-
PDF
- Split View
-
Views
-
Cite
Cite
Sri Harsha Tella, Anuhya Kommalapati, Subhashini Yaturu, Electron Kebebew, Predictors of Survival in Adrenocortical Carcinoma: An Analysis From the National Cancer Database, The Journal of Clinical Endocrinology & Metabolism, Volume 103, Issue 9, September 2018, Pages 3566–3573, https://doi.org/10.1210/jc.2018-00918
Close - Share Icon Share
Abstract
Adrenocortical carcinoma (ACC) is rare; knowledge about prognostic factors and survival outcomes is limited.
To describe predictors of survival and overall survival (OS) outcomes.
Retrospective analysis of data from the National Cancer Database (NCDB) from 2004 to 2015 on 3185 patients with pathologically confirmed ACC.
Baseline description, survival outcomes, and predictors of survival were evaluated in patients with ACC.
Median age at ACC diagnosis was 55 (range: 18 to 90) years; did not differ significantly by sex or stage of the disease at diagnosis. On multivariate analysis, increasing age, higher Charlson-Deyo comorbidity index score, high tumor grade, and no surgical therapy (all P < 0.0001); and stage IV disease (P = 0.002) and lymphadenectomy during surgery (P = 0.02) were associated with poor prognosis. Patients with stage I-III disease treated with surgical resection had significantly better median OS (63 vs 8 months; P < 0.001). In stage IV disease, better median OS occurred in patients treated with surgery (19 vs 6 months; P < 0.001), and postsurgical radiation (29 vs 10 months; P < 0.001) or chemotherapy (22 vs 13 months; P = 0.004).
OS varied with increasing age, higher comorbidity index, grade, and stage of ACC at presentation. There was improved survival with surgical resection of primary tumor, irrespective of disease stage; postsurgical chemotherapy or radiation was of benefit only in stage IV disease.
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy with dismal prognosis. Contemporary clinical data are mostly limited to institutional case series and the National Cancer Institute’s Surveillance, Epidemiology and End Results database analysis (1–4). The incidence of ACC is ∼0.7 to 2 cases per 100,000 person-years (5). It is estimated that about 200 new cases of ACC are diagnosed annually in the United States. ACC accounts for <5% of adrenal incidentalomas. The prognosis of ACC is generally poor, with a median overall survival (OS) of 3.21 years and a 5-year survival rate of 15% to 44% (1). Using the National Cancer Database (NCDB), the largest public cancer registry covering >70% of all newly diagnosed cancers in the United States and Puerto Rico, we determined the prognostic factors and survival outcomes of patients with ACC.
Methods
Data source and study population
We obtained data from the NCDB for the years 2004 to 2015. The NCDB is a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The accrual process, integrity, and quality of the NCDB has been documented (6). Facility and patient data are deidentified in the NCDB; therefore, the institutional review board of the primary author’s institution exempted the study from review.
A total of 3185 ACC cases were identified between the years 2004 and 2015 by using the International Classification of Disease for Oncology, Third Edition, code 8370/3 and site-specific code of C74.
Covariates
Covariates included for the analysis were patients’ demographics (i.e., age, sex, race), insurance type (i.e., no insurance, private, Medicare, Medicaid, or other), socioeconomic status based on income, Charlson-Deyo comorbidity index (CDCI) score, year of diagnosis, tumor characteristics (i.e., stage, grade), type of therapy received (i.e., surgery, lymphadenectomy, administration of postsurgical chemotherapy and/or radiation therapy).
Statistical analyses
We used SPSS, version 24.0 (IBM, Armonk, NY) for the statistical analyses and Cox proportional-multivariable hazards regression model to determine the prognostic factors, adjusting for the aforementioned covariables. Univariate survival analysis was performed; covariates with a significance level <0.05 were included in multivariate analysis. Descriptive statistics are expressed as median and range.
The Mann-Whitney U test was used to compare continuous variables, and the χ2 test was used to compare categorical data. We investigated the effect of surgical resection, lymphadenectomy, postsurgical chemotherapy, and postsurgical radiation on OS using multivariate analysis by Cox proportional hazards method. OS (with death due to any cause) analysis was performed separately for American Joint Committee on Cancer/International Cancer Control (AJCC/UICC) stages I-IV disease. OS was estimated by the Kaplan-Meier method. The log-rank test was used for comparison between patient groups. Values of P < 0.05 were considered statistically significant.
Results
Baseline characteristics
A total of 3185 patients with ACC diagnosed between 2004 and 2015 met the inclusion criteria in the NCDB dataset. Of these 3185 patients, 59.5% were female. The distribution by race was 86% white, 10% black, 3% Asian-Pacific Islander, and 2% others or unknown. Median age at diagnosis was 55 (range, 18 to 90) years and did not differ by sex (P = 0.68) or stage of disease at diagnosis (P = 0.63). Patient baseline characteristics are summarized in Table 1. Stage of ACC was unknown for 50% of included patients; 3%, 15%, 8%, and 24% of patients had stage I, II, III, and IV disease, respectively. The most common distant sites of metastases for stage IV disease were lungs (11.5%) and liver (11%).
Baseline Characteristics of Patients With ACC Reported to NCDB Between 2004 and 2015
| . | Overall . |
|---|---|
| Total no. of cases | 3185 |
| Age, median (range), y | 55 (18–90) |
| Female patient | 1896 (59.5) |
| Race | |
| White | 2729 (86) |
| Black | 301 (10) |
| Asian Pacific Islander | 81 (3) |
| Insurance status | |
| No insurance | 167 (5) |
| Private | 1688 (53) |
| Medicaid | 228 (7) |
| Medicare | 929 (29) |
| Median household income, USD | |
| <38,000 | 511 (16) |
| 38,000–47,999 | 720 (23) |
| 48,000–62,999 | 866 (27) |
| >63,000 | 1034 (33) |
| CDCI score | |
| 0–1 | 2983 (94) |
| 2–3 | 202 (6) |
| Stage at diagnosis | |
| I–III | 842 (27) |
| IV | 760 (24) |
| Unknown | 1583 (49) |
| Tumor grade | |
| 1–2 | 190 (6) |
| 3–4 | 451 (14) |
| Unknown | 2544 (80) |
| Therapies received | |
| Surgery | 2305 (72) |
| Laparoscopic resection | 222 (10) |
| Open resection | 709 (31) |
| Othersa | 132 (6) |
| Unknown type of surgery | 1242 (53) |
| Adjuvant chemotherapy | 734 (23) |
| Adjuvant radiation therapy | 366 (12) |
| R0 resection | 1547 (49) |
| . | Overall . |
|---|---|
| Total no. of cases | 3185 |
| Age, median (range), y | 55 (18–90) |
| Female patient | 1896 (59.5) |
| Race | |
| White | 2729 (86) |
| Black | 301 (10) |
| Asian Pacific Islander | 81 (3) |
| Insurance status | |
| No insurance | 167 (5) |
| Private | 1688 (53) |
| Medicaid | 228 (7) |
| Medicare | 929 (29) |
| Median household income, USD | |
| <38,000 | 511 (16) |
| 38,000–47,999 | 720 (23) |
| 48,000–62,999 | 866 (27) |
| >63,000 | 1034 (33) |
| CDCI score | |
| 0–1 | 2983 (94) |
| 2–3 | 202 (6) |
| Stage at diagnosis | |
| I–III | 842 (27) |
| IV | 760 (24) |
| Unknown | 1583 (49) |
| Tumor grade | |
| 1–2 | 190 (6) |
| 3–4 | 451 (14) |
| Unknown | 2544 (80) |
| Therapies received | |
| Surgery | 2305 (72) |
| Laparoscopic resection | 222 (10) |
| Open resection | 709 (31) |
| Othersa | 132 (6) |
| Unknown type of surgery | 1242 (53) |
| Adjuvant chemotherapy | 734 (23) |
| Adjuvant radiation therapy | 366 (12) |
| R0 resection | 1547 (49) |
Data are given as no. (%) unless otherwise indicated.
Others include robotic surgery, robotic surgery converted to open surgery, laparoscopic converted to open surgery.
Baseline Characteristics of Patients With ACC Reported to NCDB Between 2004 and 2015
| . | Overall . |
|---|---|
| Total no. of cases | 3185 |
| Age, median (range), y | 55 (18–90) |
| Female patient | 1896 (59.5) |
| Race | |
| White | 2729 (86) |
| Black | 301 (10) |
| Asian Pacific Islander | 81 (3) |
| Insurance status | |
| No insurance | 167 (5) |
| Private | 1688 (53) |
| Medicaid | 228 (7) |
| Medicare | 929 (29) |
| Median household income, USD | |
| <38,000 | 511 (16) |
| 38,000–47,999 | 720 (23) |
| 48,000–62,999 | 866 (27) |
| >63,000 | 1034 (33) |
| CDCI score | |
| 0–1 | 2983 (94) |
| 2–3 | 202 (6) |
| Stage at diagnosis | |
| I–III | 842 (27) |
| IV | 760 (24) |
| Unknown | 1583 (49) |
| Tumor grade | |
| 1–2 | 190 (6) |
| 3–4 | 451 (14) |
| Unknown | 2544 (80) |
| Therapies received | |
| Surgery | 2305 (72) |
| Laparoscopic resection | 222 (10) |
| Open resection | 709 (31) |
| Othersa | 132 (6) |
| Unknown type of surgery | 1242 (53) |
| Adjuvant chemotherapy | 734 (23) |
| Adjuvant radiation therapy | 366 (12) |
| R0 resection | 1547 (49) |
| . | Overall . |
|---|---|
| Total no. of cases | 3185 |
| Age, median (range), y | 55 (18–90) |
| Female patient | 1896 (59.5) |
| Race | |
| White | 2729 (86) |
| Black | 301 (10) |
| Asian Pacific Islander | 81 (3) |
| Insurance status | |
| No insurance | 167 (5) |
| Private | 1688 (53) |
| Medicaid | 228 (7) |
| Medicare | 929 (29) |
| Median household income, USD | |
| <38,000 | 511 (16) |
| 38,000–47,999 | 720 (23) |
| 48,000–62,999 | 866 (27) |
| >63,000 | 1034 (33) |
| CDCI score | |
| 0–1 | 2983 (94) |
| 2–3 | 202 (6) |
| Stage at diagnosis | |
| I–III | 842 (27) |
| IV | 760 (24) |
| Unknown | 1583 (49) |
| Tumor grade | |
| 1–2 | 190 (6) |
| 3–4 | 451 (14) |
| Unknown | 2544 (80) |
| Therapies received | |
| Surgery | 2305 (72) |
| Laparoscopic resection | 222 (10) |
| Open resection | 709 (31) |
| Othersa | 132 (6) |
| Unknown type of surgery | 1242 (53) |
| Adjuvant chemotherapy | 734 (23) |
| Adjuvant radiation therapy | 366 (12) |
| R0 resection | 1547 (49) |
Data are given as no. (%) unless otherwise indicated.
Others include robotic surgery, robotic surgery converted to open surgery, laparoscopic converted to open surgery.
Treatment by disease stage
Staging details were available for 1602 patients. The comparison is shown in Table 2. For AJCC/UICC stage I-III disease (n = 842), 96% (n = 806) of the patients had surgical resection of the primary tumor. Among the patients who had surgery, open resection was performed in 52% patients (n = 441), laparoscopic resection was performed in 21% (n = 172), ∼12% (n = 103) underwent robotic surgery; robotic/laparoscopic surgery converted to open surgery and the type of surgery was not reported in 11% (n = 90) of the patients. Negative tumor margin (R0) resection was reported for 72% (n = 608) of patients. Among the patients who had a laparoscopic resection, R0 was achieved in 76% (n = 130) of patients and 19% (n = 33) of patients had positive surgical margins. Margin status was unknown in 5% (n = 9) of patients. In the patients who underwent open resection, 80% (n = 354) and 12% (n = 53) of patients had R0 resections and positive resection margins, respectively. Eight percent of the patients had unknown margin status (Supplemental Fig. 1).
Comparative Data Between Patients With ACC With Stage I–III Disease as Compared With Those With Stage IV Disease
| . | Stage I–III . | Stage IV . | P . |
|---|---|---|---|
| Total no. of casesa | 842 (27) | 760 (24) | |
| Age, median (range), y | 57 (18–90) | 56 (18–90) | 0.63 |
| Female patients | 504 (60) | 457 (60) | 0.86 |
| Race | |||
| White | 729 (87) | 631 (83) | |
| Black | 70 (8) | 88 (12) | 0.35 |
| Asian Pacific Islander | 20 (2) | 24 (3) | |
| Insurance status | |||
| No insurance | 47 (6) | 48 (6) | |
| Private | 438 (52) | 368 (48) | <0.0001 |
| Medicaid | 52 (6) | 69 (9) | |
| Medicare | 277 (33) | 236 (31) | |
| Median household income, USD | |||
| <38,000 | 134 (16) | 133 (18) | |
| 38,000–47,999 | 186 (22) | 175 (23) | 0.86 |
| 48,000–62,999 | 241 (29) | 200 (26) | |
| >63,000 | 281 (33) | 239 (31) | |
| CDCI score | |||
| 0–1 | 793 (94) | 712 (94) | 0.74 |
| 2–3 | 49 (6) | 48 (6) | |
| Tumor grade | |||
| 1–2 | 49 (6) | 27 (4) | |
| 3–4 | 119 (14) | 103 (14) | 0.01 |
| Unknown | 674 (80) | 630 (83) | |
| Therapies received | |||
| Surgery | 806 (96) | 325 (43) | |
| Laparoscopic resection | 172 (21) | 26 (8) | |
| Open resection | 441 (55) | 217 (67) | |
| Othersb | 103 (13) | 18 (6) | <0.0001 |
| Unknown type of surgery | 90 (11) | 64 (20) | |
| Adjuvant chemotherapy | 292 (35) | 184 (24) | |
| Adjuvant radiation therapy | 180 (21) | 72 (10) | |
| R0 resectionc | 608 (72) | 182 (24) | <0.0001 |
| . | Stage I–III . | Stage IV . | P . |
|---|---|---|---|
| Total no. of casesa | 842 (27) | 760 (24) | |
| Age, median (range), y | 57 (18–90) | 56 (18–90) | 0.63 |
| Female patients | 504 (60) | 457 (60) | 0.86 |
| Race | |||
| White | 729 (87) | 631 (83) | |
| Black | 70 (8) | 88 (12) | 0.35 |
| Asian Pacific Islander | 20 (2) | 24 (3) | |
| Insurance status | |||
| No insurance | 47 (6) | 48 (6) | |
| Private | 438 (52) | 368 (48) | <0.0001 |
| Medicaid | 52 (6) | 69 (9) | |
| Medicare | 277 (33) | 236 (31) | |
| Median household income, USD | |||
| <38,000 | 134 (16) | 133 (18) | |
| 38,000–47,999 | 186 (22) | 175 (23) | 0.86 |
| 48,000–62,999 | 241 (29) | 200 (26) | |
| >63,000 | 281 (33) | 239 (31) | |
| CDCI score | |||
| 0–1 | 793 (94) | 712 (94) | 0.74 |
| 2–3 | 49 (6) | 48 (6) | |
| Tumor grade | |||
| 1–2 | 49 (6) | 27 (4) | |
| 3–4 | 119 (14) | 103 (14) | 0.01 |
| Unknown | 674 (80) | 630 (83) | |
| Therapies received | |||
| Surgery | 806 (96) | 325 (43) | |
| Laparoscopic resection | 172 (21) | 26 (8) | |
| Open resection | 441 (55) | 217 (67) | |
| Othersb | 103 (13) | 18 (6) | <0.0001 |
| Unknown type of surgery | 90 (11) | 64 (20) | |
| Adjuvant chemotherapy | 292 (35) | 184 (24) | |
| Adjuvant radiation therapy | 180 (21) | 72 (10) | |
| R0 resectionc | 608 (72) | 182 (24) | <0.0001 |
50% of patients have unknown disease stage.
Others include robotic surgery, robotic converted to open, laparoscopic converted to open.
Percentage calculated based on the patients who received surgical therapy only.
Comparative Data Between Patients With ACC With Stage I–III Disease as Compared With Those With Stage IV Disease
| . | Stage I–III . | Stage IV . | P . |
|---|---|---|---|
| Total no. of casesa | 842 (27) | 760 (24) | |
| Age, median (range), y | 57 (18–90) | 56 (18–90) | 0.63 |
| Female patients | 504 (60) | 457 (60) | 0.86 |
| Race | |||
| White | 729 (87) | 631 (83) | |
| Black | 70 (8) | 88 (12) | 0.35 |
| Asian Pacific Islander | 20 (2) | 24 (3) | |
| Insurance status | |||
| No insurance | 47 (6) | 48 (6) | |
| Private | 438 (52) | 368 (48) | <0.0001 |
| Medicaid | 52 (6) | 69 (9) | |
| Medicare | 277 (33) | 236 (31) | |
| Median household income, USD | |||
| <38,000 | 134 (16) | 133 (18) | |
| 38,000–47,999 | 186 (22) | 175 (23) | 0.86 |
| 48,000–62,999 | 241 (29) | 200 (26) | |
| >63,000 | 281 (33) | 239 (31) | |
| CDCI score | |||
| 0–1 | 793 (94) | 712 (94) | 0.74 |
| 2–3 | 49 (6) | 48 (6) | |
| Tumor grade | |||
| 1–2 | 49 (6) | 27 (4) | |
| 3–4 | 119 (14) | 103 (14) | 0.01 |
| Unknown | 674 (80) | 630 (83) | |
| Therapies received | |||
| Surgery | 806 (96) | 325 (43) | |
| Laparoscopic resection | 172 (21) | 26 (8) | |
| Open resection | 441 (55) | 217 (67) | |
| Othersb | 103 (13) | 18 (6) | <0.0001 |
| Unknown type of surgery | 90 (11) | 64 (20) | |
| Adjuvant chemotherapy | 292 (35) | 184 (24) | |
| Adjuvant radiation therapy | 180 (21) | 72 (10) | |
| R0 resectionc | 608 (72) | 182 (24) | <0.0001 |
| . | Stage I–III . | Stage IV . | P . |
|---|---|---|---|
| Total no. of casesa | 842 (27) | 760 (24) | |
| Age, median (range), y | 57 (18–90) | 56 (18–90) | 0.63 |
| Female patients | 504 (60) | 457 (60) | 0.86 |
| Race | |||
| White | 729 (87) | 631 (83) | |
| Black | 70 (8) | 88 (12) | 0.35 |
| Asian Pacific Islander | 20 (2) | 24 (3) | |
| Insurance status | |||
| No insurance | 47 (6) | 48 (6) | |
| Private | 438 (52) | 368 (48) | <0.0001 |
| Medicaid | 52 (6) | 69 (9) | |
| Medicare | 277 (33) | 236 (31) | |
| Median household income, USD | |||
| <38,000 | 134 (16) | 133 (18) | |
| 38,000–47,999 | 186 (22) | 175 (23) | 0.86 |
| 48,000–62,999 | 241 (29) | 200 (26) | |
| >63,000 | 281 (33) | 239 (31) | |
| CDCI score | |||
| 0–1 | 793 (94) | 712 (94) | 0.74 |
| 2–3 | 49 (6) | 48 (6) | |
| Tumor grade | |||
| 1–2 | 49 (6) | 27 (4) | |
| 3–4 | 119 (14) | 103 (14) | 0.01 |
| Unknown | 674 (80) | 630 (83) | |
| Therapies received | |||
| Surgery | 806 (96) | 325 (43) | |
| Laparoscopic resection | 172 (21) | 26 (8) | |
| Open resection | 441 (55) | 217 (67) | |
| Othersb | 103 (13) | 18 (6) | <0.0001 |
| Unknown type of surgery | 90 (11) | 64 (20) | |
| Adjuvant chemotherapy | 292 (35) | 184 (24) | |
| Adjuvant radiation therapy | 180 (21) | 72 (10) | |
| R0 resectionc | 608 (72) | 182 (24) | <0.0001 |
50% of patients have unknown disease stage.
Others include robotic surgery, robotic converted to open, laparoscopic converted to open.
Percentage calculated based on the patients who received surgical therapy only.
Lymphadenectomy was performed in 18% (n = 149) of patients. On subgroup analysis, 8% (n = 9),14% (n = 69), 28% (n = 71) of patients with stage I, II, and III disease, respectively, underwent lymphadenectomy. In stage I and II disease, none of the examined lymph nodes were pathologically positive, whereas in stage III disease, 15% (n = 11 of 71) of examined lymph nodes were positive for disease.
Thirty-five percent (n = 292) and 21% (n = 180) of patients received postsurgical chemotherapy and radiation, respectively. The rates of administration of postsurgical chemotherapy were much higher in the patients with positive resection margins as compared with those with R0 resections (46% vs 34%; P = 0.008). On subgroup analysis, the odds of receiving postsurgical chemotherapy increased with advanced stage: stage I, 25.9% (n = 28); stage II, 34.3% (n = 164); and stage III, 39.1% (n = 100). A similar trend was seen in incidence of postsurgical radiation therapy (44% vs 17%; P < 0.001). On subgroup analysis, 15.7% (n = 17) of patients with stage I disease, 19.7% (n = 94) of those with stage II ACC, and 27% (n = 69) of those with stage III ACC received postsurgical radiation therapy.
In patients with AJCC/UICC stage IV disease, 43% (n = 325) of the patients underwent surgical resection of the primary tumor. Among the patients who were treated surgically, open resection was performed in 67% (n = 217) of patients and laparoscopic resection was performed in 8% (n = 26). Six percent (n = 18) of the patients underwent robotic surgery; robotic or laparoscopic surgery converted to open surgery and the type of surgery was not reported to NCDB for 20% (n = 64) of the patients. R0 resections were reported for ∼24% (n = 182) of patients, which was significantly lower than in patients with stage I-III disease (P < 0.001). Among the patients who underwent laparoscopic resection, R0 was achieved in 50% (n = 13) of patients and 34% (n = 9) of patients had positive tumor margins on resection. Margin status was unknown in 15% (n = 4) of patients. In the patients who underwent open resection, 58% (n = 126) and 29% (n = 62) of patients had R0 and positive margin resections, respectively (Supplemental Fig. 2). Lymphadenectomy was performed in 17% (n = 129) of patients; of these, tissue in 55% (n = 71) was pathologically positive for disease. Postsurgical chemotherapy and radiation therapy were administered to 24% (n = 184) and 10% (n = 72) of patients, respectively.
Predictors of survival and OS analysis
On univariate analysis, age, CDCI score, insurance status, grade, disease stage, surgical therapy, lymphadenectomy during surgery, postsurgical chemotherapy, and radiation therapy were significantly associated with OS. On Cox multivariate analysis, increasing age [hazard ratio (HR), 1.01; 95% CI, 1.01 to 1.02; P < 0.001], higher (2-3) CDCI score (HR, 1.46; 95% CI, 1.23 to 1.74; P < 0.001), high tumor grade (HR, 2.34; 95% CI, 1.81 to 3.02; P < 0.001), stage IV disease (HR, 1.99; 95% CI, 1.69 to 2.35; P < 0.001), no surgical therapy (HR, 3.78; 95% CI, 3.35 to 4.26; P < 0.001), and undergoing lymphadenectomy during surgery (HR, 1.18; 95% CI, 1.03 to 1.35; P = 0.02) were associated with worse survival (Fig. 1). Sex (P = 0.68), race (P = 0.78), year of diagnosis (P = 0.19), postsurgical radiation (P = 0.66), and postsurgical chemotherapy (P = 0.25) were not significant predictors of survival (Fig. 1).
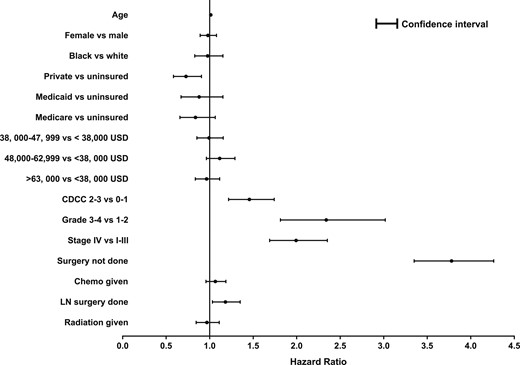
Forest plot depicting the predictors of survival in patients with ACC using a Cox proportional hazards model. CDCC, Charlson-Deyo comorbidity score; Chemo, chemotherapy; LN, lymph node.
Median survival was significantly higher for patients with stage I-III disease (61 months) as compared with those with stage IV disease (8 months; P < 0.001). On individual, stage-wise analysis, median survival was undefined in stage I disease, 81 months in stage II disease, and 36 months in stage III disease. Type of surgery (i.e., open adrenalectomy vs laparoscopic adrenalectomy) was not a prognostic factor (P = 0.26).
For stage I-III disease, patients who had surgical treatment had a better median OS than patients who had no surgical therapy (63 vs 8 months; P < 0.001; Fig. 2A). Though statistically not significant, lymphadenectomy was associated with worse survival (54 vs 63 months; P = 0.15; Fig. 2B). On unadjusted analysis, patients who had postsurgical chemotherapy (56 vs 68 months; P = 0.63; Fig. 2C) and/or postsurgical radiation therapy (56 vs 63 months; P = 0.47; Fig. 2D) had no statistically significant difference in median OS. On subgroup analysis, patients with positive tumor resection margins had a significantly better OS with postsurgical radiation (41 vs 20 months; P = 0.04; Fig. 2E), whereas no such benefit was seen with postsurgical chemotherapy (25 vs 24 months; P = 0.45; Fig. 2F).
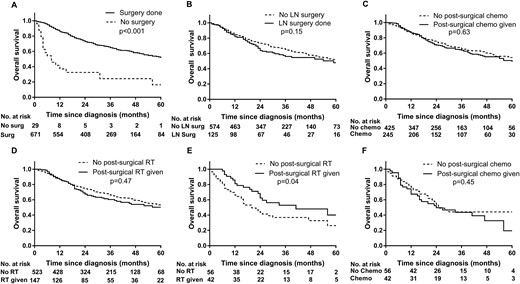
OS of patients with stage I-III ACC, based on undergoing (A) surgical therapy or (B) lymphadenectomy; or receiving (C) postsurgical chemotherapy, (D) postsurgical radiation therapy; or, patients with positive resection margins, receiving E) postsurgical radiation or (F) postsurgical chemotherapy. Chemo, chemotherapy; LN, lymph node; RT, radiation therapy; Surg, surgery.
For stage IV disease, surgical therapy was associated with a significantly longer OS as compared with no surgical intervention (19 vs 6 months; P < 0.001; Fig. 3A). The patients who had lymphadenectomy had significantly longer OS (15 vs 6 months; P < 0.001; Fig. 3B). On unadjusted analysis, patients who had postsurgical chemotherapy (22 vs 13 months; P = 0.004; Fig. 3C) and/or postsurgical radiation therapy (29 vs 10 months; P < 0.001; Fig. 3D), had significantly longer OS. On adjusted analysis, postoperative chemotherapy and/or postoperative radiation therapy were associated with improved median OS in patients who had surgical resection of their primary tumor only for positive tumor resection margins.
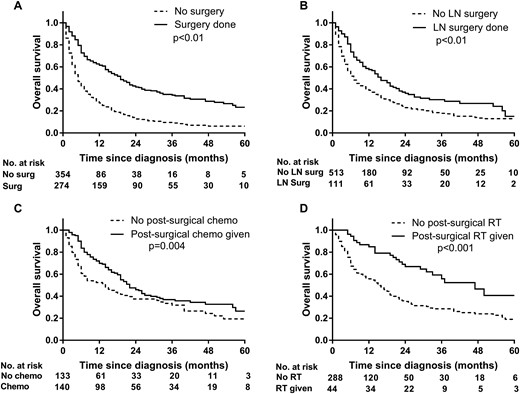
OS of patients with stage IV ACC, based on undergoing (A) surgical therapy or (B) lymphadenectomy, or receiving (C) postsurgical chemotherapy or (D) postsurgical radiation therapy. Chemo, chemotherapy; LN, lymph node; RT, radiation therapy; Surg, surgery.
Discussion
Using a national cancer registry, we examined the clinical outcomes, treatment patterns, and predictors of survival in patients with ACC. In our study of 3185 patients with pathologically confirmed ACC, high tumor grade, high baseline CDCI score, and higher stage of disease were associated with higher mortality risk. Comparable to our findings, others have previously reported that older age at diagnosis, stage of disease at diagnosis, and no surgical treatment of the primary tumor were associated with shorter survival (1, 2, 7). We found that the disease is more common in women, which has been also reported in other studies (2, 8, 9).
Surgery remains the mainstay of treatment of ACC because it is the only therapeutic approach that can be potentially curative for localized disease. In our analysis, we observed that surgical resection of the primary tumor was associated with better OS, which is in agreement with findings previously reported (10–14). We further analyzed the survival benefit based on the type of surgery. Interestingly, we did not find any statistical difference in the median OS in patients who underwent laparoscopic resection vs open adrenalectomy. This lack of difference in median OS between the procedures may be attributed to a selection bias. Of note, in stage I-III disease, the percentage of R0 resections was similar in either type of surgical approach used. This supports the European Society of Endocrine Surgeons and European Society for Medical Oncology recommendations that laparoscopic procedures could be performed for stage I-II ACC tumors if an R0 resection can be achieved (15, 16). However, it is important to note that only a few patients underwent laparoscopic surgical resection (21% of patients with stage I-III disease and 8% of patients with stage IV disease) and no definitive conclusions can be made. Because the NDCB does not have data on recurrence, we could not assess whether the rate of recurrence was different among the surgical approaches used in patients with stage I-II ACC. The current therapy pattern of preference for open adrenalectomy in stage IV disease are in line with recent American Association of Clinical Endocrinologists and the American Association of Endocrine Surgeons recommendation of preferring open adrenalectomy performed by an experienced surgeon as the procedure of choice in patients with ACC (17). Though our study could not answer the question of superiority of one modality of surgical approach and treatment over the other and does not provide data on disease recurrence, our findings show that the ability of achieving R0 resection was much higher with open adrenalectomy in both early and advanced stages of ACC.
The benefit of routine or prophylactic lymphadenectomy is controversial in the management of ACC. In our study, on Cox multivariate analysis, lymphadenectomy was associated with poor prognosis. On subgroup analysis, in stage I-III disease, lymphadenectomy was not associated with a significant difference in median OS, whereas it was associated with a survival benefit in stage IV disease. The improved survival in stage IV disease may be attributed to increased clearance of tumor burden. Our analysis demonstrates results discordant with those of a German registry-based study that showed improved survival in patients with stage I-III disease who underwent lymphadenectomy (18). One possible explanation for the different findings is the small sample size in the German registry study (n = 47) or that only patients with high risk or extensive disease underwent lymphadenectomy in our study (∼50% of patients with stage I-III disease who underwent lymphadenectomy had stage III disease). Interestingly, an analysis based on the Surveillance, Epidemiology and End Results registry showed no benefit of lymphadenectomy on OS (19). That study is, again, limited by small sample size: Only 8% of the cohort (67 of 802 patients) underwent lymphadenectomy. It is important to note that the three studies were derived from retrospective, registry-based analyses, and potential confounders such as patient selection, surgeons’ expertise, accuracy of pathologic evaluation, presence of tumor micrometastases, and lack of specific definitions on lymphadenectomy might have confounded the results.
Given the aggressiveness and high recurrence rate of ACC, postsurgical adjuvant therapy has been used in the management of the disease with conflicting results on improving OS (20). In our analysis, we did not find any benefit of postsurgical chemotherapy and/or radiation therapy in patients with stage I-III ACC who received curative-intent surgical therapy. Though this lack of association of OS with postsurgical radiation therapy is in line with the findings of previous reports (21), on subgroup analysis, radiation therapy was associated with longer survival time in the patients who had positive tumor margins. This may be partly explained by residual local tumor destructive effects of radiation thereby decreasing the tumor burden and reducing the tumor recurrence rates (22). In contrast to the benefits seen with radiation therapy in margin-positive resections in stage I-III disease, postsurgical chemotherapy did not demonstrate an OS benefit. The lack of benefit with postsurgical chemotherapy in localized or early-stage disease was demonstrated in other single-institution studies (23). On the other hand, patients with stage IV disease who were treated with postsurgical radiation and chemotherapy had OS benefit. Previous studies evaluating the benefit of postsurgical administration of chemotherapy (namely, mitotane or cytotoxic chemotherapy) reported widely inconsistent results on OS (24–28). These controversial conclusions, in large part, may be attributed to the small sample size due to the rarity of ACC, potential for selection bias, and lack of distinctive stage-wise design in some studies. Stage-specific, prospective, randomized clinical trials of ACC are needed to answer questions about the benefits of postsurgical chemotherapy with more confidence.
Our study has some limitations inherent to the retrospective study design and registry-based studies (29). Important clinical characteristics such as the location of the primary disease, tumor functional status (i.e., if secreting cortisol or androgens); Ki-67 index; mitotic count; information on disease recurrence; specific type, initiation, and duration of chemotherapy; doses and type of radiation therapy (i.e., proton vs external beam radiation therapy vs intensity-modulated radiation therapy) received by the patient; and the effect of therapy on progression-free survival are lacking. Second, lack of specific details on the use of mitotane therapy may potentially have confounded the results. Third, given the unknown disease stage of about half the patients in the sample, our statistical analysis was limited to only 50% of the cases included. Finally, lack of specific information on the management of treatment-related adverse effects such as adrenal insufficiency may potentially confound the OS outcomes. Nevertheless, NCDB provides real-world data that are not skewed by biases that arise from single-institution studies. Such a robust database is very much needed for assessing treatment use and outcomes in rare diseases such as ACC. Moreover, all data in NCDB are evaluated for integrity and undergo extensive quality monitoring, making it a powerful initiative to improve cancer care in the United States (6). To the best of our knowledge, ours is one of the largest studies to provide a focused, stage-wise analysis of management trends and survival outcomes in patients with ACC, using a large database.
Conclusion
Patients with ACC have diverse OS outcomes, which vary significantly according to age, comorbidity index, grade, and stage of the disease at presentation. Surgical resection of primary tumor is associated with longer survival in all stages of disease, whereas postsurgical chemotherapy or radiation therapy was associated with longer OS only in stage IV disease. Lymphadenectomy was associated with improved OS in stage IV disease and was not associated with survival benefit in stage I-III disease. The results of our study may aid disease prognostication and treatment decision-making.
Abbreviations:
- ACC
adrenocortical carcinoma
- AJCC/UICC
American Joint Committee on Cancer/International Cancer Control
- CDCI
Charlson-Deyo Comorbidity Index
- NCDB
National Cancer Database
- OS
overall survival
- R0
negative tumor margin
Acknowledgments
The work was presented as an oral presentation at the ENDO 2018, March 17–20, 2018, Chicago, IL.
Disclosure Summary: The authors have nothing to disclose.
References
Author notes
These authors contributed equally to this study.



