-
PDF
- Split View
-
Views
-
Cite
Cite
Herman Depypere, Dirk Timmerman, Gilbert Donders, Peter Sieprath, Steven Ramael, Jean Combalbert, Hamid R Hoveyda, Graeme L Fraser, Treatment of Menopausal Vasomotor Symptoms With Fezolinetant, a Neurokinin 3 Receptor Antagonist: A Phase 2a Trial, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 12, December 2019, Pages 5893–5905, https://doi.org/10.1210/jc.2019-00677
Close - Share Icon Share
Abstract
The thermoregulatory center in the hypothalamus is stimulated by neurokinin 3 receptor (NK3R) activation and inhibited by estrogen-negative feedback. This balance is disrupted in menopause, producing vasomotor symptoms (VMSs).
To evaluate safety and efficacy of the NK3R antagonist fezolinetant in menopausal VMSs.
Twelve-week, double-blind, randomized, placebo-controlled study.
Eight Belgian centers from September 2015 to October 2016.
Generally healthy menopausal women aged 40 to 65 years with moderate/severe VMSs.
Subjects were randomized (1:1) to 90 mg of fezolinetant twice daily or placebo for 12 weeks.
Subjects captured VMS severity and frequency using an electronic diary. The primary outcome was change from baseline to week 12 in total VMS score with fezolinetant vs placebo. Secondary outcomes included timing of changes in frequency and severity of moderate/severe VMSs and quality-of-life assessments at weeks 4, 8, and 12. Pharmacodynamic and pharmacokinetic effects were assessed, as were safety and tolerability.
Of 122 subjects screened, 87 were randomized and 80 (92%) completed the study. At week 12, fezolinetant significantly reduced total VMS score vs placebo (−26.5 vs −12.2, P < 0.001) and decreased mean frequency of moderate/severe VMSs by five episodes per day vs placebo. Severity and frequency of moderate/severe VMSs were reduced from the first day of treatment. Improvements were achieved in all quality-of-life measures. Fezolinetant was well tolerated. The most common fezolinetant-related adverse event was gastrointestinal disorder (n = 6).
Fezolinetant rapidly and significantly reduced moderate/severe VMSs, supporting its potential as an effective nonhormonal treatment option for menopausal women.
Vasomotor symptoms (VMSs) (e.g., hot flashes, night sweats) are reported by up to 80% of women in the United States during the menopausal transition (1) and persist for a median duration of 7.4 years (2). VMSs are the main menopause-related problem prompting women to seek medical treatment (3), and studies indicate that untreated VMSs impart a substantial, direct cost burden on society (4, 5). Menopausal hormone therapy (MHT; also known as hormone replacement therapy) is currently the standard treatment of VMSs, although use continues to decline (6), mainly because of perceived safety concerns, including the risks of venous thromboembolism and breast cancer (7, 8). Although the overall risk-benefit of MHT was recently reassessed (9, 10), concerns persist and women continue to seek alternatives for treatment of VMSs (11, 12).
VMSs are caused by a loss of thermoregulatory control. The thermoregulatory center in the hypothalamus is innervated by kisspeptin/neurokinin B/dynorphin (KNDy) neurons (13–18) that are stimulated by neurokinin B (NKB) (19–22) and inhibited by estrogen (23–25) as part of the feedback mechanism that supports female reproductive endocrinology and fertility (13–16, 19–25). This balance is disrupted with the decline of estrogen levels in menopause, and it has been proposed that the consequent, unregulated KNDy neuron activation is a key, underlying component of VMSs (26, 27). Thus, negative modulation of KNDy neuron signaling via antagonism of NKB signaling at its cognate receptor [neurokinin 3 receptor (NK3R)] may offer an alternative mechanism to MHT for control of VMSs (16).
Fezolinetant (ESN364) (28) is an oral NK3R antagonist that is currently in clinical development for VMSs associated with menopause. Fezolinetant selectively and reversibly blocks NKB signaling, slowing GnRH pulse frequency consistent with a decrease in KNDy neuron activity (29, 30). In this report, we present the efficacy and safety profile of fezolinetant from the first pilot/phase 2a clinical trial of an NK3 antagonist conducted according to US Food and Drug Administration (FDA) guidelines for the clinical evaluation of treatments for VMSs (31, 32). The primary objective of the study was to evaluate the effect of fezolinetant on the severity and frequency of VMSs in menopausal women. Secondary objectives were to evaluate the effect of fezolinetant on (i) time course of effects on VMSs, (ii) quality of life, (iii) pharmacodynamic effects on reproductive hormones, and (iv) safety and tolerability. Assessment of fezolinetant pharmacokinetics was an exploratory objective.
Materials and Methods
Study design
This was a 12-week, double-blind, placebo-controlled study in menopausal women, designed in accordance with FDA guidance (31) and conducted from September 2015 to October 2016 at eight Belgian clinical centers. Following a ≤4-week screening period, subjects were randomized on day 1 to receive placebo or 90 mg of fezolinetant twice daily for 12 weeks. Subjects returned to the clinic for assessment at weeks 4, 8, and 12 and were followed for 2 to 3 weeks after the end of treatment.
Randomization was based on a computer-generated schedule, balanced using randomly permuted blocks across treatment groups without stratification, and prepared by SGS Life Science Services using SAS® software (SAS Institute, Cary, NC). Subjects, investigators, other employees of the clinical site, and the sponsor were blinded to treatment assignment. To ensure blinding, the placebo was identical to active treatment with regard to appearance, taste, and smell.
All subjects provided written informed consent prior to the study. The study protocol was approved by an Independent Ethics Committee at each center. This study has been registered with EudraCT (2015-002578-20).
Subject eligibility criteria
This study enrolled women aged 40 to 65 years in good general health who had reached menopause and were experiencing moderate or severe VMSs. Menopause was defined as spontaneous amenorrhea for ≥12 consecutive months; or spontaneous amenorrhea for ≥6 months with biochemical criteria of menopause (FSH >40 IU/L); or spontaneous amenorrhea for ≥3 months with FSH >40 IU/L and estradiol (E2) <0.21 nmol/L; or bilateral oophorectomy ≥6 weeks prior to screening. Only subjects who recorded ≥7 episodes of moderate/severe VMSs (hot flashes or night sweats) per day over a period of 7 consecutive days (i.e., ≥49 moderate/severe VMS episodes) during the screening period were included in the study.
Subjects were excluded from the study if they had any medical condition that could confound results (e.g., history of drug/alcohol abuse, suicide event in the last 3 years, active liver disease/jaundice, elevated liver function enzymes, impaired kidney function). Women with a recent history (within 1 year prior to screening) of a psychological disorder according to Diagnostic and Statistical Manual of Mental Disorders (4th edition) criteria were excluded; such disorders included current major depression.
Women were also excluded if they were taking prohibited medications. These medications were defined as drugs that could interfere with the occurrence of VMSs, including selective serotonin reuptake inhibitors, selective norepinephrine reuptake inhibitors, tricyclic antidepressants, sedatives, or hypnotics. Use of hormonal medications, such as MHT, hormonal contraception, or any treatment of hot flashes (prescription, over the counter, or herbal), was not allowed during the study; these treatments were to be discontinued at least 6 weeks prior to screening.
Study drug administration
Subjects were sequentially randomized (1:1 ratio according to the randomization schedule) to receive placebo or 90 mg of fezolinetant 90 twice daily, self-administered as a single gelatin capsule with water and a light meal in the morning and in the evening. On days when site visits where scheduled, treatment was administered under fasting condition at the clinical site. Compliance was assessed by counting returned dosage units in addition to electronic patient-reported outcome (ePRO) entries upon study medication intake.
Study assessments and related outcomes
VMS severity and frequency were recorded at least twice daily from screening through follow-up visits using an ePRO diary. Data collected during the screening period were used to determine the baseline values for VMS score and severity and frequency of moderate/severe VMSs. The electronic diaries were kept by the patients from screening onward through the end-of-study visit. Patients were instructed to fill out the diaries at least twice daily for the duration of the study. The data recorded for ≥7 days during the screening period were used to assess eligibility.
As defined in FDA guidelines (31), the severity of VMSs was classified as mild (sensation of heat without sweating; at night, the subject did not wake up but later noticed damp sheets or clothing), moderate (sensation of heat with sweating; at night, subject woke up), and severe [sensation of heat with sweating, causing cessation of activity; at night, subject woke up and took action (e.g., opening window)]. As a measure of severity, the mean daily total VMS score, a composite combining both severity and frequency data, was used. The mean daily total VMS score during a given period was calculated by multiplying the number of mild, moderate, or severe VMS episodes during the period by 1, 2, or 3, respectively, summing the values and dividing by the number of days in the period (33). A higher score indicates worse symptoms; there is no maximum score.
Frequency (number of episodes) and severity score (calculated as for total VMS score) of moderate/severe VMSs were assessed at weeks 4, 8, 12, and at follow-up, taking into account only moderate and severe symptoms, as per guidelines (31). Post hoc analysis evaluated the response each day during the first week of treatment.
The ePROs included quality-of-life questionnaires to assess perceived daily interference using the Hot Flash Related Daily Interference Scale (HFRDIS) (34), sleep quality using the Leeds Sleep Evaluation Questionnaire (LSEQ) (35), changes in climacteric symptoms using the Greene Climacteric Scale (GCS) (36), and daily functional impairment using the Sheehan Disability Scale (SDS) (37, 38) (all evaluated at baseline and at weeks 4, 8, and 12).
Blood samples for pharmacokinetic assessments were collected predose at baseline and weeks 4, 8, and 12, and 3 hours postdose at week 12 into vacuum tubes containing lithium heparin (Venoject green top or equivalent). Samples were centrifuged at 4°C for 10 minutes at ≈1500 × g and plasma was stored frozen at −20°C or below until analysis. Blood samples for the assessment of hormone biomarkers (including LH, E2, FSH, and SHBG levels) were collected at screening and predose at baseline and weeks 4, 8, and 12, and 3 hours postdose at week 12 [coincident with the previously determined tmax of fezolinetant (30)], and at follow-up. After appropriate labeling, plasma samples were stored frozen at −70°C or below until analysis.
Safety and tolerability were assessed by the occurrence of treatment-emergent adverse events (TEAEs), physical examination results [including suicidal behavior using Columbia-Suicide Severity Rating Scale (39)], clinical laboratory tests, vital signs, electrocardiograms, and plasma bone density markers (bone alkaline phosphatase and carboxyl-terminal telopeptide of type I collagen). Adverse events were monitored continuously from informed consent until the last study-related activity. At regular intervals during the study, participants were asked nonleading questions to determine the occurrence of any adverse events. All adverse events reported spontaneously during the course of the study were recorded as well.
The primary endpoint was change from baseline to week 12 in daily VMS score (averaged during the course of a week). Secondary endpoints included changes from baseline over time in weekly VMS frequency and severity scores, as well as scores on the HFRDIS, LSEQ, GCS, and SDS.
Analytical methods
Fezolinetant concentration was determined in lithium-heparinized human plasma using a validated liquid chromatography method with tandem mass spectrometric detection. Plasma samples for pharmacodynamic assessments were analyzed by Barc Laboratory (Gent, Belgium) using previously validated analytical methods: FSH, LH, and SHBG levels were determined using electrochemiluminescence immunoassay analyzed on a Roche Diagnostics cobas e immunoassay analyzer; E2 levels were determined using chemiluminescent microparticle immunoassay using an LKB gamma counter or PerkinElmer Wizard.
Statistical methods
A total of 80 subjects were planned to be randomized. Given the exploratory nature of this study and the lack of information on the effect size of NK3 antagonism, no formal power calculations were performed. The sample size determination was based on general recommendations for studies using VMS diary data, as well as approximate placebo and treatment effects from previous studies related to VMSs, while still allowing for some dropouts (33, 40). Additional statistical analyses were also conducted for further insight.
The safety population, used for biomarker, pharmacokinetic, and safety analyses, included all subjects who were randomized into the study and received at least one dose of the study drug. The modified intent-to-treat (mITT) population, used for all efficacy analyses, included all randomized subjects who received at least one dose of the study medication and who had postbaseline efficacy data. A per-protocol population, used only for sensitivity analyses on the primary endpoint, included those from the mITT population who had no major protocol violations.
Descriptive statistics were used to summarize changes in VMSs from baseline over time. Differences between treatment means were analyzed using an analysis of covariance model with treatment as fixed effect and baseline as covariate (observed cases approach). Quality-of-life questionnaire (HFRDIS, GCS, SDS, and LSEQ) results were evaluated using similar analysis of covariance models as well as descriptive statistics of actual values and changes from baseline. P values for secondary endpoints were interpreted descriptively, and no adjustment for multiplicity was made. Descriptive statistics were used to summarize safety data.
Baseline VMS data were calculated from the week preceding randomization. Safety and efficacy parameters were based on observed cases. Two sensitivity analyses were performed on the primary endpoint, one in which missing values were imputed using a last observation carried forward approach and one using the per-protocol population (based on observed cases without imputation).
Statistical analyses were performed by SGS Life Sciences using SAS® software (SAS Institute; version 9.2 or higher) for statistical computations and interpreted using a two-sided significance level of 0.05.
Results
Subject disposition and baseline characteristics
Of 122 subjects screened, 87 were randomized to receive fezolinetant (n = 43; 93% completed the study) or placebo (n = 44; 91% completed the study) (Fig. 1). All subjects were compliant throughout the study, except for one (later revealed as receiving placebo) who discontinued the study because of lack of improvement of her symptoms.
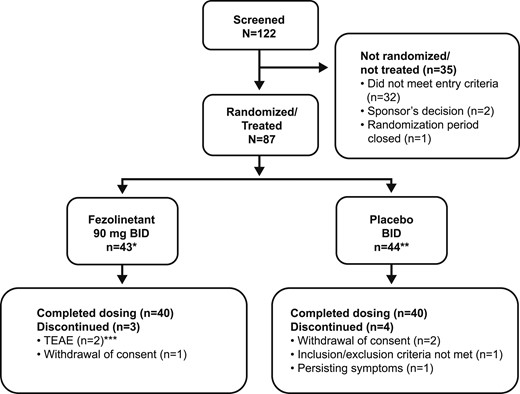
Patient disposition. *One subject randomized to receive fezolinetant treatment did not meet the inclusion criterion of ≥7 episodes of moderate or severe VMSs (hot flashes or night sweats) during a period of 7 consecutive days (i.e., ≥49 moderate/severe VMS episodes) during the screening period. This subject also did not meet the exclusion criteria relating to active liver disease/jaundice, elevated liver function enzymes, and impaired kidney function. This subject completed the trial. **Four subjects randomized to placebo did not meet inclusion or exclusion criteria. Two of these subjects did not meet inclusion criteria relating to definition of menopause, although they met criteria for VMSs. A different subject randomized to placebo did not meet the inclusion criterion of ≥7 episodes of moderate/severe VMSs (hot flashes or night sweats) during a period of 7 consecutive days (i.e., ≥49 moderate or severe VMS episodes) during the screening period. A further subject randomized to placebo did not meet the exclusion criteria relating to active liver disease/jaundice, elevated liver function enzymes, and impaired kidney function. Three of these subjects completed the trial. ***Considered possibly related to treatment (one subject with preexisting fibromyalgia experienced worsening of fibromyalgia, depression, dry mouth, headache, palpitations, diarrhea, and vomiting; one subject experienced headache and vertigo).
Demographics and baseline characteristics were well matched between the placebo and fezolinetant groups (Table 1). All subjects were women, with a mean age of 53.5 years (range, 44 to 64 years) and mean (SD) body mass index of 25.8 (5.49) kg/m2. Mean daily total VMS score at baseline was 25.8 (95% CI, 22.6, 28.9) in the placebo group and 28.8 (95% CI, 24.6, 32.9) in the fezolinetant group. At baseline, mean frequency of moderate/severe VMSs was 72.0 episodes per week (95% CI, 63.9, 80.1) in the placebo group and 80.7 episodes per week (95% CI, 70.6, 90.8) in the fezolinetant group. Mean daily moderate/severe VMS score at baseline was 24.9 (95% CI, 21.7, 28.1) in the placebo group and 27.8 (95% CI, 23.7, 32.0) in the fezolinetant group.
| . | Placebo Twice Daily (n = 44) . | Fezolinetant (90 mg) Twice Daily (n = 43) . |
|---|---|---|
| Race, n (%) | ||
| White | 44 (100) | 42 (97.7) |
| Multiple | 0 | 1 (2.3) |
| Age, y, mean (SD) | 53.7 (4.25) | 53.3 (4.03) |
| Height, cm, mean (SD) | 163.6 (6.04) | 165.7 (5.53) |
| Weight, kg, mean (SD) | 70.8 (16.27) | 69.0 (13.19) |
| Body mass index, mean kg/m2 (range) | 26.5 (6.15) | 25.1 (4.71) |
| Total VMS score, daily (measured during 1 wk) | ||
| Mean | 25.8 | 28.8 |
| 95% CI | 22.6, 28.9 | 24.6, 32.9 |
| Frequency of moderate/severe VMSs, number per wk | ||
| Mean | 72.0 | 80.7 |
| 95% CI | 63.9, 80.1 | 70.6, 90.8 |
| Moderate/severe VMS score, daily (measured during 1 wk) | ||
| Mean | 24.9 | 27.8 |
| 95% CI | 21.7, 28.1 | 23.7, 32.0 |
| . | Placebo Twice Daily (n = 44) . | Fezolinetant (90 mg) Twice Daily (n = 43) . |
|---|---|---|
| Race, n (%) | ||
| White | 44 (100) | 42 (97.7) |
| Multiple | 0 | 1 (2.3) |
| Age, y, mean (SD) | 53.7 (4.25) | 53.3 (4.03) |
| Height, cm, mean (SD) | 163.6 (6.04) | 165.7 (5.53) |
| Weight, kg, mean (SD) | 70.8 (16.27) | 69.0 (13.19) |
| Body mass index, mean kg/m2 (range) | 26.5 (6.15) | 25.1 (4.71) |
| Total VMS score, daily (measured during 1 wk) | ||
| Mean | 25.8 | 28.8 |
| 95% CI | 22.6, 28.9 | 24.6, 32.9 |
| Frequency of moderate/severe VMSs, number per wk | ||
| Mean | 72.0 | 80.7 |
| 95% CI | 63.9, 80.1 | 70.6, 90.8 |
| Moderate/severe VMS score, daily (measured during 1 wk) | ||
| Mean | 24.9 | 27.8 |
| 95% CI | 21.7, 28.1 | 23.7, 32.0 |
| . | Placebo Twice Daily (n = 44) . | Fezolinetant (90 mg) Twice Daily (n = 43) . |
|---|---|---|
| Race, n (%) | ||
| White | 44 (100) | 42 (97.7) |
| Multiple | 0 | 1 (2.3) |
| Age, y, mean (SD) | 53.7 (4.25) | 53.3 (4.03) |
| Height, cm, mean (SD) | 163.6 (6.04) | 165.7 (5.53) |
| Weight, kg, mean (SD) | 70.8 (16.27) | 69.0 (13.19) |
| Body mass index, mean kg/m2 (range) | 26.5 (6.15) | 25.1 (4.71) |
| Total VMS score, daily (measured during 1 wk) | ||
| Mean | 25.8 | 28.8 |
| 95% CI | 22.6, 28.9 | 24.6, 32.9 |
| Frequency of moderate/severe VMSs, number per wk | ||
| Mean | 72.0 | 80.7 |
| 95% CI | 63.9, 80.1 | 70.6, 90.8 |
| Moderate/severe VMS score, daily (measured during 1 wk) | ||
| Mean | 24.9 | 27.8 |
| 95% CI | 21.7, 28.1 | 23.7, 32.0 |
| . | Placebo Twice Daily (n = 44) . | Fezolinetant (90 mg) Twice Daily (n = 43) . |
|---|---|---|
| Race, n (%) | ||
| White | 44 (100) | 42 (97.7) |
| Multiple | 0 | 1 (2.3) |
| Age, y, mean (SD) | 53.7 (4.25) | 53.3 (4.03) |
| Height, cm, mean (SD) | 163.6 (6.04) | 165.7 (5.53) |
| Weight, kg, mean (SD) | 70.8 (16.27) | 69.0 (13.19) |
| Body mass index, mean kg/m2 (range) | 26.5 (6.15) | 25.1 (4.71) |
| Total VMS score, daily (measured during 1 wk) | ||
| Mean | 25.8 | 28.8 |
| 95% CI | 22.6, 28.9 | 24.6, 32.9 |
| Frequency of moderate/severe VMSs, number per wk | ||
| Mean | 72.0 | 80.7 |
| 95% CI | 63.9, 80.1 | 70.6, 90.8 |
| Moderate/severe VMS score, daily (measured during 1 wk) | ||
| Mean | 24.9 | 27.8 |
| 95% CI | 21.7, 28.1 | 23.7, 32.0 |
Efficacy
At week 12, mean daily total VMS score was 14.4 (95% CI, 9.8, 19.0) with placebo and 2.7 (95% CI, 1.4, 4.0) with fezolinetant. On the primary endpoint, fezolinetant resulted in a significantly greater reduction in daily total VMS score from baseline to week 12 (−26.5; 95% CI, −30.8, −22.2) than placebo [−12.2; 95% CI, −16.5, −7.8; least squares mean difference (LSMD) −12.3; 95% CI, −16.9, −7.8; P < 0.001]. Mean daily total VMS score was also reduced with fezolinetant compared with placebo at week 4 and week 8 (Fig. 2A). Sensitivity analyses performed on the per protocol population (observed cases) and on the mITT population using a last observation carried forward imputation method were consistent with the primary analysis and confirmed the statistically significant difference (P < 0.001) in VMS score between fezolinetant and placebo at week 12.
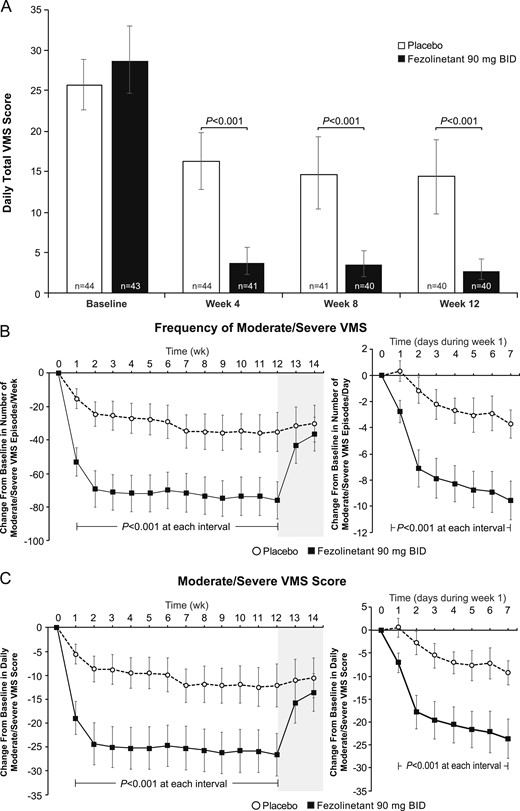
Effect of fezolinetant on VMSs over time. (A) Daily total VMS score during week 4 and week 12. (B) Frequency of moderate/severe VMSs during the duration of the study. (C) Moderate/severe VMS score during the duration of the study. Data are presented as mean ± 95% CI (mITT population, observed data).
At week 12, mean frequency of moderate/severe VMSs was 39.0 episodes per week (95% CI, 26.6, 51.5) with placebo and 5.7 episodes per week (95% CI, 2.4, 9.1) with fezolinetant. Relative to baseline, VMS frequency was reduced by 93% with fezolinetant compared with 46% with placebo. Fezolinetant resulted in a greater reduction from baseline in frequency of moderate/severe VMSs (−76.1 episodes per week; 95% CI, −87.2, −65.0) than placebo (−35.3 episodes per week; 95% CI, −46.9, −23.6; LSMD, −35.2; 95% CI, −47.6, −22.8; P < 0.001). Fezolinetant also reduced the frequency of moderate/severe VMSs more than placebo at week 4 and at all other time periods from the first day of treatment (Fig. 2B).
At week 12, the mean daily moderate/severe VMS score was 13.5 (95% CI, 8.9, 18.2) with placebo and 1.7 (95% CI, 0.7, 2.7) with fezolinetant. Fezolinetant resulted in a greater reduction from baseline in daily moderate/severe VMS score (−26.6; 95% CI, −31.1, −22.2) than placebo (−12.1; 95% CI, −16.6, −7.7; LSMD, −12.4; 95% CI, −17.0, −7.8; P < 0.001), equivalent to five fewer moderate/severe VMS episodes each day. Fezolinetant also reduced the mean daily moderate/severe VMS score more than placebo at week 4 and at all other time periods from the first day of treatment (Fig. 2C).
Fezolinetant treatment resulted in improvement from baseline in sleep quality, overall daily interference, climacteric symptoms, and function at weeks 4, 8, and 12 (Fig. 3). Of the 22 additional subdomains in the questionnaires used, 18 showed improvement from baseline at week 12 of fezolinetant treatment, with most (n = 16) improved at the first time assessed (Table 2). There was no significant impact of fezolinetant at any time point on physical symptoms and loss of interest in sex, as assessed by the GCS.

Effect of fezolinetant on quality-of-life measures. (A) LSEQ (does not include a total mean score that incorporates all four dimensions assessed). (B) HFRDIS. (C) GCS. (D) SDS. Data are presented as mean ± 95% CI (mITT population).
Effect of Fezolinetant on Subdomains of Quality-of-Life Measures (mITT Population)
| Subdomain . | Week 4 . | Week 8 . | Week 12 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LSMD for Fezolinetant vs Placebo . | 95% CI . | P Value . | LSMD for Fezolinetant vs Placebo . | 95% CI . | P Value . | LSMD for Fezolinetant vs Placebo . | 95% CI . | P Value . | |
| LSEQ | |||||||||
| Quality of sleep | 2.423 | 1.308, 3.539 | <0.001 | 2.291 | 1.331, 3.251 | <0.001 | 2.433 | 1.334, 3.532 | <0.001 |
| Getting to sleep | 1.375 | 0.651, 2.100 | <0.001 | 1.166 | 0.505, 1.827 | <0.001 | 0.895 | 0.190, 1.599 | 0.014 |
| Awake following sleep | 0.877 | −0.034, 1.789 | 0.059 | 1.457 | 0.579, 2.335 | 0.001 | 1.113 | 0.107, 2.120 | 0.031 |
| Behavior following wakening | 1.203 | 0.317, 2.088 | 0.008 | 1.597 | 0.639, 2.556 | 0.001 | 0.842 | −0.116, 1.800 | 0.084 |
| HFRDIS | |||||||||
| Overall mean score | −1.98 | −2.73, −1.23 | <0.001 | −2.21 | −3.02, −1.40 | <0.001 | −1.98 | −2.83, −1.13 | <0.001 |
| Work | −2.53 | −3.45, −1.60 | <0.001 | −2.29 | −3.15, −1.42 | <0.001 | −2.11 | −3.03, −1.20 | <0.001 |
| Social activities | −2.33 | −3.19, −1.46 | <0.001 | −2.34 | −3.21, −1.47 | <0.001 | −2.19 | −3.09, −1.29 | <0.001 |
| Leisure activities | −2.15 | −2.99, −1.31 | <0.001 | −2.37 | −3.19, −1.55 | <0.001 | −1.64 | −2.66, −0.62 | 0.002 |
| Sleep | −2.93 | −4.08, −1.78 | <0.001 | −2.71 | −3.86, −1.57 | <0.001 | −2.61 | −3.67, −1.54 | <0.001 |
| Mood | −1.74 | −2.67, −0.81 | <0.001 | −2.12 | −3.09, −1.16 | <0.001 | −1.79 | −2.69, −0.88 | <0.001 |
| Concentration | −1.92 | −2.86, −0.97 | <0.001 | −2.23 | −3.21, −1.25 | <0.001 | −1.90 | −2.91, −0.88 | <0.001 |
| Relations with others | −1.33 | −2.20, −0.46 | 0.003 | −1.85 | −2.80, −0.90 | <0.001 | −1.72 | −2.66, −0.77 | <0.001 |
| Sexuality | −1.06 | −2.25, 0.13 | 0.081 | −1.40 | −2.80, 0.00 | 0.050 | −1.51 | −2.84, −0.19 | 0.026 |
| Enjoyment of life | −1.65 | −2.51, −0.79 | <0.001 | −2.07 | −2.97, −1.18 | <0.001 | −1.83 | −2.88, −0.78 | <0.001 |
| Overall quality of life | −1.92 | −2.86, −0.97 | <0.001 | −2.51 | −3.41, −1.61 | <0.001 | −2.36 | −3.31, −1.41 | <0.001 |
| GCS | |||||||||
| Total score | −4.6 | −8.2, −1.0 | 0.013 | −6.9 | −10.7, −3.1 | <0.001 | −6.3 | −9.9, −2.8 | <0.001 |
| Loss of interest in sex | 0.0 | −0.3, 0.3 | 0.881 | −0.2 | −0.6, 0.2 | 0.383 | −0.2 | −0.6, 0.2 | 0.301 |
| Psychological | −2.5 | −4.5, −0.4 | 0.020 | −3.3 | −5.4, −1.2 | 0.003 | −3.1 | −5.2, −1.0 | 0.005 |
| Physical | −0.2 | −1.2, 0.9 | 0.732 | −0.7 | −1.9, 0.6 | 0.282 | −0.6 | −1.7, 0.5 | 0.254 |
| Vasomotor | −2.0 | −2.7, −1.4 | <0.001 | −1.8 | −2.4, −1.1 | <0.001 | −2.0 | −2.6, −1.3 | <0.001 |
| SDS | |||||||||
| Global functional impairment | −4.4 | −6.9, −2.0 | <0.001 | −5.8 | −8.0, −3.6 | <0.001 | −5.3 | −7.8, −2.8 | <0.001 |
| Work/school | −1.7 | −2.7, −0.8 | <0.001 | −2.0 | −2.8, −1.3 | <0.001 | −1.6 | −2.5, −0.8 | <0.001 |
| Social life | −1.4 | −2.2, −0.6 | <0.001 | −1.9 | −2.7, −1.1 | <0.001 | −1.6 | −2.4, −0.7 | <0.001 |
| Family life/home responsibilities | −1.3 | −2.2, −0.4 | 0.005 | −1.7 | −2.5, −0.9 | <0.001 | −1.6 | −2.4, −0.7 | <0.001 |
| Days lost | 0.0 | −0.5, 0.6 | 0.936 | 0.0 | −0.1, 0.1 | 0.884 | −0.2 | −0.4, 0.1 | 0.124 |
| Days unproductive | −0.9 | −1.8, 0.0 | 0.052 | −0.9 | −1.7, 0.0 | 0.060 | −0.8 | −1.7, 0.0 | 0.049 |
| Subdomain . | Week 4 . | Week 8 . | Week 12 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LSMD for Fezolinetant vs Placebo . | 95% CI . | P Value . | LSMD for Fezolinetant vs Placebo . | 95% CI . | P Value . | LSMD for Fezolinetant vs Placebo . | 95% CI . | P Value . | |
| LSEQ | |||||||||
| Quality of sleep | 2.423 | 1.308, 3.539 | <0.001 | 2.291 | 1.331, 3.251 | <0.001 | 2.433 | 1.334, 3.532 | <0.001 |
| Getting to sleep | 1.375 | 0.651, 2.100 | <0.001 | 1.166 | 0.505, 1.827 | <0.001 | 0.895 | 0.190, 1.599 | 0.014 |
| Awake following sleep | 0.877 | −0.034, 1.789 | 0.059 | 1.457 | 0.579, 2.335 | 0.001 | 1.113 | 0.107, 2.120 | 0.031 |
| Behavior following wakening | 1.203 | 0.317, 2.088 | 0.008 | 1.597 | 0.639, 2.556 | 0.001 | 0.842 | −0.116, 1.800 | 0.084 |
| HFRDIS | |||||||||
| Overall mean score | −1.98 | −2.73, −1.23 | <0.001 | −2.21 | −3.02, −1.40 | <0.001 | −1.98 | −2.83, −1.13 | <0.001 |
| Work | −2.53 | −3.45, −1.60 | <0.001 | −2.29 | −3.15, −1.42 | <0.001 | −2.11 | −3.03, −1.20 | <0.001 |
| Social activities | −2.33 | −3.19, −1.46 | <0.001 | −2.34 | −3.21, −1.47 | <0.001 | −2.19 | −3.09, −1.29 | <0.001 |
| Leisure activities | −2.15 | −2.99, −1.31 | <0.001 | −2.37 | −3.19, −1.55 | <0.001 | −1.64 | −2.66, −0.62 | 0.002 |
| Sleep | −2.93 | −4.08, −1.78 | <0.001 | −2.71 | −3.86, −1.57 | <0.001 | −2.61 | −3.67, −1.54 | <0.001 |
| Mood | −1.74 | −2.67, −0.81 | <0.001 | −2.12 | −3.09, −1.16 | <0.001 | −1.79 | −2.69, −0.88 | <0.001 |
| Concentration | −1.92 | −2.86, −0.97 | <0.001 | −2.23 | −3.21, −1.25 | <0.001 | −1.90 | −2.91, −0.88 | <0.001 |
| Relations with others | −1.33 | −2.20, −0.46 | 0.003 | −1.85 | −2.80, −0.90 | <0.001 | −1.72 | −2.66, −0.77 | <0.001 |
| Sexuality | −1.06 | −2.25, 0.13 | 0.081 | −1.40 | −2.80, 0.00 | 0.050 | −1.51 | −2.84, −0.19 | 0.026 |
| Enjoyment of life | −1.65 | −2.51, −0.79 | <0.001 | −2.07 | −2.97, −1.18 | <0.001 | −1.83 | −2.88, −0.78 | <0.001 |
| Overall quality of life | −1.92 | −2.86, −0.97 | <0.001 | −2.51 | −3.41, −1.61 | <0.001 | −2.36 | −3.31, −1.41 | <0.001 |
| GCS | |||||||||
| Total score | −4.6 | −8.2, −1.0 | 0.013 | −6.9 | −10.7, −3.1 | <0.001 | −6.3 | −9.9, −2.8 | <0.001 |
| Loss of interest in sex | 0.0 | −0.3, 0.3 | 0.881 | −0.2 | −0.6, 0.2 | 0.383 | −0.2 | −0.6, 0.2 | 0.301 |
| Psychological | −2.5 | −4.5, −0.4 | 0.020 | −3.3 | −5.4, −1.2 | 0.003 | −3.1 | −5.2, −1.0 | 0.005 |
| Physical | −0.2 | −1.2, 0.9 | 0.732 | −0.7 | −1.9, 0.6 | 0.282 | −0.6 | −1.7, 0.5 | 0.254 |
| Vasomotor | −2.0 | −2.7, −1.4 | <0.001 | −1.8 | −2.4, −1.1 | <0.001 | −2.0 | −2.6, −1.3 | <0.001 |
| SDS | |||||||||
| Global functional impairment | −4.4 | −6.9, −2.0 | <0.001 | −5.8 | −8.0, −3.6 | <0.001 | −5.3 | −7.8, −2.8 | <0.001 |
| Work/school | −1.7 | −2.7, −0.8 | <0.001 | −2.0 | −2.8, −1.3 | <0.001 | −1.6 | −2.5, −0.8 | <0.001 |
| Social life | −1.4 | −2.2, −0.6 | <0.001 | −1.9 | −2.7, −1.1 | <0.001 | −1.6 | −2.4, −0.7 | <0.001 |
| Family life/home responsibilities | −1.3 | −2.2, −0.4 | 0.005 | −1.7 | −2.5, −0.9 | <0.001 | −1.6 | −2.4, −0.7 | <0.001 |
| Days lost | 0.0 | −0.5, 0.6 | 0.936 | 0.0 | −0.1, 0.1 | 0.884 | −0.2 | −0.4, 0.1 | 0.124 |
| Days unproductive | −0.9 | −1.8, 0.0 | 0.052 | −0.9 | −1.7, 0.0 | 0.060 | −0.8 | −1.7, 0.0 | 0.049 |
Effect of Fezolinetant on Subdomains of Quality-of-Life Measures (mITT Population)
| Subdomain . | Week 4 . | Week 8 . | Week 12 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LSMD for Fezolinetant vs Placebo . | 95% CI . | P Value . | LSMD for Fezolinetant vs Placebo . | 95% CI . | P Value . | LSMD for Fezolinetant vs Placebo . | 95% CI . | P Value . | |
| LSEQ | |||||||||
| Quality of sleep | 2.423 | 1.308, 3.539 | <0.001 | 2.291 | 1.331, 3.251 | <0.001 | 2.433 | 1.334, 3.532 | <0.001 |
| Getting to sleep | 1.375 | 0.651, 2.100 | <0.001 | 1.166 | 0.505, 1.827 | <0.001 | 0.895 | 0.190, 1.599 | 0.014 |
| Awake following sleep | 0.877 | −0.034, 1.789 | 0.059 | 1.457 | 0.579, 2.335 | 0.001 | 1.113 | 0.107, 2.120 | 0.031 |
| Behavior following wakening | 1.203 | 0.317, 2.088 | 0.008 | 1.597 | 0.639, 2.556 | 0.001 | 0.842 | −0.116, 1.800 | 0.084 |
| HFRDIS | |||||||||
| Overall mean score | −1.98 | −2.73, −1.23 | <0.001 | −2.21 | −3.02, −1.40 | <0.001 | −1.98 | −2.83, −1.13 | <0.001 |
| Work | −2.53 | −3.45, −1.60 | <0.001 | −2.29 | −3.15, −1.42 | <0.001 | −2.11 | −3.03, −1.20 | <0.001 |
| Social activities | −2.33 | −3.19, −1.46 | <0.001 | −2.34 | −3.21, −1.47 | <0.001 | −2.19 | −3.09, −1.29 | <0.001 |
| Leisure activities | −2.15 | −2.99, −1.31 | <0.001 | −2.37 | −3.19, −1.55 | <0.001 | −1.64 | −2.66, −0.62 | 0.002 |
| Sleep | −2.93 | −4.08, −1.78 | <0.001 | −2.71 | −3.86, −1.57 | <0.001 | −2.61 | −3.67, −1.54 | <0.001 |
| Mood | −1.74 | −2.67, −0.81 | <0.001 | −2.12 | −3.09, −1.16 | <0.001 | −1.79 | −2.69, −0.88 | <0.001 |
| Concentration | −1.92 | −2.86, −0.97 | <0.001 | −2.23 | −3.21, −1.25 | <0.001 | −1.90 | −2.91, −0.88 | <0.001 |
| Relations with others | −1.33 | −2.20, −0.46 | 0.003 | −1.85 | −2.80, −0.90 | <0.001 | −1.72 | −2.66, −0.77 | <0.001 |
| Sexuality | −1.06 | −2.25, 0.13 | 0.081 | −1.40 | −2.80, 0.00 | 0.050 | −1.51 | −2.84, −0.19 | 0.026 |
| Enjoyment of life | −1.65 | −2.51, −0.79 | <0.001 | −2.07 | −2.97, −1.18 | <0.001 | −1.83 | −2.88, −0.78 | <0.001 |
| Overall quality of life | −1.92 | −2.86, −0.97 | <0.001 | −2.51 | −3.41, −1.61 | <0.001 | −2.36 | −3.31, −1.41 | <0.001 |
| GCS | |||||||||
| Total score | −4.6 | −8.2, −1.0 | 0.013 | −6.9 | −10.7, −3.1 | <0.001 | −6.3 | −9.9, −2.8 | <0.001 |
| Loss of interest in sex | 0.0 | −0.3, 0.3 | 0.881 | −0.2 | −0.6, 0.2 | 0.383 | −0.2 | −0.6, 0.2 | 0.301 |
| Psychological | −2.5 | −4.5, −0.4 | 0.020 | −3.3 | −5.4, −1.2 | 0.003 | −3.1 | −5.2, −1.0 | 0.005 |
| Physical | −0.2 | −1.2, 0.9 | 0.732 | −0.7 | −1.9, 0.6 | 0.282 | −0.6 | −1.7, 0.5 | 0.254 |
| Vasomotor | −2.0 | −2.7, −1.4 | <0.001 | −1.8 | −2.4, −1.1 | <0.001 | −2.0 | −2.6, −1.3 | <0.001 |
| SDS | |||||||||
| Global functional impairment | −4.4 | −6.9, −2.0 | <0.001 | −5.8 | −8.0, −3.6 | <0.001 | −5.3 | −7.8, −2.8 | <0.001 |
| Work/school | −1.7 | −2.7, −0.8 | <0.001 | −2.0 | −2.8, −1.3 | <0.001 | −1.6 | −2.5, −0.8 | <0.001 |
| Social life | −1.4 | −2.2, −0.6 | <0.001 | −1.9 | −2.7, −1.1 | <0.001 | −1.6 | −2.4, −0.7 | <0.001 |
| Family life/home responsibilities | −1.3 | −2.2, −0.4 | 0.005 | −1.7 | −2.5, −0.9 | <0.001 | −1.6 | −2.4, −0.7 | <0.001 |
| Days lost | 0.0 | −0.5, 0.6 | 0.936 | 0.0 | −0.1, 0.1 | 0.884 | −0.2 | −0.4, 0.1 | 0.124 |
| Days unproductive | −0.9 | −1.8, 0.0 | 0.052 | −0.9 | −1.7, 0.0 | 0.060 | −0.8 | −1.7, 0.0 | 0.049 |
| Subdomain . | Week 4 . | Week 8 . | Week 12 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LSMD for Fezolinetant vs Placebo . | 95% CI . | P Value . | LSMD for Fezolinetant vs Placebo . | 95% CI . | P Value . | LSMD for Fezolinetant vs Placebo . | 95% CI . | P Value . | |
| LSEQ | |||||||||
| Quality of sleep | 2.423 | 1.308, 3.539 | <0.001 | 2.291 | 1.331, 3.251 | <0.001 | 2.433 | 1.334, 3.532 | <0.001 |
| Getting to sleep | 1.375 | 0.651, 2.100 | <0.001 | 1.166 | 0.505, 1.827 | <0.001 | 0.895 | 0.190, 1.599 | 0.014 |
| Awake following sleep | 0.877 | −0.034, 1.789 | 0.059 | 1.457 | 0.579, 2.335 | 0.001 | 1.113 | 0.107, 2.120 | 0.031 |
| Behavior following wakening | 1.203 | 0.317, 2.088 | 0.008 | 1.597 | 0.639, 2.556 | 0.001 | 0.842 | −0.116, 1.800 | 0.084 |
| HFRDIS | |||||||||
| Overall mean score | −1.98 | −2.73, −1.23 | <0.001 | −2.21 | −3.02, −1.40 | <0.001 | −1.98 | −2.83, −1.13 | <0.001 |
| Work | −2.53 | −3.45, −1.60 | <0.001 | −2.29 | −3.15, −1.42 | <0.001 | −2.11 | −3.03, −1.20 | <0.001 |
| Social activities | −2.33 | −3.19, −1.46 | <0.001 | −2.34 | −3.21, −1.47 | <0.001 | −2.19 | −3.09, −1.29 | <0.001 |
| Leisure activities | −2.15 | −2.99, −1.31 | <0.001 | −2.37 | −3.19, −1.55 | <0.001 | −1.64 | −2.66, −0.62 | 0.002 |
| Sleep | −2.93 | −4.08, −1.78 | <0.001 | −2.71 | −3.86, −1.57 | <0.001 | −2.61 | −3.67, −1.54 | <0.001 |
| Mood | −1.74 | −2.67, −0.81 | <0.001 | −2.12 | −3.09, −1.16 | <0.001 | −1.79 | −2.69, −0.88 | <0.001 |
| Concentration | −1.92 | −2.86, −0.97 | <0.001 | −2.23 | −3.21, −1.25 | <0.001 | −1.90 | −2.91, −0.88 | <0.001 |
| Relations with others | −1.33 | −2.20, −0.46 | 0.003 | −1.85 | −2.80, −0.90 | <0.001 | −1.72 | −2.66, −0.77 | <0.001 |
| Sexuality | −1.06 | −2.25, 0.13 | 0.081 | −1.40 | −2.80, 0.00 | 0.050 | −1.51 | −2.84, −0.19 | 0.026 |
| Enjoyment of life | −1.65 | −2.51, −0.79 | <0.001 | −2.07 | −2.97, −1.18 | <0.001 | −1.83 | −2.88, −0.78 | <0.001 |
| Overall quality of life | −1.92 | −2.86, −0.97 | <0.001 | −2.51 | −3.41, −1.61 | <0.001 | −2.36 | −3.31, −1.41 | <0.001 |
| GCS | |||||||||
| Total score | −4.6 | −8.2, −1.0 | 0.013 | −6.9 | −10.7, −3.1 | <0.001 | −6.3 | −9.9, −2.8 | <0.001 |
| Loss of interest in sex | 0.0 | −0.3, 0.3 | 0.881 | −0.2 | −0.6, 0.2 | 0.383 | −0.2 | −0.6, 0.2 | 0.301 |
| Psychological | −2.5 | −4.5, −0.4 | 0.020 | −3.3 | −5.4, −1.2 | 0.003 | −3.1 | −5.2, −1.0 | 0.005 |
| Physical | −0.2 | −1.2, 0.9 | 0.732 | −0.7 | −1.9, 0.6 | 0.282 | −0.6 | −1.7, 0.5 | 0.254 |
| Vasomotor | −2.0 | −2.7, −1.4 | <0.001 | −1.8 | −2.4, −1.1 | <0.001 | −2.0 | −2.6, −1.3 | <0.001 |
| SDS | |||||||||
| Global functional impairment | −4.4 | −6.9, −2.0 | <0.001 | −5.8 | −8.0, −3.6 | <0.001 | −5.3 | −7.8, −2.8 | <0.001 |
| Work/school | −1.7 | −2.7, −0.8 | <0.001 | −2.0 | −2.8, −1.3 | <0.001 | −1.6 | −2.5, −0.8 | <0.001 |
| Social life | −1.4 | −2.2, −0.6 | <0.001 | −1.9 | −2.7, −1.1 | <0.001 | −1.6 | −2.4, −0.7 | <0.001 |
| Family life/home responsibilities | −1.3 | −2.2, −0.4 | 0.005 | −1.7 | −2.5, −0.9 | <0.001 | −1.6 | −2.4, −0.7 | <0.001 |
| Days lost | 0.0 | −0.5, 0.6 | 0.936 | 0.0 | −0.1, 0.1 | 0.884 | −0.2 | −0.4, 0.1 | 0.124 |
| Days unproductive | −0.9 | −1.8, 0.0 | 0.052 | −0.9 | −1.7, 0.0 | 0.060 | −0.8 | −1.7, 0.0 | 0.049 |
Pharmacokinetic/pharmacodynamic results
Fezolinetant plasma concentrations are summarized in Table 3.
| . | Mean (SEM) (ng/mL) . | n . |
|---|---|---|
| Trough (12 h postdose) | ||
| Week 4 | 283.7 (37.05) | 31 |
| Week 8 | 311.8 (40.19) | 35 |
| Week 12 | 288.8 (31.45) | 38 |
| Peak (3 h postdose) | ||
| Week 12 | 845.9 (55.81) | 38 |
| . | Mean (SEM) (ng/mL) . | n . |
|---|---|---|
| Trough (12 h postdose) | ||
| Week 4 | 283.7 (37.05) | 31 |
| Week 8 | 311.8 (40.19) | 35 |
| Week 12 | 288.8 (31.45) | 38 |
| Peak (3 h postdose) | ||
| Week 12 | 845.9 (55.81) | 38 |
| . | Mean (SEM) (ng/mL) . | n . |
|---|---|---|
| Trough (12 h postdose) | ||
| Week 4 | 283.7 (37.05) | 31 |
| Week 8 | 311.8 (40.19) | 35 |
| Week 12 | 288.8 (31.45) | 38 |
| Peak (3 h postdose) | ||
| Week 12 | 845.9 (55.81) | 38 |
| . | Mean (SEM) (ng/mL) . | n . |
|---|---|---|
| Trough (12 h postdose) | ||
| Week 4 | 283.7 (37.05) | 31 |
| Week 8 | 311.8 (40.19) | 35 |
| Week 12 | 288.8 (31.45) | 38 |
| Peak (3 h postdose) | ||
| Week 12 | 845.9 (55.81) | 38 |
At peak drug levels (i.e., 3 hours postdose), fezolinetant decreased plasma LH by 49.8% relative to baseline, compared with 16.4% with placebo (Fig. 4A). Plasma levels of E2, FSH, and SHBG showed little impact of fezolinetant treatment (Fig. 4B–4D). Mean levels of E2 at baseline and all subsequent time points were consistently higher for the placebo group than the fezolinetant group, possibly owing to perimenopausal subjects in the placebo group who did not meet inclusion criteria for menopause, although they satisfied the VMS inclusion criterion. Consistent with this explanation, the difference was removed when perimenopausal subjects were excluded (data not shown).
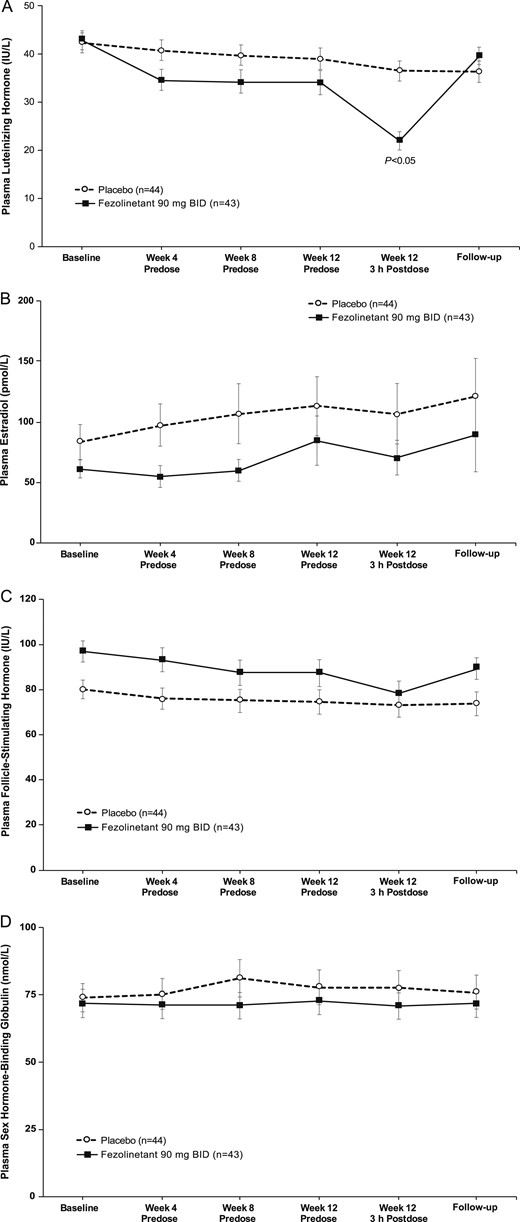
Effect of fezolinetant on plasma concentration of (A) LH, (B) E2, (C) FSH, and (D) SHBG levels. Data are presented as mean ± SE (mITT population).
Safety
Both treatments were well tolerated. Seven subjects discontinued the study early, four in the placebo group and three in the fezolinetant group. Treatment compliance was high, and other than the discontinued subjects, no subjects missed >10 study medication intakes over the trial duration. TEAEs were reported by 35 (79.5%) subjects in the placebo group and 29 (67.4%) subjects in the fezolinetant group (Table 4). All TEAEs were “mild” or “moderate” in severity. A serious TEAE of upper limb fracture was reported by one subject in the placebo group and was considered moderate in severity and not related to the study drug. No deaths were reported during the study.
Adverse Events Occurring in Two or More Fezolinetant-Treated Subjects (Safety Population)
| . | Placebo Twice Daily (n = 44) . | Fezolinetant (90 mg) Twice Daily (n = 43) . |
|---|---|---|
| TEAE, n (%) | ||
| At least one TEAE | 35 (79.5) | 29 (67.4) |
| At least one SAEa | 1 (2.3) | 0 |
| At least one TEAE leading to death | 0 | 0 |
| At least one severe TEAE | 0 | 0 |
| At least one TEAE leading to discontinuation of study drug | 0 | 2 (4.7) |
| At least one TEAE that was considered treatment related | 11 (25.0) | 13 (30.2) |
| System organ class | ||
| Preferred term, n (%) | ||
| Any TEAE | 35 (79.5) | 29 (67.4) |
| Cardiac disorders | 2 (4.5) | 3 (7.0) |
| Palpitations | 2 (4.5) | 3 (7.0) |
| Ear and labyrinth disorders | 0 | 2 (4.7) |
| Vertigo | 0 | 2 (4.7) |
| Gastrointestinal disorders | 4 (9.1) | 10 (23.3) |
| Abdominal discomfort | 1 (2.3) | 2 (4.7) |
| Diarrhea | 0 | 3 (7.0) |
| Paresthesia oral | 0 | 2 (4.7) |
| General disorders and administration site conditions | 2 (4.5) | 2 (4.7) |
| Fatigue | 0 | 2 (4.7) |
| Infections and infestation | 13 (29.5) | 11 (25.6) |
| Gastroenteritis | 2 (4.5) | 0 |
| Influenza | 1 (2.3) | 3 (7.0) |
| Nasopharyngitis | 4 (9.1) | 1 (2.3) |
| Musculoskeletal and connective tissue disorders | 9 (20.5) | 7 (16.3) |
| Arthralgia | 1 (2.3) | 2 (4.7) |
| Arthritis | 3 (6.8) | 0 |
| Back pain | 2 (4.5) | 0 |
| Fibromyalgia | 0 | 2 (4.7) |
| Nervous system disorders | 8 (18.2) | 8 (18.6) |
| Dizziness | 0 | 2 (4.7) |
| Headache | 6 (13.6) | 7 (16.3) |
| Psychiatric disorders | 1 (2.3) | 4 (9.3) |
| Depression | 0 | 2 (4.7) |
| Reproductive system and breast disorders | 6 (13.6) | 2 (4.7) |
| Postmenopausal hemorrhage | 2 (4.5) | 0 |
| Vaginal hemorrhage | 2 (4.5) | 1 (2.3) |
| . | Placebo Twice Daily (n = 44) . | Fezolinetant (90 mg) Twice Daily (n = 43) . |
|---|---|---|
| TEAE, n (%) | ||
| At least one TEAE | 35 (79.5) | 29 (67.4) |
| At least one SAEa | 1 (2.3) | 0 |
| At least one TEAE leading to death | 0 | 0 |
| At least one severe TEAE | 0 | 0 |
| At least one TEAE leading to discontinuation of study drug | 0 | 2 (4.7) |
| At least one TEAE that was considered treatment related | 11 (25.0) | 13 (30.2) |
| System organ class | ||
| Preferred term, n (%) | ||
| Any TEAE | 35 (79.5) | 29 (67.4) |
| Cardiac disorders | 2 (4.5) | 3 (7.0) |
| Palpitations | 2 (4.5) | 3 (7.0) |
| Ear and labyrinth disorders | 0 | 2 (4.7) |
| Vertigo | 0 | 2 (4.7) |
| Gastrointestinal disorders | 4 (9.1) | 10 (23.3) |
| Abdominal discomfort | 1 (2.3) | 2 (4.7) |
| Diarrhea | 0 | 3 (7.0) |
| Paresthesia oral | 0 | 2 (4.7) |
| General disorders and administration site conditions | 2 (4.5) | 2 (4.7) |
| Fatigue | 0 | 2 (4.7) |
| Infections and infestation | 13 (29.5) | 11 (25.6) |
| Gastroenteritis | 2 (4.5) | 0 |
| Influenza | 1 (2.3) | 3 (7.0) |
| Nasopharyngitis | 4 (9.1) | 1 (2.3) |
| Musculoskeletal and connective tissue disorders | 9 (20.5) | 7 (16.3) |
| Arthralgia | 1 (2.3) | 2 (4.7) |
| Arthritis | 3 (6.8) | 0 |
| Back pain | 2 (4.5) | 0 |
| Fibromyalgia | 0 | 2 (4.7) |
| Nervous system disorders | 8 (18.2) | 8 (18.6) |
| Dizziness | 0 | 2 (4.7) |
| Headache | 6 (13.6) | 7 (16.3) |
| Psychiatric disorders | 1 (2.3) | 4 (9.3) |
| Depression | 0 | 2 (4.7) |
| Reproductive system and breast disorders | 6 (13.6) | 2 (4.7) |
| Postmenopausal hemorrhage | 2 (4.5) | 0 |
| Vaginal hemorrhage | 2 (4.5) | 1 (2.3) |
Abbreviation: SAE, serious adverse event.
Upper limb fracture.
Adverse Events Occurring in Two or More Fezolinetant-Treated Subjects (Safety Population)
| . | Placebo Twice Daily (n = 44) . | Fezolinetant (90 mg) Twice Daily (n = 43) . |
|---|---|---|
| TEAE, n (%) | ||
| At least one TEAE | 35 (79.5) | 29 (67.4) |
| At least one SAEa | 1 (2.3) | 0 |
| At least one TEAE leading to death | 0 | 0 |
| At least one severe TEAE | 0 | 0 |
| At least one TEAE leading to discontinuation of study drug | 0 | 2 (4.7) |
| At least one TEAE that was considered treatment related | 11 (25.0) | 13 (30.2) |
| System organ class | ||
| Preferred term, n (%) | ||
| Any TEAE | 35 (79.5) | 29 (67.4) |
| Cardiac disorders | 2 (4.5) | 3 (7.0) |
| Palpitations | 2 (4.5) | 3 (7.0) |
| Ear and labyrinth disorders | 0 | 2 (4.7) |
| Vertigo | 0 | 2 (4.7) |
| Gastrointestinal disorders | 4 (9.1) | 10 (23.3) |
| Abdominal discomfort | 1 (2.3) | 2 (4.7) |
| Diarrhea | 0 | 3 (7.0) |
| Paresthesia oral | 0 | 2 (4.7) |
| General disorders and administration site conditions | 2 (4.5) | 2 (4.7) |
| Fatigue | 0 | 2 (4.7) |
| Infections and infestation | 13 (29.5) | 11 (25.6) |
| Gastroenteritis | 2 (4.5) | 0 |
| Influenza | 1 (2.3) | 3 (7.0) |
| Nasopharyngitis | 4 (9.1) | 1 (2.3) |
| Musculoskeletal and connective tissue disorders | 9 (20.5) | 7 (16.3) |
| Arthralgia | 1 (2.3) | 2 (4.7) |
| Arthritis | 3 (6.8) | 0 |
| Back pain | 2 (4.5) | 0 |
| Fibromyalgia | 0 | 2 (4.7) |
| Nervous system disorders | 8 (18.2) | 8 (18.6) |
| Dizziness | 0 | 2 (4.7) |
| Headache | 6 (13.6) | 7 (16.3) |
| Psychiatric disorders | 1 (2.3) | 4 (9.3) |
| Depression | 0 | 2 (4.7) |
| Reproductive system and breast disorders | 6 (13.6) | 2 (4.7) |
| Postmenopausal hemorrhage | 2 (4.5) | 0 |
| Vaginal hemorrhage | 2 (4.5) | 1 (2.3) |
| . | Placebo Twice Daily (n = 44) . | Fezolinetant (90 mg) Twice Daily (n = 43) . |
|---|---|---|
| TEAE, n (%) | ||
| At least one TEAE | 35 (79.5) | 29 (67.4) |
| At least one SAEa | 1 (2.3) | 0 |
| At least one TEAE leading to death | 0 | 0 |
| At least one severe TEAE | 0 | 0 |
| At least one TEAE leading to discontinuation of study drug | 0 | 2 (4.7) |
| At least one TEAE that was considered treatment related | 11 (25.0) | 13 (30.2) |
| System organ class | ||
| Preferred term, n (%) | ||
| Any TEAE | 35 (79.5) | 29 (67.4) |
| Cardiac disorders | 2 (4.5) | 3 (7.0) |
| Palpitations | 2 (4.5) | 3 (7.0) |
| Ear and labyrinth disorders | 0 | 2 (4.7) |
| Vertigo | 0 | 2 (4.7) |
| Gastrointestinal disorders | 4 (9.1) | 10 (23.3) |
| Abdominal discomfort | 1 (2.3) | 2 (4.7) |
| Diarrhea | 0 | 3 (7.0) |
| Paresthesia oral | 0 | 2 (4.7) |
| General disorders and administration site conditions | 2 (4.5) | 2 (4.7) |
| Fatigue | 0 | 2 (4.7) |
| Infections and infestation | 13 (29.5) | 11 (25.6) |
| Gastroenteritis | 2 (4.5) | 0 |
| Influenza | 1 (2.3) | 3 (7.0) |
| Nasopharyngitis | 4 (9.1) | 1 (2.3) |
| Musculoskeletal and connective tissue disorders | 9 (20.5) | 7 (16.3) |
| Arthralgia | 1 (2.3) | 2 (4.7) |
| Arthritis | 3 (6.8) | 0 |
| Back pain | 2 (4.5) | 0 |
| Fibromyalgia | 0 | 2 (4.7) |
| Nervous system disorders | 8 (18.2) | 8 (18.6) |
| Dizziness | 0 | 2 (4.7) |
| Headache | 6 (13.6) | 7 (16.3) |
| Psychiatric disorders | 1 (2.3) | 4 (9.3) |
| Depression | 0 | 2 (4.7) |
| Reproductive system and breast disorders | 6 (13.6) | 2 (4.7) |
| Postmenopausal hemorrhage | 2 (4.5) | 0 |
| Vaginal hemorrhage | 2 (4.5) | 1 (2.3) |
Abbreviation: SAE, serious adverse event.
Upper limb fracture.
The number of subjects who reported at least one TEAE that was considered to be related to treatment was 11 (25.0%) in the placebo group and 13 (30.2%) in the fezolinetant group. The most common treatment-related TEAEs were gastrointestinal disorders, reported by six (14.0%) subjects in the fezolinetant vs none in the placebo group. Two subjects (4.7%) in the fezolinetant group discontinued study drug because of TEAEs that were considered possibly related to study treatment: one subject with preexisting fibromyalgia reported worsening of fibromyalgia, depression, dry mouth, headache, palpitations, diarrhea, and vomiting, and the other reported headache and vertigo. None of the subjects in the placebo group discontinued the study drug because of a TEAE.
Increased values for aspartate aminotransferase (AST) were more frequent with placebo (four subjects, 9.1%) than fezolinetant (two subjects, 4.8%); increased values for alanine aminotransferase (ALT) were more frequent with fezolinetant (five subjects, 11.9%) than placebo (one subject, 2.3%). In all cases, the observed increases in ALT and AST were mild and transient and did not exceed three times the upper limit of normal. Except for one occurrence of raised ALT and AST in the placebo group, none of these abnormalities was considered clinically significant.
No relevant or consistent changes in vital signs, electrocardiograms, or bone density markers were observed at any point during the study. None of the treatment-emergent vital signs or electrocardiographic abnormalities was considered by the investigator to be clinically significant.
Discussion
To our knowledge, this is the first clinical study designed according to the FDA guidelines (31) that demonstrates a significant beneficial effect of an NK3 antagonist for the treatment of VMSs. This 12-week, multicenter, double-blind, placebo-controlled study demonstrated that fezolinetant substantially reduced the frequency of moderate/severe VMSs and moderate/severe VMS score, with improvements achieved within the first day of treatment and sustained throughout the study. Fezolinetant treatment also improved sleep quality and other subject-reported outcomes that measure impact of VMSs on quality of life. The effect of fezolinetant to lower LH levels, in step with peak levels of drug exposure, is consistent with the proposed mechanism of action of KNDy neuron inhibition. The recurrence of VMSs after stopping fezolinetant, with similar severity and frequency in the follow-up phase as in the placebo group, further confirms the potent activity and reversibility of fezolinetant treatment. Fezolinetant was well tolerated and no drug-related serious adverse events were reported.
The FDA guidelines on the conduct of clinical trials to measure drug efficacy in VMSs (31) facilitate comparison of the current trial results with those of marketed hormonal (41–43) and nonhormonal therapies (44). Fezolinetant produced a 93% reduction in VMS frequency from baseline to week 12. The incremental reduction in the number of moderate/severe VMSs with fezolinetant relative to placebo was 35.2 VMS episodes per week (95% CI, 22.8, 47.6); that is, fezolinetant-treated subjects had a reduction of approximately five episodes per day beyond that of placebo-treated subjects at week 12. In comparison, paroxetine, the only FDA-approved nonhormonal menopausal therapy, reduced VMS frequency by 6.2 to 9.2 episodes per week at week 12 relative to placebo, equivalent to an incremental benefit of <1 to 1.4 episodes per day (44). The FDA considers a reduction of two episodes per day to be a clinically meaningful difference. The reduction in VMS frequency in fezolinetant-treated women was also accompanied by improvements in subject-reported outcomes (e.g., HFRDIS, GCS, LSEQ, SDS), providing further evidence that the treatment effect was clinically meaningful. Fezolinetant demonstrated rapid improvement in symptoms, with decrease in frequency of moderate/severe VMSs from the first day of treatment, concurrent with the maximal drug concentration in plasma observed within a few hours of dosing. In comparison, studies of hormonal treatments conducted according to FDA guidelines and with a similar patient population found significant improvements in VMSs after only 2 to 4 weeks of treatment (42, 45, 46). The rapid relief of VMSs reported here with fezolinetant is consistent with findings with another NK3 antagonist, MLE4901, that was formerly in development and tested using alternative study designs (47–49).
Following hot flashes and night sweats, difficulty sleeping is the next most impactful menopausal symptom for which women seek medical attention (3). Sleep disturbances may worsen adverse impact on quality of life arising from mood changes and symptoms of depression (50–53). Additionally, the dysregulated KNDy neuron activity in menopause may have direct consequences on various autonomic functions affecting sleep, stress, and aspects of social behavior (54). In our study, fezolinetant improved sleep quality at all test intervals, and improved psychological, social, and productivity-related subject-reported outcomes. These improvements in quality of life may be relevant to decreasing the societal burden of VMSs, including overall health care resource utilization and negative effects on work productivity (5, 55).
Our clinical study found that peak fezolinetant levels were associated with a sharp decrease from baseline in plasma LH levels, but not FSH levels, consistent with the proposed mechanism of action on KNDy neurons (15, 16, 23). This direct action on the thermoregulatory pathway is independent of effects on ovarian hormones, as demonstrated by plasma levels of E2 remaining unchanged from baseline during fezolinetant treatment. This may be considered a therapeutically relevant outcome in the treatment of menopausal subjects at risk for endometrial hyperplasia with unopposed estrogen therapies (56).
Fezolinetant was well tolerated. The total number of TEAEs was higher in the placebo than in the fezolinetant treatment group, and no drug-related serious adverse events were reported. Adverse events related to vaginal bleeding, a common complaint with MHT (57), were less common with fezolinetant than with placebo. Gastrointestinal events (e.g., abdominal discomfort, diarrhea) were more common with fezolinetant than placebo, which may be attributable to the fact that NK3 receptors also are expressed in the gastrointestinal tract (58).
Strengths of this study include the design in accordance with FDA guidance for VMS treatments. A limitation is the restriction of study population to healthy menopausal women of largely common ethnicity with moderate/severe VMSs and exclusion of women receiving other treatments or with disorders that might have interfered with interpretation of study results; therefore, results may not be generalizable to all menopausal women. One further limitation is that inclusion of women with spontaneous amenorrhea for ≥3 months with FSH >40 IU/L and E2 <0.21 nmol/L may have allowed inclusion of some perimenopausal women with fluctuating hormone levels and symptoms; however, as this was a randomized trial, we expected these participants to be evenly represented in both treatment groups, balancing any impact on results across both groups. Additionally, the minimum washout for hormone therapies (6 weeks prior to screening) was less than the FDA guidance for certain hormone therapy formulations; however, this was unlikely to have affected the results because it would apply equally to both groups, and because the screening period added an additional 1 to 4 weeks of washout before study drug initiation. Although results of this study are promising, larger studies of longer duration are required to confirm efficacy and safety.
In summary, fezolinetant significantly reduced frequency and severity of moderate/severe VMSs, with response apparent from the first day of treatment. Fezolinetant was well tolerated and had no effect on E2 levels. The safety and efficacy demonstrated in this study support the potential use of fezolinetant as an effective nonhormonal treatment option for VMSs in menopausal women.
Acknowledgments
The authors thank Josephine Wolfram, Astellas Pharma Global Development, for statistical review of this manuscript.
Financial Support: This work was supported by Ogeda SA (Gosselies, Belgium). Astellas Pharma Inc. acquired 100% of the equity of Ogeda SA on 17 May 2017. Development of this manuscript, including editorial support provided by Mike Zbreski and Rosalba Satta of SuccinctChoice Medical Communications (Chicago, IL), was sponsored by Astellas Pharma Global Development.
Clinical Trial Information: EudraCT no. 2015-002578-20 (registered 29 July 2015).
Author Contributions: H.D., G.L.F., and S.R. were involved in the study design, study management, and board meetings concerning the follow-up of this study. H.D., D.T., G.D., and P.S. were involved in collection of study data. All authors had full access to the study data during development of this manuscript. G.L.F. prepared the first draft of the manuscript. All authors provided critical review and approval of the manuscript.
Additional Information
Disclosure Summary: H.D. reports personal fees from Ogeda SA during conduct of the study. D.T. received fees (paid to University Hospitals Leuven) from Astellas Pharma Inc. during the conduction of the study. S.R., J.C., H.R.H., and G.L.F. were employees of Ogeda SA when the study was conducted. G.L.F. reports grants from Region Wallon during the conduct of the study. Additionally, G.L.F. and H.R.H. have patents WO 2013/050424, WO 2014/154895, and EP15159296 licensed to Astellas Pharma Inc. The remaining authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request. Studies conducted with product indications or formulations that remain in development are assessed after study completion to determine if individual participant data can be shared. The plan to share individual participant data is based on the status of product approval or termination of the compound, in addition to other study specific criteria as described on www.clinicalstudydatarequest.com under “Sponsor Specific Details for Astellas.”
Abbreviations:
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- E2
estradiol
- ePRO
electronic patient-reported outcome
- FDA
US Food and Drug Administration
- GCS
Greene Climacteric Scale
- HFRDIS
Hot Flash Related Daily Interference Scale
- KNDy
kisspeptin/neurokinin B/dynorphin
- LSEQ
Leeds Sleep Evaluation Questionnaire
- LSMD
least squares mean difference
- MHT
menopausal hormone therapy
- mITT
modified intent-to-treat
- NK3R
neurokinin 3 receptor
- NKB
neurokinin B
- SDS
Sheehan Disability Scale
- TEAE
treatment-emergent adverse event
- VMS
vasomotor symptom



