-
PDF
- Split View
-
Views
-
Cite
Cite
Hélène David, Camille Aupiais, Baptiste Louveau, Pierre Quartier, Evelyne Jacqz-Aigrain, Jean-Claude Carel, Dominique Simon, Growth Outcomes After GH Therapy of Patients Given Long-Term Corticosteroids for Juvenile Idiopathic Arthritis, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 12, 1 December 2017, Pages 4578–4587, https://doi.org/10.1210/jc.2017-01455
Close - Share Icon Share
Abstract
Growth hormone (GH) therapy may improve statural growth outcomes in patients with severe juvenile idiopathic arthritis (JIA).
To evaluate the effect of GH treatment on adult height and to identify determinants of growth outcomes in JIA.
Data from 58 patients with JIA, including 53 receiving GH, enrolled in three prospective clinical trials between 1997 and 2002 were analyzed.
GH (0.056 mg/kg/d [interquartile range (IQR), 0.050 to 0.062]) for a median duration of 6.5 years (IQR, 4.7 to 7.9 years).
Factors associated with a favorable growth outcome (adult height − target height ≤ −1.5 standard deviations) were identified by multivariate logistic regression.
Adult height was available for 48 patients 8.6 years after GH initiation (IQR, 6.0 to 10.2 years). Height standard deviation score (SDS) increased from −2.9 (IQR, −4.4 to −1.6) at baseline to −1.7 (IQR, −3.9 to −0.1) in adulthood (P < 0.001). Median adult height was below target height [SDS, −0.2 (IQR, −1.4 to 0.4); P < 0.001]. Corrected adult height SDS was −1.3 (IQR, −3.0 to −0.2). Growth outcome was favorable in 24 (52.2%) patients. Significant independent determinants of growth outcome were age at GH initiation [adjusted odds ratio (aOR), 0.68 per additional year; 95% confidence interval (CI), 0.47 to 0.99], height at GH initiation (aOR, 2.6 per additional SDS; 95% CI, 1.15 to 5.9), and mean C-reactive protein levels during follow up (aOR, 0.51 per additional 10 mg/L; 95% CI, 0.28 to 0.92).
Long-term GH treatment significantly increased growth in patients with JIA but did not fully restore the genetic growth potential. The response showed marked interindividual variability and was weaker in patients with severe inflammation.
Juvenile idiopathic arthritis (JIA) is a chronic inflammatory joint disease that starts before 16 years of age (1). Growth retardation and short adult height were reported in about 10% of patients and were most common in systemic and polyarticular JIA (2, 3). In patients with systemic JIA treated with long-term glucocorticoid therapy, we found that adult height was below the target height in 80% of patients and below −2 standard deviation score (SDS) in 40% of patients (4).
Growth retardation is mainly due to the deleterious effects of cytokines and glucocorticoids on the growth plates and bone metabolism (5–7). The treatment of JIA has changed substantially over the past decades. In particular, the introduction of biologic therapies has changed the course of the disease by improving the control of inflammation and decreasing glucocorticoid requirements. Several studies found improved growth in patients with JIA treated with biologic agents (8–11). Nevertheless, in a recent study of 100 patients with JIA, biologic therapy failed to restore normal growth velocity in 20% of patients overall and in higher proportions of those who had systemic JIA or required more than one biologic agent (12).
Recombinant human growth hormone (GH) therapy has been investigated as an intervention to improve growth and decrease adverse metabolic outcomes in patients with severe chronic diseases. In patients with JIA, uncontrolled (13, 14) and controlled studies showed that GH treatment improved growth velocity, muscle mass, and bone mineralization (13, 15–18). Adult height has been evaluated in a single controlled study, which showed a significant improvement after 6 years of GH therapy, with a mean height gain of 1.57 standard deviations (SDs) and considerable variability in the growth response according to disease severity (19). Although this study was controlled, its small sample size of 13 patients limits its validity.
We therefore designed a study to investigate the effects of GH therapy on adult height in a larger group of patients and to identify factors associated with the growth response.
Methods
Patients
Three prospective clinical studies evaluating the effects of GH on growth in 58 patients receiving glucocorticoid therapy for systemic or polyarticular JIA have been conducted in the Department of Pediatric Endocrinology of the Robert Debré Hospital, Paris, France (Fig. 1). Two were uncontrolled studies that included patients in 1997 (n = 13) and from 2000 to 2002 (n = 15), respectively. The main inclusion criteria were JIA meeting International League of Associations for Rheumatology criteria (1), chronologic age (CA) >3 years, glucocorticoid treatment of at least 1 year in a daily dosage of prednisone-equivalent ≥0.2 mg/kg/dy, and height <−2 SDS or loss of height of 1 SD in the 2 years before inclusion or growth velocity ≤−1 SDS for CA. The third study was a randomized controlled trial in 30 patients enrolled from 1998 to 2002. The main inclusion criteria were JIA, CA >18 months and ≤ 9 years in girls or ≤11 years in boys, prepubertal status, glucocorticoid treatment started within the last 12 to 15 months in a daily dosage of prednisone-equivalent ≥0.2 mg/kg/d, and growth velocity <−1 SDS for CA over the last year. Exclusion criteria in the three studies were diabetes mellitus and other severe chronic diseases likely to affect growth (endocrine disease except well-substituted hypothyroidism, chronic renal failure, liver or heart failure, and nephrotic syndrome). In the two uncontrolled studies, sex steroid treatment within the last 6 months was also an exclusion criterion.
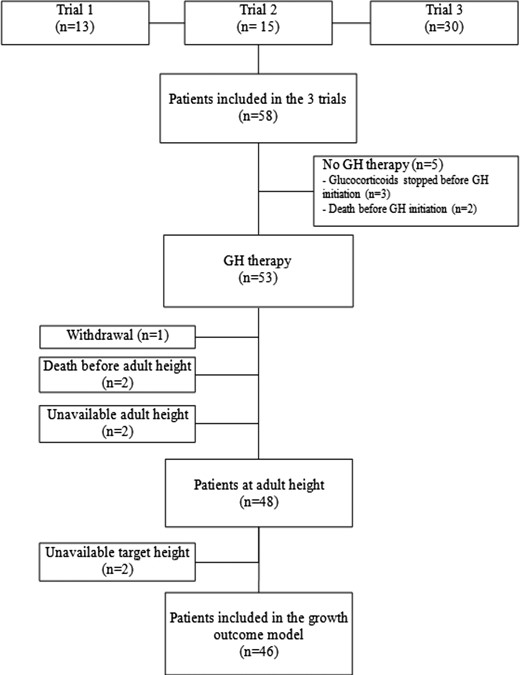
Study design and follow-up procedures
In the two uncontrolled studies (studies 1 and 2), GH (Genotonorm®, Pfizer, New York, NY) was given in a mean dosage of 0.065 mg/kg/d.
In the controlled trial (study 3), patients were randomly assigned to GH therapy (n = 15, 0.065 mg/kg/d) or no GH therapy (n = 15) for 3 years. At the end of this 3-year study, GH therapy was offered to all patients in a mean dosage of 0.085 mg/kg/d for the next 3 years. The dosage was then decreased to 0.065 mg/kg/d for the remainder of the follow-up.
GH dosage was adjusted for weight and serum insulinlike growth factor-1 (IGF-1) levels. The dosage was reduced by 20% when serum IGF-1 levels were >+2.5 SDs for sex, CA, and pubertal stage compared with normative data from our center (20). GH therapy was continued until adult height attainment, defined as growth velocity ≤1.5 cm during the last year and bone age ≥17 years in boys and ≥15 years in girls. GH therapy was discontinued after more than 6 months without glucocorticoid therapy in patients whose height was ≥−2 SDS. Patients off GH therapy continued to be followed until they attained adult height. GH treatment could be resumed if glucocorticoid therapy was resumed for at least 3 months.
Study parameters
Clinical data
The adult height of each parent and the height of each child recorded yearly during the 3 years preceding GH therapy initiation (baseline) were abstracted from the medical records. The same pediatric endocrinologist and a pediatric rheumatologist examined all the study patients at GH therapy initiation (baseline), then twice a year. The last follow-up visit occurred 1 year after the attainment of adult height. The target height was computed as [father’s height (centimeters) + mother’s height (centimeters)]/2 + 6.5 cm for boys and −6.5 cm for girls. Height and growth velocity were expressed as SDS/CA based on reference values for the French population (21). Adult height and target height were expressed as height SDS based on reference values for adult height in the French population (21). Age at menarche was abstracted from the medical records. The median height gain (centimeters, SD) before and during puberty was assessed only in the subgroup of patients who were prepubertal (Tanner stage <2) at baseline (22). Bone age was assessed on radiographs of the left hand according to Greulich and Pyle (23).
Age at JIA diagnosis and JIA type (systemic or polyarticular) were abstracted from the medical records. JIA was assessed at each visit by the pediatric rheumatologist, who recorded changes in glucocorticoid dosage and other antirheumatic drugs (immunosuppressants [methotrexate, cyclosporine] and biologics [the anti–interleukin (IL)-1 anakinra; the anti–IL-1 receptor canakinumab]; the anti–IL-6 tocilizumab; and the tumor necrosis factor-α antagonists infliximab, etanercept, and adalimumab).
Laboratory data
All laboratory tests were done at the same laboratory on samples obtained after an overnight fast. Blood tests were assessed at baseline and every 6 months. Serum IGF-1 levels (ng/mL) were measured by radioimmunoassay [IGF-I–radiometric immunoassay computed tomography (RIAACT); Cisbio, Gif-sur-Yvette, France]. The interassay coefficient of variation was <9%, and the limit of detection was 9 ng/mL. Serum concentrations were expressed as SDS for sex, CA, and pubertal stage according to normative data obtained at our center in children age >6 years (20). Mean values of the erythrocyte sedimentation rate, C-reactive protein (CRP), hemoglobin, platelets, and IGF-1 were calculated as the geometric mean of erythrocyte sedimentation rate, CRP, hemoglobin, platelets, and IGF-1 throughout follow-up.
Statistical analysis
Continuous variables were described as median and interquartile range (IQR) and categorical variables as frequencies. Variables were compared between groups by using the χ2 test or Fisher exact test for categorical variables and the nonparametric Mann-Whitney-Wilcoxon test for continuous variables. All tests were two-tailed, and P values < 0.05 were considered to indicate statistically significant differences. Changes in height SDS during follow-up [3 years before baseline (T − 3 years), at baseline (T), and at adult height attainment] and target height were compared by using tests for matched data (Wilcoxon signed-rank test or conditional logistic regression depending on conditions of validity). Bonferroni correction for multiple comparisons was applied when appropriate; this procedure set the threshold for significance at 0.017.
Adult height is within ±1.5 SDs of target height in 95% of normal children (24). Therefore, the primary outcome chosen for this study was a favorable growth outcome, defined as a corrected adult height ≤1.5 SDs. Factors associated with a favorable growth outcome were identified by univariate and multivariate logistic regression analyses. Before including a quantitative variable in the model, we assessed that it was linearly related to the log-odds. The initial multivariate model was adjusted for noncollinear covariates associated with P values < 0.20 in univariate models. Multivariate model selection was based on a stepwise procedure. Covariates associated with P values < 0.05 were kept in the final model. Odds ratios and their 95% confidence intervals were calculated. Statistical analyses were performed by using SAS statistical software, version 9.4 (SAS Institute, Cary, NC).
Ethics
The three prospective trials (NCT 00174291, NCT 00174187, and NCT 00174278) were conducted in accordance with the guidelines in the Declaration of Helsinki, Good Clinical Practice guidelines, and French legislation. Written informed consent was obtained from all parents or legal surrogates and from patients >7 years of age. The current study of data from these three studies was approved by our local institutional review board (CEERB, #2014/101), which waived the need for written informed consent.
Results
Patients
Of the 58 patients included in the three studies, 5 never received GH therapy, for the reasons shown in Fig. 1. Of the 53 patients given GH therapy, 48 (26 males) were followed until they reached their adult height [i.e., for a median of 8.6 years (IQR, 6.0 to 10.2 years)]. Adult height was unavailable for the remaining 5 patients (4 females), for the following reasons: death (n = 2, due to sudden death with bilateral pleural effusion), unavailable height measurements due to severe bone deformities (n = 2), or study withdrawal after glucocorticoid therapy discontinuation (n = 1).
Among the 48 patients whose adult height was available, 37 (77%) had systemic JIA and 11 (23%) had polyarticular JIA. Median CA at diagnosis was 3.7 years (IQR, 2.0 to 6.8 years) and median CA at glucocorticoid therapy was 4.0 years (IQR, 2.2 to 7.1 years). Over the years preceding GH initiation, growth velocity SDS was markedly decreased [−2.5 (IQR, −3.4 to −1.1), n = 37, at T − 3 years; −2.5 (IQR, −4.6 to −1.4) n = 40, at T − 2 years; and −2.9 (IQR, −4.8 to −1.2, n = 44) at T − 1 year]. Consequently, median height SDS worsened significantly during this period, from −1.1 (IQR, −2.6 to 0.4) at T − 3 years to −2.8 (IQR, −4.4 to −1.6) at baseline (T) (P < 0.001) (Fig. 2). At GH initiation, height SDS correlated negatively with CA (P < 0.01).
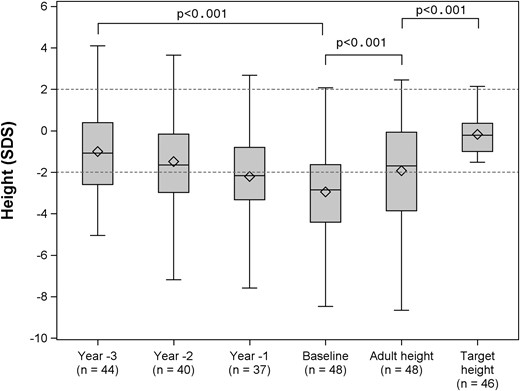
Changes in height SDS before and during GH treatment until the attainment of adult height SDS, along with target height SDS. Data are shown as 25th and 75th percentiles (box plots) and medians (rules), with whiskers at the 10th and 90th percentiles. The hatched area represents the normal range for height SDS.
Growth during follow-up
GH therapy was initiated 5.0 years (IQR, 2.4 to 8.2) after the diagnosis, at a median CA of 10.1 years (IQR, 7.3 to 13.3 years). Table 1 lists the main characteristics of the 48 patients and among them the two groups of patients treated (n = 31) or not treated (n = 17) with biologics during the follow-up. No significant difference in auxologic parameters was found between males and females at GH initiation (Supplemental Table 1).
Characteristics at Baseline and at Adult Height of Patients Treated With vs Not Treated With Biologics During Follow-up
| Variable . | Total (n = 48) . | Treatment With Biologics . | ||
|---|---|---|---|---|
| Biologics (n = 31) . | No Biologics (n = 17) . | P Valuea . | ||
| At baseline | ||||
| Male sex, n (%) | 15 (48.4) | 11 (64.7) | 0.28 | |
| CA (y) | 10.1 (7.3–13.3) | 8.6 (6.8–11.1) | 13.3 (10.3–14.8) | 0.01 |
| Glucocorticoid therapy duration (y) | 4.7 (2.3–7.5) | 4.1 (1.3–4.9) | 8.2 (4.8–10.3) | <0.01 |
| Height (SDS) | −2.9 (−4.4 to −1.6) | −2.5 (−3.6 to −1.3) | −4.2 (−5.0 to −2.9) | 0.03 |
| Bone age (y) (n = 46b) | 8.7 (5.5–10.0) | 7.5 (5.0–9.0) | 10.0 (7.8–11.3) | 0.02 |
| Target height (SDS) (n = 46b) | −0.2 (−1.0 to 0.4) | −0.3 (−1.0 to 0.3) | −0.2 (−0.9 to 0.4) | 0.78 |
| Height − target height (SDS) (n = 46b) | −2.5 (−4.1 to −1.4) | −2.2 (−3.0 to −1.3) | −3.9 (−4.6 to −2.4) | 0.03 |
| Age at biologics initiation (y) | 10.5 (8.6–13.1) | |||
| At adult height | ||||
| GH therapy duration (y) | 6.5 (4.7–8.0) | 7.0 (4.8–8.4) | 5.1 (4.7–6.6) | 0.14 |
| Glucocorticoid therapy duration (y) (n = 47c) | 12.2 (9.0–14.4) | 11.2 (8.6–14.0) | 12.6 (10.0–14.6) | 0.14 |
| CA (y) | 18.1 (17.0–20.1) | 17.9 (17.0–19.0) | 18.5 (17.6–20.8) | 0.25 |
| Bone age (y) (n = 40)d | 17.0 (16.0–18.0) | 17.0 (16.0–18.0) | 17.0 (16.0–18.0) | 0.47 |
| Adult height (SDS) | −1.7 (−3.9 to −0.1) | −1.5 (−3.0 to 0.1) | −2.6 (−4.2 to −0.4) | 0.16 |
| Corrected adult heighte(SDS) (n = 46b) | −1.3 (−3.0 to −0.2) | −0.9 (−2.3 to −0.1) | −2.0 (−3.7 to −1.0) | 0.08 |
| Height gain from baseline to adult height (SDS) | 1.0 (0.0– 2.2) | 1.0 (0.1–2.0) | 1.0 (−0.2 to 2.5) | 0.84 |
| Favorable growth outcome during GH therapy, n (%)f | 24 (52.2) (n = 46) | 19 (63.3) (n = 30) | 5 (31.3) (n = 16) | 0.06 |
| Variable . | Total (n = 48) . | Treatment With Biologics . | ||
|---|---|---|---|---|
| Biologics (n = 31) . | No Biologics (n = 17) . | P Valuea . | ||
| At baseline | ||||
| Male sex, n (%) | 15 (48.4) | 11 (64.7) | 0.28 | |
| CA (y) | 10.1 (7.3–13.3) | 8.6 (6.8–11.1) | 13.3 (10.3–14.8) | 0.01 |
| Glucocorticoid therapy duration (y) | 4.7 (2.3–7.5) | 4.1 (1.3–4.9) | 8.2 (4.8–10.3) | <0.01 |
| Height (SDS) | −2.9 (−4.4 to −1.6) | −2.5 (−3.6 to −1.3) | −4.2 (−5.0 to −2.9) | 0.03 |
| Bone age (y) (n = 46b) | 8.7 (5.5–10.0) | 7.5 (5.0–9.0) | 10.0 (7.8–11.3) | 0.02 |
| Target height (SDS) (n = 46b) | −0.2 (−1.0 to 0.4) | −0.3 (−1.0 to 0.3) | −0.2 (−0.9 to 0.4) | 0.78 |
| Height − target height (SDS) (n = 46b) | −2.5 (−4.1 to −1.4) | −2.2 (−3.0 to −1.3) | −3.9 (−4.6 to −2.4) | 0.03 |
| Age at biologics initiation (y) | 10.5 (8.6–13.1) | |||
| At adult height | ||||
| GH therapy duration (y) | 6.5 (4.7–8.0) | 7.0 (4.8–8.4) | 5.1 (4.7–6.6) | 0.14 |
| Glucocorticoid therapy duration (y) (n = 47c) | 12.2 (9.0–14.4) | 11.2 (8.6–14.0) | 12.6 (10.0–14.6) | 0.14 |
| CA (y) | 18.1 (17.0–20.1) | 17.9 (17.0–19.0) | 18.5 (17.6–20.8) | 0.25 |
| Bone age (y) (n = 40)d | 17.0 (16.0–18.0) | 17.0 (16.0–18.0) | 17.0 (16.0–18.0) | 0.47 |
| Adult height (SDS) | −1.7 (−3.9 to −0.1) | −1.5 (−3.0 to 0.1) | −2.6 (−4.2 to −0.4) | 0.16 |
| Corrected adult heighte(SDS) (n = 46b) | −1.3 (−3.0 to −0.2) | −0.9 (−2.3 to −0.1) | −2.0 (−3.7 to −1.0) | 0.08 |
| Height gain from baseline to adult height (SDS) | 1.0 (0.0– 2.2) | 1.0 (0.1–2.0) | 1.0 (−0.2 to 2.5) | 0.84 |
| Favorable growth outcome during GH therapy, n (%)f | 24 (52.2) (n = 46) | 19 (63.3) (n = 30) | 5 (31.3) (n = 16) | 0.06 |
Data are median (IQR) unless stated otherwise.
Data were analyzed using the Mann-Whitney-Wilcoxon test for nonnormally distributed continuous variables.
Data were missing for one male and one female.
Data were missing for one male.
Data were missing for four males and four females.
Adult height SDS − target height SDS.
Favorable growth outcome was defined as a corrected adult height (adult height − target height) ≤ 1.5 SDs.
Characteristics at Baseline and at Adult Height of Patients Treated With vs Not Treated With Biologics During Follow-up
| Variable . | Total (n = 48) . | Treatment With Biologics . | ||
|---|---|---|---|---|
| Biologics (n = 31) . | No Biologics (n = 17) . | P Valuea . | ||
| At baseline | ||||
| Male sex, n (%) | 15 (48.4) | 11 (64.7) | 0.28 | |
| CA (y) | 10.1 (7.3–13.3) | 8.6 (6.8–11.1) | 13.3 (10.3–14.8) | 0.01 |
| Glucocorticoid therapy duration (y) | 4.7 (2.3–7.5) | 4.1 (1.3–4.9) | 8.2 (4.8–10.3) | <0.01 |
| Height (SDS) | −2.9 (−4.4 to −1.6) | −2.5 (−3.6 to −1.3) | −4.2 (−5.0 to −2.9) | 0.03 |
| Bone age (y) (n = 46b) | 8.7 (5.5–10.0) | 7.5 (5.0–9.0) | 10.0 (7.8–11.3) | 0.02 |
| Target height (SDS) (n = 46b) | −0.2 (−1.0 to 0.4) | −0.3 (−1.0 to 0.3) | −0.2 (−0.9 to 0.4) | 0.78 |
| Height − target height (SDS) (n = 46b) | −2.5 (−4.1 to −1.4) | −2.2 (−3.0 to −1.3) | −3.9 (−4.6 to −2.4) | 0.03 |
| Age at biologics initiation (y) | 10.5 (8.6–13.1) | |||
| At adult height | ||||
| GH therapy duration (y) | 6.5 (4.7–8.0) | 7.0 (4.8–8.4) | 5.1 (4.7–6.6) | 0.14 |
| Glucocorticoid therapy duration (y) (n = 47c) | 12.2 (9.0–14.4) | 11.2 (8.6–14.0) | 12.6 (10.0–14.6) | 0.14 |
| CA (y) | 18.1 (17.0–20.1) | 17.9 (17.0–19.0) | 18.5 (17.6–20.8) | 0.25 |
| Bone age (y) (n = 40)d | 17.0 (16.0–18.0) | 17.0 (16.0–18.0) | 17.0 (16.0–18.0) | 0.47 |
| Adult height (SDS) | −1.7 (−3.9 to −0.1) | −1.5 (−3.0 to 0.1) | −2.6 (−4.2 to −0.4) | 0.16 |
| Corrected adult heighte(SDS) (n = 46b) | −1.3 (−3.0 to −0.2) | −0.9 (−2.3 to −0.1) | −2.0 (−3.7 to −1.0) | 0.08 |
| Height gain from baseline to adult height (SDS) | 1.0 (0.0– 2.2) | 1.0 (0.1–2.0) | 1.0 (−0.2 to 2.5) | 0.84 |
| Favorable growth outcome during GH therapy, n (%)f | 24 (52.2) (n = 46) | 19 (63.3) (n = 30) | 5 (31.3) (n = 16) | 0.06 |
| Variable . | Total (n = 48) . | Treatment With Biologics . | ||
|---|---|---|---|---|
| Biologics (n = 31) . | No Biologics (n = 17) . | P Valuea . | ||
| At baseline | ||||
| Male sex, n (%) | 15 (48.4) | 11 (64.7) | 0.28 | |
| CA (y) | 10.1 (7.3–13.3) | 8.6 (6.8–11.1) | 13.3 (10.3–14.8) | 0.01 |
| Glucocorticoid therapy duration (y) | 4.7 (2.3–7.5) | 4.1 (1.3–4.9) | 8.2 (4.8–10.3) | <0.01 |
| Height (SDS) | −2.9 (−4.4 to −1.6) | −2.5 (−3.6 to −1.3) | −4.2 (−5.0 to −2.9) | 0.03 |
| Bone age (y) (n = 46b) | 8.7 (5.5–10.0) | 7.5 (5.0–9.0) | 10.0 (7.8–11.3) | 0.02 |
| Target height (SDS) (n = 46b) | −0.2 (−1.0 to 0.4) | −0.3 (−1.0 to 0.3) | −0.2 (−0.9 to 0.4) | 0.78 |
| Height − target height (SDS) (n = 46b) | −2.5 (−4.1 to −1.4) | −2.2 (−3.0 to −1.3) | −3.9 (−4.6 to −2.4) | 0.03 |
| Age at biologics initiation (y) | 10.5 (8.6–13.1) | |||
| At adult height | ||||
| GH therapy duration (y) | 6.5 (4.7–8.0) | 7.0 (4.8–8.4) | 5.1 (4.7–6.6) | 0.14 |
| Glucocorticoid therapy duration (y) (n = 47c) | 12.2 (9.0–14.4) | 11.2 (8.6–14.0) | 12.6 (10.0–14.6) | 0.14 |
| CA (y) | 18.1 (17.0–20.1) | 17.9 (17.0–19.0) | 18.5 (17.6–20.8) | 0.25 |
| Bone age (y) (n = 40)d | 17.0 (16.0–18.0) | 17.0 (16.0–18.0) | 17.0 (16.0–18.0) | 0.47 |
| Adult height (SDS) | −1.7 (−3.9 to −0.1) | −1.5 (−3.0 to 0.1) | −2.6 (−4.2 to −0.4) | 0.16 |
| Corrected adult heighte(SDS) (n = 46b) | −1.3 (−3.0 to −0.2) | −0.9 (−2.3 to −0.1) | −2.0 (−3.7 to −1.0) | 0.08 |
| Height gain from baseline to adult height (SDS) | 1.0 (0.0– 2.2) | 1.0 (0.1–2.0) | 1.0 (−0.2 to 2.5) | 0.84 |
| Favorable growth outcome during GH therapy, n (%)f | 24 (52.2) (n = 46) | 19 (63.3) (n = 30) | 5 (31.3) (n = 16) | 0.06 |
Data are median (IQR) unless stated otherwise.
Data were analyzed using the Mann-Whitney-Wilcoxon test for nonnormally distributed continuous variables.
Data were missing for one male and one female.
Data were missing for one male.
Data were missing for four males and four females.
Adult height SDS − target height SDS.
Favorable growth outcome was defined as a corrected adult height (adult height − target height) ≤ 1.5 SDs.
Patients were treated with GH for a median duration of 6.5 years (IQR, 4.7 to 8.0 years). According to the study protocol, 29 (60%) patients received GH until they attained their adult height and 11 patients (22%) stopped GH therapy after glucocorticoid discontinuation. The remaining 8 (16%) patients decided to stop GH treatment because of personal reasons. The median GH dosage during follow-up was 0.056 mg/kg/d (IQR, 0.050 to 0.062 mg/kg/d). Median IGF-1 levels increased significantly, from 168 ng/mL (IQR, 114 to 223 ng/mL) at baseline to 471 ng/mL (IQR, 276 to 672 ng/mL) after the first year of GH treatment (P < 0.001) [i.e., from −1.2 SDS (IQR, −2.4 to 0.1) to 1.9 SDS (IQR, 0.6 to 3.3); P < 0.001]. Table 2 reports mean IGF-1 values during follow-up.
Laboratory Parameters and Glucocorticoid Dosages at Baseline and During Follow-up
| Variable . | At Baseline (n = 48a) . | Mean During Follow up (n = 48) . |
|---|---|---|
| ESR (mm) | 40 (18–64) | 20 (13–37) |
| CRP (mg/L) | 36 (12–71) (n = 33) | 20 (9–38) (n = 45) |
| Platelets (G/L) | 422 (324–584) (n = 34) | 353 (291–444) (n = 47) |
| Hemoglobin (g/dL) | 11.0 (10.0–11.8) (n = 34) | 11.7 (10.7–12.5) (n = 47) |
| Prednisone equivalent (mg/kg per d) | 0.4 (0.2–0.5)b | 0.2 (0.1–0.4) |
| IGF1 (ng/mL) | 168 (114–223) (n = 46) | 493 (353–585) |
| IGF1 (SDS)c | −1.2 (−2.4 to 0.1) (n = 38) | 1.0 (0.1−2.1) |
| Variable . | At Baseline (n = 48a) . | Mean During Follow up (n = 48) . |
|---|---|---|
| ESR (mm) | 40 (18–64) | 20 (13–37) |
| CRP (mg/L) | 36 (12–71) (n = 33) | 20 (9–38) (n = 45) |
| Platelets (G/L) | 422 (324–584) (n = 34) | 353 (291–444) (n = 47) |
| Hemoglobin (g/dL) | 11.0 (10.0–11.8) (n = 34) | 11.7 (10.7–12.5) (n = 47) |
| Prednisone equivalent (mg/kg per d) | 0.4 (0.2–0.5)b | 0.2 (0.1–0.4) |
| IGF1 (ng/mL) | 168 (114–223) (n = 46) | 493 (353–585) |
| IGF1 (SDS)c | −1.2 (−2.4 to 0.1) (n = 38) | 1.0 (0.1−2.1) |
Data are median (IQR). Normal values: ESR <20 mm, CRP <10 mg/L, hemoglobin 11 to 15 g/dL, platelets 150 to 450 G/L.
Abbreviation: ESR, erythrocyte sedimentation rate.
n = 48 unless stated otherwise.
Mean during the first year of follow-up.
Available only in patients age >6 years.
Laboratory Parameters and Glucocorticoid Dosages at Baseline and During Follow-up
| Variable . | At Baseline (n = 48a) . | Mean During Follow up (n = 48) . |
|---|---|---|
| ESR (mm) | 40 (18–64) | 20 (13–37) |
| CRP (mg/L) | 36 (12–71) (n = 33) | 20 (9–38) (n = 45) |
| Platelets (G/L) | 422 (324–584) (n = 34) | 353 (291–444) (n = 47) |
| Hemoglobin (g/dL) | 11.0 (10.0–11.8) (n = 34) | 11.7 (10.7–12.5) (n = 47) |
| Prednisone equivalent (mg/kg per d) | 0.4 (0.2–0.5)b | 0.2 (0.1–0.4) |
| IGF1 (ng/mL) | 168 (114–223) (n = 46) | 493 (353–585) |
| IGF1 (SDS)c | −1.2 (−2.4 to 0.1) (n = 38) | 1.0 (0.1−2.1) |
| Variable . | At Baseline (n = 48a) . | Mean During Follow up (n = 48) . |
|---|---|---|
| ESR (mm) | 40 (18–64) | 20 (13–37) |
| CRP (mg/L) | 36 (12–71) (n = 33) | 20 (9–38) (n = 45) |
| Platelets (G/L) | 422 (324–584) (n = 34) | 353 (291–444) (n = 47) |
| Hemoglobin (g/dL) | 11.0 (10.0–11.8) (n = 34) | 11.7 (10.7–12.5) (n = 47) |
| Prednisone equivalent (mg/kg per d) | 0.4 (0.2–0.5)b | 0.2 (0.1–0.4) |
| IGF1 (ng/mL) | 168 (114–223) (n = 46) | 493 (353–585) |
| IGF1 (SDS)c | −1.2 (−2.4 to 0.1) (n = 38) | 1.0 (0.1−2.1) |
Data are median (IQR). Normal values: ESR <20 mm, CRP <10 mg/L, hemoglobin 11 to 15 g/dL, platelets 150 to 450 G/L.
Abbreviation: ESR, erythrocyte sedimentation rate.
n = 48 unless stated otherwise.
Mean during the first year of follow-up.
Available only in patients age >6 years.
During GH treatment, annual growth velocity SDS/CA seemed to correlate negatively with the cumulated prednisone dosage (mg/y) during the same period (Supplemental Fig. 1). Height SDS increased significantly from baseline by a median of 1.0 SD (IQR, 0.0 to 2.2) (Table 1). This height gain did not differ significantly between boys and girls (Supplemental Table 1) or between patients treated or not treated with biologics. The median gain in height SD was ≥2.5 in 8 (16.7%) patients, ≥1.5 and <2.5 in 12 (25%) patients, and ≥0.5 and <1.5 in 11 (23%) patients. In 11 (23%) patients, height SDS was classified as stable, defined as no change >0.5 in either direction. Six (12.5%) patients continued to lose height (>−0.5 SD) during follow-up.
Patients reached their adult height at a median CA of 18.1 years (IQR, 17.0 to 20.1 years). Adult height was within the normal range (≥−2 SDS) in 52% of patients. Median adult height SDS remained significantly lower than target height SDS (P < 0.001). Median adult height was 9 cm (IQR, −17.5 to −2.2 cm) below median target height. Among the 46 patients whose target height was available, the difference between height SDS and target height SDS decreased from −2.5 (IQR, −4.1 to −1.4) at baseline to −1.3 (−3.0 to −0.2) at adult height. The growth outcome was favorable in 24 (52.2%) patients overall, in 19 of 30 (63.3%) patients treated with biologics, and in 5 of 16 (31.3%) untreated patients (P = 0.06).
Of the 48 patients, 38 (79%; 16 girls and 22 boys) were prepubertal at GH initiation. In the boys, median GH therapy duration before puberty was 4 years (IQR, 2.0 to 4.5 years), and during this period median height gain was 17.5 cm (IQR, 5.0 to 31.1 cm) [0.2 SD (IQR, −0.5 to 1.5)]. In the girls, median GH therapy duration before puberty was 2.3 years (IQR, 1.5 to 5.0 years), during which median height gain was 13.6 cm (IQR, 5.0 to 27.5 cm) [0.3 SD (IQR, −1.4 to 1.0)]. Median age at puberty was 12.8 years (IQR, 11.8 to 13.9 years) in boys and 11.9 years (IQR, 10.4 to 14.1 years) in girls. Five boys received testosterone therapy for 2.7 years (IQR, 1.9 to 3.4 years) and two girls received estrogen therapy for 2.3 years, for delayed or slowly progressing puberty. The median height gain during puberty was 26.8 cm (IQR, 22.8 to 31.2 cm) [0.4 SD (IQR, −0.1 to 1.3)] in boys and 18.9 cm (IQR, 15.3 to 25.4 cm) [1.3 SD (IQR, 0.6 to 1.6)] in girls, over a median duration of 5.3 years. Of the 16 girls who were prepubertal at GH initiation and whose age at menarche was available, 14 had a median age at menarche of 14.3 years (IQR, 13.1 to 16.8 years). Data were missing for 2 patients.
Disease activity during follow-up
Median glucocorticoid therapy duration from the diagnosis to the end of follow-up was 12.2 years (IQR, 9.0 to 14.4 years). CA at glucocorticoid therapy discontinuation was 16.3 years (IQR, 14.0 to 19.0 years). At adult height attainment, 8 (16.6%) patients were still taking glucocorticoid therapy. Biologics were used during follow-up in 31 (64.6%) patients, starting at a median CA of 10.5 years (IQR, 8.6 to 13.1 years) and for a median duration of 4.9 years (IQR, 3.2 to 7.4 years). Other immunosuppressants were used during follow-up in 36 (75%) patients. Table 2 reports data on disease activity parameters.
Factors associated with growth outcome
A favorable growth outcome was positively associated with height SDS at GH initiation and negatively associated with older age at GH initiation and with CRP values indicating greater severity of inflammation during follow-up (Table 3).
Univariate and Multivariable Odds Ratios of a Favorable Growth Outcome During GH Therapy
| Factors . | Univariate Analysis . | Multivariate Analysis (n = 42) . | ||
|---|---|---|---|---|
| Crude OR (95% CI) . | P Value . | Adjusted OR (95% CI) . | P Value . | |
| At baseline | ||||
| Girls (vs boys) | 1.44 (0.45–4.64) | 0.54 | ||
| Polyarticular JIA (vs systemic) | 1.13 (0.29–4.41) | 0.86 | ||
| Age (per additional y) | 0.67 (0.53–0.85) | <0.01 | 0.68 (0.47–0.99) | 0.04 |
| Height (per additional SDS point) | 3.21 (1.66–3.24) | <0.01 | 2.61 (1.15–5.96) | 0.02 |
| Prepubertal (vs pubertal) | 7.62 (1.42–40.80) | 0.02 | ||
| Bone age (per additional y) | 0.77 (0.61–0.96) | 0.02 | ||
| Time spent on GC treatment (per additional y) | 0.58 (0.41–0.82) | <0.01 | ||
| During follow-up | ||||
| Mean ESR (per additional 10 mm/h) | 0.66 (0.45–0.97) | 0.03 | ||
| Mean CRP values (per additional 10 mg/L) | 0.67 (0.46–0.96) | 0.03 | 0.51 (0.28–0.92) | 0.03 |
| Mean hemoglobin values (per additional g/dL) | 1.54 (0.93–2.55) | 0.09 | ||
| Mean platelets values (per additional 10 g/L) | 0.81 (0.55–1.19) | 0.29 | ||
| Mean IGF-1 values (per additional SDS point) | 1.51 (0.98–2.31) | 0.06 | ||
| Time spent on GC treatment (per additional y) | 0.71 (0.57–0.89) | <0.01 | ||
| Mean GC dose (per additional 0.1 mg/k/d) | 0.81 (0.53–1.23) | 0.32 | ||
| Combined treatment with biologics | 3.80 (1.04–13.83) | 0.04 | ||
| Combined treatment with other immunosuppressants | 1.40 (0.37–5.29) | 0.62 | ||
| Factors . | Univariate Analysis . | Multivariate Analysis (n = 42) . | ||
|---|---|---|---|---|
| Crude OR (95% CI) . | P Value . | Adjusted OR (95% CI) . | P Value . | |
| At baseline | ||||
| Girls (vs boys) | 1.44 (0.45–4.64) | 0.54 | ||
| Polyarticular JIA (vs systemic) | 1.13 (0.29–4.41) | 0.86 | ||
| Age (per additional y) | 0.67 (0.53–0.85) | <0.01 | 0.68 (0.47–0.99) | 0.04 |
| Height (per additional SDS point) | 3.21 (1.66–3.24) | <0.01 | 2.61 (1.15–5.96) | 0.02 |
| Prepubertal (vs pubertal) | 7.62 (1.42–40.80) | 0.02 | ||
| Bone age (per additional y) | 0.77 (0.61–0.96) | 0.02 | ||
| Time spent on GC treatment (per additional y) | 0.58 (0.41–0.82) | <0.01 | ||
| During follow-up | ||||
| Mean ESR (per additional 10 mm/h) | 0.66 (0.45–0.97) | 0.03 | ||
| Mean CRP values (per additional 10 mg/L) | 0.67 (0.46–0.96) | 0.03 | 0.51 (0.28–0.92) | 0.03 |
| Mean hemoglobin values (per additional g/dL) | 1.54 (0.93–2.55) | 0.09 | ||
| Mean platelets values (per additional 10 g/L) | 0.81 (0.55–1.19) | 0.29 | ||
| Mean IGF-1 values (per additional SDS point) | 1.51 (0.98–2.31) | 0.06 | ||
| Time spent on GC treatment (per additional y) | 0.71 (0.57–0.89) | <0.01 | ||
| Mean GC dose (per additional 0.1 mg/k/d) | 0.81 (0.53–1.23) | 0.32 | ||
| Combined treatment with biologics | 3.80 (1.04–13.83) | 0.04 | ||
| Combined treatment with other immunosuppressants | 1.40 (0.37–5.29) | 0.62 | ||
A favorable growth outcome was defined as a corrected adult height (adult height − target height) ≤−1.5 SDs.
Abbreviations: CI, confidence interval; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GC, glucocorticoid; OR, odds ratio.
Univariate and Multivariable Odds Ratios of a Favorable Growth Outcome During GH Therapy
| Factors . | Univariate Analysis . | Multivariate Analysis (n = 42) . | ||
|---|---|---|---|---|
| Crude OR (95% CI) . | P Value . | Adjusted OR (95% CI) . | P Value . | |
| At baseline | ||||
| Girls (vs boys) | 1.44 (0.45–4.64) | 0.54 | ||
| Polyarticular JIA (vs systemic) | 1.13 (0.29–4.41) | 0.86 | ||
| Age (per additional y) | 0.67 (0.53–0.85) | <0.01 | 0.68 (0.47–0.99) | 0.04 |
| Height (per additional SDS point) | 3.21 (1.66–3.24) | <0.01 | 2.61 (1.15–5.96) | 0.02 |
| Prepubertal (vs pubertal) | 7.62 (1.42–40.80) | 0.02 | ||
| Bone age (per additional y) | 0.77 (0.61–0.96) | 0.02 | ||
| Time spent on GC treatment (per additional y) | 0.58 (0.41–0.82) | <0.01 | ||
| During follow-up | ||||
| Mean ESR (per additional 10 mm/h) | 0.66 (0.45–0.97) | 0.03 | ||
| Mean CRP values (per additional 10 mg/L) | 0.67 (0.46–0.96) | 0.03 | 0.51 (0.28–0.92) | 0.03 |
| Mean hemoglobin values (per additional g/dL) | 1.54 (0.93–2.55) | 0.09 | ||
| Mean platelets values (per additional 10 g/L) | 0.81 (0.55–1.19) | 0.29 | ||
| Mean IGF-1 values (per additional SDS point) | 1.51 (0.98–2.31) | 0.06 | ||
| Time spent on GC treatment (per additional y) | 0.71 (0.57–0.89) | <0.01 | ||
| Mean GC dose (per additional 0.1 mg/k/d) | 0.81 (0.53–1.23) | 0.32 | ||
| Combined treatment with biologics | 3.80 (1.04–13.83) | 0.04 | ||
| Combined treatment with other immunosuppressants | 1.40 (0.37–5.29) | 0.62 | ||
| Factors . | Univariate Analysis . | Multivariate Analysis (n = 42) . | ||
|---|---|---|---|---|
| Crude OR (95% CI) . | P Value . | Adjusted OR (95% CI) . | P Value . | |
| At baseline | ||||
| Girls (vs boys) | 1.44 (0.45–4.64) | 0.54 | ||
| Polyarticular JIA (vs systemic) | 1.13 (0.29–4.41) | 0.86 | ||
| Age (per additional y) | 0.67 (0.53–0.85) | <0.01 | 0.68 (0.47–0.99) | 0.04 |
| Height (per additional SDS point) | 3.21 (1.66–3.24) | <0.01 | 2.61 (1.15–5.96) | 0.02 |
| Prepubertal (vs pubertal) | 7.62 (1.42–40.80) | 0.02 | ||
| Bone age (per additional y) | 0.77 (0.61–0.96) | 0.02 | ||
| Time spent on GC treatment (per additional y) | 0.58 (0.41–0.82) | <0.01 | ||
| During follow-up | ||||
| Mean ESR (per additional 10 mm/h) | 0.66 (0.45–0.97) | 0.03 | ||
| Mean CRP values (per additional 10 mg/L) | 0.67 (0.46–0.96) | 0.03 | 0.51 (0.28–0.92) | 0.03 |
| Mean hemoglobin values (per additional g/dL) | 1.54 (0.93–2.55) | 0.09 | ||
| Mean platelets values (per additional 10 g/L) | 0.81 (0.55–1.19) | 0.29 | ||
| Mean IGF-1 values (per additional SDS point) | 1.51 (0.98–2.31) | 0.06 | ||
| Time spent on GC treatment (per additional y) | 0.71 (0.57–0.89) | <0.01 | ||
| Mean GC dose (per additional 0.1 mg/k/d) | 0.81 (0.53–1.23) | 0.32 | ||
| Combined treatment with biologics | 3.80 (1.04–13.83) | 0.04 | ||
| Combined treatment with other immunosuppressants | 1.40 (0.37–5.29) | 0.62 | ||
A favorable growth outcome was defined as a corrected adult height (adult height − target height) ≤−1.5 SDs.
Abbreviations: CI, confidence interval; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GC, glucocorticoid; OR, odds ratio.
Discussion
This study describes growth in a group of patients with JIA given long-term glucocorticoid therapy before and during GH therapy, until they reached their adult height. GH therapy was effective in arresting the loss of height observed over the years preceding treatment initiation. GH therapy significantly increased height SDS but failed to fully restore the genetic growth potential. Thus, overall, the target height was missed by 9 cm, and only 52.2% of patients had an adult height within their target height. The main determinants of growth outcome were severity of inflammation and age and height at GH initiation.
In chronic inflammatory diseases, inflammatory cytokines and exogenous glucocorticoid exposure affect growth through systemic effects on the GH–IGF-1 axis and local effects on the growth plates. Low plasma IGF-1 levels are related to systemic GH insufficiency or to hepatic GH resistance (25, 26). Changes in binding proteins have also been reported (6, 27). At the growth plates, cytokines and glucocorticoids suppress chondrocyte proliferation, increase apoptosis, decrease expression of cartilage matrix proteins (7), and may alter GH and/or IGF-1 signaling (28).
In the first study, all patients but one were GH sufficient (14). Normal GH secretion was an inclusion criterion in the second study. In the randomized trial, an assessment of GH secretion was not required before inclusion. Patients were treated with supraphysiologic GH dosages higher than those prescribed in other growth-retarded GH-sufficient children, in whom GH dosages ranged from 0.035 to 0.050 mg/kg per day. IGF-1 levels increased significantly during the first year of GH treatment, indicating some degree of GH sensitivity and good treatment adherence. GH dosages were adjusted during follow-up to maintain IGF-1 levels within the normal range. There is no consensus so far about the optimal GH dosages for patients with JIA, and no study has compared long-term growth outcomes with different GH dosages. Many factors may influence the IGF-1 response to GH injections, such as nutrition, sex, pubertal stage, and disease-related factors. Improved knowledge of these factors should facilitate the design of more individualized treatment schedules in the future. The absolute height gain was +1 SD after 6.5 years of GH treatment. This height gain was smaller than previously reported in patients with JIA treated with GH (19).
The patients who form the basis for this study were severely ill, as shown by the predominance of systemic JIA, young age at disease onset, and long duration of glucocorticoid therapy. They fulfilled criteria for a poor prognosis in systemic JIA (29) and were at high risk for final short stature. Growth velocity was markedly decreased over the years preceding GH treatment, and height SDS worsened gradually during the course of the disease, in keeping with data from observational studies (4). The loss of height was greater in patients with long-lasting disease, as reflected by the negative correlation between age at GH onset and severity of growth impairment. After GH initiation, a significant height gain occurred until the attainment of adult height, despite the persistent inflammation and glucocorticoid therapy. Our study design does not allow us to determine whether spontaneous catch-up growth would have occurred during the course of the disease. However, spontaneous catch-up growth has rarely been reported during the natural history of severe JIA (17, 19, 30) even after disease remission and glucocorticoid therapy discontinuation (4). It can be argued that these historical groups differ from our patients and that a weakness of our study is the absence of a contemporary control group. However, given the long time needed to achieve adult height and the introduction of new therapies in recent decades, data on adult height cannot be entirely relevant to patients currently undergoing growth.
Height SDS increased significantly during GH treatment. Nevertheless, GH treatment did not fully restore the patients’ genetic height potential. Thus, our patients missed their target height by 9 cm (−1.3 SD), in keeping with results reported by Bechtold et al. (19). Whereas 64.7% of patients experienced catch-up growth, height SDS stabilized without catch-up growth in 20% of patients, and 12% obtained no benefits from GH treatment. This interindividual variability suggests that chronic disease per se may limit the efficacy of GH therapy. Greater severity of inflammation with higher CRP levels was associated with worse growth outcomes. The inverse relationship that we found between glucocorticoid dosage and growth velocity was also consistent with the effect of inflammation on growth. Recent studies reported that biologics were highly effective in systemic JIA (8, 10, 31) and that patients experienced catch-up growth with better disease control (9). Thus, the growth improvement observed in our patients was likely due to combined effects of GH treatment and steroid-sparing biologics.
Patients who were younger and taller at GH initiation had the best growth outcomes, defined as a corrected adult height ≤−1.5 SD. Young age and/or delayed bone maturation are often reported as predicting a good growth response to GH treatment and as indicating a good potential for catch-up growth. In animals, glucocorticoid exposure conserves the proliferative capacity of chondrocytes and delays growth-plate senescence, allowing catch-up growth to occur after glucocorticoid discontinuation (32, 33). On the other hand, sustained exposure to inflammatory cytokines may have irreversible adverse effects on growth plate chondrogenesis (34) that may explain the partial and inconsistent catch-up growth seen after a remission is achieved (4) and the weaker growth response to GH therapy in older patients with longer-lasting disease. The loss of height observed during JIA, unpredictable severity of the disease, and limiting effects of chronic inflammation on the growth response to GH support the initiation of GH therapy before the growth deficiency becomes severe.
Slow progression of puberty, decreased peak height velocity, and delayed menarche have been reported in patients with JIA. Inflammation contributes to delayed puberty (35, 36). In the prepubertal children in our study given GH therapy, median age at puberty onset was delayed and sex steroid therapy was required in 18% of patients. Age at menarche was delayed by about 2 years according to French normative data (37). The pubertal height gain during GH treatment was within the normal range in both sexes and extended the catch-up growth initiated during the prepubertal period. In patients with delayed puberty, added sex steroid therapy may deserve consideration to optimize the height gain during GH therapy and to limit the muscle wasting and bone loss related to inflammation. Further studies are needed to evaluate this possibility.
Previous studies are reassuring concerning glucose tolerance (38) and the disease process during GH treatment (13–19). However, long-term follow-up is crucial to evaluate potential adverse events, particularly the potential increased risk for malignancy (39, 40). National or worldwide registries of patients with JIA treated with biologics and/or GH will be helpful to collect such long-term data. In addition, more work is required to evaluate the potential impact of height gains on the quality of life and psychological health of patients with JIA and to assess the extent to which these benefits compensate for the burden of daily GH injections.
In conclusion, this study showed that height increased significantly during GH treatment in patients with JIA. However, the growth response to GH varied substantially across patients. The main factor limiting the growth response to GH treatment was disease severity, which was unpredictable. Biologic agents are more efficient in achieving tight inflammation control and thus ensuring optimal growth in patients with JIA. However, clinical monitoring of growth remains crucial to detect persistent growth failure leading to clinically significant short stature in poor responders, who may benefit from GH therapy.
Abbreviations:
- aOR
adjusted odds ratio
- CA
chronologic age
- CI
confidence interval
- CRP
C-reactive protein
- GH
growth hormone
- IGF-1
insulinlike growth factor-1
- IL
interleukin
- IQR
interquartile range
- JIA
juvenile idiopathic arthritis
- OR
odds ratio
- SD
standard deviation
- SDS
standard deviation score.
Acknowledgments
We thank Dr. Bader Meunier, Professor L. David, Dr. A. Duquesne, Dr. C. Gay, Dr. C. Job Deslandres, Professor I. Kone Paut, Dr. A.M. Prieur, and Dr F. Mazingues for recruiting the patients. We also thank the nurses of the clinical investigation center for their care to the patients included in these trials. We acknowledge the role of Professor Paul Czernichow at the early phases of these studies.
Financial Support: These clinical trials were supported by Pfizer. No funding was received for this report. Data collection, analysis, and interpretation and the decision to submit the paper for publication were the responsibility of the authors alone.
Clinical Trial Information: ClinicalTrials.gov no. NCT 00174291, NCT 00174187, NCT 00174278 (registered 15 September 2005).
Disclosure Summary: D.S. received honoraria from Pfizer as a clinical investigator in the studies that provided the data for the current study. J.-C.C. has the following conflicts of interest to declare, all outside the scope of this work: investigator in clinical trials using GH sponsored by Pfizer and Lilly and in postmarketing studies with several brands of GH, as well as support for travel to international meetings from several GH manufacturers. The remaining authors have nothing to disclose.
References
Tanner JM.



