-
PDF
- Split View
-
Views
-
Cite
Cite
Stine Linding Andersen, Stig Andersen, Zeyan Liew, Peter Vestergaard, Jørn Olsen, Maternal Thyroid Function in Early Pregnancy and Neuropsychological Performance of the Child at 5 Years of Age, The Journal of Clinical Endocrinology & Metabolism, Volume 103, Issue 2, February 2018, Pages 660–670, https://doi.org/10.1210/jc.2017-02171
Close - Share Icon Share
Abstract
Abnormal maternal thyroid function in pregnancy may impair fetal brain development, but more evidence is needed to refine and corroborate the hypothesis.
To estimate the association between maternal thyroid function in early pregnancy and neuropsychological performance of the child at 5 years of age.
Follow-up study.
A cohort of 1153 women and their children sampled from the Danish National Birth Cohort. Maternal thyroid-stimulating hormone (TSH) and free thyroxine (fT4) were measured in stored biobank sera from early pregnancy.
Child neuropsychological test results (Wechsler Intelligence Scale/Test of Everyday Attention), test of motor function (Movement Assessment Battery), and results of parent and teacher reports (Behavior Rating Inventory of Executive Function/Strengths and Difficulties Questionnaire).
Altogether 145 children (12.6%) were born to mothers with abnormal thyroid function in the early pregnancy. High maternal TSH and low fT4 were associated with lower child verbal intelligence quotient (adjusted mean difference TSH ≥ 10 mIU/L vs 0.1 to 2.49 mIU/L, −8.9 [95% confidence interval (CI), −15 to −2.4]; fT4 < 10 pmol/l vs 12.0 to 18.99 pmol/l, −13 [95% CI, −19 to −7.3]). Abnormal maternal thyroid function was also associated with adverse motor function and teacher-reported problems of executive function and behavior, and these associations were dominated by exposure to maternal hypothyroxinemia.
Maternal thyroid hormone abnormalities were associated with adverse neuropsychological function of the child at 5 years of age. For intelligence, marked hypothyroidism was important, whereas for motor function and executive and behavior problems, maternal hypothyroxinemia was predominant.
Thyroid hormones are important regulators of early brain development (1) and lack or excess of maternal thyroid hormone in early pregnancy may interfere and impair fetal brain development (2). Outcome of pregnancies prenatally exposed to maternal thyroid dysfunction is therefore a matter of concern. The potential benefits and risks associated with routine testing for undetected thyroid disease in pregnant women are debated (3), and the degree of thyroid abnormality relevant to screening and intervention remains unsolved (4). This study was conducted within the Danish National Birth Cohort (DNBC) to evaluate the association between maternal thyroid function as measured in stored biobank sera from early pregnancy and neuropsychological performance of children who at age 5 years participated in an extensive neuropsychological testing program. The program included testing of intelligence, attention, and motor function, as well as parent- and teacher-reported evaluation of executive function and behavior.
Methods and Materials
Study design and population
The DNBC enrolled about 100,000 pregnancies in 1997 to 2003 from women who lived in Denmark and were able to participate in telephone interviews in Danish during the pregnancy (5). For the investigation of maternal thyroid function in pregnancy within the DNBC, the woman’s first pregnancy in the study period was included if the woman completed the first of a series of telephone interviews in the pregnancy and gave birth to a singleton live-born child (Fig. 1).
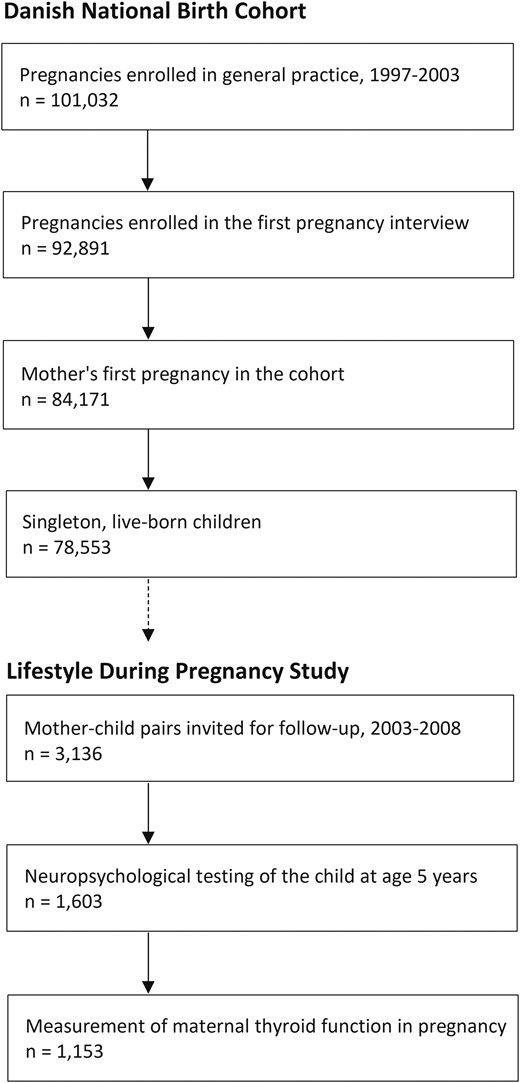
Flowchart illustrating the eligible cohort of children and the number of children invited for follow-up and participating in neuropsychological assessment, as well as the number of children with available measurement of maternal thyroid function in early pregnancy.
Participants for the current study were part of the Lifestyle During Pregnancy Study (LDPS), which is a subcohort within the DNBC (6). The LDPS sampled participants from maternal reports of alcohol intake and binge drinking in pregnancy, as described in detail elsewhere (6). In brief, the sampling procedure was based on the timing of exposure in pregnancy combined with information on the amount of alcohol intake (zero, one to four, five to eight, and nine or more drinks per week) and the number of binge episodes. Exclusion criteria were multiple pregnancies, inability to speak Danish well enough for participation, children with impaired hearing or vision likely to compromise the ability to complete the neuropsychological tests, and children with congenital disorders associated with mental retardation (e.g., Down syndrome and cerebral palsy) (6).
Among the singleton, live-born children eligible for the investigation of maternal thyroid function in pregnancy, a total of 3136 mother-child pairs were invited to participate in the LDPS when the child reached 5 years of age, and 1603 (51.1%) participated in the neuropsychological assessment (Fig. 1). At the time of enrollment in DNBC, a blood sample was drawn from the pregnant woman at the first antenatal visit in general practice (median, week 9 of pregnancy; range, week 5 to week 25; interquartile range, week 8 to week 11) (5). The blood sample was sent by mail and arrived in the Danish National Biobank within a median of 1 day from sampling, where it was stored in a −80°C freezer. Stored biobank sera for measurement of maternal thyroid function were available for 1153 (71.9%) of the 1603 mother-child pairs who participated in the follow-up assessment (Fig. 1).
All participating mothers had provided written informed consent at initial recruitment and during follow-up. The Danish Ethic Committee approved the study (N-20130054).
Exposure assessment
Maternal thyroid function was evaluated from measurement of thyroid-stimulating hormone (TSH) and free thyroxine (fT4) by a Dimension Vista automated immunoassay (Siemens Healthcare Diagnostics, Eschborn, Germany), as previously described (7, 8). The biochemical analyses were performed from January to August 2015. Maternal thyroid function was classified according to the method- and pregnancy week–specific reference ranges (2.5th and 97.5th percentiles) previously established and described for this cohort of women (7). In summary, week-specific reference ranges were used for pregnancy weeks 5 to 14, and a combined reference range for weeks 15 to 19 was applied to blood samples from week 15 and onward. Lower reference limit for TSH ranged from the highest value of 0.69 mIU/L (week 5) to the lowest value of 0.061 mIU/L (week 10), whereas the upper reference limit ranged from 3.09 (week 11) to 3.85 mIU/L (week 14). For fT4, less variation was observed and the lower reference limit ranged from 11.23 to 12.07 pmol/L, whereas the upper reference limit ranged from 17.60 to 19.16 pmol/L (7). The pregnant women were classified as having abnormal thyroid function if they had TSH and/or fT4 values below or above the week-specific reference limits, including overt hyperthyroidism or hypothyroidism defined by abnormal TSH and fT4, subclinical hyperthyroidism or hypothyroidism defined by abnormal TSH and normal fT4, or hyperthyroxinemia or hypothyroxinemia defined by abnormal fT4 with normal TSH (8).
Outcome assessment
Neuropsychological testing took place when the child was 60 to 64 months old in one of the four largest cities in Denmark from 2003 to 2008 (6). The testing was a 3-hour assessment and was performed by 10 trained psychologists who followed standardized test procedures; on a regular basis, these psychologists blindly scored several tests administered by other psychologists to calibrate agreement (6).
Intelligence was assessed by using a shorter version of the Wechsler Primary and Preschool Scale of Intelligence-Revised (WPPSI-R), including three verbal (arithmetic, information, and vocabulary) and three performance (block design, geometric design, and object assembly) subtests from which full, performance, and verbal intelligence quotients (IQs) were derived. Danish WPPSI-R norms were not available, and Swedish norms were used. Thus, the theoretical distribution of IQ with a mean of 100 and a standard deviation (SD) of 100 cannot be expected in this sample (9).
Attention was assessed by using the Test of Everyday Attention for Children at Five (TEACh-5), which has been described in detail elsewhere (10). TEACh-5 included four subtests: assessment of selective (the great balloon hunt and hide and seek II) and sustained (barking and draw a line) attention via visual and auditory stimuli. Each subscore was standardized to a mean of 0 and an SD of 1, and the mean of these was restandardized to a selective attention score and sustained attention score. Furthermore, an overall attention score was calculated as the mean of all four standardized subscores and restandardized.
Motor function was assessed from 2003 to 2006 on the same day as the neuropsychological testing and was performed by 30 physiotherapists who regularly compared and discussed their test procedures (6). Motor function was assessed by using the Movement Assessment Battery for Children (MABC), which included eight subtests covering fine and gross motor function and static and dynamic balance (11). A total score of 0 to 40 was calculated as the sum of all subtests, with a lower score indicating better performance. The total score was interpreted by using percentile norm tables, and children at or below the fifth percentile (total scores ranging from 17 to 40) were classified as having poor motor function (11).
Executive function and behavior of the child were assessed at home and in the daycare environment from questionnaires rated by the mother and a teacher. Executive function was assessed by using the Behavior Rating Inventory of Executive Function (BRIEF), which included 86 statements measuring eight aspects of executive function (12). Danish BRIEF norms were not available, and a normalizing T-score transformation for the observed scores was computed, with higher scores indicating more difficulties (13). The transformed scores were combined in the behavioral regulation index, including the scales for inhibit, shift, and emotional control, and a metacognition index, including the scales for initiate, working memory, plan/organize, organization of materials, and monitor. Furthermore, an overall global executive composite score was calculated as the mean of all eight scales. Behavior was assessed by using the Strengths and Difficulties Questionnaire (SDQ), which included 25 questions on five aspects of behavior (14). A total difficulties score was calculated as the mean of the four scales of emotional symptoms, peer problems, hyperactivity/inattention, and conduct problems, with higher scores indicating more difficulties. Furthermore, a score for internalizing problems (emotional and peer problems) and for externalizing problems (hyperactivity/inattention and conduct problems) were derived (15). The prosocial scale was not included in the current study because our focus was on problem behavior.
Covariates
Information on potential maternal and child covariates was obtained from the DNBC interviews during the pregnancy (maternal prepregnancy height and weight, smoking and alcohol intake in the pregnancy, and psychiatric disease), from the LDPS follow-up interview (parental education and marital status), and from the Danish Medical Birth Register (16) (child’s sex, 5-minutes Apgar score, gestational age at birth, and birth weight, as well as maternal age an parity). Information was also available on the age of the child at testing (1-month intervals from the age of 5 years) and on the testing psychologist.
Maternal intelligence was assessed at follow-up from two verbal subtests of the Wechsler Adult Intelligence Scale and from a nonverbal test of the Ravens’ Standard Progressive Matrices (13). Results from each subtest were weighted and equally standardized to a combined IQ (mean, 100; SD, 15).
Statistical analyses
The association between maternal thyroid function and child neuropsychological test results was evaluated by using linear and logistic regression. The main analyses were conducted with a combined categorical exposure variable of abnormal maternal thyroid function, defined as TSH and/or fT4 outside the pregnancy week–specific reference ranges (including overt and subclinical hyperthyroidism and hypothyroidism as well as hyperthyroxinemia and hypothyroxinemia). In subsequent analyses, we evaluated the association with different levels of TSH and fT4, separately, by using predefined cutoffs. Nonlinear associations were assessed by using restricted cubic splines with three knots (10th, 50th, and 90th percentiles) adjusting for pregnancy week of blood sampling.
Outcomes of WPPSI-R, TEACh-5, and BRIEF showed symmetric distribution, and results of linear regression were reported as mean difference with 95% confidence intervals (CIs). Outcomes of MABC and SDQ showed asymmetric distribution, and results were log-transformed and reported as exponentiated β values with 95% CIs. For outcomes of logistic regression, abnormal WPPSI-R and TEACh-5 results were defined as a score below the mean minus 1 SD, abnormal BRIEF as a score above the sample mean plus 1 SD, abnormal MABC as at or below the fifth percentile (11), and abnormal SDQ as a score above the sex-specific cutoffs previously established in Danish 5- to 7-year-old children (17).
The LDPS study oversampled women with a high alcohol intake during pregnancy, and analyses were weighted by sampling fraction with robust variance estimation to account for the sampling design (13). For outcomes of neuropsychological testing, child’s age at testing and testing psychologist were included in the model. Other potential confounders considered a priori were maternal IQ, age, parity, marital status, smoking and alcohol intake in pregnancy, prepregnancy body mass index (BMI), psychiatric disease, and parental education. We used the change-in-estimate method (10% cutoff) via backward elimination and identified maternal IQ and prepregnancy BMI for inclusion in the model. We observed no multicollinearity between maternal IQ and prepregnancy BMI. Results of this model strategy as compared with the fully adjusted model led to similar conclusions. We considered possible effect measure modification by child’s sex in stratified analyses and by measures of multiplicative (cross-product term) and additive (relative excess risk due to interaction) interaction. Missing values on maternal IQ (0.5%) and maternal prepregnancy BMI (2.4%) were imputed by using multiple imputation with chained equations and results of multiple imputation were reported. Complete case analysis was also performed and revealed similar results.
Statistical analyses were performed by using Stata software, version 13 (Stata Corp., College Station, TX).
Results
Altogether, 1153 children and their mothers had available data on child neuropsychological testing and data on maternal thyroid function and were included in the study. As expected, the children sampled for the follow-up study differed from the eligible cohort of live-born children in regard to maternal alcohol intake in pregnancy (Table 1). Besides that, few differences were found between children invited for follow-up, those who participated in follow-up, and children with measurement of maternal thyroid function (Table 1).
Characteristic of Eligible Cohort of Singleton Live-Born Children, Children Invited for Follow-Up, Children Who Participated in Follow-Up, and Children Who Participated in Follow-Up and Had Maternal Thyroid Function Tested in a Blood Sample From the Early Pregnancy
| Characteristic . | Eligible Cohort . | Invited for Follow-Up . | Participated in Follow-Up . | Maternal Thyroid Function . |
|---|---|---|---|---|
| Children (n) | 78,553 | 3136 | 1603 | 1153 |
| Birth year of the child | ||||
| 1997–2000 | 46,980 (59.8) | 1583 (50.5) | 834 (52.0) | 572 (49.6) |
| 2001–2003 | 31,573 (40.2) | 1553 (49.5) | 769 (48.0) | 581 (50.4) |
| Child’s sex | ||||
| Female | 38,338 (48.8) | 1534 (48.9) | 777 (48.5) | 549 (47.6) |
| Male | 40,215 (51.2) | 1602 (51.1) | 826 (51.5) | 604 (52.4) |
| Gestational age at birtha | ||||
| <37 wk | 4,147 (5.3) | 136 (4.3) | 49 (3.1) | 29 (2.5) |
| ≥37 wk | 74,228 (94.7) | 2996 (95.7) | 1551 (96.9) | 1124 (97.5) |
| Birth weighta | ||||
| <2500 g | 2,438 (3.1) | 74 (2.4) | 24 (1.5) | 13 (1.1) |
| ≥2500 g | 76,003 (96.9) | 3059 (97.6) | 1576 (98.5) | 1138 (98.9) |
| 5-min Apgar scorea | ||||
| 7–10 | 77,362 (99.3) | 3107 (99.5) | 1582 (99.4) | 1137 (99.5) |
| 0–6 | 564 (0.7) | 14 (0.5) | 9 (0.6) | 6 (0.5) |
| Maternal paritya | ||||
| Nulliparous | 38,097 (49.9) | 1636 (53.7) | 846 (54.6) | 606 (53.8) |
| Multiparous | 38,264 (50.1) | 1412 (46.3) | 705 (45.4) | 520 (46.2) |
| Maternal age | ||||
| <30 y | 38,521 (49.0) | 1519 (48.4) | 731 (45.6) | 507 (44.0) |
| ≥30 y | 40,032 (51.0) | 1617 (51.6) | 872 (54.4) | 646 (56.0) |
| Maternal prepregnancy BMIa,b | ||||
| <25 kg/m2 | 56,001 (72.5) | 2214 (71.8) | 1173 (74.8) | 837 (74.4) |
| ≥25 kg/m2 | 21,207 (27.5) | 869 (28.2) | 396 (25.2) | 288 (25.6) |
| Maternal smoking in pregnancya,b | ||||
| Yes | 21,112 (26.9) | 1086 (34.7) | 523 (32.6) | 375 (32.5) |
| No | 57,413 (73.1) | 2048 (65.3) | 1080 (67.4) | 778 (67.5) |
| Maternal alcohol intake in pregnancya,b | ||||
| 0 units/wk | 43,687 (55.6) | 1559 (49.7) | 763 (47.6) | 539 (46.8) |
| 1–4 units/wk | 34,020 (43.3) | 1249 (39.8) | 663 (41.4) | 486 (42.1) |
| ≥5 units/wk | 844 (1.1) | 328 (10.5) | 177 (11.0) | 128 (11.1) |
| Characteristic . | Eligible Cohort . | Invited for Follow-Up . | Participated in Follow-Up . | Maternal Thyroid Function . |
|---|---|---|---|---|
| Children (n) | 78,553 | 3136 | 1603 | 1153 |
| Birth year of the child | ||||
| 1997–2000 | 46,980 (59.8) | 1583 (50.5) | 834 (52.0) | 572 (49.6) |
| 2001–2003 | 31,573 (40.2) | 1553 (49.5) | 769 (48.0) | 581 (50.4) |
| Child’s sex | ||||
| Female | 38,338 (48.8) | 1534 (48.9) | 777 (48.5) | 549 (47.6) |
| Male | 40,215 (51.2) | 1602 (51.1) | 826 (51.5) | 604 (52.4) |
| Gestational age at birtha | ||||
| <37 wk | 4,147 (5.3) | 136 (4.3) | 49 (3.1) | 29 (2.5) |
| ≥37 wk | 74,228 (94.7) | 2996 (95.7) | 1551 (96.9) | 1124 (97.5) |
| Birth weighta | ||||
| <2500 g | 2,438 (3.1) | 74 (2.4) | 24 (1.5) | 13 (1.1) |
| ≥2500 g | 76,003 (96.9) | 3059 (97.6) | 1576 (98.5) | 1138 (98.9) |
| 5-min Apgar scorea | ||||
| 7–10 | 77,362 (99.3) | 3107 (99.5) | 1582 (99.4) | 1137 (99.5) |
| 0–6 | 564 (0.7) | 14 (0.5) | 9 (0.6) | 6 (0.5) |
| Maternal paritya | ||||
| Nulliparous | 38,097 (49.9) | 1636 (53.7) | 846 (54.6) | 606 (53.8) |
| Multiparous | 38,264 (50.1) | 1412 (46.3) | 705 (45.4) | 520 (46.2) |
| Maternal age | ||||
| <30 y | 38,521 (49.0) | 1519 (48.4) | 731 (45.6) | 507 (44.0) |
| ≥30 y | 40,032 (51.0) | 1617 (51.6) | 872 (54.4) | 646 (56.0) |
| Maternal prepregnancy BMIa,b | ||||
| <25 kg/m2 | 56,001 (72.5) | 2214 (71.8) | 1173 (74.8) | 837 (74.4) |
| ≥25 kg/m2 | 21,207 (27.5) | 869 (28.2) | 396 (25.2) | 288 (25.6) |
| Maternal smoking in pregnancya,b | ||||
| Yes | 21,112 (26.9) | 1086 (34.7) | 523 (32.6) | 375 (32.5) |
| No | 57,413 (73.1) | 2048 (65.3) | 1080 (67.4) | 778 (67.5) |
| Maternal alcohol intake in pregnancya,b | ||||
| 0 units/wk | 43,687 (55.6) | 1559 (49.7) | 763 (47.6) | 539 (46.8) |
| 1–4 units/wk | 34,020 (43.3) | 1249 (39.8) | 663 (41.4) | 486 (42.1) |
| ≥5 units/wk | 844 (1.1) | 328 (10.5) | 177 (11.0) | 128 (11.1) |
Numbers are n (%).
Individuals with missing values not included in the table.
Self-reported in the pregnancy.
Characteristic of Eligible Cohort of Singleton Live-Born Children, Children Invited for Follow-Up, Children Who Participated in Follow-Up, and Children Who Participated in Follow-Up and Had Maternal Thyroid Function Tested in a Blood Sample From the Early Pregnancy
| Characteristic . | Eligible Cohort . | Invited for Follow-Up . | Participated in Follow-Up . | Maternal Thyroid Function . |
|---|---|---|---|---|
| Children (n) | 78,553 | 3136 | 1603 | 1153 |
| Birth year of the child | ||||
| 1997–2000 | 46,980 (59.8) | 1583 (50.5) | 834 (52.0) | 572 (49.6) |
| 2001–2003 | 31,573 (40.2) | 1553 (49.5) | 769 (48.0) | 581 (50.4) |
| Child’s sex | ||||
| Female | 38,338 (48.8) | 1534 (48.9) | 777 (48.5) | 549 (47.6) |
| Male | 40,215 (51.2) | 1602 (51.1) | 826 (51.5) | 604 (52.4) |
| Gestational age at birtha | ||||
| <37 wk | 4,147 (5.3) | 136 (4.3) | 49 (3.1) | 29 (2.5) |
| ≥37 wk | 74,228 (94.7) | 2996 (95.7) | 1551 (96.9) | 1124 (97.5) |
| Birth weighta | ||||
| <2500 g | 2,438 (3.1) | 74 (2.4) | 24 (1.5) | 13 (1.1) |
| ≥2500 g | 76,003 (96.9) | 3059 (97.6) | 1576 (98.5) | 1138 (98.9) |
| 5-min Apgar scorea | ||||
| 7–10 | 77,362 (99.3) | 3107 (99.5) | 1582 (99.4) | 1137 (99.5) |
| 0–6 | 564 (0.7) | 14 (0.5) | 9 (0.6) | 6 (0.5) |
| Maternal paritya | ||||
| Nulliparous | 38,097 (49.9) | 1636 (53.7) | 846 (54.6) | 606 (53.8) |
| Multiparous | 38,264 (50.1) | 1412 (46.3) | 705 (45.4) | 520 (46.2) |
| Maternal age | ||||
| <30 y | 38,521 (49.0) | 1519 (48.4) | 731 (45.6) | 507 (44.0) |
| ≥30 y | 40,032 (51.0) | 1617 (51.6) | 872 (54.4) | 646 (56.0) |
| Maternal prepregnancy BMIa,b | ||||
| <25 kg/m2 | 56,001 (72.5) | 2214 (71.8) | 1173 (74.8) | 837 (74.4) |
| ≥25 kg/m2 | 21,207 (27.5) | 869 (28.2) | 396 (25.2) | 288 (25.6) |
| Maternal smoking in pregnancya,b | ||||
| Yes | 21,112 (26.9) | 1086 (34.7) | 523 (32.6) | 375 (32.5) |
| No | 57,413 (73.1) | 2048 (65.3) | 1080 (67.4) | 778 (67.5) |
| Maternal alcohol intake in pregnancya,b | ||||
| 0 units/wk | 43,687 (55.6) | 1559 (49.7) | 763 (47.6) | 539 (46.8) |
| 1–4 units/wk | 34,020 (43.3) | 1249 (39.8) | 663 (41.4) | 486 (42.1) |
| ≥5 units/wk | 844 (1.1) | 328 (10.5) | 177 (11.0) | 128 (11.1) |
| Characteristic . | Eligible Cohort . | Invited for Follow-Up . | Participated in Follow-Up . | Maternal Thyroid Function . |
|---|---|---|---|---|
| Children (n) | 78,553 | 3136 | 1603 | 1153 |
| Birth year of the child | ||||
| 1997–2000 | 46,980 (59.8) | 1583 (50.5) | 834 (52.0) | 572 (49.6) |
| 2001–2003 | 31,573 (40.2) | 1553 (49.5) | 769 (48.0) | 581 (50.4) |
| Child’s sex | ||||
| Female | 38,338 (48.8) | 1534 (48.9) | 777 (48.5) | 549 (47.6) |
| Male | 40,215 (51.2) | 1602 (51.1) | 826 (51.5) | 604 (52.4) |
| Gestational age at birtha | ||||
| <37 wk | 4,147 (5.3) | 136 (4.3) | 49 (3.1) | 29 (2.5) |
| ≥37 wk | 74,228 (94.7) | 2996 (95.7) | 1551 (96.9) | 1124 (97.5) |
| Birth weighta | ||||
| <2500 g | 2,438 (3.1) | 74 (2.4) | 24 (1.5) | 13 (1.1) |
| ≥2500 g | 76,003 (96.9) | 3059 (97.6) | 1576 (98.5) | 1138 (98.9) |
| 5-min Apgar scorea | ||||
| 7–10 | 77,362 (99.3) | 3107 (99.5) | 1582 (99.4) | 1137 (99.5) |
| 0–6 | 564 (0.7) | 14 (0.5) | 9 (0.6) | 6 (0.5) |
| Maternal paritya | ||||
| Nulliparous | 38,097 (49.9) | 1636 (53.7) | 846 (54.6) | 606 (53.8) |
| Multiparous | 38,264 (50.1) | 1412 (46.3) | 705 (45.4) | 520 (46.2) |
| Maternal age | ||||
| <30 y | 38,521 (49.0) | 1519 (48.4) | 731 (45.6) | 507 (44.0) |
| ≥30 y | 40,032 (51.0) | 1617 (51.6) | 872 (54.4) | 646 (56.0) |
| Maternal prepregnancy BMIa,b | ||||
| <25 kg/m2 | 56,001 (72.5) | 2214 (71.8) | 1173 (74.8) | 837 (74.4) |
| ≥25 kg/m2 | 21,207 (27.5) | 869 (28.2) | 396 (25.2) | 288 (25.6) |
| Maternal smoking in pregnancya,b | ||||
| Yes | 21,112 (26.9) | 1086 (34.7) | 523 (32.6) | 375 (32.5) |
| No | 57,413 (73.1) | 2048 (65.3) | 1080 (67.4) | 778 (67.5) |
| Maternal alcohol intake in pregnancya,b | ||||
| 0 units/wk | 43,687 (55.6) | 1559 (49.7) | 763 (47.6) | 539 (46.8) |
| 1–4 units/wk | 34,020 (43.3) | 1249 (39.8) | 663 (41.4) | 486 (42.1) |
| ≥5 units/wk | 844 (1.1) | 328 (10.5) | 177 (11.0) | 128 (11.1) |
Numbers are n (%).
Individuals with missing values not included in the table.
Self-reported in the pregnancy.
Altogether, 145 women (12.6%) had abnormal thyroid function in the early pregnancy blood sample (Table 2). A characteristic of children born to mothers with abnormal thyroid function was a lower maternal alcohol intake during the pregnancy and a lower educational level of the parents (Table 2). The subtypes of maternal thyroid dysfunction included overt hyperthyroidism (n = 8), subclinical hyperthyroidism (n = 16), overt hypothyroidism (n = 16), subclinical hypothyroidism (n = 35), hyperthyroxinemia (n = 11), and hypothyroxinemia (n = 59). Treatment with thyroid hormone in the early pregnancy was reported by four women, and thyroid function testing revealed normal results (n = 2), subclinical hyperthyroidism (n = 1), and isolated high fT4 (n = 1). None of the women reported use of antithyroid drugs.
Characteristics of Children Included in Study, Stratified by Maternal Thyroid Function in Early Pregnancy
| Characteristic . | No Maternal Thyroid Dysfunctiona . | Maternal Thyroid Dysfunctionb . |
|---|---|---|
| Children (n) | 1,008 | 145 |
| Birth year of the child | ||
| 1997–2000 | 503 (49.9) | 69 (47.6) |
| 2001–2003 | 505 (50.1) | 76 (52.4) |
| Child’s sex | ||
| Female | 475 (47.1) | 74 (51.0) |
| Male | 533 (52.9) | 71 (49.0) |
| Gestational age at birth | ||
| <37 wk | 25 (2.5) | 4 (2.8) |
| ≥37 wk | 983 (97.5) | 141 (97.2) |
| Birth weightc | ||
| <2500 g | 10 (1.0) | 3 (2.1) |
| ≥2500 g | 996 (99.0) | 142 (97.9) |
| 5-min Apgar scorec | ||
| 7–10 | 992 (99.4) | 145 (100.0) |
| 0–6 | 6 (0.6) | 0 (0) |
| Maternal parityc | ||
| Nulliparous | 533 (54.1) | 73 (52.1) |
| Multiparous | 453 (45.9) | 67 (47.9) |
| Maternal age | ||
| <30 y | 439 (43.6) | 68 (46.9) |
| ≥30 y | 569 (56.4) | 77 (53.1) |
| Maternal prepregnancy BMIc,d | ||
| <25 kg/m2 | 741 (75.3) | 96 (68.1) |
| ≥25 kg/m2 | 243 (24.7) | 45 (31.9) |
| Maternal smoking in pregnancyc,d | ||
| Yes | 329 (32.6) | 46 (31.7) |
| No | 679 (67.4) | 99 (68.3) |
| Maternal alcohol intake in pregnancyd | ||
| 0 units/wk | 455 (45.1) | 84 (57.9) |
| 1-4 units/wk | 439 (43.6) | 47 (32.4) |
| ≥5 units/wk | 114 (11.3) | 14 (9.7) |
| Maternal psychiatric diseased | ||
| Yes | 102 (10.1) | 13 (9.0) |
| No | 906 (89.9) | 132 (91.0) |
| Maternal IQe | ||
| Abnormal low | 150 (14.9) | 29 (20.0) |
| Normal | 858 (85.1) | 116 (80.0) |
| Parental educational levelc | ||
| <12 y | 267 (26.7) | 51 (35.2) |
| ≥12 y | 735 (73.3) | 94 (64.8) |
| Characteristic . | No Maternal Thyroid Dysfunctiona . | Maternal Thyroid Dysfunctionb . |
|---|---|---|
| Children (n) | 1,008 | 145 |
| Birth year of the child | ||
| 1997–2000 | 503 (49.9) | 69 (47.6) |
| 2001–2003 | 505 (50.1) | 76 (52.4) |
| Child’s sex | ||
| Female | 475 (47.1) | 74 (51.0) |
| Male | 533 (52.9) | 71 (49.0) |
| Gestational age at birth | ||
| <37 wk | 25 (2.5) | 4 (2.8) |
| ≥37 wk | 983 (97.5) | 141 (97.2) |
| Birth weightc | ||
| <2500 g | 10 (1.0) | 3 (2.1) |
| ≥2500 g | 996 (99.0) | 142 (97.9) |
| 5-min Apgar scorec | ||
| 7–10 | 992 (99.4) | 145 (100.0) |
| 0–6 | 6 (0.6) | 0 (0) |
| Maternal parityc | ||
| Nulliparous | 533 (54.1) | 73 (52.1) |
| Multiparous | 453 (45.9) | 67 (47.9) |
| Maternal age | ||
| <30 y | 439 (43.6) | 68 (46.9) |
| ≥30 y | 569 (56.4) | 77 (53.1) |
| Maternal prepregnancy BMIc,d | ||
| <25 kg/m2 | 741 (75.3) | 96 (68.1) |
| ≥25 kg/m2 | 243 (24.7) | 45 (31.9) |
| Maternal smoking in pregnancyc,d | ||
| Yes | 329 (32.6) | 46 (31.7) |
| No | 679 (67.4) | 99 (68.3) |
| Maternal alcohol intake in pregnancyd | ||
| 0 units/wk | 455 (45.1) | 84 (57.9) |
| 1-4 units/wk | 439 (43.6) | 47 (32.4) |
| ≥5 units/wk | 114 (11.3) | 14 (9.7) |
| Maternal psychiatric diseased | ||
| Yes | 102 (10.1) | 13 (9.0) |
| No | 906 (89.9) | 132 (91.0) |
| Maternal IQe | ||
| Abnormal low | 150 (14.9) | 29 (20.0) |
| Normal | 858 (85.1) | 116 (80.0) |
| Parental educational levelc | ||
| <12 y | 267 (26.7) | 51 (35.2) |
| ≥12 y | 735 (73.3) | 94 (64.8) |
Numbers are n (%).
TSH and fT4 between the pregnancy week–specific 2.5th and 97.5th percentiles.
TSH and/or fT4 outside the pregnancy week–specific 2.5th and 97.5th percentiles.
Individuals with missing values not included in the table.
Self-reported in the pregnancy.
Abnormal IQ defined by a score below the sample mean minus 1 SD.
Characteristics of Children Included in Study, Stratified by Maternal Thyroid Function in Early Pregnancy
| Characteristic . | No Maternal Thyroid Dysfunctiona . | Maternal Thyroid Dysfunctionb . |
|---|---|---|
| Children (n) | 1,008 | 145 |
| Birth year of the child | ||
| 1997–2000 | 503 (49.9) | 69 (47.6) |
| 2001–2003 | 505 (50.1) | 76 (52.4) |
| Child’s sex | ||
| Female | 475 (47.1) | 74 (51.0) |
| Male | 533 (52.9) | 71 (49.0) |
| Gestational age at birth | ||
| <37 wk | 25 (2.5) | 4 (2.8) |
| ≥37 wk | 983 (97.5) | 141 (97.2) |
| Birth weightc | ||
| <2500 g | 10 (1.0) | 3 (2.1) |
| ≥2500 g | 996 (99.0) | 142 (97.9) |
| 5-min Apgar scorec | ||
| 7–10 | 992 (99.4) | 145 (100.0) |
| 0–6 | 6 (0.6) | 0 (0) |
| Maternal parityc | ||
| Nulliparous | 533 (54.1) | 73 (52.1) |
| Multiparous | 453 (45.9) | 67 (47.9) |
| Maternal age | ||
| <30 y | 439 (43.6) | 68 (46.9) |
| ≥30 y | 569 (56.4) | 77 (53.1) |
| Maternal prepregnancy BMIc,d | ||
| <25 kg/m2 | 741 (75.3) | 96 (68.1) |
| ≥25 kg/m2 | 243 (24.7) | 45 (31.9) |
| Maternal smoking in pregnancyc,d | ||
| Yes | 329 (32.6) | 46 (31.7) |
| No | 679 (67.4) | 99 (68.3) |
| Maternal alcohol intake in pregnancyd | ||
| 0 units/wk | 455 (45.1) | 84 (57.9) |
| 1-4 units/wk | 439 (43.6) | 47 (32.4) |
| ≥5 units/wk | 114 (11.3) | 14 (9.7) |
| Maternal psychiatric diseased | ||
| Yes | 102 (10.1) | 13 (9.0) |
| No | 906 (89.9) | 132 (91.0) |
| Maternal IQe | ||
| Abnormal low | 150 (14.9) | 29 (20.0) |
| Normal | 858 (85.1) | 116 (80.0) |
| Parental educational levelc | ||
| <12 y | 267 (26.7) | 51 (35.2) |
| ≥12 y | 735 (73.3) | 94 (64.8) |
| Characteristic . | No Maternal Thyroid Dysfunctiona . | Maternal Thyroid Dysfunctionb . |
|---|---|---|
| Children (n) | 1,008 | 145 |
| Birth year of the child | ||
| 1997–2000 | 503 (49.9) | 69 (47.6) |
| 2001–2003 | 505 (50.1) | 76 (52.4) |
| Child’s sex | ||
| Female | 475 (47.1) | 74 (51.0) |
| Male | 533 (52.9) | 71 (49.0) |
| Gestational age at birth | ||
| <37 wk | 25 (2.5) | 4 (2.8) |
| ≥37 wk | 983 (97.5) | 141 (97.2) |
| Birth weightc | ||
| <2500 g | 10 (1.0) | 3 (2.1) |
| ≥2500 g | 996 (99.0) | 142 (97.9) |
| 5-min Apgar scorec | ||
| 7–10 | 992 (99.4) | 145 (100.0) |
| 0–6 | 6 (0.6) | 0 (0) |
| Maternal parityc | ||
| Nulliparous | 533 (54.1) | 73 (52.1) |
| Multiparous | 453 (45.9) | 67 (47.9) |
| Maternal age | ||
| <30 y | 439 (43.6) | 68 (46.9) |
| ≥30 y | 569 (56.4) | 77 (53.1) |
| Maternal prepregnancy BMIc,d | ||
| <25 kg/m2 | 741 (75.3) | 96 (68.1) |
| ≥25 kg/m2 | 243 (24.7) | 45 (31.9) |
| Maternal smoking in pregnancyc,d | ||
| Yes | 329 (32.6) | 46 (31.7) |
| No | 679 (67.4) | 99 (68.3) |
| Maternal alcohol intake in pregnancyd | ||
| 0 units/wk | 455 (45.1) | 84 (57.9) |
| 1-4 units/wk | 439 (43.6) | 47 (32.4) |
| ≥5 units/wk | 114 (11.3) | 14 (9.7) |
| Maternal psychiatric diseased | ||
| Yes | 102 (10.1) | 13 (9.0) |
| No | 906 (89.9) | 132 (91.0) |
| Maternal IQe | ||
| Abnormal low | 150 (14.9) | 29 (20.0) |
| Normal | 858 (85.1) | 116 (80.0) |
| Parental educational levelc | ||
| <12 y | 267 (26.7) | 51 (35.2) |
| ≥12 y | 735 (73.3) | 94 (64.8) |
Numbers are n (%).
TSH and fT4 between the pregnancy week–specific 2.5th and 97.5th percentiles.
TSH and/or fT4 outside the pregnancy week–specific 2.5th and 97.5th percentiles.
Individuals with missing values not included in the table.
Self-reported in the pregnancy.
Abnormal IQ defined by a score below the sample mean minus 1 SD.
Child intelligence was evaluated from full, performance, and verbal IQs (Table 3). For all measurements, mean IQ was lower in children born to mothers with thyroid dysfunction, but the mean difference attenuated in adjusted analyses (Table 3). Similarly, children born to mothers with thyroid dysfunction more frequently had abnormal low IQ, but adjustment for maternal BMI and IQ attenuated these associations (Table 3).When evaluated according to categories of TSH and fT4 (Fig. 2), a distinct pattern emerged from the association with verbal IQ. TSH of ≥ 10 mIU/L and fT4 < 10 pmol/L were associated with lower child IQ. No differences in verbal IQ were observed for maternal TSH < 0.1 mIU/L or in the range from 2.5 to 9.99 mIU/L. All women with fT4 < 10 pmol/L (Fig. 2) also had TSH ≥ 10 mIU/L and were classified as having overt hypothyroidism, whereas two of the six women with TSH ≥ 10 mIU/L had fT4 > 10 pmol/L and were classified as having overt and subclinical hypothyroidism. None of the six women with fT4 < 10 pmol/L and/or TSH ≥ 10 mIU/L reported current treatment with thyroid hormone, and the majority were subsequently diagnosed with thyroid disease during the pregnancy (n = 2) or after the pregnancy (n = 3). Among women with fT4 ranging from 10 to 12 pmol/L, 11 women were classified as having overt hypothyroidism (TSH ranging from 3.6 to 11 mIU/L), but the exclusion of this group did not change the associations observed (Fig. 2).
Association Between Maternal Thyroid Dysfunction in Early Pregnancy and WPPSI-R and TEACh-5 Scores in Their 5-Year-Old Children
| Variable . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Mean Difference . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Odds Ratio for Abnormal Scorec . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (n) . | Mean Score . | Children (n) . | Mean Score . | Crude . | Adjustedd (95% CI) . | % Abnormal . | % Abnormal . | Crude . | Adjustedd (95% CI) . | |
| WPPSI-R | ||||||||||
| Full IQ | 1003 | 105.25 | 142 | 104.73 | −3.74 | −1.56 (−4.53 to 1.41) | 15.25 | 19.01 | 1.98 | 1.34 (0.69–2.61) |
| Performance IQ | 1005 | 104.61 | 142 | 103.99 | −3.80 | −1.61 (−5.42 to 2.20) | 17.01 | 19.01 | 1.56 | 1.29 (0.68–2.45) |
| Verbal IQ | 1004 | 104.66 | 142 | 104.26 | −2.90 | −1.28 (−3.90 to 1.35) | 13.05 | 17.61 | 2.13 | 1.52 (0.75–3.10) |
| TEACh-5 | ||||||||||
| Overall attention | 1004 | −0.00 | 144 | 0.03 | −0.15 | −0.05 (−0.30 to 0.20) | 16.04 | 13.89 | 1.45 | 1.22 (0.64–2.35) |
| Sustained attention | 1001 | −0.00 | 143 | 0.06 | −0.08 | 0.02 (−0.22 to 0.25) | 16.58 | 11.89 | 0.94 | 0.73 (0.34–1.58) |
| Selective attention | 1002 | −0.00 | 144 | 0.01 | −0.11 | −0.05 (−0.31 to 0.21) | 16.07 | 15.97 | 1.32 | 1.25 (0.66–2.35) |
| Variable . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Mean Difference . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Odds Ratio for Abnormal Scorec . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (n) . | Mean Score . | Children (n) . | Mean Score . | Crude . | Adjustedd (95% CI) . | % Abnormal . | % Abnormal . | Crude . | Adjustedd (95% CI) . | |
| WPPSI-R | ||||||||||
| Full IQ | 1003 | 105.25 | 142 | 104.73 | −3.74 | −1.56 (−4.53 to 1.41) | 15.25 | 19.01 | 1.98 | 1.34 (0.69–2.61) |
| Performance IQ | 1005 | 104.61 | 142 | 103.99 | −3.80 | −1.61 (−5.42 to 2.20) | 17.01 | 19.01 | 1.56 | 1.29 (0.68–2.45) |
| Verbal IQ | 1004 | 104.66 | 142 | 104.26 | −2.90 | −1.28 (−3.90 to 1.35) | 13.05 | 17.61 | 2.13 | 1.52 (0.75–3.10) |
| TEACh-5 | ||||||||||
| Overall attention | 1004 | −0.00 | 144 | 0.03 | −0.15 | −0.05 (−0.30 to 0.20) | 16.04 | 13.89 | 1.45 | 1.22 (0.64–2.35) |
| Sustained attention | 1001 | −0.00 | 143 | 0.06 | −0.08 | 0.02 (−0.22 to 0.25) | 16.58 | 11.89 | 0.94 | 0.73 (0.34–1.58) |
| Selective attention | 1002 | −0.00 | 144 | 0.01 | −0.11 | −0.05 (−0.31 to 0.21) | 16.07 | 15.97 | 1.32 | 1.25 (0.66–2.35) |
TSH and fT4 within the gestational week–specific reference ranges.
TSH and/or fT4 outside the gestational week–specific reference ranges.
Abnormal WPPSI-R/TEACh-5 defined by a score below the sample mean minus 1 SD.
Adjusted model included maternal IQ, maternal prepregnancy BMI, child’s age at testing, and testing psychologist.
Association Between Maternal Thyroid Dysfunction in Early Pregnancy and WPPSI-R and TEACh-5 Scores in Their 5-Year-Old Children
| Variable . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Mean Difference . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Odds Ratio for Abnormal Scorec . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (n) . | Mean Score . | Children (n) . | Mean Score . | Crude . | Adjustedd (95% CI) . | % Abnormal . | % Abnormal . | Crude . | Adjustedd (95% CI) . | |
| WPPSI-R | ||||||||||
| Full IQ | 1003 | 105.25 | 142 | 104.73 | −3.74 | −1.56 (−4.53 to 1.41) | 15.25 | 19.01 | 1.98 | 1.34 (0.69–2.61) |
| Performance IQ | 1005 | 104.61 | 142 | 103.99 | −3.80 | −1.61 (−5.42 to 2.20) | 17.01 | 19.01 | 1.56 | 1.29 (0.68–2.45) |
| Verbal IQ | 1004 | 104.66 | 142 | 104.26 | −2.90 | −1.28 (−3.90 to 1.35) | 13.05 | 17.61 | 2.13 | 1.52 (0.75–3.10) |
| TEACh-5 | ||||||||||
| Overall attention | 1004 | −0.00 | 144 | 0.03 | −0.15 | −0.05 (−0.30 to 0.20) | 16.04 | 13.89 | 1.45 | 1.22 (0.64–2.35) |
| Sustained attention | 1001 | −0.00 | 143 | 0.06 | −0.08 | 0.02 (−0.22 to 0.25) | 16.58 | 11.89 | 0.94 | 0.73 (0.34–1.58) |
| Selective attention | 1002 | −0.00 | 144 | 0.01 | −0.11 | −0.05 (−0.31 to 0.21) | 16.07 | 15.97 | 1.32 | 1.25 (0.66–2.35) |
| Variable . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Mean Difference . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Odds Ratio for Abnormal Scorec . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (n) . | Mean Score . | Children (n) . | Mean Score . | Crude . | Adjustedd (95% CI) . | % Abnormal . | % Abnormal . | Crude . | Adjustedd (95% CI) . | |
| WPPSI-R | ||||||||||
| Full IQ | 1003 | 105.25 | 142 | 104.73 | −3.74 | −1.56 (−4.53 to 1.41) | 15.25 | 19.01 | 1.98 | 1.34 (0.69–2.61) |
| Performance IQ | 1005 | 104.61 | 142 | 103.99 | −3.80 | −1.61 (−5.42 to 2.20) | 17.01 | 19.01 | 1.56 | 1.29 (0.68–2.45) |
| Verbal IQ | 1004 | 104.66 | 142 | 104.26 | −2.90 | −1.28 (−3.90 to 1.35) | 13.05 | 17.61 | 2.13 | 1.52 (0.75–3.10) |
| TEACh-5 | ||||||||||
| Overall attention | 1004 | −0.00 | 144 | 0.03 | −0.15 | −0.05 (−0.30 to 0.20) | 16.04 | 13.89 | 1.45 | 1.22 (0.64–2.35) |
| Sustained attention | 1001 | −0.00 | 143 | 0.06 | −0.08 | 0.02 (−0.22 to 0.25) | 16.58 | 11.89 | 0.94 | 0.73 (0.34–1.58) |
| Selective attention | 1002 | −0.00 | 144 | 0.01 | −0.11 | −0.05 (−0.31 to 0.21) | 16.07 | 15.97 | 1.32 | 1.25 (0.66–2.35) |
TSH and fT4 within the gestational week–specific reference ranges.
TSH and/or fT4 outside the gestational week–specific reference ranges.
Abnormal WPPSI-R/TEACh-5 defined by a score below the sample mean minus 1 SD.
Adjusted model included maternal IQ, maternal prepregnancy BMI, child’s age at testing, and testing psychologist.
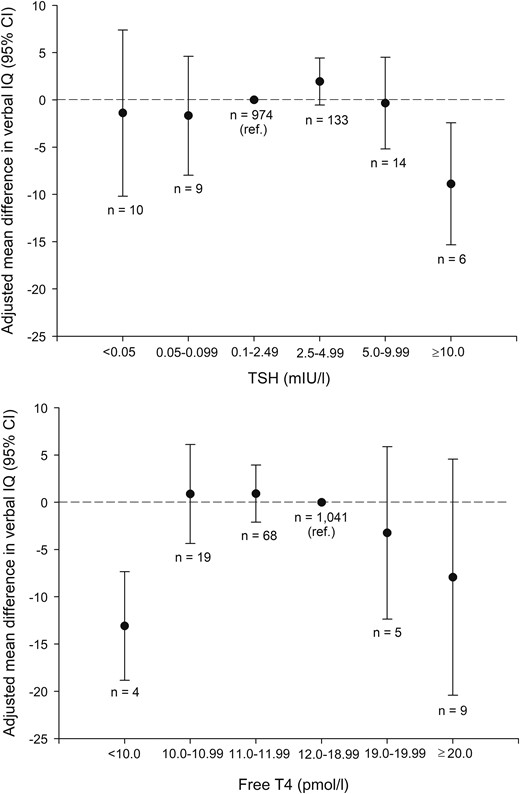
Adjusted mean difference with 95% confidence interval in child verbal IQ by maternal TSH (top) and fT4 (bottom) measured in an early pregnancy blood sample. Reference group was maternal TSH in the range from 0.1 to 2.49 mIU/L (top) and maternal fT4 in the range from 12.0 to 18.99 (bottom). The adjusted model included maternal IQ, maternal prepregnancy BMI, child’s age at testing, and testing psychologist. In the bottom panel, all women with fT4 < 10 pmol/L (n = 4) had TSH ≥ 10 mIU/L. Among women with fT4 in the range from 10 to 12 pmol/L, 11 women had overt hypothyroidism; the exclusion of this group revealed the following associations: fT4 10.0 to 10.99 pmol/L (n = 14): adjusted mean difference, 1.14 (95% CI, −4.91 to 7.18), fT4 11.0 to 11.99 pmol/L (n = 62): adjusted mean difference, 1.10 (95% CI, −2.17 to 4.37).
Other measures of IQ showed similar trends though nonsignificant [adjusted mean difference in full IQ: TSH ≥ 10 mIU/l, −8.2 (95% CI, −19.2 to 2.8), fT4 < 10 pmol/L, −10.6 (95% CI, −24.3 to 3.04); performance IQ: TSH ≥ 10 mIU/L, −5.9 (95% CI, −21.8 to 9.9), fT4 < 10 pmol/L, −5.3 (95% CI, −26.4 to 15.8)]. The association between maternal thyroid function and child verbal IQ was also evaluated by using restricted cubic splines (Supplemental Fig. 1). This analysis showed a significant association with TSH, which was robust for a change in the number and location of knots, whereas the model of fT4 was nonsignificant.
Child attention was evaluated from overall, sustained, and selective attention, with lower scores indicating poorer performance, and showed no association (Table 3).
Child motor function was evaluated only in a subgroup of participants (n = 487). The total score was a median of 7.5 both in children born to mothers with thyroid dysfunction (n = 60) and in nonexposed children (n = 427); adjusted exponentiated β was 1.07 (95% CI, 0.75 to 1.54). However, 16.7% of children born to mothers with thyroid dysfunction were classified as having an abnormal score compared with 9.8% among nonexposed children: Crude odds ratio (OR) was 6.21 (95% CI, 2.41 to 16.0), and adjusted OR was 5.82 (95% CI, 2.33 to 14.6). Most exposed children with abnormal score had been exposed to maternal hypothyroxinemia.
Child executive function and behavior were evaluated from parent and teacher reports of the BRIEF and the SDQ questionnaires, with higher scores indicating more difficulties (Tables 4 and 5). No association was observed with parent reports. On the other hand, BRIEF and SDQ showed more difficulties in children born to mothers with thyroid dysfunction when evaluated by their teacher. Evaluation of exposed cases with abnormal score indicated that the associations were predominated by exposure to maternal hypothyroxinemia, which showed associations with teacher-reported abnormal BRIEF scores [behavioral regulation index: adjusted OR, 3.43 (95% CI, 1.44 to 8.18); metacognition index: adjusted OR, 4.70 (95% CI, 2.05 to 10.7)], and SDQ scores [internalizing problems: adjusted OR, 2.36 (95% CI, 0.84 to 6.65); externalizing problems: adjusted OR, 4.33 (95% CI, 1.69 to 11.1)]. Stratification by child’s sex revealed differences in the stratum-specific estimates for internalizing problems in the SDQ teacher report [adjusted ORs for abnormal score, 6.86 (95% CI, 2.53 to 18.61) in girls and 1.33 (95% CI, 0.51 to 3.47) in boys], with significant deviation from multiplicative and additive interaction (Supplemental Table 1). Significant interaction was not observed for other outcomes.
Association Between Maternal Thyroid Dysfunction in Early Pregnancy and BRIEF Scores in Their 5-Year-Old Children
| Variable . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Mean Difference . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Odds Ratio for Abnormal Scorec . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (n) . | Mean Score . | Children (n) . | Mean Score . | Crude . | Adjustedd (95% CI) . | % Abnormal . | % Abnormal . | Crude . | Adjustedd (95% CI) . | |
| Parent report | ||||||||||
| General executive composite | 1006 | 50.07 | 144 | 49.56 | 0.84 | 0.21 (−2.51 to 2.93) | 16.10 | 15.97 | 1.37 | 1.29 (0.67–2.49) |
| Behavioral regulation index | 1006 | 50.10 | 144 | 49.56 | 0.90 | 0.31 (−2.15 to 2.78) | 15.51 | 12.50 | 1.21 | 1.07 (0.53–2.15) |
| Metacognition index | 1,006 | 50.07 | 144 | 49.66 | 0.74 | 0.13 (−2.69 to 2.95) | 15.11 | 18.06 | 1.51 | 1.39 (0.73–2.66) |
| Teacher report | ||||||||||
| General executive composite | 879 | 49.83 | 127 | 51.22 | 4.76 | 4.13 (1.01–7.25) | 15.59 | 19.69 | 3.09 | 2.76 (1.48–5.16) |
| Behavioral regulation index | 882 | 49.83 | 128 | 51.30 | 4.31 | 3.85 (0.86–6.84) | 14.97 | 22.66 | 3.13 | 2.88 (1.57–5.27) |
| Metacognition index | 879 | 49.88 | 127 | 51.06 | 4.54 | 3.89 (0.76–7.02) | 15.24 | 18.11 | 2.72 | 2.38 (1.24–4.58) |
| Variable . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Mean Difference . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Odds Ratio for Abnormal Scorec . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (n) . | Mean Score . | Children (n) . | Mean Score . | Crude . | Adjustedd (95% CI) . | % Abnormal . | % Abnormal . | Crude . | Adjustedd (95% CI) . | |
| Parent report | ||||||||||
| General executive composite | 1006 | 50.07 | 144 | 49.56 | 0.84 | 0.21 (−2.51 to 2.93) | 16.10 | 15.97 | 1.37 | 1.29 (0.67–2.49) |
| Behavioral regulation index | 1006 | 50.10 | 144 | 49.56 | 0.90 | 0.31 (−2.15 to 2.78) | 15.51 | 12.50 | 1.21 | 1.07 (0.53–2.15) |
| Metacognition index | 1,006 | 50.07 | 144 | 49.66 | 0.74 | 0.13 (−2.69 to 2.95) | 15.11 | 18.06 | 1.51 | 1.39 (0.73–2.66) |
| Teacher report | ||||||||||
| General executive composite | 879 | 49.83 | 127 | 51.22 | 4.76 | 4.13 (1.01–7.25) | 15.59 | 19.69 | 3.09 | 2.76 (1.48–5.16) |
| Behavioral regulation index | 882 | 49.83 | 128 | 51.30 | 4.31 | 3.85 (0.86–6.84) | 14.97 | 22.66 | 3.13 | 2.88 (1.57–5.27) |
| Metacognition index | 879 | 49.88 | 127 | 51.06 | 4.54 | 3.89 (0.76–7.02) | 15.24 | 18.11 | 2.72 | 2.38 (1.24–4.58) |
TSH and fT4 within the gestational week–specific reference ranges.
TSH and/or fT4 outside the gestational week–specific reference ranges.
Abnormal BRIEF defined by a score above the sample mean plus 1 SD.
Adjusted model included maternal IQ and maternal prepregnancy BMI.
Association Between Maternal Thyroid Dysfunction in Early Pregnancy and BRIEF Scores in Their 5-Year-Old Children
| Variable . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Mean Difference . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Odds Ratio for Abnormal Scorec . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (n) . | Mean Score . | Children (n) . | Mean Score . | Crude . | Adjustedd (95% CI) . | % Abnormal . | % Abnormal . | Crude . | Adjustedd (95% CI) . | |
| Parent report | ||||||||||
| General executive composite | 1006 | 50.07 | 144 | 49.56 | 0.84 | 0.21 (−2.51 to 2.93) | 16.10 | 15.97 | 1.37 | 1.29 (0.67–2.49) |
| Behavioral regulation index | 1006 | 50.10 | 144 | 49.56 | 0.90 | 0.31 (−2.15 to 2.78) | 15.51 | 12.50 | 1.21 | 1.07 (0.53–2.15) |
| Metacognition index | 1,006 | 50.07 | 144 | 49.66 | 0.74 | 0.13 (−2.69 to 2.95) | 15.11 | 18.06 | 1.51 | 1.39 (0.73–2.66) |
| Teacher report | ||||||||||
| General executive composite | 879 | 49.83 | 127 | 51.22 | 4.76 | 4.13 (1.01–7.25) | 15.59 | 19.69 | 3.09 | 2.76 (1.48–5.16) |
| Behavioral regulation index | 882 | 49.83 | 128 | 51.30 | 4.31 | 3.85 (0.86–6.84) | 14.97 | 22.66 | 3.13 | 2.88 (1.57–5.27) |
| Metacognition index | 879 | 49.88 | 127 | 51.06 | 4.54 | 3.89 (0.76–7.02) | 15.24 | 18.11 | 2.72 | 2.38 (1.24–4.58) |
| Variable . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Mean Difference . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Odds Ratio for Abnormal Scorec . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (n) . | Mean Score . | Children (n) . | Mean Score . | Crude . | Adjustedd (95% CI) . | % Abnormal . | % Abnormal . | Crude . | Adjustedd (95% CI) . | |
| Parent report | ||||||||||
| General executive composite | 1006 | 50.07 | 144 | 49.56 | 0.84 | 0.21 (−2.51 to 2.93) | 16.10 | 15.97 | 1.37 | 1.29 (0.67–2.49) |
| Behavioral regulation index | 1006 | 50.10 | 144 | 49.56 | 0.90 | 0.31 (−2.15 to 2.78) | 15.51 | 12.50 | 1.21 | 1.07 (0.53–2.15) |
| Metacognition index | 1,006 | 50.07 | 144 | 49.66 | 0.74 | 0.13 (−2.69 to 2.95) | 15.11 | 18.06 | 1.51 | 1.39 (0.73–2.66) |
| Teacher report | ||||||||||
| General executive composite | 879 | 49.83 | 127 | 51.22 | 4.76 | 4.13 (1.01–7.25) | 15.59 | 19.69 | 3.09 | 2.76 (1.48–5.16) |
| Behavioral regulation index | 882 | 49.83 | 128 | 51.30 | 4.31 | 3.85 (0.86–6.84) | 14.97 | 22.66 | 3.13 | 2.88 (1.57–5.27) |
| Metacognition index | 879 | 49.88 | 127 | 51.06 | 4.54 | 3.89 (0.76–7.02) | 15.24 | 18.11 | 2.72 | 2.38 (1.24–4.58) |
TSH and fT4 within the gestational week–specific reference ranges.
TSH and/or fT4 outside the gestational week–specific reference ranges.
Abnormal BRIEF defined by a score above the sample mean plus 1 SD.
Adjusted model included maternal IQ and maternal prepregnancy BMI.
Association Between Maternal Thyroid Dysfunction in Early Pregnancy and SDQ Scores in Their 5-Year-Old Children
| Variable . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Exponentiated β . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Odds Ratio for Abnormal Scorec . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (n) . | Median Score . | Children (n) . | Median Score . | Crude . | Adjustedd (95% CI) . | % Abnormal . | % Abnormal . | Crude . | Adjustedd (95% CI) . | |
| Parent report | ||||||||||
| Total difficulties score | 1001 | 6 | 145 | 6 | 1.06 | 1.03 (0.86–1.23) | 9.59 | 8.97 | 0.95 | 0.90 (0.36–2.26) |
| Internalizing problems | 1000 | 2 | 145 | 2 | 1.16 | 1.16 (0.95–1.42) | 6.40 | 11.03 | 1.57 | 1.50 (0.67–3.37) |
| Externalizing problems | 1001 | 4 | 144 | 4 | 1.03 | 0.99 (0.83–1.19) | 11.79 | 11.11 | 1.38 | 1.29 (0.59–2.82) |
| Teacher report | ||||||||||
| Total difficulties score | 884 | 4 | 128 | 5 | 1.45 | 1.40 (1.09–1.81) | 8.94 | 12.50 | 3.51 | 3.07 (1.48–6.34) |
| Internalizing problems | 884 | 1 | 128 | 2 | 1.56 | 1.50 (1.14–1.96) | 9.16 | 14.06 | 2.94 | 2.66 (1.32–5.36) |
| Externalizing problems | 884 | 2 | 128 | 2 | 1.47 | 1.42 (1.09–1.85) | 9.95 | 12.50 | 2.69 | 2.42 (1.16–5.04) |
| Variable . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Exponentiated β . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Odds Ratio for Abnormal Scorec . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (n) . | Median Score . | Children (n) . | Median Score . | Crude . | Adjustedd (95% CI) . | % Abnormal . | % Abnormal . | Crude . | Adjustedd (95% CI) . | |
| Parent report | ||||||||||
| Total difficulties score | 1001 | 6 | 145 | 6 | 1.06 | 1.03 (0.86–1.23) | 9.59 | 8.97 | 0.95 | 0.90 (0.36–2.26) |
| Internalizing problems | 1000 | 2 | 145 | 2 | 1.16 | 1.16 (0.95–1.42) | 6.40 | 11.03 | 1.57 | 1.50 (0.67–3.37) |
| Externalizing problems | 1001 | 4 | 144 | 4 | 1.03 | 0.99 (0.83–1.19) | 11.79 | 11.11 | 1.38 | 1.29 (0.59–2.82) |
| Teacher report | ||||||||||
| Total difficulties score | 884 | 4 | 128 | 5 | 1.45 | 1.40 (1.09–1.81) | 8.94 | 12.50 | 3.51 | 3.07 (1.48–6.34) |
| Internalizing problems | 884 | 1 | 128 | 2 | 1.56 | 1.50 (1.14–1.96) | 9.16 | 14.06 | 2.94 | 2.66 (1.32–5.36) |
| Externalizing problems | 884 | 2 | 128 | 2 | 1.47 | 1.42 (1.09–1.85) | 9.95 | 12.50 | 2.69 | 2.42 (1.16–5.04) |
TSH and fT4 within the gestational week–specific reference ranges.
TSH and/or fT4 outside the gestational week–specific reference ranges.
Abnormal SDQ defined by sex-specific cutoffs previously established in Danish 5-year-old children (17).
Adjusted model included maternal IQ and maternal prepregnancy BMI.
Association Between Maternal Thyroid Dysfunction in Early Pregnancy and SDQ Scores in Their 5-Year-Old Children
| Variable . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Exponentiated β . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Odds Ratio for Abnormal Scorec . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (n) . | Median Score . | Children (n) . | Median Score . | Crude . | Adjustedd (95% CI) . | % Abnormal . | % Abnormal . | Crude . | Adjustedd (95% CI) . | |
| Parent report | ||||||||||
| Total difficulties score | 1001 | 6 | 145 | 6 | 1.06 | 1.03 (0.86–1.23) | 9.59 | 8.97 | 0.95 | 0.90 (0.36–2.26) |
| Internalizing problems | 1000 | 2 | 145 | 2 | 1.16 | 1.16 (0.95–1.42) | 6.40 | 11.03 | 1.57 | 1.50 (0.67–3.37) |
| Externalizing problems | 1001 | 4 | 144 | 4 | 1.03 | 0.99 (0.83–1.19) | 11.79 | 11.11 | 1.38 | 1.29 (0.59–2.82) |
| Teacher report | ||||||||||
| Total difficulties score | 884 | 4 | 128 | 5 | 1.45 | 1.40 (1.09–1.81) | 8.94 | 12.50 | 3.51 | 3.07 (1.48–6.34) |
| Internalizing problems | 884 | 1 | 128 | 2 | 1.56 | 1.50 (1.14–1.96) | 9.16 | 14.06 | 2.94 | 2.66 (1.32–5.36) |
| Externalizing problems | 884 | 2 | 128 | 2 | 1.47 | 1.42 (1.09–1.85) | 9.95 | 12.50 | 2.69 | 2.42 (1.16–5.04) |
| Variable . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Exponentiated β . | No Thyroid Dysfunctiona . | Thyroid Dysfunctionb . | Odds Ratio for Abnormal Scorec . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (n) . | Median Score . | Children (n) . | Median Score . | Crude . | Adjustedd (95% CI) . | % Abnormal . | % Abnormal . | Crude . | Adjustedd (95% CI) . | |
| Parent report | ||||||||||
| Total difficulties score | 1001 | 6 | 145 | 6 | 1.06 | 1.03 (0.86–1.23) | 9.59 | 8.97 | 0.95 | 0.90 (0.36–2.26) |
| Internalizing problems | 1000 | 2 | 145 | 2 | 1.16 | 1.16 (0.95–1.42) | 6.40 | 11.03 | 1.57 | 1.50 (0.67–3.37) |
| Externalizing problems | 1001 | 4 | 144 | 4 | 1.03 | 0.99 (0.83–1.19) | 11.79 | 11.11 | 1.38 | 1.29 (0.59–2.82) |
| Teacher report | ||||||||||
| Total difficulties score | 884 | 4 | 128 | 5 | 1.45 | 1.40 (1.09–1.81) | 8.94 | 12.50 | 3.51 | 3.07 (1.48–6.34) |
| Internalizing problems | 884 | 1 | 128 | 2 | 1.56 | 1.50 (1.14–1.96) | 9.16 | 14.06 | 2.94 | 2.66 (1.32–5.36) |
| Externalizing problems | 884 | 2 | 128 | 2 | 1.47 | 1.42 (1.09–1.85) | 9.95 | 12.50 | 2.69 | 2.42 (1.16–5.04) |
TSH and fT4 within the gestational week–specific reference ranges.
TSH and/or fT4 outside the gestational week–specific reference ranges.
Abnormal SDQ defined by sex-specific cutoffs previously established in Danish 5-year-old children (17).
Adjusted model included maternal IQ and maternal prepregnancy BMI.
Discussion
This nationwide cohort study combined neuropsychological test results from a large group of children with the measurement of maternal thyroid function in stored biobank sera from early pregnancy. Results corroborate and extend the hypothesis of adverse neuropsychological function in children prenatally exposed to maternal thyroid function abnormalities. Interestingly, associations differed in magnitude by subtype of maternal thyroid dysfunction and areas of child neuropsychological function.
An individual’s ability to acquire and apply knowledge and skills can be assessed by the use of verbal and nonverbal intelligence tests. IQ is a frequently used standardized method for measuring intellectual ability, and numerous association studies have been conducted on genetic and environmental risk factors. We evaluated a spectrum of abnormalities in maternal thyroid function and different levels of TSH and fT4 and observed a distinct association with lack of maternal thyroid hormones. In fact, verbal IQ was lower in children born to mothers whose thyroid function testing indicated marked hypothyroidism in early pregnancy. Cognitive development after in utero exposure to maternal hypothyroidism has been considered for decades, and the scientific interest has extended beyond the consequences of overt hypothyroidism. Thus, the potential adverse effect of smaller aberrations in maternal function has been a major topic of debate (4). Studies within this field not only differ in regard to definition and type of maternal thyroid dysfunction but also show considerable disparities concerning the age of the child at testing and type of neurocognitive outcome. A major study published in 1999 by Haddow et al. (18) showed that 48 children born to mothers with undiagnosed hypothyroidism (defined by TSH >98th percentile; range, ∼5 to 90 mIU/L) in pregnancy had a lower IQ at age 7 to 9 years compared with 124 controls and 14 children whose mother had hypothyroidism but received treatment in the pregnancy. Similar to our study, this study (18) evaluated full, performance, and verbal IQ from the Wechsler intelligence scale and found lower performance with all three measurements.
We can only speculate on underlying developmental abnormalities in children with low verbal IQ. Evidence from experimental studies has shown developmental effects of maternal hypothyroidism on the fetal brain and involvement of different areas of the brain (19, 20). Abnormalities in the development of hippocampus have been described both in rats (21) and in human offspring (22) exposed to maternal hypothyroidism, and children with abnormal hippocampal development had impaired memory (22). Interestingly, the size of the hippocampus correlated with verbal, but not performance, IQ in male children (23).
We also observed an association between low maternal fT4 and child verbal IQ, and all cases with maternal fT4 <10 pmol/L in our population had high TSH and were classified as having overt hypothyroidism. The association with fT4 showed a nonsignificant U-shaped trend, which was not robust in our sample. Results from the Generation R study showed that both maternal high and low fT4 were associated with lower nonverbal IQ and altered gray matter and cortex volume at 6 to 8 years of age (24). On the other hand, this study showed no association with maternal TSH (24). Numerous studies have examined the association between subclinical hypothyroidism or hypothyroxinemia and child IQ (20, 25). However, results of randomized controlled trials have not shown improved cognitive outcomes after treatment of such abnormalities (26, 27). Notably, we found that children whose mothers had early pregnancy TSH in the range from 2.5 to 5 mIU/L and from 5 to 10 mIU/L did not have lower IQ when compared with those whose maternal TSH was <2.5 mIU/L. Clinical guidelines on the management of thyroid disease in pregnant women have recently revised the recommendation on TSH upper limit in the first trimester of pregnancy, raising it from 2.5 to 4.0 mIU/L (3). Our results support this change in the upper limit of TSH.
We had no information on thyroid autoantibodies, and we could not directly evaluate the impact of thyroid autoimmunity per se. However, individuals with elevated TSH are in general more likely to have thyroid peroxidase and/or thyroglobulin autoantibodies (28), and the observation that children whose mother had TSH in the range from 5 to 10 mIU/L did not have lower IQ may argue against thyroid autoimmunity as a major determinant of the associations observed.
We found an association between maternal thyroid function and child motor function, executive function, and behavior at 5 years of age, and, in contrast to IQ, these associations were dominated by exposure to isolated changes in maternal fT4. Previous studies have shown an association between maternal hypothyroxinemia and delayed child motor function at 2.5 years of age or earlier (29–32). For outcomes of executive function and behavior, our findings were confined to teacher reports. A recent study from the Netherlands (33) evaluated the association between maternal hypothyroxinemia and outcomes of SDQ at 5 to 6 years of age and found no association with parent reports but did find an association with teacher-reported symptoms of hyperactivity/inattention. Similarly, a study from the Generation R cohort observed an association between maternal hypothyroxinemia and symptoms of attention-deficit/hyperactivity disorder (34), whereas another study within the same cohort reported an association with markers of thyroid autoimmunity (35). Our finding of an association between maternal thyroid function and internalizing problems in the child should be a topic for future studies, including the interaction with child’s sex.
A strength of our study was the measurement of maternal thyroid function in stored biobank sera for the detection of undiagnosed thyroid disease in early pregnancy. Assessment of maternal thyroid function in our study was based on a single measurement in early pregnancy and may not reflect maternal thyroid status throughout the pregnancy. Still, major deviations were likely to be detected and correct (36). We were able to identify women currently treated for thyroid disease from self-report but had no information on redeemed prescriptions of drugs. The exclusion of women (n = 4) who reported current treatment with thyroid hormone did not change results. Mother-child pairs were selected from a nationwide cohort, and all analyses were weighted by sampling fraction to account for the slightly skewed selection, as evaluated from maternal alcohol intake. Participants with results of both maternal thyroid function testing and child neuropsychological testing were similar to all women selected for the follow-up study. Still, the number of participants was limited considering the evaluation of subtypes of maternal thyroid function.
Neuropsychological testing was performed by trained psychologists, and the children were 5 years of age. Yet, we acknowledge that the stability of cognitive test scores may be low even at this age (37), and it is unknown whether the changes will fade or persist and affect long-term outcomes. We had information on several potential confounders, including maternal IQ, but unmeasured or residual confounding may persist. We considered maternal IQ a potential confounder because it associates with child IQ and also potentially with our exposure variable via socioeconomic status and therefore different environmental exposures, which are known risk factors for thyroid disease. We have noticed that most previous investigations did not include information on maternal IQ, and further studies considering maternal IQ are warranted. We had no information on paternal IQ, which may be associated with the outcomes under study (38, 39). It remains uncertain whether measurement of paternal intelligence would be associated with our exposure, and a study on an alternative fetal exposure showed that paternal IQ was not significant after adjustment for maternal IQ.
Conclusion
Measurement of maternal thyroid function in biobank sera from pregnant women in a Danish nationwide birth cohort showed that marked maternal hypothyroidism in early pregnancy was associated with lower child verbal IQ, whereas no association was observed with smaller abnormalities in maternal thyroid function. On the other hand, isolated deviations in maternal fT4 was associated with child motor function, executive function, and problem behavior. Further investigations are needed to elucidate whether the detection and treatment of maternal thyroid abnormalities will improve child outcomes.
Abbreviations:
- BMI
body mass index
- BRIEF
Behavior Rating Inventory of Executive Function
- CI
confidence interval
- DNBC
Danish National Birth Cohort
- fT4
free thyroxine
- IQ
intelligence quotient
- LDPS
Lifestyle During Pregnancy Study
- MABC
Movement Assessment Battery for Children
- OR
odds ratio
- SD
standard deviation
- SDQ
Strengths and Difficulties Questionnaire
- TEACh-5
Test of Everyday Attention for Children at Five
- TSH
thyroid-stimulating hormone
- WPPSI-R
Wechsler Primary and Preschool Scale of Intelligence-Revised.
Acknowledgments
We acknowledge Professor Peter Laurberg, Department of Endocrinology, Aalborg University Hospital, Denmark, who died on 20 June 2016. Professor Laurberg conceptualized and initiated the study, participated in the design, and was responsible for the biochemical analyses.
Financial Support: This work was supported by the Obel Family Foundation. This research has been conducted using the Danish National Biobank resource. The Danish National Biobank is supported by the Novo Nordisk Foundation. The Danish National Research Foundation has established the Danish Epidemiology Science Centre, which initiated and created the Danish National Birth Cohort. The cohort is furthermore a result of a major grant from this foundation. Additional support for the Danish National Birth Cohort is obtained from the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Augustinus Foundation, and the Health Foundation. Z.L. was supported by the National Institutes of Health/National Institute of Environmental Health Sciences Pathway to Independence Award (K99ES026729).
Disclosure Summary: The authors have nothing to disclose.
References
Niclasen J. SDQ cut off scores for danish 5-7-year-olds. http://www.sdqinfo.com/norms/SDQ_Danish_cut_off_scores_5_7_year_olds.pdf. Accessed 23 June 2017.



