-
PDF
- Split View
-
Views
-
Cite
Cite
Shanlee M Davis, Najiba Lahlou, Matthew Cox-Martin, Karen Kowal, Philip S Zeitler, Judith L Ross, Oxandrolone Treatment Results in an Increased Risk of Gonadarche in Prepubertal Boys With Klinefelter Syndrome, The Journal of Clinical Endocrinology & Metabolism, Volume 103, Issue 9, September 2018, Pages 3449–3455, https://doi.org/10.1210/jc.2018-00682
Close - Share Icon Share
Abstract
Klinefelter syndrome (KS) is a common genetic condition in which males have an extra X chromosome. KS is associated with testosterone deficiency, neurodevelopmental delays, and cardiometabolic disorders. There has been recent interest in prepubertal androgen treatment; however, the effects on puberty and gonadal function are unknown.
To compare onset of puberty and testicular function in prepubertal boys treated with 2 years of oxandrolone (Ox) vs placebo (Pl).
Double-blind, randomized, controlled trial.
Single tertiary care referral center.
Eighty prepubertal boys with KS; mean age: 8.0 ± 2.2 years (range: 4 to 12).
Ox 0.05 mg/kg vs identical-appearing Pl capsule given for 2 years.
Onset of gonadarche (testicular volume ≥4 mL) and onset of pubarche (Tanner 2 pubic hair); change in testicular hormone concentrations.
Ox-treated group had 20.5 times higher odds of reaching gonadarche (OR 95% CI: 6.5, 77.8) and 28.1 times higher odds of reaching pubarche (OR 95% CI: 8.8, 110.4) during the 2-year study period after adjusting for baseline age. Gonadarche and pubarche both occurred at a younger age in the Ox group (gonadarche: 9.8 ± 1.5 vs 12.1 ± 1.0 years, P < 0.001; pubarche: 10.2 ± 1.1 vs 11.6 ± 1.3 years, P = 0.02). Serum concentrations of testicular hormones and gonadotropins were not different between groups.
Two years of Ox treatment in prepubertal boys with KS results in an increased risk of early gonadarche, on average 2 years earlier than in Pl-treated boys. Ox did not affect serum concentrations of testicular hormones.
Klinefelter syndrome (KS) is a common genetic condition affecting phenotypic boys who are born with an additional X chromosome, typically with a karyotype 47,XXY (1). It affects ∼1 in every 650 boys but is widely underdiagnosed, and interventions aimed at improving the manifestations in KS are understudied (2). Recently, there has been increased interest within the scientific and lay communities to give prepubertal boys with KS androgen treatment, with benefits cited in cognitive, behavioral, psychosocial, and cardiometabolic profiles (3–7). These reports have not addressed the effect of exogenous androgens on endogenous gonadal function, a critical safety outcome.
Testicular function in KS has been studied from infancy through adulthood, although studies with longitudinal components have historically had low sample sizes limiting generalizability (8). There is general consensus that testicular function is compromised in KS from midpuberty through adulthood (9, 10). Whether testicular function is impaired in younger prepubertal boys is not resolved (11). We have previously reported hormone concentrations in a cohort of 93 prepubertal boys with KS (12). This cohort participated in a 2-year, randomized, controlled trial of the low-dose, orally available androgen, oxandrolone (Ox), with improvements in body composition, fasting triglycerides, measures of anxiety, and visual-motor integration compared with placebo (Pl), but no significant changes in cognition or motor function (3, 6). Ox was well tolerated with no serious adverse events; however, growth acceleration and bone age advancement was noted despite Ox being a nonaromatizable androgen and not expected to have an effect on the growth plate. We hypothesized Ox may have an impact on testicular function. Therefore, the objective of this work was to compare onset of puberty and testicular function in prepubertal boys treated with Ox compared with Pl.
Methods
Study design
Details of the study protocol and enrollment flow diagram have previously been published and are summarized here (3, 6). This was randomized clinical trial of 2 years of oral Ox vs Pl in boys with KS age 4.0 to 12.9 years. Inclusion criteria included a karyotype 47,XXY, 48,XXYY, or 48,XXXY with <50% mosaicism for a 46,XY cell line in blood. Exclusion criteria included treatment with exogenous androgens in the previous year. A total of 93 participants were recruited through national advertisements. For this analysis, participants who had pubic hair or testicular volume ≥4 mL on baseline exam were also excluded. All participants provided assent, and written informed consent was obtained from parents. After enrollment, participants were randomized 1:1 to Ox 0.06 mg/kg/d rounded to the nearest 1.25 mg (minimum: 1.25 mg/d; maximum: 3.75 mg/d) or an identical-appearing Pl capsule. All investigators and participants were blinded to both allocation and randomization. Study visits occurred every 6 months for 2 years with study assessments, including a physical exam with anthropometric measurements, fasting blood draw, bone age x-ray, and a neuropsychiatric assessment battery. A predetermined dose-reduction safety protocol was used, and an independent Data and Safety Monitoring Board reviewed safety data annually. The study was approved by the local Human Subjects Committee and was registered on ClinicalTrials.gov (no. NCT00348946).
Pubertal assessment
Pubic hair and breast tissue were assessed according to Tanner staging at every visit by an experienced pediatric endocrine clinician. Testicular volume was estimated by palpation using the Prader orchidometer. Testis volume smaller than the smallest measurement on the Prader orchidometer (1 mL) was estimated as 0.5 mL. Stretched penile length and penile width were measured to the nearest 0.25 cm. Pubarche was defined as ≥Tanner 2 pubic hair. Gonadarche was defined as testicular volume of either testis ≥4 mL. Premature gonadarche was defined as gonadarche prior to age 9 years.
Laboratory assessments
A fasting, morning blood sample was obtained at all study visits and immediately processed according to protocol. Total testosterone (TT) was measured by high-pressure liquid chromatography tandem mass spectrometry with interassay and intra-assay coefficient of variation (CV) of <10% (Esoterix Laboratories, Calabasas Hills, CA). LH, FSH, inhibin B (INHB), and anti-Müllerian hormone (AMH) were measured simultaneously from frozen serum. Normal ranges by age and pubertal stage were determined from 304 boys 4.0 to 13.0 years of age without an endocrine or metabolic disorder. The LH and FSH immunoassays (TRFIA AutoDelfia Wallac; PerkinElmer, Courtaboeuf, France) had limit of quantification of 0.024 and 0.033 IU/L, respectively, with interassay and intra-assay CV of <5% for both assays. The INHB enzyme-linked immunosorbent assay (AnschLabs, Webster, TX) had a limit of quantification of 2.2 pg/mL, interassay CV of 9.7% and 4.8% at a concentration of 43 and 93 pg/mL, respectively, and an intra-assay CV of 4.6% and 2.8% at concentrations of 53 and 96 pg/mL, respectively. The assay had no cross-reactivity with activins or inhibin A. AMH was measured with an enzyme-linked immunosorbent assay (AnschLabs) down to 0.2 pmol/L. The interassay CV was <9% at concentrations of 7 to 110 pmol/L with an intra-assay CV <4% above 5 pmol/L. Values below the limit of detection for any hormone were assigned the lower limit of quantification for the assay.
Statistical analyses
As we previously reported, there were no differences between completers and noncompleters in this study; analyses were performed without imputation of missing data (6). The a priori primary outcomes were onset of gonadarche and pubarche in prepubertal boys. Therefore, only boys who were both pregonadarchal and prepubarchal at baseline were included in this analysis. Baseline comparisons of age, testicular volumes, penile length, and hormones were done using Welch t tests for parametric variables and Wilcoxon rank sum tests for nonparametric variables. A mixed-effect logistical regression model was used to compare primary outcomes between groups over time while adjusting for age. Univariate regression analyses were conducted to identify baseline factors that were independently predictive of gonadarche. Descriptive statistics were then used to compare subgroups. Paired t tests or Wilcoxon tests were used to compare unadjusted testicular hormone concentrations, testicular volume, and stretched penile length from the beginning to the end of the study period, and two-sided t tests were used to compare change scores between treatment groups. Results were considered statistically significant at an α of 0.05. Statistical analyses were performed using GraphPad Prism, version 7.0 for Mac (GraphPad Software, La Jolla, CA) and R: A Language and Environment for Statistical Computing, version 3.3.1 for Windows (R Foundation for Statistical Computing, Vienna, Austria).
Results
Eighty participants (43 Ox, 37 Pl) were prepubertal on baseline exam and included in this analysis. One participant each had karyotype 48,XXYY, 48,XXXY, and 47,XXY/46,XY mosaicism, and the remaining 77 participants were nonmosaic 47,XXY. Baseline characteristics for all subjects are presented in Table 1; the Pl group was significantly older than the Ox group. After adjusting for baseline age, the Ox-treated group had 20.5 times higher odds of reaching gonadarche (OR 95% CI: 6.5, 77.8) and 28.1 times higher odds of reaching pubarche (OR 95% CI: 8.8, 110.4) during the 2-year study period compared with Pl. Gonadarche and pubarche both occurred at a younger age in the Ox group (gonadarche: 9.8 ± 1.5 vs 12.1 ± 1.0 years, P < 0.001; pubarche: 10.2 ± 1.1 vs 11.6 ± 1.3 years, P = 0.02).
| . | Ox (n = 43) . | Pl (n = 37) . | P Valuea . |
|---|---|---|---|
| Age, y | 6.6 ± 2.0 | 7.7 ± 2.4 | 0.03 |
| Bone age, y | 6.1 ± 2.0 | 7.0 ± 2.3 | 0.06 |
| Testicular volume, mL | 1.0 ± 0.5 | 1.1 ± 0.6 | 0.30 |
| Testicular volume z score | –1.6 ± 1.1 | −1.4 ± 1.1 | 0.43 |
| Stretched penile length, cm | 4.0 ± 0.8 | 4.1 ± 0.8 | 0.29 |
| Stretched penile length z score | −1.8 ± 0.7 | −1.7 ± 0.7 | 0.55 |
| LH, mIU/mL | 0.03 (0.01, 0.05) | 0.03 (0.01, 0.07) | 0.70 |
| FSH, mIU/mL | 0.28 (0.07, 0.46) | 0.36 (0.27, 0.83) | 0.86 |
| TT, ng/dL | 3 (2, 5) | 3 (2.5, 5.5) | 0.12 |
| INHB, pg/mL | 92 (65, 140) | 77 (45, 106) | 0.10 |
| AMH, pmol/L | 883 (603, 1323) | 699 (427, 1281) | 0.27 |
| . | Ox (n = 43) . | Pl (n = 37) . | P Valuea . |
|---|---|---|---|
| Age, y | 6.6 ± 2.0 | 7.7 ± 2.4 | 0.03 |
| Bone age, y | 6.1 ± 2.0 | 7.0 ± 2.3 | 0.06 |
| Testicular volume, mL | 1.0 ± 0.5 | 1.1 ± 0.6 | 0.30 |
| Testicular volume z score | –1.6 ± 1.1 | −1.4 ± 1.1 | 0.43 |
| Stretched penile length, cm | 4.0 ± 0.8 | 4.1 ± 0.8 | 0.29 |
| Stretched penile length z score | −1.8 ± 0.7 | −1.7 ± 0.7 | 0.55 |
| LH, mIU/mL | 0.03 (0.01, 0.05) | 0.03 (0.01, 0.07) | 0.70 |
| FSH, mIU/mL | 0.28 (0.07, 0.46) | 0.36 (0.27, 0.83) | 0.86 |
| TT, ng/dL | 3 (2, 5) | 3 (2.5, 5.5) | 0.12 |
| INHB, pg/mL | 92 (65, 140) | 77 (45, 106) | 0.10 |
| AMH, pmol/L | 883 (603, 1323) | 699 (427, 1281) | 0.27 |
Data are represented in mean ± SD score or median (25th, 75th percentiles).
t test or Wilcoxin rank sum test.
| . | Ox (n = 43) . | Pl (n = 37) . | P Valuea . |
|---|---|---|---|
| Age, y | 6.6 ± 2.0 | 7.7 ± 2.4 | 0.03 |
| Bone age, y | 6.1 ± 2.0 | 7.0 ± 2.3 | 0.06 |
| Testicular volume, mL | 1.0 ± 0.5 | 1.1 ± 0.6 | 0.30 |
| Testicular volume z score | –1.6 ± 1.1 | −1.4 ± 1.1 | 0.43 |
| Stretched penile length, cm | 4.0 ± 0.8 | 4.1 ± 0.8 | 0.29 |
| Stretched penile length z score | −1.8 ± 0.7 | −1.7 ± 0.7 | 0.55 |
| LH, mIU/mL | 0.03 (0.01, 0.05) | 0.03 (0.01, 0.07) | 0.70 |
| FSH, mIU/mL | 0.28 (0.07, 0.46) | 0.36 (0.27, 0.83) | 0.86 |
| TT, ng/dL | 3 (2, 5) | 3 (2.5, 5.5) | 0.12 |
| INHB, pg/mL | 92 (65, 140) | 77 (45, 106) | 0.10 |
| AMH, pmol/L | 883 (603, 1323) | 699 (427, 1281) | 0.27 |
| . | Ox (n = 43) . | Pl (n = 37) . | P Valuea . |
|---|---|---|---|
| Age, y | 6.6 ± 2.0 | 7.7 ± 2.4 | 0.03 |
| Bone age, y | 6.1 ± 2.0 | 7.0 ± 2.3 | 0.06 |
| Testicular volume, mL | 1.0 ± 0.5 | 1.1 ± 0.6 | 0.30 |
| Testicular volume z score | –1.6 ± 1.1 | −1.4 ± 1.1 | 0.43 |
| Stretched penile length, cm | 4.0 ± 0.8 | 4.1 ± 0.8 | 0.29 |
| Stretched penile length z score | −1.8 ± 0.7 | −1.7 ± 0.7 | 0.55 |
| LH, mIU/mL | 0.03 (0.01, 0.05) | 0.03 (0.01, 0.07) | 0.70 |
| FSH, mIU/mL | 0.28 (0.07, 0.46) | 0.36 (0.27, 0.83) | 0.86 |
| TT, ng/dL | 3 (2, 5) | 3 (2.5, 5.5) | 0.12 |
| INHB, pg/mL | 92 (65, 140) | 77 (45, 106) | 0.10 |
| AMH, pmol/L | 883 (603, 1323) | 699 (427, 1281) | 0.27 |
Data are represented in mean ± SD score or median (25th, 75th percentiles).
t test or Wilcoxin rank sum test.
Testicular hormone concentrations throughout the study are presented in Fig. 1. LH, FSH, and TT all increased in both Ox and Pl groups (paired t tests or Wilcoxon tests, P < 0.001 for all), whereas biomarkers of Sertoli cell function (INHB and AMH) did not significantly change over time (P > 0.05). Treatment with Ox did not significantly alter any of these testicular hormone concentrations; in particular, it did not suppress Sertoli cell function. Testicular volume and stretched penile length increased significantly more in the Ox group (Fig. 2). As we previously reported, height SD score and bone age significantly increased in the treated group; however, change in body mass index (BMI) SD score did not differ between groups (6).
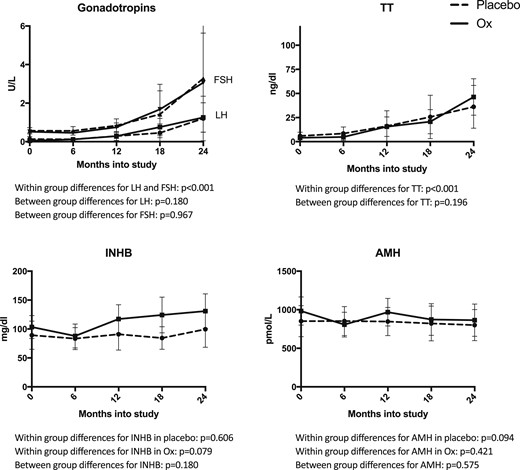
Testicular hormone concentrations over the study period for all subjects. LH, FSH, and TT significantly increased during the 2-y study period in both Ox (solid) and Pl (dashed) (P < 0.001 for all), but Ox treatment did not significantly alter this (P > 0.05). INHB and AMH concentrations were stable during the study and also did not significantly differ between treatment groups (P > 0.05). Points and error bars represent mean and 95% CI, respectively.
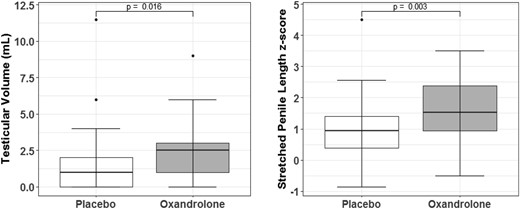
Change scores between baseline and final visit for testicular volume and stretched penile length z scores were compared between treatment groups. Mean testicular volume increased to 3.6 ± 2.2 mL in the Ox group compared with 2.2 ± 1.8 mL in the Pl group (P = 0.016). Stretched penile length increased to 6.2 ± 1.5 cm in the Ox group and 5.4 ± 1.4 cm in the Pl group, with corresponding z scores to adjust for the age differences depicted in the figure on the right (P = 0.003).
Baseline and 2-year pubertal and hormonal characteristics for all the subjects that reached gonadarche during the study period (16 Ox, 8 Pl) are presented in Table 2. Patterns for testicular volumes and hormone concentrations in relation to gonadarche appeared similar between groups (Fig. 3). Within the Ox group, baseline characteristics independently associated with reaching gonadarche within the 2-year study period included older age, greater testicular volume, and higher LH, TT, and INHB.
Comparison Between Treatment Groups in the 24 Boys Who Reached Gonadarche During the Study
| . | Ox (n = 16) . | Pl (n = 8) . | P Value . |
|---|---|---|---|
| At baseline | |||
| Age, y | 8.3 ± 1.8 | 10.5 ± 0.9 | 0.003a |
| Bone age | 7.8 ± 1.7 | 9.4 ± 0.9 | 0.023a |
| Bone age delay | −0.5 ± 1.1 | −1.1 ± 1.0 | 0.16 |
| BMI SD score | 0.5 ± 1.1 | 0.1 ± 1.2 | 0.38 |
| FSH, mIU/mL | 0.54 (0.41–0.75) | 0.84 (0.02–0.70) | 0.39 |
| LH, mIU/mL | 0.05 (0.02–0.07) | 0.18 (0.67–2.2) | 0.18 |
| TT, ng/dL | 4.5 (3.0–6.8) | 7.0 (4.5–13.0) | 0.11 |
| AMH, pmol/L | 993 ± 467 | 860 ± 581 | 0.65 |
| INHB, ng/mL | 130 ± 111 | 154 ± 65 | 0.52 |
| At the time of gonadarche | |||
| Months into study | 17.8 ± 7.1 | 18.1 ± 7.2 | 0.92 |
| Age, y (range) | 9.8 ± 1.5 (7.7–11.8) | 12.1 ± 1.0 (10.0–12.0) | <0.001a |
| Bone age | 9.8 ± 1.8 | 10.9 ± 0.8 | 0.07 |
| Bone age delay | 0.0 ± 1.2 | −1.2 ± 1.2 | 0.035a |
| BMI SD score | 0.5 ± 1.1 | 0.0 ± 1.2 | 0.32 |
| FSH, mIU/mL | 0.56 (0.44–1.1) | 0.90 (0.62–4.0) | 0.10 |
| LH, mIU/mL | 0.50 (0.17–1.2) | 1.1 (0.67–2.2) | 0.06 |
| TT, ng/dL | 46.1 ± 52.9 | 84.9 ± 61.8 | 0.15 |
| AMH, pmol/L | 878 ± 544 | 860 ± 581 | 0.94 |
| INHB, ng/mL | 160 ± 83 | 184 ± 115 | 0.60 |
| . | Ox (n = 16) . | Pl (n = 8) . | P Value . |
|---|---|---|---|
| At baseline | |||
| Age, y | 8.3 ± 1.8 | 10.5 ± 0.9 | 0.003a |
| Bone age | 7.8 ± 1.7 | 9.4 ± 0.9 | 0.023a |
| Bone age delay | −0.5 ± 1.1 | −1.1 ± 1.0 | 0.16 |
| BMI SD score | 0.5 ± 1.1 | 0.1 ± 1.2 | 0.38 |
| FSH, mIU/mL | 0.54 (0.41–0.75) | 0.84 (0.02–0.70) | 0.39 |
| LH, mIU/mL | 0.05 (0.02–0.07) | 0.18 (0.67–2.2) | 0.18 |
| TT, ng/dL | 4.5 (3.0–6.8) | 7.0 (4.5–13.0) | 0.11 |
| AMH, pmol/L | 993 ± 467 | 860 ± 581 | 0.65 |
| INHB, ng/mL | 130 ± 111 | 154 ± 65 | 0.52 |
| At the time of gonadarche | |||
| Months into study | 17.8 ± 7.1 | 18.1 ± 7.2 | 0.92 |
| Age, y (range) | 9.8 ± 1.5 (7.7–11.8) | 12.1 ± 1.0 (10.0–12.0) | <0.001a |
| Bone age | 9.8 ± 1.8 | 10.9 ± 0.8 | 0.07 |
| Bone age delay | 0.0 ± 1.2 | −1.2 ± 1.2 | 0.035a |
| BMI SD score | 0.5 ± 1.1 | 0.0 ± 1.2 | 0.32 |
| FSH, mIU/mL | 0.56 (0.44–1.1) | 0.90 (0.62–4.0) | 0.10 |
| LH, mIU/mL | 0.50 (0.17–1.2) | 1.1 (0.67–2.2) | 0.06 |
| TT, ng/dL | 46.1 ± 52.9 | 84.9 ± 61.8 | 0.15 |
| AMH, pmol/L | 878 ± 544 | 860 ± 581 | 0.94 |
| INHB, ng/mL | 160 ± 83 | 184 ± 115 | 0.60 |
Mean ± SD or median [interquartile range (IQR)]. P value is calculated with a t test or Mann-Whitney U test between Ox and Pl groups.
Significant at P < 0.05.
Comparison Between Treatment Groups in the 24 Boys Who Reached Gonadarche During the Study
| . | Ox (n = 16) . | Pl (n = 8) . | P Value . |
|---|---|---|---|
| At baseline | |||
| Age, y | 8.3 ± 1.8 | 10.5 ± 0.9 | 0.003a |
| Bone age | 7.8 ± 1.7 | 9.4 ± 0.9 | 0.023a |
| Bone age delay | −0.5 ± 1.1 | −1.1 ± 1.0 | 0.16 |
| BMI SD score | 0.5 ± 1.1 | 0.1 ± 1.2 | 0.38 |
| FSH, mIU/mL | 0.54 (0.41–0.75) | 0.84 (0.02–0.70) | 0.39 |
| LH, mIU/mL | 0.05 (0.02–0.07) | 0.18 (0.67–2.2) | 0.18 |
| TT, ng/dL | 4.5 (3.0–6.8) | 7.0 (4.5–13.0) | 0.11 |
| AMH, pmol/L | 993 ± 467 | 860 ± 581 | 0.65 |
| INHB, ng/mL | 130 ± 111 | 154 ± 65 | 0.52 |
| At the time of gonadarche | |||
| Months into study | 17.8 ± 7.1 | 18.1 ± 7.2 | 0.92 |
| Age, y (range) | 9.8 ± 1.5 (7.7–11.8) | 12.1 ± 1.0 (10.0–12.0) | <0.001a |
| Bone age | 9.8 ± 1.8 | 10.9 ± 0.8 | 0.07 |
| Bone age delay | 0.0 ± 1.2 | −1.2 ± 1.2 | 0.035a |
| BMI SD score | 0.5 ± 1.1 | 0.0 ± 1.2 | 0.32 |
| FSH, mIU/mL | 0.56 (0.44–1.1) | 0.90 (0.62–4.0) | 0.10 |
| LH, mIU/mL | 0.50 (0.17–1.2) | 1.1 (0.67–2.2) | 0.06 |
| TT, ng/dL | 46.1 ± 52.9 | 84.9 ± 61.8 | 0.15 |
| AMH, pmol/L | 878 ± 544 | 860 ± 581 | 0.94 |
| INHB, ng/mL | 160 ± 83 | 184 ± 115 | 0.60 |
| . | Ox (n = 16) . | Pl (n = 8) . | P Value . |
|---|---|---|---|
| At baseline | |||
| Age, y | 8.3 ± 1.8 | 10.5 ± 0.9 | 0.003a |
| Bone age | 7.8 ± 1.7 | 9.4 ± 0.9 | 0.023a |
| Bone age delay | −0.5 ± 1.1 | −1.1 ± 1.0 | 0.16 |
| BMI SD score | 0.5 ± 1.1 | 0.1 ± 1.2 | 0.38 |
| FSH, mIU/mL | 0.54 (0.41–0.75) | 0.84 (0.02–0.70) | 0.39 |
| LH, mIU/mL | 0.05 (0.02–0.07) | 0.18 (0.67–2.2) | 0.18 |
| TT, ng/dL | 4.5 (3.0–6.8) | 7.0 (4.5–13.0) | 0.11 |
| AMH, pmol/L | 993 ± 467 | 860 ± 581 | 0.65 |
| INHB, ng/mL | 130 ± 111 | 154 ± 65 | 0.52 |
| At the time of gonadarche | |||
| Months into study | 17.8 ± 7.1 | 18.1 ± 7.2 | 0.92 |
| Age, y (range) | 9.8 ± 1.5 (7.7–11.8) | 12.1 ± 1.0 (10.0–12.0) | <0.001a |
| Bone age | 9.8 ± 1.8 | 10.9 ± 0.8 | 0.07 |
| Bone age delay | 0.0 ± 1.2 | −1.2 ± 1.2 | 0.035a |
| BMI SD score | 0.5 ± 1.1 | 0.0 ± 1.2 | 0.32 |
| FSH, mIU/mL | 0.56 (0.44–1.1) | 0.90 (0.62–4.0) | 0.10 |
| LH, mIU/mL | 0.50 (0.17–1.2) | 1.1 (0.67–2.2) | 0.06 |
| TT, ng/dL | 46.1 ± 52.9 | 84.9 ± 61.8 | 0.15 |
| AMH, pmol/L | 878 ± 544 | 860 ± 581 | 0.94 |
| INHB, ng/mL | 160 ± 83 | 184 ± 115 | 0.60 |
Mean ± SD or median [interquartile range (IQR)]. P value is calculated with a t test or Mann-Whitney U test between Ox and Pl groups.
Significant at P < 0.05.
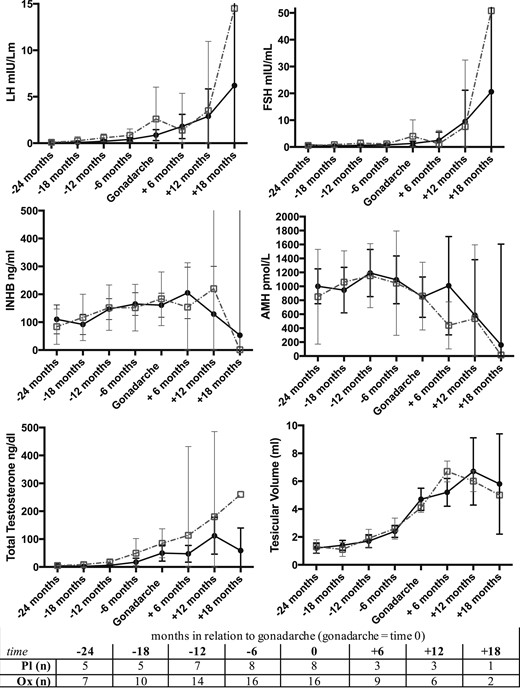
Means and 95% CIs for testicular volume and hormone concentrations in relation to gonadarche (time 0) between Ox (black solid line) and Pl (gray dashed line). The number of subjects (n) for each time interval are indicated in the table. There were no statistical differences between groups using t tests at each time point (+18 mos excluded due to too few of subjects).
Among boys under 9 years of age for the duration of the study, 5 of 22 boys treated with Ox (23%) experienced gonadarche before 9 years of age, whereas no one in the Pl group had gonadarche before 9 years of age (Fischer exact P = 0.049). These five boys reached gonadarche at a mean age of 8.0 ± 0.7 years (range: 7.7 to 8.2 years), mean bone age of 8.1 ± 0.7 years (range: 7.0 to 9.0 years), and mean duration in the study of 22.8 months (range: 18 to 24 months). Mean gonadotropins and testosterone were in the pubertal range at the time of gonadarche. No participants with a baseline bone age ≤5 years had gonadarche during the 2-year study period (negative predictive value: 1.0, 95% CI: 0.77 to 1.0). A bone age >5 years at the start of treatment yielded a positive predictive value of 0.63 (95% CI: 0.24 to 0.91) for early gonadarche within 2 years of Ox treatment. Height SD score at the time of gonadarche was 2.1 ± 1.3, and their predicted adult heights according to Greulich-Pyle prediction tables were all above 74 inches. Therefore, there was no concern for precocious puberty leading to short stature. None of these boys had worsening in anxiety or depression scores on parent or self-reported measures (data not shown).
Discussion
In this analysis of a 2-year, randomized, Pl-controlled trial in boys with KS, we observed that treatment with Ox led to earlier gonadarche and pubarche. Nearly a quarter of boys treated prior to age 9 experienced gonadarche during the 2-year study period. However, Ox did not affect testicular function during puberty as assessed by testicular volumes and serum testosterone, LH, FSH, INHB, and AMH concentrations. Predicted adult height and psychosocial measures were not compromised in the Ox group, including in the five boys who experienced early gonadarche.
Ox is an anabolic steroid with a much less virilizing effect compared with testosterone. It is a weak agonist of the androgen receptor, so pubic hair development could be a direct effect of Ox binding to the androgen receptor on epithelial hair follicles. An alternative hypothesis is that pubic hair development is secondary to endogenous androgen production, as supported by the observation of increasing testicular volume, serum LH, serum testosterone, and bone age advancement in the Ox-treated boys. As Ox is not aromatized to estrogen, bone age advancement would not be an expected effect of Ox directly (13). Serum hormone concentrations did not differ in the treated vs untreated groups as would be expected based on the higher likelihood of gonadarche and pubarche in the treated group; however, our study did not follow many participants beyond the initial signs of puberty and may be underpowered to detect differences in hormone concentrations.
Studies of Ox in children have been primarily focused on three populations: girls with Turner syndrome (>8 years of age), boys with constitutional delay of growth and puberty (>12 years of age), and children with substantial burn injuries (all ages). In girls with Turner syndrome, a meta-analysis concluded there is a dose-dependent relationship between Ox >0.06 mg/kg/d and virilization (hirsutism, voice deepening, clitoromegaly) (14). Importantly, the large majority of these girls have gonadal dysgenesis. Therefore, it is not possible to evaluate the effect of Ox on central puberty in this cohort. For adolescent boys with constitutional delay, Ox 2.5 mg daily resulted in increased testicular volume, serum testosterone concentrations, and pubic hair development compared with untreated controls, in addition to greater bone age advancement (15). A small study of boys >13 years with constitutional delay of puberty suggested that Ox (n = 5) may result in temporary suppression of the pituitary-gonadal axis followed by an increase in serum LH and testosterone with corresponding increase in testicular volume (16). In another study that included younger boys with growth delay (9 to 14 years), 40 of the 46 participants entered puberty within the follow-up period (mean of 9 months) (17). These data are consistent with our study results, although notably endogenous puberty is typically desired in boys with constitutional delay of puberty. In contrast, use of Ox in burn victims does not seem to effect virilization or testicular function despite the use of higher doses. In one study of 70 burn victims treated for 1 year with 0.1 mg/kg/d of Ox starting at a mean age of 8, virilization was not observed in any subjects (18). In addition, TT concentrations were not different between Ox and control groups. Testicular volumes and pubertal status were not reported in this study, and pre- and postpubertal subjects of both sexes were analyzed together, potentially masking effects unique to prepubertal boys. In addition, given that our cohort experienced gonadarche at a median of 18 months into Ox treatment, it is possible the effects on the gonadal axis would not yet be apparent with only 1 year of treatment.
The recognition that chronic prepubertal sex steroid exposure may result in early activation of the hypothalamic-pituitary-gonadal axis is not unique. Poorly controlled congenital adrenal hyperplasia and McCune Albright syndrome, resulting in chronically elevated testosterone and estrogen concentrations, respectively, are well known to lead to earlier central puberty (19, 20). However, both of these conditions are associated with significant bone age advancement, and central puberty often commences when the bone age reaches a pubertal age. In contrast, the boys treated with Ox in our study reached gonadarche at a significantly younger bone age than would be expected. Prolonged prepubertal exposure to Ox and other sex steroids may interrupt the normal suppression of the hypothalamic-pituitary-gonadal axis in at least a subset of children, and this may be a useful model in studying disorders of pubertal development and/or treatment of delayed puberty.
Does early gonadarche or pubarche matter? Our participants were not followed beyond these very early signs of puberty, so we cannot determine if puberty progresses or if earlier gonadarche or pubarche have sequelae. Although there are ample data pointing toward increased depression in girls with precocious puberty, there are very few data on mental health in boys with precocious puberty (21). Our data do not suggest any ill effects on psychosocial functioning or predicted final height in this small cohort. Although we cannot discern if early gonadarche is problematic, this risk should be discussed with parents if prepubertal androgen treatment is being considered. In addition, given our findings, all studies using Ox in prepubertal children should rigorously evaluate signs of puberty, hormone concentrations, and potential sequelae of precocious puberty.
An inherent weakness in this study is that assessing pubertal status and gonadal function in prepubertal boys with KS is not straightforward. For this analysis, we defined gonadarche at ≥4 mL; however, as boys with KS have smaller testes throughout the lifespan, this may not reflect the commencement of central puberty in KS. In addition, relying on morning LH levels to define puberty may underclassify central puberty, and in some boys with KS, LH may be elevated into the pubertal range even at a very young age secondary to primary hypogonadism rather than true puberty. Another limitation is, due to the young age of our cohort and defined follow-up period of 2 years, we were unable to assess the effects of Ox on fertility. Importantly, serum measures of Sertoli cell function were not different between groups. In addition, the natural history of testicular function in KS demonstrates markedly reduced spermatozoa starting in infancy and progressing throughout childhood and adolescence, even without androgen treatment (8). We did not assess adrenal androgen concentrations that may contribute to pubarche. An additional limitation of this study is the small number of subjects who experienced gonadarche or pubarche, making it difficult to draw conclusions about risk factors for these outcomes. Strengths of this study include that it is the largest prepubertal cohort of boys with KS, as well as the randomized design, the 2-year follow-up period, and the multimodel safety assessments.
In conclusion, 2 years of Ox treatment in prepubertal boys with KS results in increased risk of early gonadarche, on average 2 years earlier than in Pl-treated boys. The underlying mechanism of this finding warrants further investigation, as it potentially has implications for other populations with pubertal disorders. Ox increases penile length and does not seem to impair the natural history of testicular function in boys with KS. For now, caution should be used in treating prepubertal boys with KS due to a possible risk of precocious puberty. As studies have suggested potential benefits of prepubertal androgens on neurodevelopment and cardiometabolism in KS, further studies are needed to fully evaluate the effects of exogenous androgens on testicular function in boys with KS, including optimization of formulation, dose, and duration.
Abbreviations:
- AMH
anti-Müllerian hormone
- BMI
body mass index
- CV
coefficient of variation
- INHB
inhibin B
- KS
Klinefelter syndrome
- Ox
oxandrolone
- Pl
placebo
- TT
total testosterone
Acknowledgments
Financial Support: This work was supported by the National Institute of Neurologic Disorders and Stroke (NS050597 to J.L.R.), the Institut de Recherche Endocrinienne et Metabolique, Paris (to N.L.), the National Institute Diabetes and Digestive and Kidney Diseases (2T32DK63687011A1), and the National Institute for Child Health and Human Development (K23HD092588-01 to S.M.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Clinical Trial Information: ClinicalTrials.gov no. NCT00348946 (registered 6 July 2006).
Disclosure Summary: The authors have nothing to disclose.



