response to treatment
Initially, a 60 mg dose of denosumab was administered subcutaneously at six-month intervals in six patients but failed to maintain the decrease in serum BTMs observed at three months at the six-month evaluation time point (Fig. 1). For example, serum ALP activity, although decreased at three months after the injection, returned to baseline values after six months, in contrast to the additional decrease observed in the patients who received a second denosumab injection after three months. Changes in serum P1NP levels were similar (Fig. 1). Linear mixed model analysis (Tables 3 and 4) showed a substantial difference over time for ALP (P = 0.007) and P1NP (P = 0.025), but not for CTX (P = 0.162), between patients who received denosumab at three-month intervals compared with those who received the agent at six-month intervals. The response of ALP and P1NP values did not differ between patients with FD/MAS and those with FD.
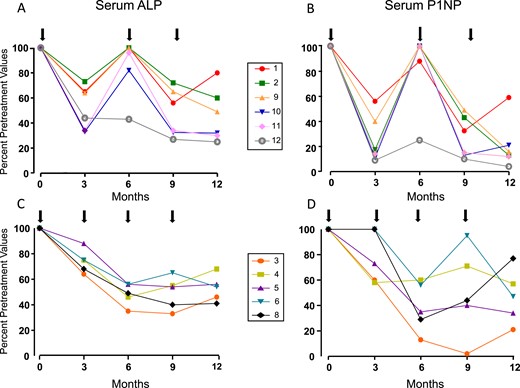
(A–D) Percentage changes in serum ALP and P1NP levels with subcutaneous injections of 60 mg of denosumab (black arrows) in 12 patients with FD/MAS. Normal ranges: ALP 40 to 98 IU/L and P1NP <59 ng/mL. Percentage changes consistently show the different trend in changes in BTMs with denosumab given at 6-mo intervals compared with 3-mo intervals. Patients with 6-mo schedules clearly show an initial decrease in bone turnover after 3 mo, with values of ALP and P1NP returning to baseline after 6 mo. Patients with 3-mo schedules show a similar decrease after 3 mo, but further decrease of ALP and P1NP levels after 6-mo of treatment. Numbers in boxes correspond to individual patient numbers shown in Table 1.
Results From Linear Mixed Model Analysis After 9 Months of Treatment With Denosumab
| BTM . | Subtype . | Time . | Mean . | 95% CI . | Significance . |
|---|---|---|---|---|---|
| ALP | Polyostotic | T1 | 228.5 | 130–327 | P = 0.083 |
| T2 | 172.6 | 73–272 | |||
| T3 | 138.0 | 40–236 | |||
| MAS | T1 | 252.8 | 113–392 | ||
| T2 | 95.7 | −48–237 | |||
| T3 | 167.8 | 28 –307 | |||
| P1NP | Polyostotic | T1 | 409.8 | 138–682 | P = 0.110 |
| T2 | 380.0 | 103–657 | |||
| T3 | 230.6 | −46–507 | |||
| MAS | T1 | 335.5 | −50–721 | ||
| T2 | 42.0 | −343– 427 | |||
| T3 | 223.0 | −162 –608 | |||
| CTX | Polyostotic | T1 | 357 | 143 –572 | P = 0.010 |
| T2 | 511 | 287–736 | |||
| T3 | 292 | 67 –516 | |||
| MAS | T1 | 521 | 217 –825 | ||
| T2 | 129 | −206 –464 | |||
| T3 | 603 | 300 –907 |
| BTM . | Subtype . | Time . | Mean . | 95% CI . | Significance . |
|---|---|---|---|---|---|
| ALP | Polyostotic | T1 | 228.5 | 130–327 | P = 0.083 |
| T2 | 172.6 | 73–272 | |||
| T3 | 138.0 | 40–236 | |||
| MAS | T1 | 252.8 | 113–392 | ||
| T2 | 95.7 | −48–237 | |||
| T3 | 167.8 | 28 –307 | |||
| P1NP | Polyostotic | T1 | 409.8 | 138–682 | P = 0.110 |
| T2 | 380.0 | 103–657 | |||
| T3 | 230.6 | −46–507 | |||
| MAS | T1 | 335.5 | −50–721 | ||
| T2 | 42.0 | −343– 427 | |||
| T3 | 223.0 | −162 –608 | |||
| CTX | Polyostotic | T1 | 357 | 143 –572 | P = 0.010 |
| T2 | 511 | 287–736 | |||
| T3 | 292 | 67 –516 | |||
| MAS | T1 | 521 | 217 –825 | ||
| T2 | 129 | −206 –464 | |||
| T3 | 603 | 300 –907 |
No statistically significant differences in serum ALP and P1NP values between patients with polyostotic FD and patients with MAS are shown. Serum CTX values differed significantly between patients with MAS and polyostotic FD, although these values should be interpreted with care, because blood samples for serum CTX levels was collected in the nonfasting state.
Results From Linear Mixed Model Analysis After 9 Months of Treatment With Denosumab
| BTM . | Subtype . | Time . | Mean . | 95% CI . | Significance . |
|---|---|---|---|---|---|
| ALP | Polyostotic | T1 | 228.5 | 130–327 | P = 0.083 |
| T2 | 172.6 | 73–272 | |||
| T3 | 138.0 | 40–236 | |||
| MAS | T1 | 252.8 | 113–392 | ||
| T2 | 95.7 | −48–237 | |||
| T3 | 167.8 | 28 –307 | |||
| P1NP | Polyostotic | T1 | 409.8 | 138–682 | P = 0.110 |
| T2 | 380.0 | 103–657 | |||
| T3 | 230.6 | −46–507 | |||
| MAS | T1 | 335.5 | −50–721 | ||
| T2 | 42.0 | −343– 427 | |||
| T3 | 223.0 | −162 –608 | |||
| CTX | Polyostotic | T1 | 357 | 143 –572 | P = 0.010 |
| T2 | 511 | 287–736 | |||
| T3 | 292 | 67 –516 | |||
| MAS | T1 | 521 | 217 –825 | ||
| T2 | 129 | −206 –464 | |||
| T3 | 603 | 300 –907 |
| BTM . | Subtype . | Time . | Mean . | 95% CI . | Significance . |
|---|---|---|---|---|---|
| ALP | Polyostotic | T1 | 228.5 | 130–327 | P = 0.083 |
| T2 | 172.6 | 73–272 | |||
| T3 | 138.0 | 40–236 | |||
| MAS | T1 | 252.8 | 113–392 | ||
| T2 | 95.7 | −48–237 | |||
| T3 | 167.8 | 28 –307 | |||
| P1NP | Polyostotic | T1 | 409.8 | 138–682 | P = 0.110 |
| T2 | 380.0 | 103–657 | |||
| T3 | 230.6 | −46–507 | |||
| MAS | T1 | 335.5 | −50–721 | ||
| T2 | 42.0 | −343– 427 | |||
| T3 | 223.0 | −162 –608 | |||
| CTX | Polyostotic | T1 | 357 | 143 –572 | P = 0.010 |
| T2 | 511 | 287–736 | |||
| T3 | 292 | 67 –516 | |||
| MAS | T1 | 521 | 217 –825 | ||
| T2 | 129 | −206 –464 | |||
| T3 | 603 | 300 –907 |
No statistically significant differences in serum ALP and P1NP values between patients with polyostotic FD and patients with MAS are shown. Serum CTX values differed significantly between patients with MAS and polyostotic FD, although these values should be interpreted with care, because blood samples for serum CTX levels was collected in the nonfasting state.
| BTM . | Doses . | Time . | Mean . | 95% CI . | Significance . |
|---|---|---|---|---|---|
| ALP | 3-mo | T1 | 270.7 | 163 –378 | 0.007 |
| T2 | 173.5 | 66 –281 | |||
| T3 | 118.5 | 11 –226 | |||
| 6–mo | T1 | 202.5 | 95 –310 | ||
| T2 | 116.7 | 6 –228 | |||
| T3 | 177.3 | 70–285 | |||
| P1NP | 3-mo | T1 | 450.3 | 144 –757 | 0.025 |
| T2 | 319.7 | 13 –626 | |||
| T3 | 91.4 | −222–404 | |||
| 6–mo | T1 | 319.7 | 13–626 | ||
| T2 | 175.1 | −149 –499 | |||
| T3 | 342.7 | 36 –649 | |||
| CTX | 3-mo | T1 | 484 | 238 –731 | 0.162 |
| T2 | 555 | 308– 801 | |||
| T3 | 375 | 106 –643 | |||
| 6-mo | T1 | 339 | 93– 586 | ||
| T2 | 129 | −170 –428 | |||
| T3 | 431 | 185– 678 |
| BTM . | Doses . | Time . | Mean . | 95% CI . | Significance . |
|---|---|---|---|---|---|
| ALP | 3-mo | T1 | 270.7 | 163 –378 | 0.007 |
| T2 | 173.5 | 66 –281 | |||
| T3 | 118.5 | 11 –226 | |||
| 6–mo | T1 | 202.5 | 95 –310 | ||
| T2 | 116.7 | 6 –228 | |||
| T3 | 177.3 | 70–285 | |||
| P1NP | 3-mo | T1 | 450.3 | 144 –757 | 0.025 |
| T2 | 319.7 | 13 –626 | |||
| T3 | 91.4 | −222–404 | |||
| 6–mo | T1 | 319.7 | 13–626 | ||
| T2 | 175.1 | −149 –499 | |||
| T3 | 342.7 | 36 –649 | |||
| CTX | 3-mo | T1 | 484 | 238 –731 | 0.162 |
| T2 | 555 | 308– 801 | |||
| T3 | 375 | 106 –643 | |||
| 6-mo | T1 | 339 | 93– 586 | ||
| T2 | 129 | −170 –428 | |||
| T3 | 431 | 185– 678 |
A statistically significant difference over time for ALP and P1NP between patients who received denosumab at 3-mo intervals compared with those with 6-mo intervals is shown.
Reference ranges: ALP 40–98 IU/L, P1NP <59 ng/mL, CTX <573 pg/mL.
| BTM . | Doses . | Time . | Mean . | 95% CI . | Significance . |
|---|---|---|---|---|---|
| ALP | 3-mo | T1 | 270.7 | 163 –378 | 0.007 |
| T2 | 173.5 | 66 –281 | |||
| T3 | 118.5 | 11 –226 | |||
| 6–mo | T1 | 202.5 | 95 –310 | ||
| T2 | 116.7 | 6 –228 | |||
| T3 | 177.3 | 70–285 | |||
| P1NP | 3-mo | T1 | 450.3 | 144 –757 | 0.025 |
| T2 | 319.7 | 13 –626 | |||
| T3 | 91.4 | −222–404 | |||
| 6–mo | T1 | 319.7 | 13–626 | ||
| T2 | 175.1 | −149 –499 | |||
| T3 | 342.7 | 36 –649 | |||
| CTX | 3-mo | T1 | 484 | 238 –731 | 0.162 |
| T2 | 555 | 308– 801 | |||
| T3 | 375 | 106 –643 | |||
| 6-mo | T1 | 339 | 93– 586 | ||
| T2 | 129 | −170 –428 | |||
| T3 | 431 | 185– 678 |
| BTM . | Doses . | Time . | Mean . | 95% CI . | Significance . |
|---|---|---|---|---|---|
| ALP | 3-mo | T1 | 270.7 | 163 –378 | 0.007 |
| T2 | 173.5 | 66 –281 | |||
| T3 | 118.5 | 11 –226 | |||
| 6–mo | T1 | 202.5 | 95 –310 | ||
| T2 | 116.7 | 6 –228 | |||
| T3 | 177.3 | 70–285 | |||
| P1NP | 3-mo | T1 | 450.3 | 144 –757 | 0.025 |
| T2 | 319.7 | 13 –626 | |||
| T3 | 91.4 | −222–404 | |||
| 6–mo | T1 | 319.7 | 13–626 | ||
| T2 | 175.1 | −149 –499 | |||
| T3 | 342.7 | 36 –649 | |||
| CTX | 3-mo | T1 | 484 | 238 –731 | 0.162 |
| T2 | 555 | 308– 801 | |||
| T3 | 375 | 106 –643 | |||
| 6-mo | T1 | 339 | 93– 586 | ||
| T2 | 129 | −170 –428 | |||
| T3 | 431 | 185– 678 |
A statistically significant difference over time for ALP and P1NP between patients who received denosumab at 3-mo intervals compared with those with 6-mo intervals is shown.
Reference ranges: ALP 40–98 IU/L, P1NP <59 ng/mL, CTX <573 pg/mL.
These results indicated that the 6-month denosumab regimen used in the treatment of osteoporosis is inadequate for inducing a sustained reduction of biochemical markers of bone turnover in patients with FD and all patients were subsequently treated with a 3-month schedule of 60 mg of denosumab. In one patient (patient 7, Fig. 2) with severe disease, serum ALP activity decreased from 538 IU/L reaching a plateau of 321 IU/L after 18 months of treatment with denosumab given at 6-month and later 3-month intervals. Continuation of treatment at a dose of 120 mg every 3 months resulted in a further decrease of serum ALP activity to 100 IU/L, a near-normal value. This patient was not included in the further analysis of the pharmacodynamic responses to denosumab treatment.
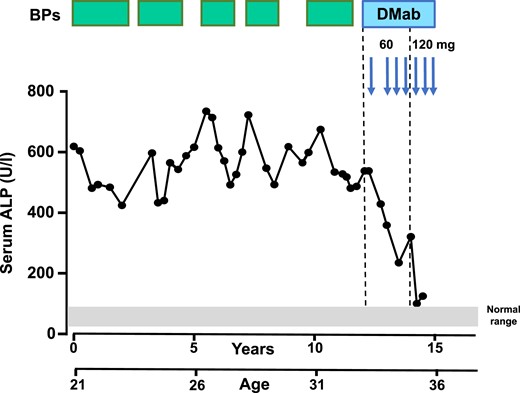
Sequential changes in serum ALP levels in a patient with polyostotic FD (patient 7) during treatment with bisphosphonates (BPs, green boxes) and denosumab (DMab, blue arrows). The gray bar represents the normal laboratory reference range of serum ALP (40 to 98 IU/L). This patient was treated initially with denosumab at a 6-mo interval followed by three 3-mo injections and three 3-mo doses of 120 mg resulting in normalization of bone turnover and reduction in pain complaints. Note the improved response with subcutaneous injections of 60 mg of denosumab and the normalization of serum ALP levels with subsequent injections of 120 mg of denosumab.
In the 11 patients who received 60 mg of denosumab, serum ALP activity decreased from 212 ± 39 IU/L to 79 ± 6 IU/L (P = 0.004) at the end of the observation period (median, 15.5 months). Similarly, serum P1NP values decreased from 346 ± 111 ng/mL to 56 ± 17 ng/mL (P = 0.023) (Fig. 3). Serum CTX decreased from 412 ± 251 pg/mL to 315 ± 318 pg/mL, a nonsignificant decrease (P = 0.403).
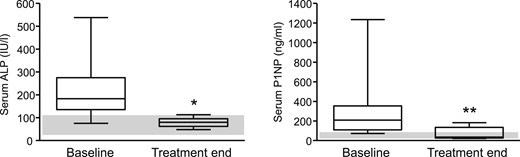
Box plots of median and range of serum ALP (left panel, normal range 40 to 98 IU/L) and serum P1NP (right panel, normal range <59 ng/mL) levels of 11 patients with FD/MAS before and at the end of treatment with denosumab. The gray bars indicate normal ranges for serum ALP and P1NP values. *P = 0.004, **P = 0.023.
At the end of the observation period, of the 10 patients with increased serum ALP activity at baseline, values normalized in 7 (70%) (range final serum ALP: 48 to 113 IU/L), whereas serum P1NP levels that were increased in all patients reached the normal range in 9 of 12 (75%) (range final serum P1NP: 21 to 183 ng/mL). There were no statistically significant differences in response between patients with FD and those with FD/MAS, but numbers were small.
There was no correlation between pretreatment SBS and changes in biochemical parameters of bone turnover under treatment with denosumab. There was, however, a significant correlation between pretreatment and final values of serum ALP activity (Spearman ρ = 0.71, P = 0.015).
Clinical response to treatment
Ten of the 12 patients treated with denosumab reported improvement of pain, 6 of whom reporting complete disappearance of pain. In 3 of the 10 responders, pain decreased early after the first denosumab injection, in 4 it did not change after 3 months, and in 3 patients pain increased transiently (not requiring additional analgesics) at 3 months. Thereafter, pain progressively decreased in all. In 5 of the 6 patients who became pain-free this response was observed 6 to 12 months after the start of treatment. In the 1 patient (patient 7, Fig. 2) although pain decreased substantially with treatment, it disappeared completely after 730 days concurrently with the administration of the highest denosumab dose. These results suggest that longer treatment periods may be needed for optimal clinical benefit in the majority of the patients. Of 8 patients who were using analgesics prior to start of denosumab treatment (paracetamol, n = 5; NSAIDs, n = 3; morphine, n = 2), 5 patients used a lesser dose and/or less frequent and 3 stopped their use. In these patients, reduction of pain symptoms was associated with increased mobility and function, although this was not quantified with validated questionnaires. In 1 patient with severe disease of the right lower limb, in addition to the reported reduction in pain, local tenderness and skin temperature were also reduced over the affected femur. In the patient with craniofacial FD (patient 5) pain levels remained unchanged after the first 3 months and increased after decompression surgery of the optic nerve. Although bone turnover normalized after treatment with denosumab in this patient, postsurgical headaches persisted. No other patient had a surgical procedure during the period of observation.
Adverse events
Treatment was well tolerated and none of the patients developed symptomatic or asymptomatic hypocalcemia. One patient (patient 1) discontinued treatment after one year because of a skin rash thought to be possibly related to denosumab. She was started on bisphosphonates that could not, however, maintain the decreased level of BTMs achieved with denosumab although these did not increase above pretreatment levels nor were there any changes in serum calcium. In two patients with MAS (patients 10 and 12), treatment was associated with transient, asymptomatic, mild decrease in serum phosphate concentration to nadir values of 0.63 mmol/L (normal range, 0.8 to 1.5mmol/L), associated with an increase in intact PTH concentrations to 11.2 and 20.7 pmol/L, respectively, in the absence of changes in serum calcium. Both patients had a decreased tubular phosphate reabsorption in relation to glomerular filtration rate, 0.53 and 0.7, respectively, and increased FGF-23 levels prior to treatment with denosumab. Both were using active vitamin D metabolites. Serum phosphate and PTH concentrations normalized by increasing the dose of vitamin D supplementation and no phosphate supplements were required. There were no cases of osteonecrosis of the jaw or atypical femoral fractures during the period of observation.
Discussion
Data from this case series of 12 patients with mostly polyostotic FD/MAS pretreated with bisphosphonates demonstrated that treatment with denosumab is promising and effective in reducing bone turnover, is relatively safe and well tolerated, and although not quantitively measured, probably also effective in reducing pain, thus representing a potential alternative therapeutic option in patients with FD with inadequate responses to bisphosphonates. Administration of 60 mg of denosumab at six-month intervals was not sufficient to sustain the initial beneficial effect on BTMs, whereas this was achieved in all but one patient with the use of 60 mg of denosumab at three-month intervals.
Although there is as yet no approved medical treatment to control pain and disease activity as reflected by increased BTMs in FD/MAS, various regimens of different bisphosphonates are commonly used in the management of patients with FD/MAS-related bone pain and/or high rates of bone turnover. Several open-label studies, mostly using intravenous pamidronate and olpadronate, have demonstrated reduction of bone turnover and bone pain in adults and children with FD (7, 9–12, 14, 29, 30). However, it is clear from these studies that some patients, and especially those with a higher skeletal disease burden, do not respond properly to treatment with these agents, necessitating alternative treatment options. The only randomized controlled trial using daily oral alendronate in six-month cycles for two years has also failed to demonstrate a beneficial effect compared with placebo (13), although in this study serum ALP activity did not decrease, despite the decrease in CTX, perhaps suggesting that higher doses or shorter interval schedules may have been required to achieve an adequate biochemical response (12). Thus, despite the success of bisphosphonate treatment reported in open studies, it appears that some are refractory to treatment with these agents, demonstrating persistence of pain and inadequate suppression of bone turnover markers, necessitating alternative treatment options.
The upregulation of RANKL expression in skeletal lesions as well as the increased circulating levels of the cytokine that were positively correlated with SBS in patients with FD (4, 31) provide a strong rationale for further exploring the use of the RANKL inhibitor denosumab in the management of severely affected patients with insufficient responses to bisphosphonates. Further support for the potential beneficial effect of this agent is provided by the findings of improvement in radiographic features as well as arrest of development of new lesions in a transgenic mouse model treated with anti-RANKL antibody (32). In addition, published case reports of five individual patients with FD treated with different schedules and doses of denosumab (15–18) described early clinical improvement in the form of reduction of pain in four of the five patients, combined with dramatic reduction in biochemical markers of bone turnover (15–18).
The present case series addresses the issue of denosumab dosing in FD and showed that the dose of denosumab as used in the treatment of osteoporosis (60 mg once every six months) is insufficient to maintain the treatment-induced reduction of BTMs in patients with FD. In contrast, the same dose of 60 mg used at a shorter three-month interval led to a decrease in the levels of BTMs by more than 50% of baseline values leading to their normalization in a substantial number of patients, in some of whom bisphosphonates had failed to do so even after long-term use and high cumulative doses. In our previous study of a larger group of patients with FD/MAS treated with bisphosphonates, we found an association of clinical flares with increased bone turnover markers and abatement of pain symptoms associated in the majority of patients with normalization of these markers in the majority of patients however this has not been the experience in other groups (12).
The relation between baseline and final values of serum ALP activity further indicated that some patients, particularly those with extensive disease, may require higher doses of denosumab to normalize bone turnover as illustrated in the patient who required 120 mg every three months to achieve normalization of bone turnover markers. However, other patients with high SBS (patients 9 to 12) did respond adequately to the 60 mg three-month regimen. Important for clinical practice is the observation that the decrease in biochemical markers of bone turnover reached usually their nadir values within the first six months of treatment with denosumab and, thus, a decision about changing the dose regimen can be made if necessary early in the course of treatment. The pathophysiology of pain in FD is complex and still ill-understood, although several mechanisms have been suggested to play a role in the process (33). The relation between BTMs and pain remains to date elusive, although in a previous study from our group including a larger group of patients with FD/MAS treated with bisphosphonates, we did find an association of clinical flares with increased BTMs and abatement of pain symptoms associated with decrease or normalization of these markers after treatment with bisphosphonates in the majority of patients (12). However, this has not been the experience of other groups.
Of clinical relevance in our case series was the reduction of bone pain in 10 of 12 patients treated with denosumab and even disappearance of pain at some point during treatment in half of the patients. Although pain was not formally assessed using a validated instrument, it was systemically evaluated and recorded by the treating physicians (N.A.T.H., N.M.A.-D.) during the entire period of observation. In 4 of the 5 denosumab-treated patients reported in the literature, reduction of pain was observed early after the start of treatment (15–18). With the three-month treatment regimen, the beneficial effect on pain persisted for the whole observation period. Earlier, pharmacodynamic studies of denosumab have shown dramatic reductions of serum CTX within days after a subcutaneous injection and there is no reason to believe that the response would be different in patients with fibrous dysplasia, however, this remains to be shown (34, 35). Our protocol with blood sampling every three months including for CTX preclude any conclusion about an association between a decrease of bone resorption and the improvement of FD-related pain bone symptoms as missing the maximum early decrease in this marker.
Response to treatment was shown to be irrespective of disease severity as observed by comparing treatment between patients. For example, patient 2 had a low SBS of 0.58 and P1NP levels that were less than two times increased but severe pain, for which she required treatment with paracetamol, NSAIDs and morphine prior to start of denosumab treatment. Patient 12 had the highest SBS (64.4) and a near 14-fold increase in P1NP (812 ng/mL), but only mild FD-related pain symptoms. Denosumab treatment resulted in normalization of BTMs and reduction in pain and improved function in both patients, which allowed patient 2 to discontinue morphine, and only use paracetamol and occasional NSAIDs. Findings from this relatively small case series of adult patients with FDS/MAS of variable skeletal burden suggest, therefore, that a dose of 60 mg administered every three months is associated with a positive clinical and biochemical outcome in the majority of patients irrespective of SBS, and that there is thus so far no rationale for initially using higher doses of denosumab or shorter intervals of administration of this agent in more severely affected patients.
An important question precluded by the design of this study is the optimal duration of denosumab therapy and further management once a clinical and biochemical remission is achieved and it may be deemed reasonable to discontinue treatment. The dilemma arises from the well-established quickly reversible antiresorptive effect of denosumab (in contrast to the long-term antiresorptive effect of bisphosphonates) observed after discontinuation of treatment of osteoporosis, with transient increases in BTMs above pretreatment levels, also described as rebound phenomenon, that are thought to be the result of rapid, synchronous upregulation of osteoclastogenesis as also reported in the treatment of a child with FD, who was pretreated at some stage with intravenous pamidronate (15, 36). This response is intriguing because it was reported recently that patients treated with bisphosphonates followed by denosumab do not generally show a rebound of bone turnover markers after discontinuation of the latter (37). Data from our case series show an increase of BTMs at six months but not above pretreatment levels in the six patients initially treated at six-month intervals compared with a sustained decrease in those treated at three-month intervals. Levels of BTMs increased rapidly but not above pretreatment values in the patient who discontinued denosumab (patient 1), and there was no evidence for hypercalcemia. This suggests a different response from the previously reported pediatric case who demonstrated a dramatic rebound in BTMs and severe hypercalcemia and is likely to be the result of treatment with bisphosphonates prior to starting treatment with denosumab (15). However, our patients were only treated for a relatively short period and without assessment of clinical and biochemical responses after ceasing denosumab apart from patient 1. Whereas this may suggest a different response from that observed in the first reported pediatric case who demonstrated a dramatic rebound in BTMs exceeding pretreatment levels and severe hypercalcemia after discontinuation of denosumab, other factors should be taken in consideration in pediatric cases, such as the higher bone remodeling activity in children and the higher monthly frequency and possible cumulative dose administered over seven months in the child reported (15). It is also of note that in our case series all patients had received long-term treatment with bisphosphonates prior to starting treatment with denosumab, shown to provide some degree of protection against severe rebounds after discontinuation of treatment in patients with osteoporosis (37). We were not in a position to evaluate the effect of discontinuation of denosumab in our case series, because all but one patient were still receiving denosumab every three months with sustained clinical and biochemical effects at the time of analysis of the data.
Until additional data become available from randomized controlled trials, caution should be exercised in the treatment of patients with FD/MAS with denosumab. If such treatment is deemed necessary, it should be administered by physicians with expertise in the management of patients with metabolic bone disorders. A point of caution with the use of antiresorptive agents in patients with FD, which equally applies to the use of bisphosphonates or denosumab, is that attention should be given to correction of any vitamin D deficiency and/or FGF-23–related hypophosphatemia that may potentially aggravate the underlying osteomalacia that is a feature of FD/MAS, before starting antiresorptive therapy.
Although data from this case series are promising, a number of safety issues still need to be addressed before this potent antiresorptive could be advocated for widespread use in FD/MAS Until additional data become available from randomized controlled trials, the use of denosumab is thus not recommended outside specialist centers with expertise in the management of patients with metabolic bone disorders, preferably in the context of clinical trials.
We believe that data presented in this case series, together with findings from published case reports provide sufficient background and rationale for the design of a randomized controlled study on the efficacy and tolerability of denosumab in patients with FD/MAS refractory or not tolerating treatment with bisphosphonates and for whom no alternative therapeutic option is currently available.
Acknowledgments
Financial Support: B.C.J.M. was supported by a grant from the Bontius Foundation, Netherlands, a nonprofit institution supporting research within the Leiden University Medical Center. No funding was provided by any pharmaceutical company.
Disclosure Summary: S.E.P. received consulting and speaking fees from Amgen and UCB and consulting fees from Axsome, Gador. and Radius Health. N.M.A.-D. received consulting fees for scientific advisory board meetings for Amgen, Netherlands. The other authors do not have any disclosures to report.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations:
- 25-OHD
25-OH vitamin D
- ALP
alkaline phosphatase
- BTM
bone turnover marker
- FD
fibrous dysplasia
- FGF
fibroblast growth factor
- MAS
McCune-Albright syndrome
- NSAID
nonsteroidal anti-inflammatory drug
- P1NP
terminal propeptide of type 1 procollagen
- RANKL
receptor activator of nuclear factor-κB ligand
- SBS
skeletal bone score



