-
PDF
- Split View
-
Views
-
Cite
Cite
Brett Barrett, Colin P Hawkes, Amber Isaza, Andrew J Bauer, The Effects of Amiodarone on Thyroid Function in Pediatric and Young Adult Patients, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 11, November 2019, Pages 5540–5546, https://doi.org/10.1210/jc.2019-00990
Close - Share Icon Share
Abstract
Amiodarone is used in patients with arrhythmias, but thyroid dysfunction [amiodarone-induced thyrotoxicosis (AIT) or amiodarone-induced hypothyroidism (AIH)] is a common adverse effect. As the onset of AIT and AIH has not been studied in children, the timing of dysfunction and long-term monitoring are not known in this population.
To describe the incidence and timing of amiodarone-induced thyroid dysfunction in children and adolescents, with a secondary aim to identify risk factors for amiodarone-induced thyroid dysfunction, and to identify variance in thyroid hormone surveillance and treatment.
Retrospective review of thyroid dysfunction in children and young adults treated with amiodarone between 2007 and 2018.
Children’s Hospital of Philadelphia.
Children and young adults treated with amiodarone.
Prevalence of amiodarone-induced thyroid dysfunction.
Of 484 patients, 190 had thyroid-function testing; 17.3% were found to have subclinical hypothyroidism, and 13.7% testing developed hypothyroidism. Hyperthyroidism occurred in 2.1%. In patients with subclinical hypothyroidism, 63% returned to normal thyroid function without thyroid hormone replacement. Only 26% of patients with hypothyroidism had spontaneous normalization of thyroid function. Twenty-five percent of AIT patients had spontaneous normalization of thyroid function.
This study looks at a pediatric and young-adult population in an effort to describe the natural history of amiodarone-induced thyroid dysfunction. Based on our data, we recommend that a complete thyroid-function panel be obtained within the first week and then at weekly intervals for the first 5 weeks after initiation. The majority of thyroid dysfunction was noted within the first 35 days of treatment.
Amiodarone is a potent antiarrhythmic agent that is a structural analog of the thyroid hormone. The antiarrhythmic effect is believed to be mediated through blocking membrane ion channels via alteration in the lipid bilayer cell membrane (1). The most common side effect of amiodarone use is thyroid dysfunction, with alterations in thyroid hormone production and metabolism or direct thyroid gland toxicity (1–3). Amiodarone-induced side effects to the thyroid may occur within days of administration of the medication and may persist for weeks to months after discontinuation as a result of its high iodine content and the lipophilic structure of the drug (4). The clearance half-life of amiodarone is between 40 and 100 days (5).
Amiodarone contains two iodine atoms per molecule, and the hepatic metabolism of 100 mg releases ∼3 mg inorganic iodine into the systemic circulation (6). Approximately 150 µg of dietary iodine is required per day for thyroid hormone production, 10- to 100-fold less than the iodine burden associated with the daily therapeutic use of amiodarone (4, 7). In comparison, the average daily iodine content of a typical American diet is ∼0.3 mg/day (2, 7).
When excess iodine is ingested, thyroid hormone production may either decrease or increase depending on the individual’s baseline thyroid gland and iodine status at the time of exposure. For patients with a healthy thyroid gland, exposure to excess iodine most commonly results in decreased thyroid hormone production, secondary to downregulation of the sodium-iodine symporter, as well as thyroid peroxidase (TPO), a protective reflex known as the Wolff-Chaikoff effect (5). For patients with a history of thyroiditis, excess exposure to iodine may lead to the inability to escape from the Wolff-Chaikoff effect. In those with iodine deficiency or autonomous function, there may be increased thyroid hormone production, a phenomenon known as the Jod-Basedow effect. Both of these phenomena are reversible after removal of excess iodine exposure; however, because of the accumulation of iodine in the thyroid and lipid stores, amiodarone-induced hypothyroidism (AIH) and amiodarone-induced thyrotoxicosis (AIT) may persist for months after the discontinuation of amiodarone, and medical therapy with either thyroid hormone-replacement or anti-thyroid medications may be required (8–10).
In adults, AIH is more common in iodine-sufficient populations, females, and the setting of Hashimoto’s thyroiditis, where amiodarone administration is associated with an increased incidence of overt hypothyroidism (TSH > 10 mIU/L) (11, 12). The onset of AIH is early, typically occurring within 6 months after starting amiodarone (1, 5). Remission from hypothyroidism may occur for patients without a history of underlying thyroid disease. However, for patients with evidence of thyroid autoimmunity, it is more common for the hypothyroidism to persist. Overall, up to 25% of patients taking amiodarone will develop AIH with no correlation to the administered dose (1, 9, 10).
In contrast to AIH, AIT is more common in iodine-deficient areas and occurs more frequently in males. AIT may occur within the first few weeks after the commencement of amiodarone; however, it is more common for the onset to occur months after the initiation of amiodarone (1, 9, 10). There are two types of AIT: type I is associated with increased thyroid hormone production, and type II occurs secondary to destructive thyroiditis, with an unregulated release of a preformed thyroid hormone into the bloodstream. Patients with a history of autoimmune hyperthyroidism or multinodular goiter are at increased risk for developing type I AIT, and patients who develop type II AIT often have no previous history of thyroid disease (1, 5).
The intrinsic side effects of amiodarone may also affect thyroid hormone levels via alterations in thyroid hormone metabolism, transport, and action. Many of these changes occur within 7 to 10 days of initiating amiodarone therapy. Amiodarone inhibits 5′-monodeiodination (T4, T3 production, as well as clearance of. This results in decreased plasma levels of T3 and increased rT3, respectively. Decreased T3 production within the pituitary gland likely explains the concomitant early increase in TSH levels (5). In cardiac tissue, the main metabolite of amiodarone, desethylamiodarone, inhibits T3 receptor binding to nuclear receptors and decreases expression of thyroid hormone-related genes. Electrophysiological changes associated with chronic amiodarone administration resemble changes induced by hypothyroidism (5, 9, 13). The acute, amiodarone-induced thyroid hormone dysfunction typically persists for weeks; however, similar to other side effects, it may persist for months.
In adult patients, there are a number of large studies, review articles, as well as guidelines for the screening of amiodarone-induced side effects (1–3, 5, 8–10, 12–14). The current North American Society of Pacing and Electrophysiology adult guidelines recommend that patients should be screened with a TSH, free T4 (FT4), and total T3 checked before initiation of therapy. These tests should be rechecked every 3–6 months, based on clinical findings (13). The American Thyroid Association and American Association of Clinical Endocrinologists also published guidelines for adults on amiodarone therapy, which were similar, recommending thyroid-function testing at baseline and 1, 3, and every 3 to 6 months thereafter (15). One prospective study on adult patients living in an iodine-sufficient region reported an incidence of AIH of 6.9% and AIT of 12.1%. All cases of AIH occurred early in the course of amiodarone therapy. The development of AIH was associated with a history of pre-existent thyroid disease. The relative risk for women with TPO and/or thyroglobulin (TG) autoantibodies before treatment was 13.5% (11). Another recently published adult study retrospectively reviewed the frequency at which overt thyroid dysfunction was preceded by subclinical thyroid dysfunction in subjects placed on amiodarone therapy. AIT occurred in 14.5%, and AIH developed in 10.9% of patients after initiation of amiodarone therapy (8).
Although the prevalence and natural history of amiodarone-induced thyroid dysfunction have been described in adult patients, little is known regarding the risk of amiodarone-induced thyroid disease in pediatric patients, including little to no data on the incidence, timing, and type of amiodarone-induced alterations to thyroid hormone metabolism and thyroid function, as well as a lack of data to determine if and/or when thyroid hormone screening and thyroid hormone-replacement therapy may be beneficial (16–19). Furthermore, there are no clear evidence-based guidelines for the screening of pediatric patients for thyroid dysfunction after the initiation of amiodarone therapy. The primary aim of our study was to describe the incidence and timing of amiodarone-induced alterations in the thyroid hormone metabolism and thyroid function in children and adolescents at our center, with secondary aims to identify any risk factors for the development of amiodarone-induced thyroid dysfunction and the variance in thyroid hormone surveillance and management of pediatric patients with amiodarone-induced thyroid dysfunction and to recommend guidelines for prospective surveillance and treatment.
Methods
Patients and methods
All patients treated with amiodarone at the Children’s Hospital of Philadelphia between 1 January 2007 and 23 January 2018 were included in this retrospective chart review. Patients with primary thyroid disease on medication before initiation of amiodarone were excluded. This study was approved by the Children’s Hospital of Philadelphia Institutional Review Board.
We extracted demographic data, including patients’ sex, race, ethnicity, date of birth, start and end of amiodarone therapy, and time of death (when applicable). We also collected all available thyroid-function test results (TSH, T4, FT4, T3, and rT3), thyroid autoantibody results [anti-TPO, anti-TG, thyroid-stimulating immunoglobulin (TSI), and TSH receptor antibody], and possible therapy for AIT/AIH (levothyroxine, methimazole, propylthiouracil, and prednisone).
Primary hypothyroidism was defined as TSH > 10 mIU/L, subclinical hypothyroidism as TSH > 5 and < 10 mIU/L, and hyperthyroidism as a TSH < 0.1 mIU/L, associated with an elevated FT4, T4, or T3. Exposure time to amiodarone was defined as acute (<10 days), subacute (≥10 days but <3 months), and chronic (≥3 months) after initiation of amiodarone therapy.
Statistical analysis
Baseline descriptive statistics, demographics, and clinical characteristics are presented as means ± SD for normally distributed data or median (range) for non-normally distributed data. Counts with percentages for categorical variables are presented. All statistical analysis was performed using the Statistical Package for the Social Sciences 21.0 (IBM, Armonk, NY).
Results
Of the 484 total patients, 190 had accompanying thyroid-function testing and were not on levothyroxine therapy before initiation of amiodarone. Sixty-three patients were found to have thyroid dysfunction. Four patients did not have continued thyroid monitoring to resolution of thyroid dysfunction or start of treatment, and these four patients were excluded from the data analysis (Fig. 1 and Table 1).
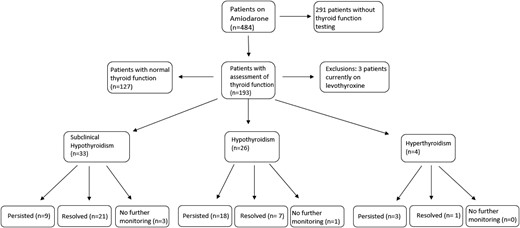
Patients developing subclinical hypothyroidism, AIH, and AIT and resolution.
Demographics, Outcomes, and Clinical Characteristics in All Included Patients and in Those Who Developed Thyroid Dysfunction
| Demographics . | All Patients (n = 484) . | Patients Who Developed Thyroid Dysfunction (n = 63) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 300 (62) | 42 (66.7) |
| Race groups, n (%) | ||
| Black | 106 (22) | 13 (20.6) |
| White | 261 (54) | 28 (44.4) |
| Other | 110 (22.7) | 22 (34.9) |
| Unknown | 7 (1.4) | |
| Ethnicity, n (%) | ||
| Latino | 52 (10.7) | 8 (14.8) |
| Age, median (IQR) | 1.35 (1.4–15.1) | |
| Age started amiodarone, median (IQR) | n/a | 0.7 (0.1–10.4) |
| Died, n (%) | n/a | 14 (22.2) |
| Thyroid outcome, n (%) | ||
| Hyperthyroidism | 4 (6.4) | |
| Hypothyroidism | 26 (41.3) | |
| Subclinical hypothyroidism | 33 (53.3) | |
| Days on amiodarone before developing thyroid dysfunction, median (IQR) | 9 (3–20) | |
| Resolved, n (%) | 29 (49.2) |
| Demographics . | All Patients (n = 484) . | Patients Who Developed Thyroid Dysfunction (n = 63) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 300 (62) | 42 (66.7) |
| Race groups, n (%) | ||
| Black | 106 (22) | 13 (20.6) |
| White | 261 (54) | 28 (44.4) |
| Other | 110 (22.7) | 22 (34.9) |
| Unknown | 7 (1.4) | |
| Ethnicity, n (%) | ||
| Latino | 52 (10.7) | 8 (14.8) |
| Age, median (IQR) | 1.35 (1.4–15.1) | |
| Age started amiodarone, median (IQR) | n/a | 0.7 (0.1–10.4) |
| Died, n (%) | n/a | 14 (22.2) |
| Thyroid outcome, n (%) | ||
| Hyperthyroidism | 4 (6.4) | |
| Hypothyroidism | 26 (41.3) | |
| Subclinical hypothyroidism | 33 (53.3) | |
| Days on amiodarone before developing thyroid dysfunction, median (IQR) | 9 (3–20) | |
| Resolved, n (%) | 29 (49.2) |
Abbreviations: IQR, interquartile range; n/a, not applicable.
Demographics, Outcomes, and Clinical Characteristics in All Included Patients and in Those Who Developed Thyroid Dysfunction
| Demographics . | All Patients (n = 484) . | Patients Who Developed Thyroid Dysfunction (n = 63) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 300 (62) | 42 (66.7) |
| Race groups, n (%) | ||
| Black | 106 (22) | 13 (20.6) |
| White | 261 (54) | 28 (44.4) |
| Other | 110 (22.7) | 22 (34.9) |
| Unknown | 7 (1.4) | |
| Ethnicity, n (%) | ||
| Latino | 52 (10.7) | 8 (14.8) |
| Age, median (IQR) | 1.35 (1.4–15.1) | |
| Age started amiodarone, median (IQR) | n/a | 0.7 (0.1–10.4) |
| Died, n (%) | n/a | 14 (22.2) |
| Thyroid outcome, n (%) | ||
| Hyperthyroidism | 4 (6.4) | |
| Hypothyroidism | 26 (41.3) | |
| Subclinical hypothyroidism | 33 (53.3) | |
| Days on amiodarone before developing thyroid dysfunction, median (IQR) | 9 (3–20) | |
| Resolved, n (%) | 29 (49.2) |
| Demographics . | All Patients (n = 484) . | Patients Who Developed Thyroid Dysfunction (n = 63) . |
|---|---|---|
| Sex, n (%) | ||
| Male | 300 (62) | 42 (66.7) |
| Race groups, n (%) | ||
| Black | 106 (22) | 13 (20.6) |
| White | 261 (54) | 28 (44.4) |
| Other | 110 (22.7) | 22 (34.9) |
| Unknown | 7 (1.4) | |
| Ethnicity, n (%) | ||
| Latino | 52 (10.7) | 8 (14.8) |
| Age, median (IQR) | 1.35 (1.4–15.1) | |
| Age started amiodarone, median (IQR) | n/a | 0.7 (0.1–10.4) |
| Died, n (%) | n/a | 14 (22.2) |
| Thyroid outcome, n (%) | ||
| Hyperthyroidism | 4 (6.4) | |
| Hypothyroidism | 26 (41.3) | |
| Subclinical hypothyroidism | 33 (53.3) | |
| Days on amiodarone before developing thyroid dysfunction, median (IQR) | 9 (3–20) | |
| Resolved, n (%) | 29 (49.2) |
Abbreviations: IQR, interquartile range; n/a, not applicable.
Thyroid function
Of those who had thyroid-function testing and met inclusion criteria, 17.3% (33/190) were found to have subclinical hypothyroidism, and 13.7% (26/190) developed hypothyroidism. Three of the 26 patients who were included in the hypothyroid group had preceding subclinical hypothyroidism. The median time of onset for subclinical AIH and AIH, respectively, was 7 (2 to 23) and 11 (6 to 17) days (Fig. 2).
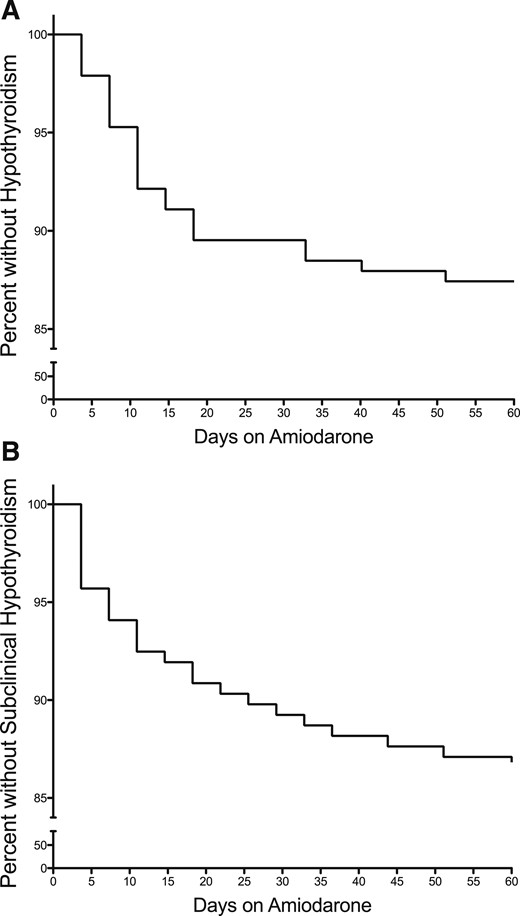
Percent of patients without (A) overt hypothyroidism or (B) subclinical hypothyroidism after initiation of amiodarone treatment.
Of those with subclinical hypothyroidism, 63% (21/33) became euthyroid without thyroid hormone replacement. Nine patients had persistent elevation in TSH > 5 and < 10 mIU/L, and two of those, with respective ages 2 months and 13 years, were started on levothyroxine with TSH levels of 7.4 and 7.8 mIU/L. Three patients did not undergo additional monitoring and were excluded from the analysis.
For patients with hypothyroidism (TSH > 10 mIU/L), only 26.9% (7/26) of patients with hypothyroidism had a TSH level that spontaneously reverted back to the normal range. The median peak TSH of this group was 12.1 mIU/L (11.7 to 18.3), and thyroid function normalized within 30 days (18 to 67). Of the remaining 69% (18/26) of patients with hypothyroidism, 15 were started on levothyroxine-replacement therapy, and three did not have a TSH that normalized documented in the chart. The median peak TSH at the time of levothyroxine initiation was 14.2 mIU/L (11.8 to 22). One patient with hypothyroidism was not monitored further and was excluded from the analysis (Fig. 1 and Table 2).
| Diagnosis . | Age Starting Amiodarone Therapy in Years . | Duration of Treatment at Time of Peak TSH . | Peak TSH, mIU/L . | Time to Normalization of Thyroid Function, Days . |
|---|---|---|---|---|
| SVT | 0.19 | Day 2 | 23.2 | 13 |
| Coarctation of aorta | 0.01 | Day 3 | 11.3 | 90 |
| TOF | 0.01 | Day 15 | 12.1 | 172 |
| Myocarditis | 1.37 | Day 16 | 13.5 | 44 |
| TAPVR | 0.1 | Day 39 | 11.8 | 23 |
| RVOT | 0.85 | Day 7 | 11.6 | 10 |
| LVOT | 0.12 | Day 49 | 15.5 | 30 |
| Diagnosis . | Age Starting Amiodarone Therapy in Years . | Duration of Treatment at Time of Peak TSH . | Peak TSH, mIU/L . | Time to Normalization of Thyroid Function, Days . |
|---|---|---|---|---|
| SVT | 0.19 | Day 2 | 23.2 | 13 |
| Coarctation of aorta | 0.01 | Day 3 | 11.3 | 90 |
| TOF | 0.01 | Day 15 | 12.1 | 172 |
| Myocarditis | 1.37 | Day 16 | 13.5 | 44 |
| TAPVR | 0.1 | Day 39 | 11.8 | 23 |
| RVOT | 0.85 | Day 7 | 11.6 | 10 |
| LVOT | 0.12 | Day 49 | 15.5 | 30 |
Abbreviations: LVOT, left-ventricular outflow tract; RVOT, right-ventricular outflow tract; SVT, supraventricular tachycardia; TAPVR, total anomalous pulmonary venous return; TOF, tetralogy of fallot.
| Diagnosis . | Age Starting Amiodarone Therapy in Years . | Duration of Treatment at Time of Peak TSH . | Peak TSH, mIU/L . | Time to Normalization of Thyroid Function, Days . |
|---|---|---|---|---|
| SVT | 0.19 | Day 2 | 23.2 | 13 |
| Coarctation of aorta | 0.01 | Day 3 | 11.3 | 90 |
| TOF | 0.01 | Day 15 | 12.1 | 172 |
| Myocarditis | 1.37 | Day 16 | 13.5 | 44 |
| TAPVR | 0.1 | Day 39 | 11.8 | 23 |
| RVOT | 0.85 | Day 7 | 11.6 | 10 |
| LVOT | 0.12 | Day 49 | 15.5 | 30 |
| Diagnosis . | Age Starting Amiodarone Therapy in Years . | Duration of Treatment at Time of Peak TSH . | Peak TSH, mIU/L . | Time to Normalization of Thyroid Function, Days . |
|---|---|---|---|---|
| SVT | 0.19 | Day 2 | 23.2 | 13 |
| Coarctation of aorta | 0.01 | Day 3 | 11.3 | 90 |
| TOF | 0.01 | Day 15 | 12.1 | 172 |
| Myocarditis | 1.37 | Day 16 | 13.5 | 44 |
| TAPVR | 0.1 | Day 39 | 11.8 | 23 |
| RVOT | 0.85 | Day 7 | 11.6 | 10 |
| LVOT | 0.12 | Day 49 | 15.5 | 30 |
Abbreviations: LVOT, left-ventricular outflow tract; RVOT, right-ventricular outflow tract; SVT, supraventricular tachycardia; TAPVR, total anomalous pulmonary venous return; TOF, tetralogy of fallot.
Hyperthyroidism occurred in 2.1% (4/190), and there were no patients with subclinical hyperthyroidism. In patients with hyperthyroidism, only 25% (1/4) of patients had a spontaneous return to normal thyroid function with 75% (3/4) of patients treated with methimazole (Fig. 1). Only two of these patient had antibody testing or imaging. One patient had positive TSI, along with thyroid ultrasound, consistent with Graves’ disease. The other patient had negative TSI testing.
Duration of amiodarone therapy was taken into account. Those with either subacute or chronic amiodarone therapy were more likely to develop thyroid dysfunction than those with acute therapy. Most commonly, patients from the subacute therapy group developed thyroid dysfunction, with >50% of each type of thyroid dysfunction coming from the subacute therapy group (Table 3).
Clinical Features of Patients Who Developed Each Type of Thyroid Dysfunction
| . | Hyperthyroidism (n = 4) . | Hypothyroidism (n = 26) . | Subclinical Hypothyroidism (n = 33) . |
|---|---|---|---|
| Duration of amiodarone treatment, n (%) | |||
| Acute, <10 days | 1 (25) | 3 (11.5) | 6 (18.2) |
| Subacute, ≥10 days to <3 months | 2 (50) | 16 (61.5) | 18 (54.6) |
| Chronic, ≥3 months | 1 (25) | 7 (26.9) | 9 (27.3) |
| Days on amiodarone before thyroid dysfunction, mean (SD) | 9.00 (11.8) | 19.38 (32.0) | 16.09 (20.6) |
| Resolved, n (%) | 1 (25) | 7 (26.9) | 21 (63.6) |
| . | Hyperthyroidism (n = 4) . | Hypothyroidism (n = 26) . | Subclinical Hypothyroidism (n = 33) . |
|---|---|---|---|
| Duration of amiodarone treatment, n (%) | |||
| Acute, <10 days | 1 (25) | 3 (11.5) | 6 (18.2) |
| Subacute, ≥10 days to <3 months | 2 (50) | 16 (61.5) | 18 (54.6) |
| Chronic, ≥3 months | 1 (25) | 7 (26.9) | 9 (27.3) |
| Days on amiodarone before thyroid dysfunction, mean (SD) | 9.00 (11.8) | 19.38 (32.0) | 16.09 (20.6) |
| Resolved, n (%) | 1 (25) | 7 (26.9) | 21 (63.6) |
Clinical Features of Patients Who Developed Each Type of Thyroid Dysfunction
| . | Hyperthyroidism (n = 4) . | Hypothyroidism (n = 26) . | Subclinical Hypothyroidism (n = 33) . |
|---|---|---|---|
| Duration of amiodarone treatment, n (%) | |||
| Acute, <10 days | 1 (25) | 3 (11.5) | 6 (18.2) |
| Subacute, ≥10 days to <3 months | 2 (50) | 16 (61.5) | 18 (54.6) |
| Chronic, ≥3 months | 1 (25) | 7 (26.9) | 9 (27.3) |
| Days on amiodarone before thyroid dysfunction, mean (SD) | 9.00 (11.8) | 19.38 (32.0) | 16.09 (20.6) |
| Resolved, n (%) | 1 (25) | 7 (26.9) | 21 (63.6) |
| . | Hyperthyroidism (n = 4) . | Hypothyroidism (n = 26) . | Subclinical Hypothyroidism (n = 33) . |
|---|---|---|---|
| Duration of amiodarone treatment, n (%) | |||
| Acute, <10 days | 1 (25) | 3 (11.5) | 6 (18.2) |
| Subacute, ≥10 days to <3 months | 2 (50) | 16 (61.5) | 18 (54.6) |
| Chronic, ≥3 months | 1 (25) | 7 (26.9) | 9 (27.3) |
| Days on amiodarone before thyroid dysfunction, mean (SD) | 9.00 (11.8) | 19.38 (32.0) | 16.09 (20.6) |
| Resolved, n (%) | 1 (25) | 7 (26.9) | 21 (63.6) |
There was a high mortality rate among our population. Fourteen of the 63 (22%) patients who developed abnormal thyroid function died of their primary cardiac disease or complications related to cardiac disease. Of those with normal thyroid function, mortality was similar. Twenty-seven of the 127 (21%) patients with normal thyroid function died.
Discussion
This study examines a pediatric and young-adult population in an effort to describe the natural history of amiodarone-induced thyroid dysfunction from a large cohort of patients at a single pediatric referral center. We found that 33% (63/190) of patients developed thyroid dysfunction while on amiodarone. Of those with thyroid-function testing performed, 17.3% developed subclinical hypothyroidism, and 13.7% developed AIH. Just 2.1% of patients developed AIT, with average time to develop AIT after initiation of amiodarone of 9 days. Our data showed significant variation in practice regarding thyroid-function screening and surveillance after initiation of amiodarone treatment, with only 40% of our cohort having thyroid-function studies obtained.
Despite the study being conducted at a single children’s hospital, there was still substantial variation in the timing and consistency for monitoring thyroid function before and after initiation of amiodarone therapy. The variation in timing and consistency of monitoring amiodarone-induced thyroid dysfunction have been previously reported by Moffett et al. (18) in a retrospective review of children on amiodarone therapy, where only 12.2% of children on amiodarone had a full thyroid-function evaluation with TSH, T3, and T4 or FT4 measurement. Our data show better compliance, with ∼40% of patients undergoing thyroid-function testing; however, not all of the thyroid-function testing included a complete set of thyroid hormone levels that would allow for full assessment of the thyroid axis. This lower-than-expected percentage may be, in part, a result of the majority of patients being on amiodarone therapy for short periods of time, in many cases, <48 hours; the under-reporting of thyroid-dysfunction risk in children treated with amiodarone; and the lack of national guidelines for monitoring thyroid function in pediatric patients treated with amiodarone.
One-third of our population developed thyroid dysfunction after initiation of amiodarone, 17.3% developed subclinical hypothyroidism, and 13.7% developed AIH. Previous studies in adults show slightly lower rates of AIH. In two adult studies, the incidences of AIH were 6.9% and 10.9% (8, 11). In our study, younger age and female sex were risk factors for developing overt hypothyroidism. Overt hypothyroidism was also more likely to be persistent than subclinical hypothyroidism. This high resolution rate of subclinical hypothyroidism may reflect the normal rise in TSH after initiation of amiodarone, secondary to decreased T3 production, which often resolves after 1 month or more of treatment (5). We also found that children and young adults developed AIH and subclinical hypothyroidism more quickly than adults, which may reflect greater sensitivity to iodine-induced thyroid dysfunction. The median time of onset for subclinical AIH and AIH after initiation of amiodarone, respectively, was 7 (2 to 23) and 11 (6 to 17) days in our study. Adult studies have shown rates of subclinical AIH and AIH of 1.1 and 1.7 years, respectively (8).
Only 2.1% of our patients developed AIT. Previous studies in adults have shown much higher rates of AIT. Two previous adult studies showed incidences of AIT to be 12.1% and 14.5% (8, 11). This observation that AIT may be more common in older patients treated with amiodarone is supported by the fact that AIT occurred in an older set of patients in our study (median 17.9 years). The distinction between type I and type II AIT is difficult, given the lack of antibody testing. However, one patient had positive TSI testing, along with thyroid ultrasound, consistent with Graves’, which would be seen in type I AIT.
The majority (87.3%) of patients who developed thyroid dysfunction did so within the first 5 weeks of initiation of amiodarone treatment. One possible explanation for this may be an age-related increased sensitivity to iodine-induced primary thyroid dysfunction and an inability to escape the Wolff-Chaikoff effect in young and often critically ill children. This has previously been described in neonates exposed to iodine, as a result of cardiac surgery (20), and in preterm neonates in general (21).
Given the high incidence of thyroid dysfunction and its early onset in pediatric patients, we recommend that a complete thyroid-function panel be measured at baseline and then at weekly intervals for the first 5 weeks after initiation of amiodarone. As shown in Fig. 2, the majority of thyroid dysfunction would be identified within the first 35 days of treatment. If thyroid dysfunction is not detected in the first 5 weeks, then thyroid-function testing could then be performed less frequently, such as every 3 months, unless signs or symptoms of thyroid dysfunction were noted. If thyroid dysfunction is detected outside of the newborn period, then TPO and TG antibodies should also be obtained, as a result of the higher risk of AIH when antibodies are present. This approach would help identify patients who may be at risk for persistent, overt hypothyroidism (TSH > 10 mIU/L), where levothyroxine therapy would be of benefit. rT3 testing may be used as a marker for severity of illness/risk of mortality or as a marker of compliance for patients on prolonged amiodarone therapy (5).
The importance of thyroid function in neurocognitive development and cardiac function is well known. Severe cognitive impairment has long been associated with persistent hypothyroidism. The prevention of neurocognitive deficits through the early initiation of treatment is of great importance in this population (22). Adverse cardiac pathology has also been associated with both hypothyroidism and hyperthyroidism. Hypothyroidism has been associated with dyslipidemia, poor cardiac output, increased systemic vascular resistance, and decreased arterial compliance. Impaired cardiac muscle relaxation, decreased heart rate, and decreased stroke volume contribute to heart failure in hypothyroidism. Hyperthyroidism has many opposite effects and may lead to high-output heart failure. Hyperthyroidism is also associated with atrial fibrillation and pulmonary hypertension. Early and effective treatment of thyroid dysfunction can prevent poor cardiac outcomes (23).
This study is limited by the restrictions of a retrospective data review. There was substantial variation in monitoring and surveillance of thyroid function while on amiodarone, making systematic collection and analysis of data challenging. In addition, many patients did not have thyroid-function testing follow-up beyond the onset of thyroid dysfunction, and there was often a lack of TPO and TG antibody testing when thyroid dysfunction was discovered. A correlation between amiodarone dosing and thyroid dysfunction was not evaluated, as this was different for each patient, often based on weight and clinical response to therapy.
In summary, this study provides important information on the incidence and natural history of thyroid dysfunction in children treated with amiodarone. Based on the high incidence and early onset of dysfunction in these patients, we recommend early and regular testing of thyroid function in patients treated with amiodarone. This is especially important in young children where neurocognitive and cardiac outcomes may be adversely affected by undetected, severe hypothyroidism. Future prospective studies systematically monitoring thyroid function in pediatric patients treated with amiodarone are needed to define more accurately the monitoring and treatment recommendation in this patient population.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations:
- AIH
amiodarone-induced hypothyroidism
- AIT
amiodarone-induced thyrotoxicosis
- FT4
free thyroxine
- TG
thyroglobulin
- TPO
thyroid peroxidase
- TSI
thyroid-stimulating immunoglobulin



