-
PDF
- Split View
-
Views
-
Cite
Cite
Kwadwo Antwi, Guillaume Nicolas, Melpomeni Fani, Tobias Heye, Francois Pattou, Ashley Grossman, Philippe Chanson, Jean Claude Reubi, Aurel Perren, Beat Gloor, Deborah R Vogt, Damian Wild, Emanuel Christ, 68Ga-Exendin-4 PET/CT Detects Insulinomas in Patients With Endogenous Hyperinsulinemic Hypoglycemia in MEN-1, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 12, December 2019, Pages 5843–5852, https://doi.org/10.1210/jc.2018-02754
Close - Share Icon Share
Abstract
Surgical intervention is advised in patients with multiple endocrine neoplasia type-1 (MEN-1) and nonfunctioning pancreatic neuroendocrine tumors (PanNETs) with a size ≥20 mm. Functioning PanNETs, such as in patients with endogenous hyperinsulinemic hypoglycemia (EHH) due to (one or multiple) insulinomas, should be treated surgically independent of size. Preoperative localization of insulinomas is critical for surgery.
To evaluate the feasibility and sensitivity of 68Ga-DOTA-exendin-4 positron emission tomography (PET)/CT in the detection of clinically relevant lesions in patients with MEN-1 and EHH in combination with MRI.
Post hoc subgroup analysis of a larger prospective imaging study with 52 patients with EHH.
Six of 52 consecutive patients with EHH and genetically proven MEN-1 mutation were included.
All patients received one 68Ga-DOTA-exendin-4 PET/CT and one MRI scan within 3 to 4 days. Thereafter, surgery was performed based on all imaging results.
Lesion-based sensitivity of PET/CT and MRI for detection of clinically relevant lesions was calculated. Readers were unaware of other results. The reference standard was surgery with histology and treatment outcome. True positive (i.e., clinically relevant lesions) was defined as PanNETs ≥20 mm or insulinoma.
In six patients, 37 PanNETs were confirmed by histopathology. Sensitivity (95% CI) in the detection of clinically relevant lesions for combined PET/CT plus MRI, MRI, and PET/CT was 92.3% (64% to 99.8%), 38.5% (13.9% to 68.4%), and 84.6% (54.6% to 98.1%), respectively (P = 0.014 for the comparison of PET/CT plus MRI vs MRI). Postsurgery, EHH resolved in all patients.
68Ga-DOTA-exendin-4 PET/CT is feasible in patients with MEN-1 and EHH. The combination with MRI is superior to MRI alone in the detection of insulinomas and may guide the surgical strategy.
Multiple endocrine neoplasia type 1 (MEN-1) is an autosomal dominant inherited tumor syndrome caused by heterozygous mutations in the MEN-1 tumor suppressor gene with an incidence of 1:50,000. More than 80% of these patients develop pancreatic neuroendocrine tumors (PanNETs), either functioning (F-PanNETs) or nonfunctioning (NF-PanNETs), which are a major cause of premature death due to malignant progression (1, 2). The most common F-PanNETs in MEN-1 are duodenal gastrinomas causing Zollinger–Ellison syndrome and pancreatic insulinomas causing endogenous hyperinsulinemic hypoglycemia (EHH) (3). Insulinomas can cause life-threatening EHH, and surgery is the cornerstone of therapy, as current medical treatments remain insufficient, do not provide a permanent cure, and thus should be reserved for the perioperative period or for patients who cannot undergo surgery (4).
Older studies have proposed aggressive resection of every tumor identified by conventional imaging studies in the presence of biochemically proven EHH (5–7). However, pancreatic surgery is associated with significant mortality and long-term morbidity (8, 9). NF-PanNETs <20 mm rarely develop metastases (10, 11), and current studies have not shown any survival benefit for patients with NF-PanNETs ≤20 mm who received surgery in comparison with patients who had conservative management, that is, watchful waiting (12). Consequently, in patients with nonmetastatic PanNETs all international guidelines recommend the resection of symptomatic insulinomas with the aim of eliminating life-threatening EHH, but resection of NF-PanNETs only ≥20 mm due to the increased risk for malignant progression (12–14). For NF-PanNETs <20 mm, all international guidelines suggest a conservative management strategy (13).
In clinical routine, different imaging modalities are used for the localization of insulinomas: contrast-enhanced CT, contrast-enhanced MRI which is more sensitive than CT for this indication (15, 16)], endoscopic ultrasound, and somatostatin receptor imaging [Octreoscan® or 68Ga-DOTATOC/68Ga-DOTATATE/68Ga-DOTANOC positron emission tomography (PET)/CT]. Especially in the context of MEN-1, when multiple other PanNETs are detected, these investigations remain insufficient due to mainly two reasons. First, the small size of insulinomas [usually <20 mm at presentation (15, 17)] makes them susceptible to motion artifacts, such as respiratory motion, cardiac pulsation, and bowel peristalsis, and thus limits their detectability (18). Second, morphological imaging as well as somatostatin receptor imaging is not able to differentiate insulinomas from NF-PanNETs. To regionalize an insulinoma, a calcium stimulation test is recommended to avoid “blind” pancreatic resections (19, 20).
In general, gastroenteropancreatic neuroendocrine neoplasms are known to overexpress the somatostatin receptor subtype 2 at a high density in 70% to 100% of the cases, except in benign insulinomas, where the expression is lower. Glucagon-like peptide-1 (GLP-1) receptors (GLP-1Rs), alternatively, are expressed at high density in sporadic insulinomas (21). Exendin-4 is a synthetic U.S. Food and Drug Administration–approved GLP-1 analog primarily established for the treatment of type 2 diabetes mellitus by targeting GLP-1Rs. The GLP-1 pathway is important in islet regeneration (22, 23) by regulating inhibition of β-cell apoptosis (24) and stimulation of β-cell proliferation and insulin secretion. Previous studies have shown that GLP-1R imaging can be used as a very sensitive, noninvasive method to localize benign sporadic insulinomas using radiolabeled exendin-4 single-photon emission CT/CT or PET/CT (25–31), where GLP-1R PET/CT performs significantly better than single-photon emission CT/CT (28). Nonetheless, as opposed to benign insulinomas, malignant insulinomas often lack GLP-1Rs and, conversely, often overexpress the somatostatin type 2 receptor (32).
In the context of MEN-1, the biological characteristics (benign or malignant) of insulin-secreting NETs is not well explored. A recent study with GLP-1R imaging using 68Ga-DOTA-exendin-4 PET/CT in heterozygous MEN-1 knockout mice demonstrated its potential for lesion detection in the MEN-1 pancreas (33). This was related to increased GLP-1R expression in early tumorigenesis. However, in humans with MEN-1 and EHH, in vivo or in vitro GLP-1R expression has not yet been examined. In view of the always multiple PanNETs visualized on conventional imaging in the context of patients with MEN-1, the exact localization of insulin-secreting PanNETs is critical for the surgical strategy to avoid unnecessary morbidity due to extensive surgery.
In this retrospective analysis, the combination of 68Ga-DOTA-exendin-4 ([Nle14,Lys40(Ahx-DOTA-68Ga)NH2]exendin-4) PET/CT and MRI was analyzed to evaluate its potential role in patients with MEN-1 mutations and confirmed EHH. We hypothesized that GLP-1R imaging in the context of MEN-1 is feasible and may be able to guide surgical strategy in patients with PanNETs in the context of EHH and multiple pancreatic lesions.
Materials and Methods
Study design and patients
Between January 2014 and March 2017, 52 patients with biochemically proven EHH highly suspicious for an insulinoma were prospectively recruited from different centers in Europe and the United States (ClinicalTrials.gov no. NCT02127541). Six of the 52 patients (female, 4 patients; male, 2 patients; median age, 34.5 years; interquartile range, 18.8 to 46 years) were identified to have a genetically proven germline mutation in the MEN-1 gene and were included in this post hoc analysis.
The patients fulfilled the inclusion criteria, which were age ≥18 years, biochemically proven EHH with neuroglycopenic symptoms, a positive Whipple triad, and negative results on sulfonylurea screening. Exclusion criteria were evidence of a malignant insulinoma on conventional imaging, pregnancy or breastfeeding women, and renal insufficiency (serum creatinine >140 µmol/L). None of the patients had bariatric surgery or other known causes for EHH (34).
The study was approved by the Regional Scientific Ethics Committee, and patients provided written consent in accordance with provisions of the Declaration of Helsinki prior to study participation.
Procedures
All patients received one 68Ga-DOTA-exendin-4 PET/CT and a standardized contrast-enhanced MRI scan within 3 to 4 days. The reference standard was successful surgery with histopathological and immunohistochemical confirmation of PanNETs including insulinoma and normalization of blood glucose levels after surgery.
A PET/CT scan was performed on different scanners in the supine position: PET/16-detector CT scanner (Discovery ST; GE Healthcare), PET/64-detector CT scanner (Discovery ST; GE Healthcare), and PET/128-detector CT scanner (Biograph mCT-X RT Pro edition). Calibration of PET scanners and their cross-calibration were performed. One bed position of the upper abdomen was acquired during 8 minutes, 2.5 hours after the IV injection of 68Ga-DOTA-exendin-4. PET images were reconstructed using an ordered-subsets expectation maximization algorithm with three iterations and 25 subsets. Low-dose CT (120 kVp, 30 to 100 mA) was used in all patients for attenuation correction and to provide an anatomic reference.
Abdominal MRI acquisition was performed on a commercially available 3T system (Magnetom Prisma; Siemens Healthcare) in the supine position using a multichannel body surface coil. The protocol aimed for a high spatial resolution and robustness with regard to breathing and motion artifacts. The body surface coil was placed firmly across the abdomen, and patients were asked to breathe calm and shallow to avoid excessive abdominal excursion during breathing.
The protocol included standard sequences: (i) coronal half-Fourier acquisition single-shot turbo spin-echo (HASTE) localizer, (ii) coronal breath-hold T2-weighted HASTE, (iii) transverse breath-hold T2-weighted HASTE, (iv) transverse, fat-suppressed T2-weighted turbo spin-echo images in breath hold, (v) breath-hold in- and out-of-phase T1-weighted gradient-echo sequence, (vi) free breathing echo planar diffusion-weighted images in the transverse plane using five sequence ii values (0, 50, 200, 400, and 800), (vii) transverse, free breathing (respiratory-triggered, navigator-echo technique), and (viii) fat-suppressed, T2-weighted spin-echo sequences images using periodically rotated overlapping parallel lines with enhanced reconstruction (BLADE).
Detailed acquisition parameters for non–contrast-enhanced PET/CT and standardized contrast-enhanced MRI have been described elsewhere (28).
Evaluation
Only the pancreas was regarded as a potential tumor site. To localize tumors in the pancreas in a standardized manner, the pancreas was categorized into three regions, namely, head, body, and tail, with the portal vein and the superior mesenteric artery serving as anatomic landmarks (Fig. 1).
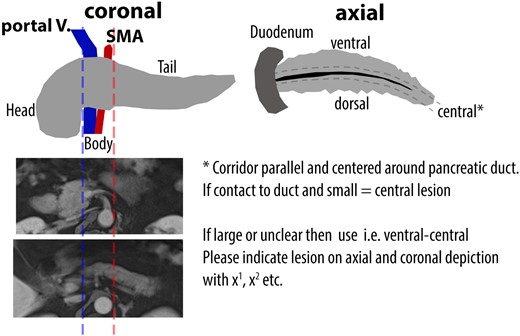
Schema of the pancreas which was used for standardized reading by blinded board-certified nuclear medicine physicians and board-certified radiologists.
PET/CT and MRI scans were independently assessed in random order by three board-certified nuclear medicine physicians for PET/CT scans and three different board-certified radiologists for MRI, each with >10 years of experience in PET/CT or MRI reading. All readers were unaware of the patient’s identity, other imaging results, or the patient’s clinical history. The number of lesions that could be clearly identified as a single focus was determined for each patient through majority reading.
A dual-accredited radiologist/nuclear medicine physician correlated lesions from blinded reading of 68Ga-DOTA-exendin-4 PET/CT and standardized contrast-enhanced MRI, which was used for the sensitivity analysis of combined 68Ga-DOTA-exendin-4 PET/CT and MRI in the detection of clinically relevant lesions.
A 68Ga-DOTA-exendin-4 PET-positive lesion was defined as an insulinoma. On contrast-enhanced MRI, a lesion with a clear tumor-to-background ratio visualized in at least two sequences was defined as a PanNET (Fig. 2).
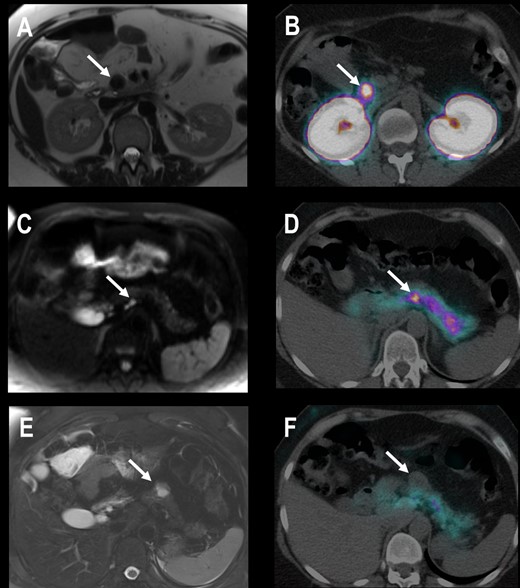
Patient 4 with EHH and three suspicious lesions in MRI. (A) Transaxial contrast-enhanced T1-weighted MRI shows a 20-mm hypointense lesion in the uncinate process of the pancreatic head. (B) Transaxial PET/CT 2.5 h after injection of 68Ga-DOTA-exendin-4 shows an intensive focal uptake in the same lesion. (C) Transaxial diffusion weighted MRI shows a 4-mm hyperintense lesion in the pancreatic body that is 68Ga-DOTA-exendin-4 positive (D). (E) Transaxial T2-weighted MRI shows a 25-mm lesion in the pancreatic tail without 68Ga-DOTA-exendin-4 uptake (F). Histological evaluation revealed one insulinoma in the uncinated process and pancreatic body (both 68Ga-DOTA-exendin-4 PET positive) and an NF-PanNET of 25 mm in the pancreatic tail. All other tumors were no insulinomas and <20 mm and therefore not clinically relevant. EHH resolved after surgery.
The reference standard for tumor size was from the surgical specimens and from MRI imaging.
68Ga-DOTA-exendin-4 uptake was quantified using maximum standardized uptake values (SUVmax).
Histopathologic diagnosis was made at the local referring institution where surgery was performed. In case of controversial findings, a central histological reading was available at the tertiary institution (Institute of Pathology, University of Bern).
Statistical analysis
Diagnostic performance in the detection of clinically relevant lesions was quantified on a per-lesion basis as sensitivity, specificity, and diagnostic accuracy. The reference standard was the histopathological diagnosis of surgically removed tissue. Data were assessed according to European Neuroendocrine Tumor Society (ENETS) recommendations, namely, to resect clinically relevant lesions, that is insulinomas independent of size and (if possible) only NF-PanNETs ≥20 mm with the aim of a pancreas-preserving surgery approach (13). Findings were defined as follows:
For MRI, which can measure tumor size but not discriminate between NF-PanNETs and insulinomas (Fig. 3):
True positive: detected NF-PanNETs and insulinomas ≥20 mm
True negative: all NF-PanNETs <20 mm (defined as clinically not relevant lesion)
False negative: not detected NF-PanNETs and insulinomas ≥20 mm; all insulinomas <20 mm
False positive: all detected lesions ≥20 mm that were not confirmed by surgery/histology
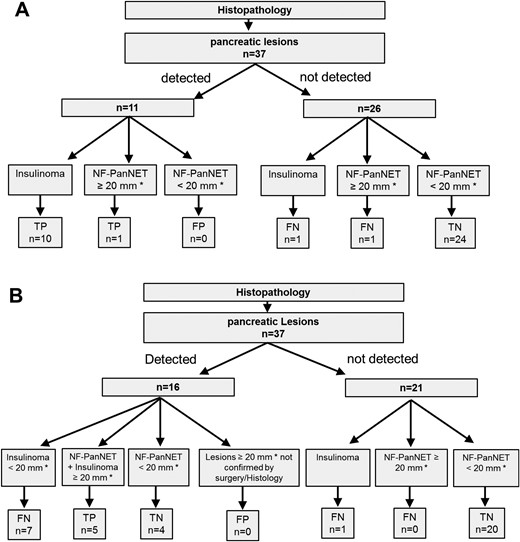
Flowchart with (A) 68Ga-DOTA-exendin-4 PET/CT and (B) MRI reading results. According to ENETS guidelines, true positive was defined as PanNETs ≥ 20 mm or insulinomas (i.e., clinically relevant lesions). MRI is not able to distinguish between functional and nonfunctional tumors, and thus the only criteria justifying resection of tumor in the pancreas is a size of ≥ 20 mm. As a result, insulinomas < 20 mm are classified as false negative in MRI reading. *Diameter measured with MRI. FN, false negative; FP, false positive; TN, true negative; TP, true positive.
For 68Ga-DOTA-exendin PET/CT, which cannot measure tumor size but can likely discriminate between NF-PanNETs and insulinomas (Fig. 3):
True positive: detected insulinomas of any size and detected NF-PanNETs ≥20 mm
True negative: not detected NF-PanNETs <20 mm
False negative: not detected NF-PanNETs ≥20 mm and not detected insulinomas of any size
False positive: detected NF-PanNETs <20 mm
In case of discordant findings for combined 68Ga-DOTA-exendin-4 PET/CT and MRI, that is, true positive with one imaging method and false negative with the other, the combined finding is rated as true positive.
All analyses were conducted using the statistical software package R (R Core Team, 2018), version 3.5.0, using two-sided statistical tests. CIs are calculated at a 1-α level of 95%. P values should be interpreted as a continuous measure of evidence against the corresponding null hypothesis (i.e., no difference between imaging methods) and not as confirmatory (“significant” vs “not significant”).
Sensitivity, specificity, and overall diagnostic accuracy for each imaging method in the detection of clinically relevant lesions were calculated with an exact binomial 95% CI using the R package epiR. The imaging methods were tested for a difference in the respective measure using mixed-effects logistic regression models (GLMM).
The models included the respective outcome as the binary endpoint (e.g., for sensitivity the outcome was true positive, 0/1), patient as random effect, and the imaging method as the main predictor. For the objective of identification of all clinically relevant tumor findings, 68Ga-DOTA-exendin-4 PET/CT and MRI were compared with both methods combined. P values for the comparisons are reported. No adjustment for multiple testing was made. Cross tabulations of the findings based on the imaging methods and the results of histopathology are given.
Results
Surgery and histopathological evaluation
All six patients received surgery based on all available imaging results and following the recommendations of current international guidelines. Table 1 summarizes the individual surgical procedures.
| Patient . | Tumor No. . | MRIa . | MRIb . | GLP-1R PET/CTa . | GLP-1R PET/CTb . | Localization . | Surgery Type . | Tumor Size (mm) . | Histological Diagnosis/Immunohistochemistry . | Grading . | Blood Glucose . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Positive | TP | Positive | TP | Body | Enucleation | 20 | Insulinoma/insulin | G2/Ki-67 = 5% | Normalization |
| 2 | 2 | Positive | FN | Positive | TP | Tail | Extended left resection | 14 | Insulinoma/insulin | G1/Ki-67 < 2% | Normalization |
| 3 | Positive | FN | Positive | TP | Tail | 11 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 4 | Positive | FN | Positive | TP | Tail | 6 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 5 | Positive | TN | Negative | TN | Body | 9 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 6 | Negative | FN | Negative | FN | Body/tail | 1 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 7 | Negative | TN | Negative | TN | Body/tail | 4 | NF-PanNET/ChA/Syn | NA | |||
| 8 | Negative | TN | Negative | TN | Body/tail | 5 | NF-PanNET/ChA/Syn | NA | |||
| 3 | 9 | Positive | FN | Positive | TP | Head | Completion pancreatectomy | 17 | Insulinoma/insulin | G1 | Normalization |
| 10 | Negative | TN | Negative | TN | Head | 10 | NF-PanNET/ChA/Syn | NA | |||
| 11 | Negative | TN | Negative | TN | Head | 9 | NF-PanNET/ChA/Syn | NA | |||
| 4 | 12 | Positive | TP | Negative | FN | Tail | Left resection/uncus enucleation | 25 | NF-PanNET/ChA/Syn | G2/Ki-67 = 3% | Normalization |
| 13 | Negative | TN | Negative | TN | Head | 17 | NF-PanNET/gastrin | G1/Ki-67 < 2% | |||
| 14 | Positive | TP | Positive | TP | Uncus | 20 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 15 | Positive | FN | Positive | TP | Body | 4 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 16 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 17 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 18 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 19 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 20 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 21 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 5 | 22 | Positive | TP | Positive | TP | Body | Exended left resection | 21 | NF-PanNET/ChA/Syn | G2/Ki-67 = 3% | Normalization |
| 23 | Positive | FN | Positive | TP | Tail | 7 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 24 | Positive | FN | Positive | TP | Tail | 11 | Insulinoma/insulin | G2/Ki-67 = 3% | |||
| 25 | Positive | TN | Negative | TN | Tail | 19 | NF-PanNET/ChA/Syn | G2/Ki-67 = 3.5% | |||
| 26 | Negative | TN | Negative | TN | Body/tail | 7 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 27 | Negative | TN | Negative | TN | Body/tail | 3 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 28 | Negative | TN | Negative | TN | Body/tail | 5 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 29 | Negative | TN | Negative | TN | Body/tail | 4 | NF-PanNET/ChA/Syn | NA | |||
| 6 | 30 | Positive | TP | Positive | TP | Body | Exended left resection | 20 | Insulinoma/insulin | G2/Ki-67 = 2%–5% | Normalization |
| 31 | Negative | TN | Negative | TN | Body | 5 | NF-PanNET/gastrin | G1/Ki-67 < 2% | |||
| 32 | Positive | TN | Negative | TN | Tail | 15 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 33 | Negative | TN | Negative | TN | Tail | 9 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 34 | Negative | TN | Negative | TN | Tail | 0.8 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 35 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 36 | Negative | TN | Negative | TN | Tail | 1.1 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 37 | Negative | TN | Negative | TN | Tail | 0.5 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% |
| Patient . | Tumor No. . | MRIa . | MRIb . | GLP-1R PET/CTa . | GLP-1R PET/CTb . | Localization . | Surgery Type . | Tumor Size (mm) . | Histological Diagnosis/Immunohistochemistry . | Grading . | Blood Glucose . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Positive | TP | Positive | TP | Body | Enucleation | 20 | Insulinoma/insulin | G2/Ki-67 = 5% | Normalization |
| 2 | 2 | Positive | FN | Positive | TP | Tail | Extended left resection | 14 | Insulinoma/insulin | G1/Ki-67 < 2% | Normalization |
| 3 | Positive | FN | Positive | TP | Tail | 11 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 4 | Positive | FN | Positive | TP | Tail | 6 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 5 | Positive | TN | Negative | TN | Body | 9 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 6 | Negative | FN | Negative | FN | Body/tail | 1 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 7 | Negative | TN | Negative | TN | Body/tail | 4 | NF-PanNET/ChA/Syn | NA | |||
| 8 | Negative | TN | Negative | TN | Body/tail | 5 | NF-PanNET/ChA/Syn | NA | |||
| 3 | 9 | Positive | FN | Positive | TP | Head | Completion pancreatectomy | 17 | Insulinoma/insulin | G1 | Normalization |
| 10 | Negative | TN | Negative | TN | Head | 10 | NF-PanNET/ChA/Syn | NA | |||
| 11 | Negative | TN | Negative | TN | Head | 9 | NF-PanNET/ChA/Syn | NA | |||
| 4 | 12 | Positive | TP | Negative | FN | Tail | Left resection/uncus enucleation | 25 | NF-PanNET/ChA/Syn | G2/Ki-67 = 3% | Normalization |
| 13 | Negative | TN | Negative | TN | Head | 17 | NF-PanNET/gastrin | G1/Ki-67 < 2% | |||
| 14 | Positive | TP | Positive | TP | Uncus | 20 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 15 | Positive | FN | Positive | TP | Body | 4 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 16 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 17 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 18 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 19 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 20 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 21 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 5 | 22 | Positive | TP | Positive | TP | Body | Exended left resection | 21 | NF-PanNET/ChA/Syn | G2/Ki-67 = 3% | Normalization |
| 23 | Positive | FN | Positive | TP | Tail | 7 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 24 | Positive | FN | Positive | TP | Tail | 11 | Insulinoma/insulin | G2/Ki-67 = 3% | |||
| 25 | Positive | TN | Negative | TN | Tail | 19 | NF-PanNET/ChA/Syn | G2/Ki-67 = 3.5% | |||
| 26 | Negative | TN | Negative | TN | Body/tail | 7 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 27 | Negative | TN | Negative | TN | Body/tail | 3 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 28 | Negative | TN | Negative | TN | Body/tail | 5 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 29 | Negative | TN | Negative | TN | Body/tail | 4 | NF-PanNET/ChA/Syn | NA | |||
| 6 | 30 | Positive | TP | Positive | TP | Body | Exended left resection | 20 | Insulinoma/insulin | G2/Ki-67 = 2%–5% | Normalization |
| 31 | Negative | TN | Negative | TN | Body | 5 | NF-PanNET/gastrin | G1/Ki-67 < 2% | |||
| 32 | Positive | TN | Negative | TN | Tail | 15 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 33 | Negative | TN | Negative | TN | Tail | 9 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 34 | Negative | TN | Negative | TN | Tail | 0.8 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 35 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 36 | Negative | TN | Negative | TN | Tail | 1.1 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 37 | Negative | TN | Negative | TN | Tail | 0.5 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% |
Abbreviations: Cha, chromogranin; FN, false negative; NA, not available; Syn, synaptophysin; TN, true negative; TP, true positive.
Results of scan reading. Readers were unaware of other results when reading the scans.
Evaluation according to ENETS recommendation with true-positive results in case of symptomatic insulinomas and NF-PanNETs ≥20 mm.
| Patient . | Tumor No. . | MRIa . | MRIb . | GLP-1R PET/CTa . | GLP-1R PET/CTb . | Localization . | Surgery Type . | Tumor Size (mm) . | Histological Diagnosis/Immunohistochemistry . | Grading . | Blood Glucose . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Positive | TP | Positive | TP | Body | Enucleation | 20 | Insulinoma/insulin | G2/Ki-67 = 5% | Normalization |
| 2 | 2 | Positive | FN | Positive | TP | Tail | Extended left resection | 14 | Insulinoma/insulin | G1/Ki-67 < 2% | Normalization |
| 3 | Positive | FN | Positive | TP | Tail | 11 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 4 | Positive | FN | Positive | TP | Tail | 6 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 5 | Positive | TN | Negative | TN | Body | 9 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 6 | Negative | FN | Negative | FN | Body/tail | 1 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 7 | Negative | TN | Negative | TN | Body/tail | 4 | NF-PanNET/ChA/Syn | NA | |||
| 8 | Negative | TN | Negative | TN | Body/tail | 5 | NF-PanNET/ChA/Syn | NA | |||
| 3 | 9 | Positive | FN | Positive | TP | Head | Completion pancreatectomy | 17 | Insulinoma/insulin | G1 | Normalization |
| 10 | Negative | TN | Negative | TN | Head | 10 | NF-PanNET/ChA/Syn | NA | |||
| 11 | Negative | TN | Negative | TN | Head | 9 | NF-PanNET/ChA/Syn | NA | |||
| 4 | 12 | Positive | TP | Negative | FN | Tail | Left resection/uncus enucleation | 25 | NF-PanNET/ChA/Syn | G2/Ki-67 = 3% | Normalization |
| 13 | Negative | TN | Negative | TN | Head | 17 | NF-PanNET/gastrin | G1/Ki-67 < 2% | |||
| 14 | Positive | TP | Positive | TP | Uncus | 20 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 15 | Positive | FN | Positive | TP | Body | 4 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 16 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 17 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 18 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 19 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 20 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 21 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 5 | 22 | Positive | TP | Positive | TP | Body | Exended left resection | 21 | NF-PanNET/ChA/Syn | G2/Ki-67 = 3% | Normalization |
| 23 | Positive | FN | Positive | TP | Tail | 7 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 24 | Positive | FN | Positive | TP | Tail | 11 | Insulinoma/insulin | G2/Ki-67 = 3% | |||
| 25 | Positive | TN | Negative | TN | Tail | 19 | NF-PanNET/ChA/Syn | G2/Ki-67 = 3.5% | |||
| 26 | Negative | TN | Negative | TN | Body/tail | 7 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 27 | Negative | TN | Negative | TN | Body/tail | 3 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 28 | Negative | TN | Negative | TN | Body/tail | 5 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 29 | Negative | TN | Negative | TN | Body/tail | 4 | NF-PanNET/ChA/Syn | NA | |||
| 6 | 30 | Positive | TP | Positive | TP | Body | Exended left resection | 20 | Insulinoma/insulin | G2/Ki-67 = 2%–5% | Normalization |
| 31 | Negative | TN | Negative | TN | Body | 5 | NF-PanNET/gastrin | G1/Ki-67 < 2% | |||
| 32 | Positive | TN | Negative | TN | Tail | 15 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 33 | Negative | TN | Negative | TN | Tail | 9 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 34 | Negative | TN | Negative | TN | Tail | 0.8 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 35 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 36 | Negative | TN | Negative | TN | Tail | 1.1 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 37 | Negative | TN | Negative | TN | Tail | 0.5 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% |
| Patient . | Tumor No. . | MRIa . | MRIb . | GLP-1R PET/CTa . | GLP-1R PET/CTb . | Localization . | Surgery Type . | Tumor Size (mm) . | Histological Diagnosis/Immunohistochemistry . | Grading . | Blood Glucose . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Positive | TP | Positive | TP | Body | Enucleation | 20 | Insulinoma/insulin | G2/Ki-67 = 5% | Normalization |
| 2 | 2 | Positive | FN | Positive | TP | Tail | Extended left resection | 14 | Insulinoma/insulin | G1/Ki-67 < 2% | Normalization |
| 3 | Positive | FN | Positive | TP | Tail | 11 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 4 | Positive | FN | Positive | TP | Tail | 6 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 5 | Positive | TN | Negative | TN | Body | 9 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 6 | Negative | FN | Negative | FN | Body/tail | 1 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 7 | Negative | TN | Negative | TN | Body/tail | 4 | NF-PanNET/ChA/Syn | NA | |||
| 8 | Negative | TN | Negative | TN | Body/tail | 5 | NF-PanNET/ChA/Syn | NA | |||
| 3 | 9 | Positive | FN | Positive | TP | Head | Completion pancreatectomy | 17 | Insulinoma/insulin | G1 | Normalization |
| 10 | Negative | TN | Negative | TN | Head | 10 | NF-PanNET/ChA/Syn | NA | |||
| 11 | Negative | TN | Negative | TN | Head | 9 | NF-PanNET/ChA/Syn | NA | |||
| 4 | 12 | Positive | TP | Negative | FN | Tail | Left resection/uncus enucleation | 25 | NF-PanNET/ChA/Syn | G2/Ki-67 = 3% | Normalization |
| 13 | Negative | TN | Negative | TN | Head | 17 | NF-PanNET/gastrin | G1/Ki-67 < 2% | |||
| 14 | Positive | TP | Positive | TP | Uncus | 20 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 15 | Positive | FN | Positive | TP | Body | 4 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 16 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 17 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 18 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 19 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 20 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 21 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | NA | |||
| 5 | 22 | Positive | TP | Positive | TP | Body | Exended left resection | 21 | NF-PanNET/ChA/Syn | G2/Ki-67 = 3% | Normalization |
| 23 | Positive | FN | Positive | TP | Tail | 7 | Insulinoma/insulin | G1/Ki-67 < 2% | |||
| 24 | Positive | FN | Positive | TP | Tail | 11 | Insulinoma/insulin | G2/Ki-67 = 3% | |||
| 25 | Positive | TN | Negative | TN | Tail | 19 | NF-PanNET/ChA/Syn | G2/Ki-67 = 3.5% | |||
| 26 | Negative | TN | Negative | TN | Body/tail | 7 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 27 | Negative | TN | Negative | TN | Body/tail | 3 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 28 | Negative | TN | Negative | TN | Body/tail | 5 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 29 | Negative | TN | Negative | TN | Body/tail | 4 | NF-PanNET/ChA/Syn | NA | |||
| 6 | 30 | Positive | TP | Positive | TP | Body | Exended left resection | 20 | Insulinoma/insulin | G2/Ki-67 = 2%–5% | Normalization |
| 31 | Negative | TN | Negative | TN | Body | 5 | NF-PanNET/gastrin | G1/Ki-67 < 2% | |||
| 32 | Positive | TN | Negative | TN | Tail | 15 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 33 | Negative | TN | Negative | TN | Tail | 9 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 34 | Negative | TN | Negative | TN | Tail | 0.8 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 35 | Negative | TN | Negative | TN | Tail | 1 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 36 | Negative | TN | Negative | TN | Tail | 1.1 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% | |||
| 37 | Negative | TN | Negative | TN | Tail | 0.5 | NF-PanNET/ChA/Syn | G1/Ki-67 < 2% |
Abbreviations: Cha, chromogranin; FN, false negative; NA, not available; Syn, synaptophysin; TN, true negative; TP, true positive.
Results of scan reading. Readers were unaware of other results when reading the scans.
Evaluation according to ENETS recommendation with true-positive results in case of symptomatic insulinomas and NF-PanNETs ≥20 mm.
In six patients a total of 37 PanNETs were confirmed by histopathology with a median number of lesions per patient of 7.5 and interquartile range (IQR) of 2.5 to 8.5 (see Table 1). This included 11 (30%) insulinomas with a median size of 11 mm (range, 1 to 20 mm) and 26 (70%) NF-PanNETs with a median size of 4 mm (range, 0.5 to 25 mm). The tumor grade was determined histologically using mitotic rates and Ki-67 indices (ENETS proposal for grading gastroenteropancreatic NETs) (35) and was available in 25 lesions: 19 lesions were classified as G1 tumors and six lesions were classified as G2 tumors. No G3 tumors were identified. Twenty-two lesions were not detected by the imaging procedures. Median tumor size of all lesions was 6 mm (range, 0.5 to 25 mm).
Table 2 summarizes the diagnostic performance of 68Ga-DOTA-exendin-4 PET/CT and MRI for detection of all clinically relevant tumor findings.
Comparison of Combined 68Ga-DOTA-Exendin-4 PET/CT Plus MRI and 68Ga-Exendin-4 PET/CT and MRI Alone in the Detection of Clinically Relevant PanNETs (Defined as NF-PanNETs ≥20 mm or Insulinomas) in MEN-1 Patients With EHH Plus Neuroglycopenia and Multiple Pancreatic Tumors
| . | 68Ga-DOTA-Exendin-4 PET/CT Plus MRI . | 68Ga-DOTA-Exendin-4 PET/CT . | Test for Superioritya . | MRI . | Test for Superiorityb . |
|---|---|---|---|---|---|
| Clinically relevant lesion: sensitivity | 92.3% (64.0–99.8) | 84.6% (54.6–98.1) | 0.537 | 38.5% (13.9–68.4) | 0.014 |
| Clinically relevant lesion: specificity | 100% (85.8–100) | 100% (85.8–100) | 1.000 | 100% (85.8–100) | 1.000 |
| Clinically relevant lesion: accuracy | 97.3% (85.8–99.9) | 94.6% (81.8–99.3) | 0.802 | 78.4% (61.8–90.2) | 0.059 |
| . | 68Ga-DOTA-Exendin-4 PET/CT Plus MRI . | 68Ga-DOTA-Exendin-4 PET/CT . | Test for Superioritya . | MRI . | Test for Superiorityb . |
|---|---|---|---|---|---|
| Clinically relevant lesion: sensitivity | 92.3% (64.0–99.8) | 84.6% (54.6–98.1) | 0.537 | 38.5% (13.9–68.4) | 0.014 |
| Clinically relevant lesion: specificity | 100% (85.8–100) | 100% (85.8–100) | 1.000 | 100% (85.8–100) | 1.000 |
| Clinically relevant lesion: accuracy | 97.3% (85.8–99.9) | 94.6% (81.8–99.3) | 0.802 | 78.4% (61.8–90.2) | 0.059 |
Diagnostic performance is the majority reading (95% CI) of three independent readers.
P values for comparison of combined 68Ga-DOTA-exendin-4 PET/CT plus MRI and 68Ga-DOTA-exendin-4 PET/CT alone.
P values for comparison of combined 68Ga-DOTA-exendin-4 PET/CT plus MRI and MRI alone.
Comparison of Combined 68Ga-DOTA-Exendin-4 PET/CT Plus MRI and 68Ga-Exendin-4 PET/CT and MRI Alone in the Detection of Clinically Relevant PanNETs (Defined as NF-PanNETs ≥20 mm or Insulinomas) in MEN-1 Patients With EHH Plus Neuroglycopenia and Multiple Pancreatic Tumors
| . | 68Ga-DOTA-Exendin-4 PET/CT Plus MRI . | 68Ga-DOTA-Exendin-4 PET/CT . | Test for Superioritya . | MRI . | Test for Superiorityb . |
|---|---|---|---|---|---|
| Clinically relevant lesion: sensitivity | 92.3% (64.0–99.8) | 84.6% (54.6–98.1) | 0.537 | 38.5% (13.9–68.4) | 0.014 |
| Clinically relevant lesion: specificity | 100% (85.8–100) | 100% (85.8–100) | 1.000 | 100% (85.8–100) | 1.000 |
| Clinically relevant lesion: accuracy | 97.3% (85.8–99.9) | 94.6% (81.8–99.3) | 0.802 | 78.4% (61.8–90.2) | 0.059 |
| . | 68Ga-DOTA-Exendin-4 PET/CT Plus MRI . | 68Ga-DOTA-Exendin-4 PET/CT . | Test for Superioritya . | MRI . | Test for Superiorityb . |
|---|---|---|---|---|---|
| Clinically relevant lesion: sensitivity | 92.3% (64.0–99.8) | 84.6% (54.6–98.1) | 0.537 | 38.5% (13.9–68.4) | 0.014 |
| Clinically relevant lesion: specificity | 100% (85.8–100) | 100% (85.8–100) | 1.000 | 100% (85.8–100) | 1.000 |
| Clinically relevant lesion: accuracy | 97.3% (85.8–99.9) | 94.6% (81.8–99.3) | 0.802 | 78.4% (61.8–90.2) | 0.059 |
Diagnostic performance is the majority reading (95% CI) of three independent readers.
P values for comparison of combined 68Ga-DOTA-exendin-4 PET/CT plus MRI and 68Ga-DOTA-exendin-4 PET/CT alone.
P values for comparison of combined 68Ga-DOTA-exendin-4 PET/CT plus MRI and MRI alone.
Comparison of MRI with histopathology
An MRI reading is based on majority readings by three radiologists. In the detection of clinically relevant lesions, defined as PanNETs ≥20 mm, MRI showed 5 true-positive, 24 true-negative, 8 false-negative, and no false-positive findings. MRI did not detect 22 tumors, which had a median size of 2.1 mm (range, 0.5 to 17 mm).
Comparison of 68Ga-DOTA-exendin-4 PET/CT with histopathology
Lesion detection on 68Ga-DOTA-exendin-4 PET/CT is based on majority readings by three nuclear medicine physicians. SUVmax was determined for all detected insulinomas and ranged from 4.3 to 43 with a median SUVmax (IQR) of 22.4 (9.1 to 34.9).
68Ga-DOTA-exendin-4 PET/CT identified 11 of 37 (30%) tumors with a median size of 14 mm (range, 4 to 21 mm), of which 10 of 11 (91%) were insulinomas and 1 of 11 (9%) was a NF-PanNET with a diameter of 21 mm. 68Ga-DOTA-exendin-4 PET/CT did not detect 26 tumors (median size of 4 mm; range, 0.5 to 25 mm). This included 1 of 26 (4%) insulinomas (tumor size 1 mm) and 2 of 26 (8%) and 25 of 26 (88%) NF-PanNETs (median tumor size 4 mm; range, 0.5 to 25 mm).
In the detection of clinically relevant lesions, 68Ga-DOTA-exendin-4 PET/CT was true positive in 11 of 37 (30%), true negative in 24 of 37 (65%), false negative in 2 of 37 (5%), and false positive in 0 of 37 (0%).
Surgical strategy and outcome
Surgical planning was based on all available imaging results and performed in all six patients. Symptoms of EHH ceased in all patients after surgery with no recurrence within a follow-up period of 3 months. Median time between study imaging and surgery was 3.5 months (IQR, 2.8 to 9 months).
Side effects
Nausea and sporadic vomiting are known side effects of 68Ga-DOTA-exendin-4 and have been described elsewhere (28). All patients received an exogenous glucose (1000 mL, 10%) infusion for 5 hours starting just before injection of the radiotracer and the flow was adapted as needed.
Discussion
To our knowledge, this is the first study investigating the potential role of GLP-1R imaging in patients with EHH and a known MEN-1 germline mutation. The main findings of this study can be summarized as follows: (i) Overexpression of GLP-1R is present in insulinomas in patients with EHH in the context of MEN-1 syndrome and, therefore, GLP-1R imaging is feasible and helpful in this context to guide selective and pancreas-sparing surgery. (ii) Conventional imaging alone may be insufficient, as it detects less than half of the pancreatic lesions in MEN-1 patients. Combination of 68Ga-DOTA-exendin-4 PET/CT and MRI is highly beneficial to detect clinically relevant lesions in MEN-1 patients, as morphological imaging alone cannot differentiate between insulin-secreting and non–insulin-secreting PanNETs.
Our data confirm that preoperative GLP-1R PET/CT in the context of an MEN-1 mutation is useful. This finding is further substantiated by the fact that in vivo assessment of GLP-1R density using SUVmax measurements yielded similar results in MEN-1 insulinomas as seen in sporadic benign insulinoma cases [median SUVmax (IQR), 22.4 (9.1 to 34.9) vs 20 (12.5 to 35.3)] (28), indicating that insulinomas in the context of MEN-1 show a similar GLP-1R overexpression. So far this feature has only been proven in a conventional heterozygous knockout mouse model of Men1 (33). These MEN-1 mice exhibited an overexpression of GLP-1R in microadenomas, consistent with our findings in humans and suggesting that 68Ga-DOTA-exendin-4 has a potential role for the detection of insulinomas in patients with MEN-1 and EHH.
On histopathological examination, 37 PanNETs were diagnosed in six patients, consistent with previous histopathological studies (36). Among them, 22 lesions were not detected by MRI or GLP-1R imaging, mainly because there were no insulinomas or they had a size below imaging resolution. The small size of the undetected lesions, the limited spatial resolution of MRI, as well as the susceptibility of MRI to motion artifacts and bowel peristaltic movement could be an explanation as to why the lesions were missed in MRI. However, for the detected tumors ≥20 mm in which according to ENETS guidelines resection due to increased malignancy potential is recommended (13), MRI had a sensitivity of 100%.
Insulin-secreting NETs in the context of MEN-1 are typically smaller lesions within multiple PanNETs (36). It is, therefore, highly relevant that GLP-1R imaging detected insulinomas up to a minimal size of 4 mm (Table 1), corroborating the high sensitivity of this investigation. Additionally, that all six patients were cured of their hypoglycemia following surgery suggests that the clinically relevant insulin-secreting NETs were successfully treated by GLP-1R imaging-guided surgery.
Morphological assessment of PanNETs (i.e., size) in patients with MEN-1 is critical to determine the potential for a malignant course of the disease (12). However, conventional morphological imaging is not able to distinguish between functional and nonfunctional tumors, and thus the only criteria justifying resection of a tumor is based on its size. Because insulinomas in the context of MEN-1 are often small (36), MRI produces a high number of false-negative findings (8 lesions) and a low number of true-positive findings (5 lesions) in comparison with GLP-1R PET/CT, which showed only 2 false-negative findings and a higher number of true-positive findings (11 lesions). The combination of both imaging modalities reduces the high rate of false-negative findings to 1 lesion and increases the rate of true-positive findings to 12, resulting in a sensitivity of 92.3%. Consequently, in clinical routine practice the combination of both modalities is advocated in patients with MEN-1 and EHH. Identification of PanNETs <20 mm with native low-dose CT was inferior compared with MRI (results not shown). However, contrast-enhanced CT in combination with GLP-1R imaging as a one-stop shot PET/CT procedure is likely to perform as well as MRI plus GLP-1R PET because contrast medium-enhanced CT is able to detect lesions with a threshold size of ≥ 20 mm. Another alternative is the use of an integrated PET/MRI scanner.
68Ga-DOTA-exendin-4 PET/CT was positive in one NF-PanNET (patient 5/tumor no. 22) with high 68Ga-DOTA-exendin-4 uptake (SUVmax of 34.9). The diameter of this tumor was 21 mm, and MRI detected it as well.
This is an intriguing finding. It suggests that NF-PanNETs can sometimes express the GLP-1R at a high density, consistent with previous findings in the literature (21). However, well-differentiated NETs, such as gastrinomas and pancreatic polypeptide-secreting tumors, were not detected by GLP-1R imaging in this study, confirming previous findings that the GLP-1R receptor is mainly overexpressed in insulinomas (21).
This study has limitations. (i) It was a retrospective analysis of prospectively collected data. As in all retrospective studies, they are prone to possible bias. In particular, there were not always enough reliable data on the intraoperative tumor localization. (ii) Histological assessment was not a priori centralized, and proliferative activity of the tumors could have an influence on the overexpression of the GLP-1R (32); this was not evaluated in all tumors. Furthermore, an additional limit maybe that positivity for insulin staining is not always associated with significant EHH, especially in patients with MEN-1. However, that all patients were cured of hypoglycemia following the surgical intervention suggests that the clinically relevant lesions were surgically removed. (iii) A low-dose native CT scan is not per se a diagnostic procedure, because it is unable to document clearly the size of lesions as well as important anatomical structures (e.g., main pancreatic duct), which is important for planning the surgical strategy. In an ideal setting contrast-enhanced PET/CT or contrast-enhanced PET/MRI should be used.
In conclusion, GLP-1R PET/CT is a useful and reliable imaging technique to selectively identify insulinomas within the multiple PanNETs in patients with MEN-1. The careful interpretation of a morphological modality (MRI) in combination with a functional imaging technique (GLP-1R PET/CT) may guide the surgical procedure and avoid unnecessary pancreatic resections. “Blind” pancreatic resections should be history.
Acknowledgments
We thank all of the patients who participated in the trial, the referring physicians and the local investigators who contributed to the trial, and the technicians who did the labelling and the scans. We specially thank Astrid Roesler (Clinical Trial Unit, Department of Clinical Research, University Hospital Basel and University of Basel, Basel, Switzerland) for monitoring the study.
Financial Support: This work was supported by Swiss National Science Foundation Grant 320030-152938 and by Desirée and Niels Yde’s Foundation Grant 389-12, both of which had no role in study design, data collection, analysis, interpretation, or writing of the report.
Clinical Trial Information: ClinicalTrials.gov no. NCT02127541 (registered 6 January 2014).
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Abbreviations:
- EHH
endogenous hyperinsulinemic hypoglycemia
- ENETS
European Neuroendocrine Tumor Society
- F-PanNET
functioning pancreatic neuroendocrine tumor
- GLP-1
glucagon-like peptide-1
- GLP-1R
GLP-1 receptor
- HASTE
half-Fourier acquisition single-shot turbo spin-echo
- IQR
interquartile range
- MEN-1
multiple endocrine neoplasia type-1
- NET
neuroendocrine tumor
- NF-PanNET
nonfunctioning pancreatic neuroendocrine tumor
- PanNET
pancreatic neuroendocrine tumor
- PET
positron emission tomography
- SUVmax
maximum standardized uptake values
References and Notes
Author notes
D.W. and E.C. contributed equally as last authors.



