-
PDF
- Split View
-
Views
-
Cite
Cite
Kris Poppe, Candice Autin, Flora Veltri, Pierre Kleynen, Lidia Grabczan, Serge Rozenberg, Lieveke Ameye, Thyroid Autoimmunity and Intracytoplasmic Sperm Injection Outcome: A Systematic Review and Meta-Analysis, The Journal of Clinical Endocrinology & Metabolism, Volume 103, Issue 5, May 2018, Pages 1755–1766, https://doi.org/10.1210/jc.2017-02633
Close - Share Icon Share
Abstract
Since 2010, three meta-analyses have been published on the impact of thyroid autoimmunity (TAI) on pregnancy outcomes in infertile women treated with assisted reproductive technology (ART). The initially observed high risk for miscarriage became very low in the most recent meta-analysis, published in 2016.
To investigate whether the lower risk for miscarriage in the latest meta-analysis was associated with the increased use of intracytoplasmic sperm injection (ICSI) in recent studies.
MEDLINE was searched from January 1990 to May 2017.
Data from case-control and cohort studies on ART (in vitro fertilization/ICSI) pregnancy outcomes in women with and without TAI. Only studies in which women were treated with ICSI were included.
Four studies were retained, including 1855 ICSI cycles (290 with and 1565 without TAI). In women with a clinical pregnancy (114 ICSI cycles with TAI and 651 without), there was no difference in miscarriage or live birth rates: respective combined odds ratios, 0.95 [95% confidence interval (CI), 0.48 to 1.87] and 1.12 (95% CI, 0.62 to 2.03), respectively. Age did not differ between women with and without TAI: combined mean difference of 0.13 years (95% CI, −0.51 to 0.76 years), but serum thyroid-stimulating hormone was higher in women with TAI: combined mean difference of 0.20 mIU/L (95% CI, 0.07 to 0.33 mIU/L).
Infertile women with TAI treated with ICSI had no increased risk for a first-trimester miscarriage compared with women without TAI.
Thyroid autoimmunity (TAI) is the most frequent cause of subclinical hypothyroidism (SCH) in women of reproductive age and is important in relation to several aspects of infertility, such as the cause, the ovarian stimulation procedure (OS), and the pregnancy outcome after assisted reproductive technology (ART) (1). In relation to the cause of infertility, the prevalence of TAI is higher in women with polycystic ovarian syndrome (PCOS) and idiopathic infertility (∼25%) compared with that in fertile women (∼10%) (2–5). Women with TAI treated with OS cannot adequately increase their production of thyroid hormone (THs) to the strain it induces compared with women without TAI (6, 7). Finally, the presence of TAI has been associated with an increased first-trimester miscarriage rate (MR) and decreased live birth rate (LBR) (8, 9).
Reasons for the increased MR remain speculative and might be due to a higher age, a higher serum thyroid-stimulating hormone (TSH) level, an underlying immune imbalance, the presence of other autoimmune problems (e.g., anticardiolipin antibodies), or a combination of factors (4, 10). Three meta-analyses investigated the impact of TAI on pregnancy outcomes after ART (8–10). No effect on the implantation and clinical pregnancy rates was observed, but the first-trimester MR was increased and LBR was decreased. Of note, in a meta-analysis published in 2011 (9), the odds ratio (OR) of miscarriage was 3.15, but in the most recent meta-analysis it was only 1.44 (10). The reasons that the OR decreased can be multiple, including differences in the study design (prospective vs retrospective), the inclusion/exclusion criteria (treatment with TH [levothyroxine (LT4)] or corticosteroids), the OS protocol (agonists vs antagonists, long vs short), and the diagnosis of TAI [only thyroid peroxidase antibodies (TPO-abs) vs both TPO-abs and thyroglobulin antibodies (Tg-abs)]. However, it is remarkable that in the most recent studies included in the 2016 meta-analysis, women were treated mainly with intracytoplasmic sperm injection (ICSI) (10–15). ICSI was developed to treat men with infertility or women with idiopathic infertility, or when conventional in vitro fertilization (IVF) has failed (16).
To investigate whether the increased use of ICSI for ART treatment contributed to the lower OR of miscarriage in women with TAI in the meta-analysis performed in 2016 (10) as compared with the high OR in earlier meta-analyses, we performed a meta-analysis that included studies in which all women with and without TAI were treated with ICSI.
Methods
Search strategy and selection criteria
The meta-analysis was reported conforming to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines (17). MEDLINE was used to complete the search of the 2016 meta-analysis by Busnelli et al. (10) that identified all the comparative studies published from January 1990 to November 2015 in the English-language literature on ART (IVF/ICSI) pregnancy outcomes in women with and without TAI. The search was completed from December 2015 to September 2017, and the following heading terms were used: "thyroid autoimmunity, autoantibodies," "infertility, ART, IVF, ICSI," and "pregnancy, miscarriage, outcome."
We included studies in which women, with and without TAI, of infertile couples were treated with ICSI only; furthermore, only the first ICSI cycle was taken into account. Studies that included women treated with IVF or intrauterine insemination only, or both techniques, were excluded (in some of these studies there was also a medical treatment arm with LT4 or corticosteroids). We collected information regarding study characteristics (author name, year and country of publication, study design, sample size), intervention characteristics (ICSI, IVF, or both and the OS protocol), and patients’ characteristics [age, cause of infertility, serum TSH levels, and the levels of thyroid antibodies (TPO-abs and Tg-abs)].
Two reviewers (K.P., L.A.) screened and extracted the data independently. Figure 1 illustrates the study selection process with a flowchart.
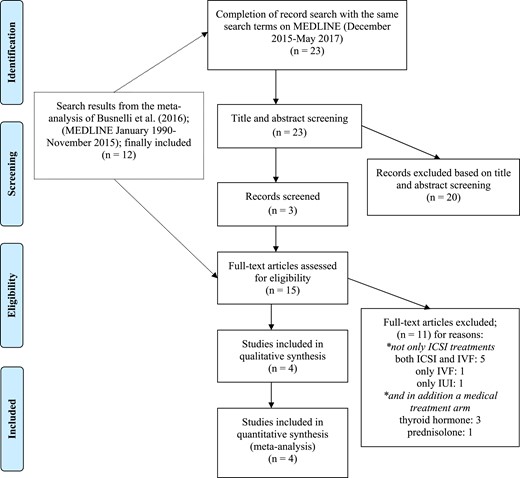
Flowchart of the study selection process. IUI, intrauterine insemination.
Data analysis
The following were collected as primary outcomes: oocyte retrieval, fertilization rates (FRs), implantation rates, clinical pregnancy rates, MRs, and LBRs. Secondary outcomes were age and basal serum concentrations of TSH. The overall impact of the TAI status on a continuous variable (age, TSH, number of oocytes retrieved) was assessed by the difference in means for the two groups of patients, and the overall effect of TAI on a binary variable (fertilization, implantation, clinical pregnancy, miscarriage, live birth) was assessed by a combined odds ratio (OR). By using the summary data published in the eligible studies, individual differences in means and individual ORs were estimated together with their variances. Combined estimates of the effects were calculated through the use of fixed-effects models (in case no heterogeneity could be detected) or random-effects models. Inference testing was performed to test the null hypothesis of combined differences in means equal to zero or of combined ORs equal to 1 from the reported 95% confidence intervals (CIs). Heterogeneity was tested by using a χ2 test. Because of the small number of studies, the risk of bias could not be assessed. The quality of the four studies was assessed by the Newcastle-Ottawa scale (18). All analyses were performed by using the package Meta in R software, version 3.0.2 (R Project for Statistical Computing).
Results
In addition to the three studies that were extracted from the meta-analysis by Busnelli et al. (10), we included the study by Sakar et al. (15). Finally, in four studies included in this meta-analysis, women were treated with ICSI only (11, 12, 14, 15). Studies that included women treated with IVF only (19), intrauterine insemination only (20), or both techniques were excluded (21–25). Furthermore, in some of the excluded studies in which women were not treated with ICSI only, there was also a medical treatment arm with LT4 (26–28) or corticosteroids (13).
Study characteristics
The study results from two prospective and two retrospective studies were analyzed (n = 1855 women); 290 (16%) women had TAI and 1565 (84%) did not have TAI. The studies were published from 2013 to 2015 but started enrolling patients in 1997 (12). The percentage of couples in which the infertility cause was male varied from 25% in the study by Lukaszuk et al. (14) to 100% in that by Tan et al. (12). Serum TSH cutoff values were limited to 3.0 mIU/L (12) and 2.5 mIU/L in the study by Karacan et al. (11) and Lukaszuk et al. (14). In the study by Sakar et al. (15), no details were provided. In all studies besides that by Lukaszuk et al. (14), TAI was defined by the measurement of both TPO-abs and Tg-abs. The presence of TAI varied between 13% in the studies by Karacan et al. (11) and Tan et al. (12) to ∼19% in the studies by Lukaszuk et al. (14) and Sakar et al. (15). Concerning the OS protocol, gonadotropin-releasing hormone (GnRH) antagonists in the study by Karacan et al. (11) and Sakar et al. (15), and no details were given in the study by Tan et al. (12). Finally, the study by Lukaszuk et al. used GnRH agonists (14). The numbers of transferred embryos were similar in all studies. The study characteristics in the four studies are summarized in Table 1.
Overview of the Study Characteristics in the Four Studies Included in the Meta-Analysis
| First Author (Year), Country (Reference) . | Study Population, % Male Infertility . | Study Type, Period of Recruitment . | Definition of SCH TSH Assay . | Thyroid Antibodies, Defining TAI Antibody Assays, Cutoff for TAI Positivity . | No. (%) TAI+ Patients, Age (y) a . | No. (%) TAI– Patients, Age (y) a . | ART Details . | Outcomes . | Quality of Evidence b . |
|---|---|---|---|---|---|---|---|---|---|
| Karacan et al. (2013), Turkey (11) | Women with different causes of infertility, 44% male | Prospective cohort, 2009–2012 | TSH > 2.5 mIU/L, enzyme-linked fluorescence assay (Vidas; BioMerieux, France) | TPO-abs and Tg-abs, electro-chemiluminescence immunoassay, TPO-abs > 35 IU/mL, Tg-abs > 115 IU/mL | 34 (13.4), 34.2 ± 4.3 | 219 (86.6), 33.1 ± 3.8 | First cycle, GnRH antagonist protocol ET day 5; number: 1.4 ± 0.8 | NOR, FR, IR, CPR, MR, OPR | 8 |
| Tan et al. (2014), Germany (12) | Women with infertile men, 100% male | Retrospective cohort, 1997–2006 | TSH > 3 mIU/L, automated chemiluminescence immunoassay (ADVIA Centaur, Siemens, Germany) | TPO-abs and Tg-abs, immunometric assay, TPO-abs > 100 IU/mL, Tg-abs > 100 IU/mL | 110 (13.2), 31.5 ± 4.0 | 725 (86.8), 31.3 ± 4.4 | First cycle, long protocol ET day ?; number: 1–3 | CPR, MR, LBR | 7 |
| Lukaszuk et al. (2015), Poland (14) | Women with different causes of infertility, 25% male | Retrospective cohort, 2010–2012 | TSH > 2.5 mIU/L, electrochemiluminescent immunometric assay (Cobas 6000, Roche Diagnostics, Poland) | TPO-abs, electrochemiluminescence immunoassay, TPO-abs > 34 IU/mL | 114 (18.7), 35 (33–38) | 495 (81.3), 34 (32–37) | First cycle, long GnRH agonist protocol ET day 5; number: 2 (1–3) | NOR, FR, IR, CPR, MR, LBR | 7 |
| Sakar et al. (2015), Turkey (15) | Women with different causes of infertility, 50% male | Prospective case-control, 2013–2014 | ? ELISA method (Beckman Coulter, Ireland, Inc.) | TPO-abs and Tg-abs, Coulter diagnostic kits (Beckman Coulter Ireland, Inc.) for TPO-abs, electrochemiluminescence immunoassay method by Cobas 6000 diagnostic kits (Roche Pharma, Germany) for Tg-abs, TPO-abs > 35 IU/mL, Tg-abs > 78 ng/mL | 49 (19.5), 30.7 ± 3.8 | 202 (80.5), 29.5 ± 3.4 | First cycle, GnRH antagonist protocol ET day 3; number: 1 | NOR, FR, IR, CPR, MR, OPR | 7 |
| First Author (Year), Country (Reference) . | Study Population, % Male Infertility . | Study Type, Period of Recruitment . | Definition of SCH TSH Assay . | Thyroid Antibodies, Defining TAI Antibody Assays, Cutoff for TAI Positivity . | No. (%) TAI+ Patients, Age (y) a . | No. (%) TAI– Patients, Age (y) a . | ART Details . | Outcomes . | Quality of Evidence b . |
|---|---|---|---|---|---|---|---|---|---|
| Karacan et al. (2013), Turkey (11) | Women with different causes of infertility, 44% male | Prospective cohort, 2009–2012 | TSH > 2.5 mIU/L, enzyme-linked fluorescence assay (Vidas; BioMerieux, France) | TPO-abs and Tg-abs, electro-chemiluminescence immunoassay, TPO-abs > 35 IU/mL, Tg-abs > 115 IU/mL | 34 (13.4), 34.2 ± 4.3 | 219 (86.6), 33.1 ± 3.8 | First cycle, GnRH antagonist protocol ET day 5; number: 1.4 ± 0.8 | NOR, FR, IR, CPR, MR, OPR | 8 |
| Tan et al. (2014), Germany (12) | Women with infertile men, 100% male | Retrospective cohort, 1997–2006 | TSH > 3 mIU/L, automated chemiluminescence immunoassay (ADVIA Centaur, Siemens, Germany) | TPO-abs and Tg-abs, immunometric assay, TPO-abs > 100 IU/mL, Tg-abs > 100 IU/mL | 110 (13.2), 31.5 ± 4.0 | 725 (86.8), 31.3 ± 4.4 | First cycle, long protocol ET day ?; number: 1–3 | CPR, MR, LBR | 7 |
| Lukaszuk et al. (2015), Poland (14) | Women with different causes of infertility, 25% male | Retrospective cohort, 2010–2012 | TSH > 2.5 mIU/L, electrochemiluminescent immunometric assay (Cobas 6000, Roche Diagnostics, Poland) | TPO-abs, electrochemiluminescence immunoassay, TPO-abs > 34 IU/mL | 114 (18.7), 35 (33–38) | 495 (81.3), 34 (32–37) | First cycle, long GnRH agonist protocol ET day 5; number: 2 (1–3) | NOR, FR, IR, CPR, MR, LBR | 7 |
| Sakar et al. (2015), Turkey (15) | Women with different causes of infertility, 50% male | Prospective case-control, 2013–2014 | ? ELISA method (Beckman Coulter, Ireland, Inc.) | TPO-abs and Tg-abs, Coulter diagnostic kits (Beckman Coulter Ireland, Inc.) for TPO-abs, electrochemiluminescence immunoassay method by Cobas 6000 diagnostic kits (Roche Pharma, Germany) for Tg-abs, TPO-abs > 35 IU/mL, Tg-abs > 78 ng/mL | 49 (19.5), 30.7 ± 3.8 | 202 (80.5), 29.5 ± 3.4 | First cycle, GnRH antagonist protocol ET day 3; number: 1 | NOR, FR, IR, CPR, MR, OPR | 7 |
Abbreviations: CPR, clinical pregnancy rate; ELISA, enzyme-linked immunosorbent assay; ET, embryo transfer; NOR, number of oocytes retrieved; OPR, ongoing pregnancy rate; TAI+, positive for thyroid antibodies; TAI–, negative for thyroid antibodies.
Age expressed as mean ± standard deviation or median (interquartile range).
Based on the Newcastle-Ottawa scale (18).
Overview of the Study Characteristics in the Four Studies Included in the Meta-Analysis
| First Author (Year), Country (Reference) . | Study Population, % Male Infertility . | Study Type, Period of Recruitment . | Definition of SCH TSH Assay . | Thyroid Antibodies, Defining TAI Antibody Assays, Cutoff for TAI Positivity . | No. (%) TAI+ Patients, Age (y) a . | No. (%) TAI– Patients, Age (y) a . | ART Details . | Outcomes . | Quality of Evidence b . |
|---|---|---|---|---|---|---|---|---|---|
| Karacan et al. (2013), Turkey (11) | Women with different causes of infertility, 44% male | Prospective cohort, 2009–2012 | TSH > 2.5 mIU/L, enzyme-linked fluorescence assay (Vidas; BioMerieux, France) | TPO-abs and Tg-abs, electro-chemiluminescence immunoassay, TPO-abs > 35 IU/mL, Tg-abs > 115 IU/mL | 34 (13.4), 34.2 ± 4.3 | 219 (86.6), 33.1 ± 3.8 | First cycle, GnRH antagonist protocol ET day 5; number: 1.4 ± 0.8 | NOR, FR, IR, CPR, MR, OPR | 8 |
| Tan et al. (2014), Germany (12) | Women with infertile men, 100% male | Retrospective cohort, 1997–2006 | TSH > 3 mIU/L, automated chemiluminescence immunoassay (ADVIA Centaur, Siemens, Germany) | TPO-abs and Tg-abs, immunometric assay, TPO-abs > 100 IU/mL, Tg-abs > 100 IU/mL | 110 (13.2), 31.5 ± 4.0 | 725 (86.8), 31.3 ± 4.4 | First cycle, long protocol ET day ?; number: 1–3 | CPR, MR, LBR | 7 |
| Lukaszuk et al. (2015), Poland (14) | Women with different causes of infertility, 25% male | Retrospective cohort, 2010–2012 | TSH > 2.5 mIU/L, electrochemiluminescent immunometric assay (Cobas 6000, Roche Diagnostics, Poland) | TPO-abs, electrochemiluminescence immunoassay, TPO-abs > 34 IU/mL | 114 (18.7), 35 (33–38) | 495 (81.3), 34 (32–37) | First cycle, long GnRH agonist protocol ET day 5; number: 2 (1–3) | NOR, FR, IR, CPR, MR, LBR | 7 |
| Sakar et al. (2015), Turkey (15) | Women with different causes of infertility, 50% male | Prospective case-control, 2013–2014 | ? ELISA method (Beckman Coulter, Ireland, Inc.) | TPO-abs and Tg-abs, Coulter diagnostic kits (Beckman Coulter Ireland, Inc.) for TPO-abs, electrochemiluminescence immunoassay method by Cobas 6000 diagnostic kits (Roche Pharma, Germany) for Tg-abs, TPO-abs > 35 IU/mL, Tg-abs > 78 ng/mL | 49 (19.5), 30.7 ± 3.8 | 202 (80.5), 29.5 ± 3.4 | First cycle, GnRH antagonist protocol ET day 3; number: 1 | NOR, FR, IR, CPR, MR, OPR | 7 |
| First Author (Year), Country (Reference) . | Study Population, % Male Infertility . | Study Type, Period of Recruitment . | Definition of SCH TSH Assay . | Thyroid Antibodies, Defining TAI Antibody Assays, Cutoff for TAI Positivity . | No. (%) TAI+ Patients, Age (y) a . | No. (%) TAI– Patients, Age (y) a . | ART Details . | Outcomes . | Quality of Evidence b . |
|---|---|---|---|---|---|---|---|---|---|
| Karacan et al. (2013), Turkey (11) | Women with different causes of infertility, 44% male | Prospective cohort, 2009–2012 | TSH > 2.5 mIU/L, enzyme-linked fluorescence assay (Vidas; BioMerieux, France) | TPO-abs and Tg-abs, electro-chemiluminescence immunoassay, TPO-abs > 35 IU/mL, Tg-abs > 115 IU/mL | 34 (13.4), 34.2 ± 4.3 | 219 (86.6), 33.1 ± 3.8 | First cycle, GnRH antagonist protocol ET day 5; number: 1.4 ± 0.8 | NOR, FR, IR, CPR, MR, OPR | 8 |
| Tan et al. (2014), Germany (12) | Women with infertile men, 100% male | Retrospective cohort, 1997–2006 | TSH > 3 mIU/L, automated chemiluminescence immunoassay (ADVIA Centaur, Siemens, Germany) | TPO-abs and Tg-abs, immunometric assay, TPO-abs > 100 IU/mL, Tg-abs > 100 IU/mL | 110 (13.2), 31.5 ± 4.0 | 725 (86.8), 31.3 ± 4.4 | First cycle, long protocol ET day ?; number: 1–3 | CPR, MR, LBR | 7 |
| Lukaszuk et al. (2015), Poland (14) | Women with different causes of infertility, 25% male | Retrospective cohort, 2010–2012 | TSH > 2.5 mIU/L, electrochemiluminescent immunometric assay (Cobas 6000, Roche Diagnostics, Poland) | TPO-abs, electrochemiluminescence immunoassay, TPO-abs > 34 IU/mL | 114 (18.7), 35 (33–38) | 495 (81.3), 34 (32–37) | First cycle, long GnRH agonist protocol ET day 5; number: 2 (1–3) | NOR, FR, IR, CPR, MR, LBR | 7 |
| Sakar et al. (2015), Turkey (15) | Women with different causes of infertility, 50% male | Prospective case-control, 2013–2014 | ? ELISA method (Beckman Coulter, Ireland, Inc.) | TPO-abs and Tg-abs, Coulter diagnostic kits (Beckman Coulter Ireland, Inc.) for TPO-abs, electrochemiluminescence immunoassay method by Cobas 6000 diagnostic kits (Roche Pharma, Germany) for Tg-abs, TPO-abs > 35 IU/mL, Tg-abs > 78 ng/mL | 49 (19.5), 30.7 ± 3.8 | 202 (80.5), 29.5 ± 3.4 | First cycle, GnRH antagonist protocol ET day 3; number: 1 | NOR, FR, IR, CPR, MR, OPR | 7 |
Abbreviations: CPR, clinical pregnancy rate; ELISA, enzyme-linked immunosorbent assay; ET, embryo transfer; NOR, number of oocytes retrieved; OPR, ongoing pregnancy rate; TAI+, positive for thyroid antibodies; TAI–, negative for thyroid antibodies.
Age expressed as mean ± standard deviation or median (interquartile range).
Based on the Newcastle-Ottawa scale (18).
We assess the differences in patient characteristics and pregnancy outcomes for patients with and without TAI.
Age, serum TSH, and number of oocytes retrieved
Age did not significantly differ between women with and without TAI: combined mean difference of 0.13 years (95% CI, −0.51 to 0.76 years) (Fig. 2A). Women with TAI had higher serum TSH levels and more oocytes retrieved: combined mean difference of 0.20 (95% CI, 0.07 to 0.33) (Fig. 2B) and 0.82 (95% CI, 0.20 to 1.44) (Fig. 2C), respectively.
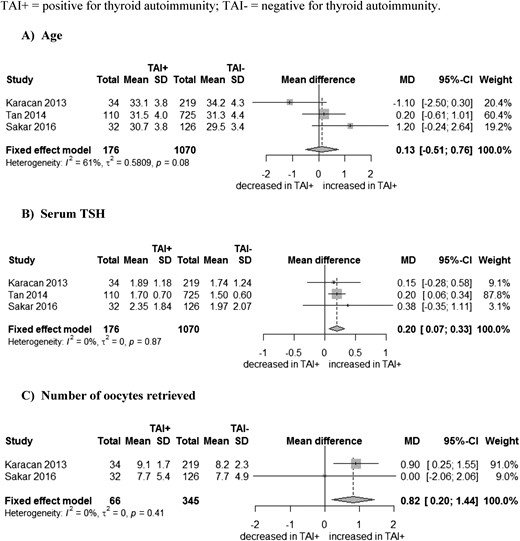
Association between (A) thyroid antibodies (TAI) and age, (B) serum TSH, and (C) number of oocytes retrieved. SD, standard deviation; TAI+, positive for thyroid antibodies; TAI-, negative for thyroid antibodies.
Fertilization, implantation, and clinical pregnancy rates
Three studies assessed the FR (11, 14, 15): 7396 oocytes in total, 1196 oocytes in women with TAI, and 6200 oocytes in women without TAI. Women with and without TAI had similar FRs: combined OR of 1.02 (95% CI, 0.89 to 1.16) (Fig. 3A). Two studies investigated the implantation rate: 1776 transferred embryos in total, 295 in women with TAI, and 1481 in women without TAI (11, 14). There was no statistical evidence for a difference in implantation rates between women with and without TAI: combined OR of 0.98 (95% CI, 0.73 to 1.32) (Fig. 3B). Clinical pregnancy was assessed in all four studies. There was no statistical evidence for an association between the presence of TAI and clinical pregnancy: combined OR of 0.91 (95% CI, 0.70 to 1.18) (Fig. 3C).
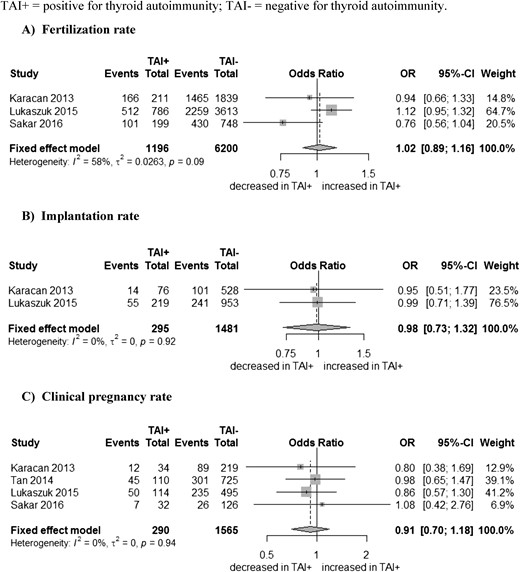
Association between (A) thyroid antibodies (TAI) and fertilization, (B) implantation, and (C) clinical pregnancy rates. SD, standard deviation; TAI+, positive for thyroid antibodies; TAI-, negative for thyroid antibodies.
MRs and LBRs in women with a clinical pregnancy
In the four studies, 765 ICSI cycles resulted in a clinical pregnancy: 114 in women with TAI and 651 in women without. All four studies reported the MRs. There was no statistical evidence for a difference in MR between women with and without TAI: respective combined OR of 0.95 (95% CI, 0.48 to 1.87) (Fig. 4A). Two studies reported LBRs: 632 ICSI cycles total, 95 in women with TAI, and 536 in women without (12, 14). No association between the presence of TAI and LBR was found: combined OR of 1.12 (95% CI, 0.62 to 2.03) (Fig. 4B). The other two studies reported the ongoing pregnancy rate (11, 15). If we use the ongoing pregnancy rates as a proxy for the LBRs and combine all four studies, the combined OR was 1.10 (95% CI, 0.63 to 1.90) (Fig. 4C).
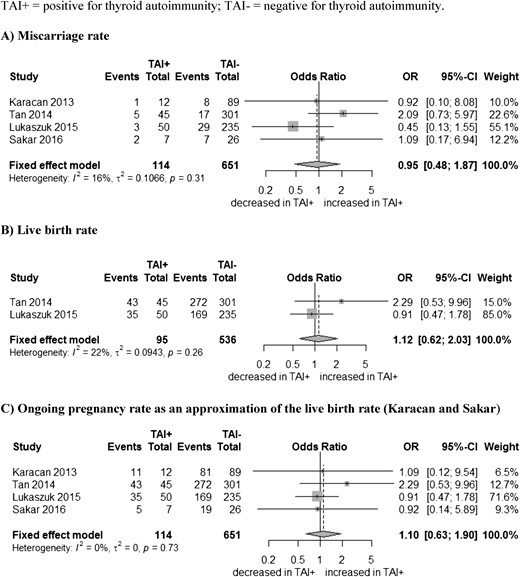
In women with a clinical pregnancy: association between (A) thyroid antibodies (TAI) and MRs, (B) live births, and (C) ongoing pregnancy rates as an approximation of the LBRs. SD, standard deviation; TAI+, positive for thyroid antibodies; TAI-, negative for thyroid antibodies.
Discussion
The most important result of our meta-analysis was the absence of an increased MR when ICSI was used in infertile women with TAI compared with that in women without TAI. Although causality cannot be proven, some arguments are in favor of a relationship between ICSI and pregnancy outcomes that goes beyond that of a simple association. ICSI can bias the impeding effect of thyroid antibodies in the follicular fluid, as mentioned by Monteleone et al. (29). In their study, they measured Tg-abs and TPO-abs in follicular fluid from women with and without TAI; in those with TAI, the TPO-abs levels in the follicular fluid correlated for 50% with those in the serum, which was not the case in women without TAI. Lower oocyte FRs, grade A embryos (≥6 cells and ≤25% fragmentation on day 3), clinical pregnancy rates, and increased early MRs were observed in women with TAI. Furthermore, all successful pregnancies in women with TAI were achieved with ICSI, whereas this was the case in only 40% of pregnant women without TAI (29).
Kelkar et al. (30) reported that human antizona pellucida antibodies recognize antigens within murine thyroid tissue, and Lee et al. (31) showed that the zona pellucida may be a target for antithyroid antibodies. The antibodies may hamper the interaction among the oocyte, the sperm cell, and the normal preimplantation embryogenesis process, and that might be overcome with ICSI (31, 32). In a case-control study, Weghofer et al. (33) reported that in women with TAI, the embryo quality was significantly lower than that in women without TAI. The definition of embryo quality differs between studies, but meanwhile, efforts toward standardization are being made (34). In summary, embryos can be classified as poor, fair, or good according to expansion, inner cell mass, and trophectoderm criteria. The presence of TAI can affect embryo quality via two pathways: one independent of thyroid function and a second via thyroid dysfunction.
TAI is known to be the strongest predictor and most important cause of SCH (1, 35, 36). Cramer et al. (37) reported that elevated serum TSH levels can predict poor fertilization, reflecting the importance of TH in oocyte physiology. Scoccia et al. (38) also showed that hypothyroid women who were optimally treated with LT4 had embryo quality similar to that of euthyroid women. A weakness of that study was the absence of TAI status. In another interventional study by Kim et al. (39) among women with SCH (TSH levels >4.0 mIU/L), LT4 treatment increased the number of top-quality embryos compared with that in the placebo group, but the study did not correlate embryo quality with TAI status as well.
Although the precise interaction among TH, TAI, and embryo quality remains poorly understood, two reviews provide some hypotheses (40, 41). TSH and triiodothyronine receptors are present in human granulosa cells, and triiodothyronine and thyroxine hormone are present in the follicular fluid; alterations in TSH levels may therefore negatively influence oocyte quality, especially in women with SCH (40). Data in animal models show that TH synergize with follicle-stimulating hormone to exert direct stimulatory effects on granulosa cell functions, including morphologic differentiation and luteinizing hormone/human chorionic gonadotropin receptor formation, and thus hypothyroidism may result in reduced oocyte quality (42).
Embryo quality also correlates negatively with the number of oocytes retrieved (33). In our meta-analysis, the number of oocytes retrieved was higher in women with TAI, but no study reported on embryo quality (11, 15). Other studies found no impact of TAI (43), thyroid function (38, 39), and LT4 treatment (44) on the number of oocytes retrieved.
Three studies included in our analysis showed no effect of TAI on the FR (11, 14, 15). Some studies observed decreased FR in women with TAI (29, 43). In one observational study, TSH levels were inversely proportional to the FR (37), but that was not the case in another study (45). Scoccia et al. (38) found that the FRs among euthyroid women were better than those in hypothyroid women treated with LT4, and in the meta-analysis by Velkeniers et al. (44) LT4 treatment improved FR in women with SCH. Especially when serum TSH levels are >4.0 mIU/L, oocyte quality and FRs were impaired for reasons that have been explained earlier in the discussion (40, 42).
The ICSI method has been used in ART for >20 years, and high FRs have been reported since its implementation (46). Embryo quality is one of the most important factors for successful implantations and ongoing pregnancies (47, 48). The effect of TAI on the implantation phase remains controversial because only a few studies considered this outcome (49). Two studies included in our meta-analysis showed no impact of TAI on the implantation rate (11, 14). In women with idiopathic infertility, it has been speculated that the presence of TAI was associated with implantation failure through a direct action of antibodies on the endometrium, or as a consequence of an underlying autoimmune imbalance (4). However, in the study by Kilic et al. (27) in a cohort of women with idiopathic infertility with and without TAI, there was no difference in the endometrial volume (an approximation of the implantation chance) (27). Zhong et al. (43) reported lower implantation rates in women with TAI, but the authors did not report on thyroid function. In one study in women with SCH (serum TSH >4.0 mIU/L), implantation rates were improved in the LT4-treated group (39); however, this was not the case in another study, although LT4 might have been underdosed (38).
Strengths of our meta-analysis are the systematic and rigorous selection in identifying women treated with ICSI only, and three of the four studies measured both types of thyroid antibodies (11, 12, 15). In most studies, the diagnosis of TAI was based on the presence of TPO-abs only (1, 4, 8). In two prospective studies determining the presence of TAI in pregnant and in infertile women, only positivity for Tg-abs was more frequent than for TPO-abs (50, 51). In the study by Unuane et al. (50), women with only Tg-abs (5%) also had higher mean serum TSH levels than women with TPO-abs, and in a recent study in China, women with Tg-abs had increased MRs compared with women with TPO-abs (52). Not testing for Tg-abs may lead to misclassification, modify study conclusions, and impair the management of pregnant women. The importance of Tg-abs is now also noticed in the recent guidelines on the management of thyroid disorders during pregnancy (53).
Our study has also several limitations. The numbers of studies and patients included were rather low because of the strict inclusion criteria. Serum TSH levels were always measured before the ICSI cycles, and no information was therefore available on changes in TSH after OS (potentially permanent when TAI was present) (6, 7).
Another limitation was the absence of individual patient data; as a result we could not take into account confounders with a potential impact on MR/LBR. One confounder could have been the type of OS because GnRH antagonists lead to a significant increase in TSH levels, which was not the case for agonists (54). Furthermore, the cumulative LBR per cycle was significantly higher in GnRH antagonist protocols than in those using agonists (55). In our meta-analysis, and despite the use of GnRH agonists and antagonists, pregnancy outcomes were similar between women with and without TAI. Another confounder could have been the presence of other autoimmune antibodies known to be more prevalent in women with TAI and to be associated with an increased MR (1). Among the studies in our meta-analysis, one excluded the presence of anticardiolipin antibodies and lupus anticoagulant, and another excluded lupus anticoagulant and rheumatoid factor; despite that, no impact of TAI on the outcomes was observed (11, 12, 14, 15).
According to Tan et al. (12), the increased MR in women with TAI as described in previous meta-analyses might have been attributed to the inclusion of women with different causes of infertility (PCOS, endometriosis, or idiopathic infertility), and therefore, they selected only women of couples with male infertility, treated with ICSI (12). The opposite of their statement might be true because ICSI treatments are known to be particularly successful in case of male infertility (56, 57). Furthermore, in the other studies in our meta-analysis, women had different causes of infertility; despite that, no impact of TAI on the outcomes was observed (11, 14, 15). Another argument provided by Tan et al. (12) for their negative study results were the similar ages between women with and without TAI—older age is a known risk factor for MR (9, 10). In the other studies in our meta-analysis, ages were similar between women with and without TAI, which a priori might have strengthened their hypothesis. However, in the meta-regression analysis by Busnelli et al. (10), the increased MR persisted after correction for age.
Another explanation for the increased MR in previous meta-analyses was the higher mean serum TSH in women with TAI (8, 10). In the studies included in this meta-analysis, a serum TSH cutoff of 2.5 and 3.0 mIU/L was used to define SCH, based on guidelines from 2012 (58). In our study, mean serum TSH levels were also higher in women with TAI, but always within the normal limits (<4.0 mIU/L). That two of the included studies (11, 14) used a TSH cutoff of 2.5 mIU/L as the upper limit and that Tan et al. (12) used a cutoff of 3.0 mIU/L might have dampened the detrimental impact of TAI as compared with a cutoff ≥4.0 mIU/L in previous studies (8, 9). However, no effect of basal TSH levels was present on the estimated association TAI - MR in the meta-analysis by Busnelli et al. (10).
In the setting of infertility, the knowledge of the TAI status is important to better predict the potential development of thyroid dysfunction induced by OS (6, 7) and to allow adapting the dosage of LT4 in hypothyroid women before OS. Indeed, a study by Busnelli et al. (7) showed that 80% of hypothyroid women pregnant after OS and with TAI had to increase their dosage of LT4 within the first 7 weeks of pregnancy, compared with 40% without TAI. Women with TAI are also more prone to develop SCH during pregnancy, even though they can be euthyroid during the first trimester of pregnancy (59).
Finally, and on the basis of the results of our meta-analysis, it would be indispensable to be aware of the women’s TAI status in order to discuss the potential advantages of ICSI over IVF. According to a recent statement of the American Society for Reproductive Medicine, there is good evidence that TAI and/or SCH (defined as a serum TSH level ≥4.0 mIU/L) is associated with miscarriage, and there is fair evidence for the association with infertility (60). LT4 treatment may improve pregnancy outcomes in women undergoing ART, especially if the serum TSH level is ≥2.5 mIU/L with TAI or >4.0 mIU/L in general (44, 60).
Also worth mentioning is that in most studies showing a beneficial effect of LT4 on the outcomes, that treatment was started before the ART procedure (44). The statement from the American Society for Reproductive Medicine also mentioned that the available data support the routine measurement of TSH in infertile women attempting pregnancy, but not that of TPO-abs, unless TSH levels are ≥2.5 mIU/L (60). The recent American Thyroid Association guidelines on thyroid disorders and pregnancy stated that there is insufficient evidence to recommend for or against universal screening for abnormal TSH concentrations before conception, except in women planning ART or those known to be positive for TPO-abs (53). However, no position was taken on the testing of antibodies as such. Clinicians should know that TAI is present in ∼25% of infertile women with PCOS and idiopathic infertility compared with only ∼10% of women in the general population (2, 3). Furthermore, it is noteworthy that in one study, the universal screening (TSH and TPO-abs) of pregnant women during the first trimester was cost-effective (61). Figure 5 provides a proposed flowchart for the management of thyroid disorders in women of infertile couples, when they are at the stage of infertility workup (49, 53). However, it remains to be proven whether the use of this algorithm could improve pregnancy outcomes or is cost-effective.
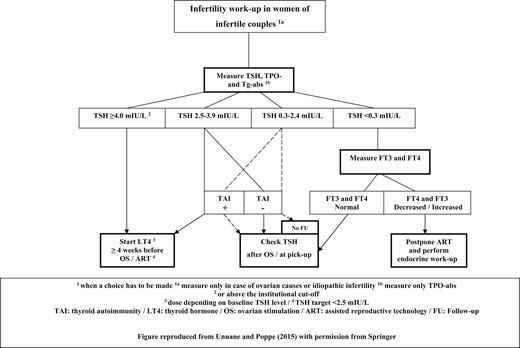
Algorithm to screen/manage thyroid disorders in women of infertile couples. Reproduced with permission from Unuane and Poppe (49) with permission from Springer.
The number of ICSI treatments increased from 2008 to 2012, especially in women with idiopathic infertility (62), but we don’t suggest that ICSI be used in all infertile women because it is not more effective in nonmale causes of infertility than IVF (63). However, because ICSI may overcome the detrimental impact of TAI on embryo quality, the presence of TAI may become a new indication independent of the cause of infertility (29).
We now need well-designed studies of women with TAI who are treated with IVF vs ICSI in a head-to-head design. The outcome data should then also be corrected for confounders, such as age, the type of OS, the types of thyroid antibodies, and thyroid function. Finally, a better understanding of the ovarian follicle unit is needed, through research in line with two recent studies (64, 65).
In conclusion, women with TAI who become pregnant after an ICSI treatment had a risk for a first-trimester miscarriage that was similar to that in women without TAI. Previous meta-analyses did not adjust for the type of ART treatment, which might have explained the high miscarriage risk in women with TAI. Our observations may therefore suggest proposing ICSI as the preferred ART treatment in women with TAI who are part of an infertile couple. Screening for the presence of TAI and thyroid dysfunction should be a part of the infertility workup in order to select female candidates for an ICSI treatment, to treat thyroid dysfunction before pregnancy, to preserve optimal in vitro oocyte development and embryo quality, and to prevent thyroid dysfunction-related pregnancy complications.
Abbreviations:
- ART
assisted reproductive technology
- CI
confidence interval
- FR
fertilization rate
- GnRH
gonadotropin-releasing hormone
- ICSI
intracytoplasmic sperm injection
- IVF
in vitro fertilization
- LBR
live birth rate
- LT4
levothyroxine
- MR
miscarriage rate
- OS
ovarian stimulation procedure
- PCOS
polycystic ovarian syndrome
- SCH
subclinical hypothyroidism
- TAI
thyroid autoimmunity
- Tg-abs
thyroglobulin antibodies
- TH
thyroid hormones
- TPO-abs
thyroid peroxidase antibodies
- TSH
thyroid-stimulating hormone
Acknowledgments
Financial Support: No funding was received to support this work.
Disclosure Summary: K.P. reports speaker fees the IBSA Institut Biochimique SA (satellite meeting of the European Thyroid Association) in 2016 and the Berlin-Chemie AG company (ETA educational thyroid meeting) in 2017. The remaining authors have nothing to disclose.



