-
PDF
- Split View
-
Views
-
Cite
Cite
Hanna Huopio, Päivi J. Miettinen, Jorma Ilonen, Päivi Nykänen, Riitta Veijola, Päivi Keskinen, Kirsti Näntö-Salonen, Jagadish Vangipurapu, Joose Raivo, Alena Stančáková, Jonna Männistö, Teemu Kuulasmaa, Mikael Knip, Timo Otonkoski, Markku Laakso, Clinical, Genetic, and Biochemical Characteristics of Early-Onset Diabetes in the Finnish Population, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 8, 1 August 2016, Pages 3018–3026, https://doi.org/10.1210/jc.2015-4296
Close - Share Icon Share
Major advances have been made in the classification and genetics of monogenic diabetes in infancy.
The objective of the study was to characterize different forms of diabetes diagnosed during the first year of life.
Patients diagnosed with diabetes before the age of 1 year in 10 Finnish hospitals from 1980 to 2014 were included.
The study was conducted at Kuopio University Hospital and University of Eastern Finland.
Patients were identified through diagnosis-based searches from hospital registries including 93 children, of whom 64 participated.
DNA sample for sequencing, serum sample, and medical records interventions were included.
Incidence of diabetes during the first year of life, sequencing results, human leukocyte antigen (HLA) genotypes, and islet autoantibodies were measured.
The incidence of diabetes diagnosed during the first 12 months was 4.4/100 000/year. Three novel and 11 previously described mutations were found in 22 patients from 15 families in the KCNJ11, ABCC8, INS, GCK, FOXP, STAT3, and RFX6 genes. Positive islet autoantibodies were observed in 40.0% of the patients diagnosed during the first 0–6 months of life vs 70.8% of the patients diagnosed between ages of 7 to 12 months. A total of 85.7% of the patients carrying protective HLA genotypes were mutation-positive compared to 7.7% of the patients having high-risk genotypes (P = .001).
Mutations in the K-ATP channel and INS genes were the most common cause of early diagnosed monogenic diabetes. After 6 months of age, patients with diabetes had high HLA risk genotypes and islet autoantibodies, reflecting the autoimmune character of diabetes in that age group.
Targeted sequencing of 104 genes associated with glucose metabolism, incidence of different HLA genotypes and diabetes-associated autoantibodies were analyzed in patients diagnosed with diabetes during the first year of life.
Monogenic diabetes of infancy represents approximately 1–4% of all cases of diabetes diagnosed in children (1–3). The overall incidence of monogenic diabetes of infancy has been estimated to be less than 2 in 100 000, although there is variation across different populations (4–6). Neonatal diabetes (NDM) is usually diagnosed before the age of 6 months, and after that age type 1 diabetes is more likely. Both permanent (PNDM) and transient forms of NDM exist, and approximately 50% of the cases require lifelong treatment (7, 8). In most cases, diabetes is isolated but some rare forms are associated with extrapancreatic syndromic features (9).
Major advances in the sequencing technologies have allowed the classification of monogenic diabetes into different subgroups. At least 20 different genes have been associated with monogenic diabetes. These genes either impair the development or function of pancreatic beta cells or lead to beta cell destruction. Activating mutations in the K-ATP channel genes ABCC8 and KCNJ11 encoding sulfonylurea receptor 1 and potassium inward rectifier (Kir6.2) proteins, are the most common causes accounting for approximately 40% of all cases (10, 11). Heterozygous mutations in the INS gene are the second most common causes of neonatal diabetes (12). Together, these three genes account for at least 50–75% of cases with PNDM (13). Genetic etiology of the patients with NDM at very young age remains unknown in about 40% of cases (13). The identification of the genetic etiology of different forms of diabetes helps clinical decision-making and the choice of appropriate treatment. For example, about 90% of the patients with K-ATP channel mutations can be successfully treated with sulfonylurea (SU) instead of insulin (14). Knowing the genetic basis also helps to predict the clinical course of the disease which is of essential importance in genetic counseling.
Finland has the highest prevalence and incidence of childhood autoimmune type 1 diabetes (T1D) in the world. This has been interpreted as a consequence of environment changes (15). Finns also have a high prevalence of some recessive diseases because of the enrichment of specific rare genetic variants due to the population “bottlenecks” (16). This was also observed in our previous studies of congenital hyperinsulinism (CHI), where two founder mutations in ABCC8 were responsible for most the CHI cases (17, 18).
In the current study, we characterized patients with diabetes diagnosed during the first year of life to identify subtypes of diabetes of infancy in Finns. Furthermore, we investigated the characteristics of monogenic diabetes and type 1 diabetes in order to differentiate these two conditions in the clinical context.
Materials and Methods
Study participants.
The study population was identified by diagnosis-based searches (Disease classification 1969, 1980 and ICD-10 since 1996), from the existing hospital registries of all five University Hospitals and the largest central hospitals in Finland. A total of 93 children were diagnosed with diabetes before the age of 1 year between the years 1980–2014. All patients were invited to the clinical examination by a letter including information on the study protocol and blood sampling for DNA and islet autoantibodies. A total of 64 patients were willing to participate (30 diagnosed between 0–6 months and 34 between 7 to 12 months). Systematic collection of data was performed by the local physicians from medical records. Islet autoantibodies and HLA haplotypes were analyzed by the Finnish Pediatric Diabetes Register.
Targeted next-generation sequencing
Genomic DNA was extracted from blood samples (n = 64) by automated QIAcube System and QIAamp DNA Blood Mini Kits (Qiagen Inc). Haloplex Target Enrichment System (Agilent Technologies) was used to capture regions of interest for next generation sequencing. Online design tool SureDesign (https://earray.chem.agilent.com/suredesign/) was used for capture of probe design. Target regions consisted of exons, UTRs and 10bp flanking regions of 104 diabetes associated genes from the RefSeq database (GRCh37/hg19) (Supplemental Table 1). Total length of the target regions was 484 566 kbp and involved 1418 regions. The design yielded 18 838 amplicons covering 481,37 kbp of target regions. Library preparation was performed using HaloPlex Target Enrichment Kit by following the manufacturer's instructions. Paired-end sequencing (2 × 300 bp) was performed on MiSeq instrument (Illumina) using the MiSeq Reagent Kits v3 (600 cycles).
Data analysis and variant calling
In-house developed analysis pipeline was used for the analysis of raw fastq files generated by the MiSeq-sequencer. Cutadapt (https://code.google.com/p/cutadapt/) software was applied for Illumina sequencing adapter removal and read trimming. Reads shorter than 20bp were abandoned. Remaining reads were mapped to human reference genome hg19 using BWA-MEM algorithm (http://bio-bwa.sourceforge.net/). Variant calling (SNVs and indels) was performed using four different variant callers: GATK HaplotypeCaller (https://www.broadinstitute.org/gatk/), SAMTools mpileup (http://samtools.sourceforge.net/), Atlas2 (http://sourceforge.net/projects/atlas2/) and Platypus (http://www.well.ox.ac.uk/platypus). All called variants were annotated using SnpEff (http://snpeff.sourceforge.net/), ANNOVAR (http://annovar.openbioinformatics.org/) and different public databases (eg, 1000 Genomes, dbSNP and ClinVar). Variants had to meet the following conditions to be included in the downstream analysis: 1) located within the exonic or splicing regions, 2) have high or moderate effect on gene function, and 3) have unknown or variant allele frequency below 2% in the 1000 genomes variant database. The alignments at variant positions were visually inspected using the Integrative Genomics Viewer (https://www.broadinstitute.org/igv/). Variants that met these conditions and passed visual inspection were annotated using Condel (http://bg.upf.edu/fannsdb/). All variants were bidirectionally confirmed by Sanger sequencing using the BigDye Terminator v1.1 Cycle Sequencing Kit on 3500xL Genetic Analyzer (Thermo Fisher Scientific Inc, MA, USA) according to the manufacturer's instructions.
HLA genotyping.
HLA genotypes were determined as previously reported (19). The patients (n = 58 in this analysis) were classified into five different risk groups for type 1 diabetes (0 = decreased, 1 = neutral risk, 2 = slightly increased risk, 3 = moderately increased risk, and 4 = strongly increased risk genotypes) based on different HLA-DR/DQ haplotype combinations (20)(Supplemental Table 2).
Islet autoantibodies.
Islet autoantibodies (insulin autoantibodies), antibodies to glutamic acid decarboxylase (GADA), islet antigen-2 (IA-2A), zinc transporter 8 (ZnT8A), and islet cell antibodies (ICA), were analyzed at the time of diagnosis as previously reported (n = 39 in this analysis) (21). Positivity for two or more autoantibodies was considered to indicate type 1 diabetes.
Statistical analysis.
All statistical analyses were performed using SPSS 19 statistical software (SPSS). Data are shown as the mean, SD and range. Differences between the variables of interest were analyzed using the nonparametric Mann-Whitney and χ2 tests, when appropriate.
Ethical considerations.
The study was approved by the local Ethics Committee of the Kuopio University Hospital and the University of Eastern Finland, and it was conducted in accordance with the Helsinki Declaration. All adult study participants or parents of underaged children gave written informed consent. The study has adhered to the STROBE guidelines for observational studies.
Results
Patients and clinical characteristics.
A total of 93 Finnish patients were diagnosed with diabetes during the first year of life over a 35-year period (1980–2014) (Figure 1). The incidence of diabetes in this age group was 4.4/100 000/y but that could be an underestimation because our study might have missed some patients. The incidence of neonatal diabetes (diabetes diagnosed during the first six months) was 1.5/100 000/y. Four cases (13%) had transient neonatal diabetes.
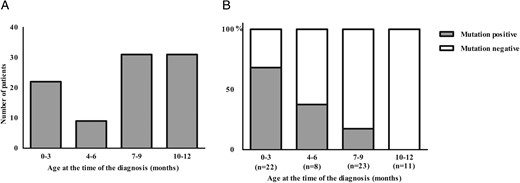
(A) Finnish patients diagnosed with diabetes before the age of 1 year between the years 1980 and 2014 (n = 93).
(B) Incidence of mutation-positive and mutation-negative patients in different age groups (n = 64).
Clinical characteristics of the patients participating in the current study categorized according to the age at the time of diabetes diagnosis are shown in Table 1. Of all 64 patients 30 (47%) and 34 (53%) of patients were diagnosed during the first 6 months and 7–12 months, respectively. Patients diagnosed during the first 6 months had a lower birth weight (P = .001) and birth weight SDS (P = .005). Plasma glucose level (P < .023) and the occurrence of ketoacidosis (37% vs 74%, P = .002) at diagnosis were significantly lower in the patients diagnosed during the first 6 months compared to those diagnosed during 7–12 months. Of the patients diagnosed within the first 6 months 12 (40%) were mutation negative. Of them 5 (42%) were DAA-positive, 4 (33%) were DAA-negative and the DAA-analysis was missing in 3 cases (25%). HLA analysis was performed to all except one patient. Eight (67%) belonged to high or moderately increased risk group and 3 (25%) to neutral or decreased risk group. We additionally compared the difference between mutation positive and mutation negative patients in each age group. In the age group 0–6 months no difference with respect to any parameters was observed but in the age group from 7 to 12 months patients who were mutation negative had significantly more often HLA risk alleles (P = .001), positive islet autoantibodies (P = .004) and ketoacidosis (P < .001) than patients who were mutation positive.
Clinical Characteristics of the Patients Categorized According to the Age at Time of the Diabetes Diagnosis
| . | ≤6 Months . | >6 Months . | P . | ||||
|---|---|---|---|---|---|---|---|
| n = 30 . | n = 34 . | ||||||
| Male n = 13 Female n = 17 . | Male n = 12 Female n = 22 . | ||||||
| Mean . | sd . | Range . | Mean . | sd . | Range . | ||
| Age at diagnosis (months) | 1.9 | 2.4 | (0 − 6) | 9.0 | 1.3 | (7–11) | <.001 |
| Current age (y) | 9.8 | 9.1 | (0 − 34.0) | 12.7 | 7.5 | (2.0–32.0) | .064 |
| Gestational age (weeks) | 38.3 | 2.9 | (28.7–41.7) | 39.5 | 1.5 | (34.6–42.0) | .070 |
| Birth weight (g) | 2855 | 830 | (1000–4300) | 3510 | 475 | (2560 − 4450) | .001 |
| Birth weight (SDS) | −1.3 | 1.6 | (−4.9 to 1.2) | −0.2 | 1.0 | (−2.3 to 1.8) | .005 |
| Plasma glucose at diagnosis (mmol/liter) | 27.4 | 16.2 | (6.5–62.8) | 35.6 | 11.8 | (17.6–70.4) | .023 |
| Ketoacidosis at diagnosis (pH < 7.30) | 11 (37%) | 25 (74%) | .002 | ||||
| Current treatment | .003 | ||||||
| Multiple-dose insulin injections | 9 (30%) | 12 (35%) | |||||
| Insulin pump | 11 (37%) | 22 (65%) | |||||
| Sulfonylurea | 4 (13%) | 0 (0%) | |||||
| No treatment | 6 (20%) | 0 (0%) | |||||
| HLA-DR-DQ genotype analysis (n = 58) | 24 (80%) | 34 (100%) | .012 | ||||
| HLA class 0 (decreased risk genotype) | 5 (21%) | 2 (6%) | |||||
| HLA class 4 (strongly increased risk genotype) | 2 (8%) | 11 (32%) | |||||
| Islet autoantibody analysis (n = 39) | 15 (50%) | 24 (71%) | .057 | ||||
| Positive islet autoantibodiesa | 6 (40%) | 17 (71%) | |||||
| Autoimmune disease other than diabetesb | 5 (17%) | 7 (21%) | .688 | ||||
| Mutation positivity | 18 (60%) | 4 (12%) | <.001 | ||||
| . | ≤6 Months . | >6 Months . | P . | ||||
|---|---|---|---|---|---|---|---|
| n = 30 . | n = 34 . | ||||||
| Male n = 13 Female n = 17 . | Male n = 12 Female n = 22 . | ||||||
| Mean . | sd . | Range . | Mean . | sd . | Range . | ||
| Age at diagnosis (months) | 1.9 | 2.4 | (0 − 6) | 9.0 | 1.3 | (7–11) | <.001 |
| Current age (y) | 9.8 | 9.1 | (0 − 34.0) | 12.7 | 7.5 | (2.0–32.0) | .064 |
| Gestational age (weeks) | 38.3 | 2.9 | (28.7–41.7) | 39.5 | 1.5 | (34.6–42.0) | .070 |
| Birth weight (g) | 2855 | 830 | (1000–4300) | 3510 | 475 | (2560 − 4450) | .001 |
| Birth weight (SDS) | −1.3 | 1.6 | (−4.9 to 1.2) | −0.2 | 1.0 | (−2.3 to 1.8) | .005 |
| Plasma glucose at diagnosis (mmol/liter) | 27.4 | 16.2 | (6.5–62.8) | 35.6 | 11.8 | (17.6–70.4) | .023 |
| Ketoacidosis at diagnosis (pH < 7.30) | 11 (37%) | 25 (74%) | .002 | ||||
| Current treatment | .003 | ||||||
| Multiple-dose insulin injections | 9 (30%) | 12 (35%) | |||||
| Insulin pump | 11 (37%) | 22 (65%) | |||||
| Sulfonylurea | 4 (13%) | 0 (0%) | |||||
| No treatment | 6 (20%) | 0 (0%) | |||||
| HLA-DR-DQ genotype analysis (n = 58) | 24 (80%) | 34 (100%) | .012 | ||||
| HLA class 0 (decreased risk genotype) | 5 (21%) | 2 (6%) | |||||
| HLA class 4 (strongly increased risk genotype) | 2 (8%) | 11 (32%) | |||||
| Islet autoantibody analysis (n = 39) | 15 (50%) | 24 (71%) | .057 | ||||
| Positive islet autoantibodiesa | 6 (40%) | 17 (71%) | |||||
| Autoimmune disease other than diabetesb | 5 (17%) | 7 (21%) | .688 | ||||
| Mutation positivity | 18 (60%) | 4 (12%) | <.001 | ||||
Significant P values (P < .05) shown in bold.
Positivity for two or more islet autoantibodies.
Hypothyroidism, hyperthyroidism, celiac disease, autoimmune enteropathy , rheumatoid arthritis or alopecia.
Clinical Characteristics of the Patients Categorized According to the Age at Time of the Diabetes Diagnosis
| . | ≤6 Months . | >6 Months . | P . | ||||
|---|---|---|---|---|---|---|---|
| n = 30 . | n = 34 . | ||||||
| Male n = 13 Female n = 17 . | Male n = 12 Female n = 22 . | ||||||
| Mean . | sd . | Range . | Mean . | sd . | Range . | ||
| Age at diagnosis (months) | 1.9 | 2.4 | (0 − 6) | 9.0 | 1.3 | (7–11) | <.001 |
| Current age (y) | 9.8 | 9.1 | (0 − 34.0) | 12.7 | 7.5 | (2.0–32.0) | .064 |
| Gestational age (weeks) | 38.3 | 2.9 | (28.7–41.7) | 39.5 | 1.5 | (34.6–42.0) | .070 |
| Birth weight (g) | 2855 | 830 | (1000–4300) | 3510 | 475 | (2560 − 4450) | .001 |
| Birth weight (SDS) | −1.3 | 1.6 | (−4.9 to 1.2) | −0.2 | 1.0 | (−2.3 to 1.8) | .005 |
| Plasma glucose at diagnosis (mmol/liter) | 27.4 | 16.2 | (6.5–62.8) | 35.6 | 11.8 | (17.6–70.4) | .023 |
| Ketoacidosis at diagnosis (pH < 7.30) | 11 (37%) | 25 (74%) | .002 | ||||
| Current treatment | .003 | ||||||
| Multiple-dose insulin injections | 9 (30%) | 12 (35%) | |||||
| Insulin pump | 11 (37%) | 22 (65%) | |||||
| Sulfonylurea | 4 (13%) | 0 (0%) | |||||
| No treatment | 6 (20%) | 0 (0%) | |||||
| HLA-DR-DQ genotype analysis (n = 58) | 24 (80%) | 34 (100%) | .012 | ||||
| HLA class 0 (decreased risk genotype) | 5 (21%) | 2 (6%) | |||||
| HLA class 4 (strongly increased risk genotype) | 2 (8%) | 11 (32%) | |||||
| Islet autoantibody analysis (n = 39) | 15 (50%) | 24 (71%) | .057 | ||||
| Positive islet autoantibodiesa | 6 (40%) | 17 (71%) | |||||
| Autoimmune disease other than diabetesb | 5 (17%) | 7 (21%) | .688 | ||||
| Mutation positivity | 18 (60%) | 4 (12%) | <.001 | ||||
| . | ≤6 Months . | >6 Months . | P . | ||||
|---|---|---|---|---|---|---|---|
| n = 30 . | n = 34 . | ||||||
| Male n = 13 Female n = 17 . | Male n = 12 Female n = 22 . | ||||||
| Mean . | sd . | Range . | Mean . | sd . | Range . | ||
| Age at diagnosis (months) | 1.9 | 2.4 | (0 − 6) | 9.0 | 1.3 | (7–11) | <.001 |
| Current age (y) | 9.8 | 9.1 | (0 − 34.0) | 12.7 | 7.5 | (2.0–32.0) | .064 |
| Gestational age (weeks) | 38.3 | 2.9 | (28.7–41.7) | 39.5 | 1.5 | (34.6–42.0) | .070 |
| Birth weight (g) | 2855 | 830 | (1000–4300) | 3510 | 475 | (2560 − 4450) | .001 |
| Birth weight (SDS) | −1.3 | 1.6 | (−4.9 to 1.2) | −0.2 | 1.0 | (−2.3 to 1.8) | .005 |
| Plasma glucose at diagnosis (mmol/liter) | 27.4 | 16.2 | (6.5–62.8) | 35.6 | 11.8 | (17.6–70.4) | .023 |
| Ketoacidosis at diagnosis (pH < 7.30) | 11 (37%) | 25 (74%) | .002 | ||||
| Current treatment | .003 | ||||||
| Multiple-dose insulin injections | 9 (30%) | 12 (35%) | |||||
| Insulin pump | 11 (37%) | 22 (65%) | |||||
| Sulfonylurea | 4 (13%) | 0 (0%) | |||||
| No treatment | 6 (20%) | 0 (0%) | |||||
| HLA-DR-DQ genotype analysis (n = 58) | 24 (80%) | 34 (100%) | .012 | ||||
| HLA class 0 (decreased risk genotype) | 5 (21%) | 2 (6%) | |||||
| HLA class 4 (strongly increased risk genotype) | 2 (8%) | 11 (32%) | |||||
| Islet autoantibody analysis (n = 39) | 15 (50%) | 24 (71%) | .057 | ||||
| Positive islet autoantibodiesa | 6 (40%) | 17 (71%) | |||||
| Autoimmune disease other than diabetesb | 5 (17%) | 7 (21%) | .688 | ||||
| Mutation positivity | 18 (60%) | 4 (12%) | <.001 | ||||
Significant P values (P < .05) shown in bold.
Positivity for two or more islet autoantibodies.
Hypothyroidism, hyperthyroidism, celiac disease, autoimmune enteropathy , rheumatoid arthritis or alopecia.
Altogether 12 different mutations were detected in 20 patients in 13 families (Figure 1B, Tables 2 and 3) in the targeted sequencing of the 104 genes (Supplemental Table 1). In addition, the cases with gene variants GCK-G261R and STAT3-K392R have been previously published (Table 2, cases 19 and 22) (22, 23). Three novel mutations, ABCC8 p.T588I (c.1763C>T), GCK-del p.S212 (c.635_637delCCT), and RFX6 frame shift p.H293L(c.878_879delAC), were found in three different families. The ABCC8-T588I mutation was detected in case 6 who presented with hyperglycemia (plasma glucose 12 mmol/liter) during the first day. Remission of diabetes occurred during the neonatal period, and she has been normoglycemic ever since. The same mutation was also found in four other family members with diabetes in three consecutive generations. All of them were diagnosed with diabetes at adult age and treated with oral medication. The deletion of codon 212 in GCK was detected in a hyperglycemic neonate (case 18) and later in five family members in three generations who were previously diagnosed with diabetes or gestational diabetes.
Gene Mutations Associated With Monogenic Diabetes Diagnosed Before Age 1 Year in the Finnish Population and Major Clinical Findings of the Mutation Carriers
| Gene/ . | Het/ . | Gender . | Case . | Family . | Age 1 . | H . | BW . | Age 2 . | P-Gluc . | DKA . | . | . | Family . | IAA . | HLA . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutation | Hom | (M/F) | No. | No. | (y) | (weeks) | (g) | (months) | (mmol/liter) | (+/−) | Treatment | Medical History | History | (n) | Genotype | Ref. |
| KCNJ11 | ||||||||||||||||
| R50Q | Het | M | 1 | 1 | 12 | 38 | 2285 | 0 | 17.4 | + | No | - | No | 0 | NA | 26 |
| Q52 L | Het | F | 2 | 2 | 2 | 40 | 3510 | 6 | 40.0 | − | SU | Craniosynostosis | No | 0 | 1 | 28 |
| R201H | Het | M | 3 | 3 | 6 | 38 | 2660 | 0 | 21.7 | − | SU | - | Yes | NA | 1 | 10 |
| R201H | Het | F | 4 | 3 | 34 | 41 | 2260 | 0 | 19.1 | − | SU + insulin | - | Yes | NA | 3 | |
| R201H | Het | F | 5 | 4 | 2 | 41 | 3300 | 6 | 52.9 | + | SU | - | No | NA | 4 | |
| ABCC8 | ||||||||||||||||
| T588I | Het | F | 6 | 5 | 6 | 40 | 3380 | 0 | 12.0 | − | No | - | Yes | NA | 2 | |
| R825W | Het | F | 7 | 6 | 0 | 29 | 1500 | 0 | 16.8 | − | No | RDS, periventricular cyst | No | NA | NA | 27 |
| R1182W | Het | M | 8 | 7 | 21 | 41 | 3770 | 2 | 50.0 | + | MDI | - | Yes | NA | 3 | 27 |
| Insulin | ||||||||||||||||
| A24D | Het | M | 9 | 8 | 2 | 41 | 2520 | 1 | 28.0 | − | Insulin pump | - | No | NA | 1 | 12 |
| C96R | Het | M | 10 | 9 | 11 | 40 | 3650 | 7 | 62.8 | − | MDI | - | Yes | 0 | 3 | 25 |
| C96R | Het | M | 11 | 9 | 32 | 31 | 3100 | 8 | 19.3 | − | MDI | - | Yes | NA | 1 | |
| C96R | Het | F | 12 | 9 | 9 | 39 | 3400 | 9 | 17.6 | − | MDI | - | Yes | 0 | 0 | |
| C96R | Het | M | 13 | 9 | 5 | 39 | 3590 | 3 | 14.9 | − | MDI | - | Yes | 0 | 0 | |
| C96R | Het | F | 14 | 9 | 1 | 37 | 2950 | 4 | 9.3 | − | MDI | - | Yes | NA | 0 | |
| C109Y | Het | F | 15 | 10 | 8 | 39 | 3120 | 9 | 51.5 | − | MDI | Hypothyroidism | Yes | 0 | 0 | 29 |
| C109Y | Het | F | 16 | 10 | 31 | 40 | 3400 | 2 | 62.8 | + | MDI | Hypothyroidism | Yes | 0 | 0 | |
| C109Y | Het | M | 17 | 10 | 0 | 38 | 3380 | 3 | 13.0 | − | MDI | - | Yes | NA | NA | |
| Glucokinase | ||||||||||||||||
| del212 | Het | F | 18 | 11 | 9 | 38 | 2220 | 0 | 11.3 | − | No | - | Yes | 0 | 0 | |
| G261R | Het | F | 19 | 12 | 12 | 39 | 2960 | 5 | 6.5 | − | No | - | Yes | 31 | ||
| RFX6 | − | |||||||||||||||
| H293Lfs | Hom | M | 20 | 13 | 0 | 38 | 1800 | 0 | 11.0 | − | Insulin pump | Phenotypea | No | NA | NA | |
| FOXP3 | ||||||||||||||||
| R337Q | Hom | M | 21 | 14 | 20 | 40 | 4300 | 0 | 57.9 | + | MDI | Phenotypeb | Yes | 1 | 3 | 30 |
| STAT3 | ||||||||||||||||
| K392R | Het | F | 22 | 15 | 15 | 34 | 1380 | 0 | NA | − | MDI | Phenotypec | No | 3 | 3 | 23 |
| Gene/ . | Het/ . | Gender . | Case . | Family . | Age 1 . | H . | BW . | Age 2 . | P-Gluc . | DKA . | . | . | Family . | IAA . | HLA . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutation | Hom | (M/F) | No. | No. | (y) | (weeks) | (g) | (months) | (mmol/liter) | (+/−) | Treatment | Medical History | History | (n) | Genotype | Ref. |
| KCNJ11 | ||||||||||||||||
| R50Q | Het | M | 1 | 1 | 12 | 38 | 2285 | 0 | 17.4 | + | No | - | No | 0 | NA | 26 |
| Q52 L | Het | F | 2 | 2 | 2 | 40 | 3510 | 6 | 40.0 | − | SU | Craniosynostosis | No | 0 | 1 | 28 |
| R201H | Het | M | 3 | 3 | 6 | 38 | 2660 | 0 | 21.7 | − | SU | - | Yes | NA | 1 | 10 |
| R201H | Het | F | 4 | 3 | 34 | 41 | 2260 | 0 | 19.1 | − | SU + insulin | - | Yes | NA | 3 | |
| R201H | Het | F | 5 | 4 | 2 | 41 | 3300 | 6 | 52.9 | + | SU | - | No | NA | 4 | |
| ABCC8 | ||||||||||||||||
| T588I | Het | F | 6 | 5 | 6 | 40 | 3380 | 0 | 12.0 | − | No | - | Yes | NA | 2 | |
| R825W | Het | F | 7 | 6 | 0 | 29 | 1500 | 0 | 16.8 | − | No | RDS, periventricular cyst | No | NA | NA | 27 |
| R1182W | Het | M | 8 | 7 | 21 | 41 | 3770 | 2 | 50.0 | + | MDI | - | Yes | NA | 3 | 27 |
| Insulin | ||||||||||||||||
| A24D | Het | M | 9 | 8 | 2 | 41 | 2520 | 1 | 28.0 | − | Insulin pump | - | No | NA | 1 | 12 |
| C96R | Het | M | 10 | 9 | 11 | 40 | 3650 | 7 | 62.8 | − | MDI | - | Yes | 0 | 3 | 25 |
| C96R | Het | M | 11 | 9 | 32 | 31 | 3100 | 8 | 19.3 | − | MDI | - | Yes | NA | 1 | |
| C96R | Het | F | 12 | 9 | 9 | 39 | 3400 | 9 | 17.6 | − | MDI | - | Yes | 0 | 0 | |
| C96R | Het | M | 13 | 9 | 5 | 39 | 3590 | 3 | 14.9 | − | MDI | - | Yes | 0 | 0 | |
| C96R | Het | F | 14 | 9 | 1 | 37 | 2950 | 4 | 9.3 | − | MDI | - | Yes | NA | 0 | |
| C109Y | Het | F | 15 | 10 | 8 | 39 | 3120 | 9 | 51.5 | − | MDI | Hypothyroidism | Yes | 0 | 0 | 29 |
| C109Y | Het | F | 16 | 10 | 31 | 40 | 3400 | 2 | 62.8 | + | MDI | Hypothyroidism | Yes | 0 | 0 | |
| C109Y | Het | M | 17 | 10 | 0 | 38 | 3380 | 3 | 13.0 | − | MDI | - | Yes | NA | NA | |
| Glucokinase | ||||||||||||||||
| del212 | Het | F | 18 | 11 | 9 | 38 | 2220 | 0 | 11.3 | − | No | - | Yes | 0 | 0 | |
| G261R | Het | F | 19 | 12 | 12 | 39 | 2960 | 5 | 6.5 | − | No | - | Yes | 31 | ||
| RFX6 | − | |||||||||||||||
| H293Lfs | Hom | M | 20 | 13 | 0 | 38 | 1800 | 0 | 11.0 | − | Insulin pump | Phenotypea | No | NA | NA | |
| FOXP3 | ||||||||||||||||
| R337Q | Hom | M | 21 | 14 | 20 | 40 | 4300 | 0 | 57.9 | + | MDI | Phenotypeb | Yes | 1 | 3 | 30 |
| STAT3 | ||||||||||||||||
| K392R | Het | F | 22 | 15 | 15 | 34 | 1380 | 0 | NA | − | MDI | Phenotypec | No | 3 | 3 | 23 |
Abbreviations: age 1 = current age; age 2 = age at diagnosis; DKA, diabetic ketoacidosis at diagnosis; F, female; H, gestational weeks; het, heterozygote; hom, homozygote; hem, hemizygote; IAA, number of positive islet autoantibodies; M, male; MDI, multiple-dose injection insulin treatment; SU, sulfonylurea treatment.
Phenotype: duodenal and biliary atresia, tracheoesophageal fistula, Meckel's diverticulum.
Phenotype: immune dysregulation, polyendocrinopathy, autoimmune enteropathy.
Phenotype: early-onset allergies, growth failure, coeliac disease, rudimentary pancreas, pulmonary fibrosis.
Gene Mutations Associated With Monogenic Diabetes Diagnosed Before Age 1 Year in the Finnish Population and Major Clinical Findings of the Mutation Carriers
| Gene/ . | Het/ . | Gender . | Case . | Family . | Age 1 . | H . | BW . | Age 2 . | P-Gluc . | DKA . | . | . | Family . | IAA . | HLA . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutation | Hom | (M/F) | No. | No. | (y) | (weeks) | (g) | (months) | (mmol/liter) | (+/−) | Treatment | Medical History | History | (n) | Genotype | Ref. |
| KCNJ11 | ||||||||||||||||
| R50Q | Het | M | 1 | 1 | 12 | 38 | 2285 | 0 | 17.4 | + | No | - | No | 0 | NA | 26 |
| Q52 L | Het | F | 2 | 2 | 2 | 40 | 3510 | 6 | 40.0 | − | SU | Craniosynostosis | No | 0 | 1 | 28 |
| R201H | Het | M | 3 | 3 | 6 | 38 | 2660 | 0 | 21.7 | − | SU | - | Yes | NA | 1 | 10 |
| R201H | Het | F | 4 | 3 | 34 | 41 | 2260 | 0 | 19.1 | − | SU + insulin | - | Yes | NA | 3 | |
| R201H | Het | F | 5 | 4 | 2 | 41 | 3300 | 6 | 52.9 | + | SU | - | No | NA | 4 | |
| ABCC8 | ||||||||||||||||
| T588I | Het | F | 6 | 5 | 6 | 40 | 3380 | 0 | 12.0 | − | No | - | Yes | NA | 2 | |
| R825W | Het | F | 7 | 6 | 0 | 29 | 1500 | 0 | 16.8 | − | No | RDS, periventricular cyst | No | NA | NA | 27 |
| R1182W | Het | M | 8 | 7 | 21 | 41 | 3770 | 2 | 50.0 | + | MDI | - | Yes | NA | 3 | 27 |
| Insulin | ||||||||||||||||
| A24D | Het | M | 9 | 8 | 2 | 41 | 2520 | 1 | 28.0 | − | Insulin pump | - | No | NA | 1 | 12 |
| C96R | Het | M | 10 | 9 | 11 | 40 | 3650 | 7 | 62.8 | − | MDI | - | Yes | 0 | 3 | 25 |
| C96R | Het | M | 11 | 9 | 32 | 31 | 3100 | 8 | 19.3 | − | MDI | - | Yes | NA | 1 | |
| C96R | Het | F | 12 | 9 | 9 | 39 | 3400 | 9 | 17.6 | − | MDI | - | Yes | 0 | 0 | |
| C96R | Het | M | 13 | 9 | 5 | 39 | 3590 | 3 | 14.9 | − | MDI | - | Yes | 0 | 0 | |
| C96R | Het | F | 14 | 9 | 1 | 37 | 2950 | 4 | 9.3 | − | MDI | - | Yes | NA | 0 | |
| C109Y | Het | F | 15 | 10 | 8 | 39 | 3120 | 9 | 51.5 | − | MDI | Hypothyroidism | Yes | 0 | 0 | 29 |
| C109Y | Het | F | 16 | 10 | 31 | 40 | 3400 | 2 | 62.8 | + | MDI | Hypothyroidism | Yes | 0 | 0 | |
| C109Y | Het | M | 17 | 10 | 0 | 38 | 3380 | 3 | 13.0 | − | MDI | - | Yes | NA | NA | |
| Glucokinase | ||||||||||||||||
| del212 | Het | F | 18 | 11 | 9 | 38 | 2220 | 0 | 11.3 | − | No | - | Yes | 0 | 0 | |
| G261R | Het | F | 19 | 12 | 12 | 39 | 2960 | 5 | 6.5 | − | No | - | Yes | 31 | ||
| RFX6 | − | |||||||||||||||
| H293Lfs | Hom | M | 20 | 13 | 0 | 38 | 1800 | 0 | 11.0 | − | Insulin pump | Phenotypea | No | NA | NA | |
| FOXP3 | ||||||||||||||||
| R337Q | Hom | M | 21 | 14 | 20 | 40 | 4300 | 0 | 57.9 | + | MDI | Phenotypeb | Yes | 1 | 3 | 30 |
| STAT3 | ||||||||||||||||
| K392R | Het | F | 22 | 15 | 15 | 34 | 1380 | 0 | NA | − | MDI | Phenotypec | No | 3 | 3 | 23 |
| Gene/ . | Het/ . | Gender . | Case . | Family . | Age 1 . | H . | BW . | Age 2 . | P-Gluc . | DKA . | . | . | Family . | IAA . | HLA . | . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutation | Hom | (M/F) | No. | No. | (y) | (weeks) | (g) | (months) | (mmol/liter) | (+/−) | Treatment | Medical History | History | (n) | Genotype | Ref. |
| KCNJ11 | ||||||||||||||||
| R50Q | Het | M | 1 | 1 | 12 | 38 | 2285 | 0 | 17.4 | + | No | - | No | 0 | NA | 26 |
| Q52 L | Het | F | 2 | 2 | 2 | 40 | 3510 | 6 | 40.0 | − | SU | Craniosynostosis | No | 0 | 1 | 28 |
| R201H | Het | M | 3 | 3 | 6 | 38 | 2660 | 0 | 21.7 | − | SU | - | Yes | NA | 1 | 10 |
| R201H | Het | F | 4 | 3 | 34 | 41 | 2260 | 0 | 19.1 | − | SU + insulin | - | Yes | NA | 3 | |
| R201H | Het | F | 5 | 4 | 2 | 41 | 3300 | 6 | 52.9 | + | SU | - | No | NA | 4 | |
| ABCC8 | ||||||||||||||||
| T588I | Het | F | 6 | 5 | 6 | 40 | 3380 | 0 | 12.0 | − | No | - | Yes | NA | 2 | |
| R825W | Het | F | 7 | 6 | 0 | 29 | 1500 | 0 | 16.8 | − | No | RDS, periventricular cyst | No | NA | NA | 27 |
| R1182W | Het | M | 8 | 7 | 21 | 41 | 3770 | 2 | 50.0 | + | MDI | - | Yes | NA | 3 | 27 |
| Insulin | ||||||||||||||||
| A24D | Het | M | 9 | 8 | 2 | 41 | 2520 | 1 | 28.0 | − | Insulin pump | - | No | NA | 1 | 12 |
| C96R | Het | M | 10 | 9 | 11 | 40 | 3650 | 7 | 62.8 | − | MDI | - | Yes | 0 | 3 | 25 |
| C96R | Het | M | 11 | 9 | 32 | 31 | 3100 | 8 | 19.3 | − | MDI | - | Yes | NA | 1 | |
| C96R | Het | F | 12 | 9 | 9 | 39 | 3400 | 9 | 17.6 | − | MDI | - | Yes | 0 | 0 | |
| C96R | Het | M | 13 | 9 | 5 | 39 | 3590 | 3 | 14.9 | − | MDI | - | Yes | 0 | 0 | |
| C96R | Het | F | 14 | 9 | 1 | 37 | 2950 | 4 | 9.3 | − | MDI | - | Yes | NA | 0 | |
| C109Y | Het | F | 15 | 10 | 8 | 39 | 3120 | 9 | 51.5 | − | MDI | Hypothyroidism | Yes | 0 | 0 | 29 |
| C109Y | Het | F | 16 | 10 | 31 | 40 | 3400 | 2 | 62.8 | + | MDI | Hypothyroidism | Yes | 0 | 0 | |
| C109Y | Het | M | 17 | 10 | 0 | 38 | 3380 | 3 | 13.0 | − | MDI | - | Yes | NA | NA | |
| Glucokinase | ||||||||||||||||
| del212 | Het | F | 18 | 11 | 9 | 38 | 2220 | 0 | 11.3 | − | No | - | Yes | 0 | 0 | |
| G261R | Het | F | 19 | 12 | 12 | 39 | 2960 | 5 | 6.5 | − | No | - | Yes | 31 | ||
| RFX6 | − | |||||||||||||||
| H293Lfs | Hom | M | 20 | 13 | 0 | 38 | 1800 | 0 | 11.0 | − | Insulin pump | Phenotypea | No | NA | NA | |
| FOXP3 | ||||||||||||||||
| R337Q | Hom | M | 21 | 14 | 20 | 40 | 4300 | 0 | 57.9 | + | MDI | Phenotypeb | Yes | 1 | 3 | 30 |
| STAT3 | ||||||||||||||||
| K392R | Het | F | 22 | 15 | 15 | 34 | 1380 | 0 | NA | − | MDI | Phenotypec | No | 3 | 3 | 23 |
Abbreviations: age 1 = current age; age 2 = age at diagnosis; DKA, diabetic ketoacidosis at diagnosis; F, female; H, gestational weeks; het, heterozygote; hom, homozygote; hem, hemizygote; IAA, number of positive islet autoantibodies; M, male; MDI, multiple-dose injection insulin treatment; SU, sulfonylurea treatment.
Phenotype: duodenal and biliary atresia, tracheoesophageal fistula, Meckel's diverticulum.
Phenotype: immune dysregulation, polyendocrinopathy, autoimmune enteropathy.
Phenotype: early-onset allergies, growth failure, coeliac disease, rudimentary pancreas, pulmonary fibrosis.
Disease-Associated Mutations Found in Targeted Sequencing of 104 Glucose Metabolism-Associated Genes in the Finnish Patients With Monogenic Diabetes Diagnosed During the First Year of Life
| Gene . | Chr . | Pos HG19 . | Mutation . | Condel . | dbSNP ID . | Novel . | Ref. . |
|---|---|---|---|---|---|---|---|
| RFX6 | 6 | 117237381–117237382 | Frame shift p.His293Leufs (c.878_879delAC) NM_173560.3 | rs762966411 | Yes | ||
| GCK | 7 | 44189401–44189403 | Deletion p.Ser212del (c.635_637delCCT) NM_000162.3 | rs193922314 | Yes | ||
| KCNJ11 | 11 | 17409490 | p.Arg50Gln (c.149G>A) NM_000525.3 | 0.562–0.619D | rs80356611 | No | 26 |
| 17409483–17409484 | p.Gln52Leu (c.155–156AG>TT) NM_000525.3 | 0.552–0.623D | rs193929337 | No | 28 | ||
| 17409037 | p.Arg201His (c.602G>A) NM_000525.3 | 0.700D | rs80356624 | No | 10 | ||
| ABCC8 | 11 | 17452415 | p.Thr588Ile (c.1763C>T) NM_000352.4 | 0.538D | Yes | ||
| 17434943 | p.Arg825Trp (c.2473C>T) NM_000352.4 | 0.652D | rs779736828 | No | 27 | ||
| 17426072 | p.Arg1182Trp (c.3544C>T) NM_000352.4 | 0.716–0.819D | rs797045209 | No | 27 | ||
| INS | 11 | 2182131 | p.Ala24Asp (c.71C>A) NM_000207.2 | 0.635D | rs80356663 | No | 12 |
| 2181129 | p.Cys96Arg (c.286T>C) NM_000207.2 | 0.721D | No | 25 | |||
| 2181089 | p.Cys109Tyr (c.326G>A) NM_000207.2 | 0.731D | No | 29 | |||
| FOXP3 | X | 49109621 | Arg337Glnp.Arg337Gln (c.1010G>A) NM_014009.3 | 0.695D | No | 30 |
| Gene . | Chr . | Pos HG19 . | Mutation . | Condel . | dbSNP ID . | Novel . | Ref. . |
|---|---|---|---|---|---|---|---|
| RFX6 | 6 | 117237381–117237382 | Frame shift p.His293Leufs (c.878_879delAC) NM_173560.3 | rs762966411 | Yes | ||
| GCK | 7 | 44189401–44189403 | Deletion p.Ser212del (c.635_637delCCT) NM_000162.3 | rs193922314 | Yes | ||
| KCNJ11 | 11 | 17409490 | p.Arg50Gln (c.149G>A) NM_000525.3 | 0.562–0.619D | rs80356611 | No | 26 |
| 17409483–17409484 | p.Gln52Leu (c.155–156AG>TT) NM_000525.3 | 0.552–0.623D | rs193929337 | No | 28 | ||
| 17409037 | p.Arg201His (c.602G>A) NM_000525.3 | 0.700D | rs80356624 | No | 10 | ||
| ABCC8 | 11 | 17452415 | p.Thr588Ile (c.1763C>T) NM_000352.4 | 0.538D | Yes | ||
| 17434943 | p.Arg825Trp (c.2473C>T) NM_000352.4 | 0.652D | rs779736828 | No | 27 | ||
| 17426072 | p.Arg1182Trp (c.3544C>T) NM_000352.4 | 0.716–0.819D | rs797045209 | No | 27 | ||
| INS | 11 | 2182131 | p.Ala24Asp (c.71C>A) NM_000207.2 | 0.635D | rs80356663 | No | 12 |
| 2181129 | p.Cys96Arg (c.286T>C) NM_000207.2 | 0.721D | No | 25 | |||
| 2181089 | p.Cys109Tyr (c.326G>A) NM_000207.2 | 0.731D | No | 29 | |||
| FOXP3 | X | 49109621 | Arg337Glnp.Arg337Gln (c.1010G>A) NM_014009.3 | 0.695D | No | 30 |
Abbreviations: Chr, chromosome; ID, identification; Pos, Position; Ref., reference.
Disease-Associated Mutations Found in Targeted Sequencing of 104 Glucose Metabolism-Associated Genes in the Finnish Patients With Monogenic Diabetes Diagnosed During the First Year of Life
| Gene . | Chr . | Pos HG19 . | Mutation . | Condel . | dbSNP ID . | Novel . | Ref. . |
|---|---|---|---|---|---|---|---|
| RFX6 | 6 | 117237381–117237382 | Frame shift p.His293Leufs (c.878_879delAC) NM_173560.3 | rs762966411 | Yes | ||
| GCK | 7 | 44189401–44189403 | Deletion p.Ser212del (c.635_637delCCT) NM_000162.3 | rs193922314 | Yes | ||
| KCNJ11 | 11 | 17409490 | p.Arg50Gln (c.149G>A) NM_000525.3 | 0.562–0.619D | rs80356611 | No | 26 |
| 17409483–17409484 | p.Gln52Leu (c.155–156AG>TT) NM_000525.3 | 0.552–0.623D | rs193929337 | No | 28 | ||
| 17409037 | p.Arg201His (c.602G>A) NM_000525.3 | 0.700D | rs80356624 | No | 10 | ||
| ABCC8 | 11 | 17452415 | p.Thr588Ile (c.1763C>T) NM_000352.4 | 0.538D | Yes | ||
| 17434943 | p.Arg825Trp (c.2473C>T) NM_000352.4 | 0.652D | rs779736828 | No | 27 | ||
| 17426072 | p.Arg1182Trp (c.3544C>T) NM_000352.4 | 0.716–0.819D | rs797045209 | No | 27 | ||
| INS | 11 | 2182131 | p.Ala24Asp (c.71C>A) NM_000207.2 | 0.635D | rs80356663 | No | 12 |
| 2181129 | p.Cys96Arg (c.286T>C) NM_000207.2 | 0.721D | No | 25 | |||
| 2181089 | p.Cys109Tyr (c.326G>A) NM_000207.2 | 0.731D | No | 29 | |||
| FOXP3 | X | 49109621 | Arg337Glnp.Arg337Gln (c.1010G>A) NM_014009.3 | 0.695D | No | 30 |
| Gene . | Chr . | Pos HG19 . | Mutation . | Condel . | dbSNP ID . | Novel . | Ref. . |
|---|---|---|---|---|---|---|---|
| RFX6 | 6 | 117237381–117237382 | Frame shift p.His293Leufs (c.878_879delAC) NM_173560.3 | rs762966411 | Yes | ||
| GCK | 7 | 44189401–44189403 | Deletion p.Ser212del (c.635_637delCCT) NM_000162.3 | rs193922314 | Yes | ||
| KCNJ11 | 11 | 17409490 | p.Arg50Gln (c.149G>A) NM_000525.3 | 0.562–0.619D | rs80356611 | No | 26 |
| 17409483–17409484 | p.Gln52Leu (c.155–156AG>TT) NM_000525.3 | 0.552–0.623D | rs193929337 | No | 28 | ||
| 17409037 | p.Arg201His (c.602G>A) NM_000525.3 | 0.700D | rs80356624 | No | 10 | ||
| ABCC8 | 11 | 17452415 | p.Thr588Ile (c.1763C>T) NM_000352.4 | 0.538D | Yes | ||
| 17434943 | p.Arg825Trp (c.2473C>T) NM_000352.4 | 0.652D | rs779736828 | No | 27 | ||
| 17426072 | p.Arg1182Trp (c.3544C>T) NM_000352.4 | 0.716–0.819D | rs797045209 | No | 27 | ||
| INS | 11 | 2182131 | p.Ala24Asp (c.71C>A) NM_000207.2 | 0.635D | rs80356663 | No | 12 |
| 2181129 | p.Cys96Arg (c.286T>C) NM_000207.2 | 0.721D | No | 25 | |||
| 2181089 | p.Cys109Tyr (c.326G>A) NM_000207.2 | 0.731D | No | 29 | |||
| FOXP3 | X | 49109621 | Arg337Glnp.Arg337Gln (c.1010G>A) NM_014009.3 | 0.695D | No | 30 |
Abbreviations: Chr, chromosome; ID, identification; Pos, Position; Ref., reference.
The homozygous deletion (c.878_879delAC) leading to a frame shift in the RFX6 gene was detected in case 20 who presented with neonatal diabetes and intestinal and biliary defects (tracheoesophageal fistula, duodenal atresia, jejunal duplication, Meckel's diverticulum, biliary atresia). Both parents were heterozygous for the deletion and were normoglycemic. The same deletion in heterozygous form was also detected in one patient diagnosed with diabetes at the age of 10 months (Supplemental Table 3) who is likely to have T1D associated with other autoimmunity signs ie, rheumatoid arthritis (RA), positive celiac disease-associated autoantibodies. To assess the influence of the heterozygosity for this deletion on glucose metabolism we screened this gene variant in middle-aged and elderly participants of the population-based Metabolic Syndrome In Men (METSIM) cohort (24). Twenty nine of the 7585 METSIM participants carried this deletion in a heterozygous form. No differences were observed in fasting plasma glucose concentration, insulin secretion nor in insulin sensitivity between the mutation carriers and noncarriers. However, the 2-hour plasma glucose concentration measured in an oral glucose tolerance test (OGTT) was higher in the heterozygous carriers of the RFX6 mutation than in the noncarriers (P = .025 in nondiabetic subjects, P = 0.032 in nondiabetic and newly-diagnosed type 2 diabetic subjects, Supplemental Table 4).
Additionally, nine previously known missense variants (KCNJ11-R50Q, KCNJ11-Q52L, KCNJ11-R201H, ABCC8-R825W, ABCC8-R1182W, INS-A24D, INS-C96R, INS-C109Y, FOXP3-R337Q) were detected (10, 12, 25–30). Though the cases with gene variants GCK-G261R and STAT3-K392R have been previously published, they are included in the current list of findings (Table 2, cases 19 and 22) (22, 23). The incidence of mutation-positive and mutation-negative patients in different age groups are shown in Figure 1.
Additionally, several heterozygous variants in 104 genes were detected by targeted sequencing which were classified as deleterious by the Condel program and could accordingly have an effect on glucose levels. However, the causality of these variants for diabetes remains uncertain (Supplemental Table 3).
Close to 60% (59%) of mutation positive and 98% of mutation-negative patients were on insulin treatment. Four patients with KCNJ11 mutations were on (SU) treatment (glibenclamide) with doses varying between 0.06 and 1 mg/kg/d. Two patients with GCK (cases 18 and 19), one patient with KCNJ11 (case 1), and two patients with ABCC8 mutations (cases 6 and 7) had transient form of the disease and did not need any treatment.
Human leukocyte antigen (HLA) genotypes were analyzed in 58 cases (Table 1, Figure 2). The decreased risk-associated HLA genotype was more common in mutation-positive than in mutation-negative patients (38 vs 2%), whereas the strongly increased risk associated genotype was more common in mutation-negative than in mutation-positive patients (29 vs 6%)(P < .001). A total of 23/39 cases were positive for at least two islet antibodies and seven patients for one islet antibody. The prevalence of islet antibody positivity was 11 vs 73% in mutation-positive and mutation-negative patients, respectively (P = .001). Islet antibody positivity increased with age, so that in the group of patients diagnosed before 6 months of age 40% were positive whereas the corresponding rate was 71% in patients diagnosed after 6 months (Table 1, Figure 3).
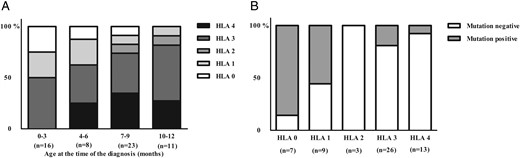
Distribution of different HLA genotypes in the Finnish patients diagnosed with diabetes before the age of 1 year (n = 58) (A) according to the age of diagnosis and (B) according to the mutation positivity.
(0 = decreased, 1 = neutral risk, 2 = slightly increased risk, 3 moderately increased risk, and 4 strongly increased risk genotypes.)
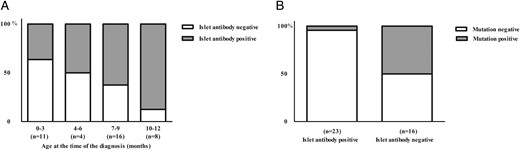
Incidence of islet autoantibodies in the Finnish patients diagnosed with diabetes before the age of 1 year (n = 39) shown as (A) according to the age of diagnosis and (B) according to the mutation positivity.
Discussion
The main aim of our study was to characterize clinical, genetic, and laboratory findings in Finnish patients diagnosed with diabetes during their first 12 months of life. The sequencing of 104 genes associated with the risk of diabetes or hyperglycemia, in addition to the genes previously associated with neonatal diabetes, showed that mutations in INS and K-ATP channel genes are the major causes of monogenic diabetes of infancy in Finns. We also found a novel mutation in RFX6 gene that was detected in a single patient diagnosed with syndromic neonatal diabetes. Additionally, our study demonstrated that after the age of 6 months, type 1 diabetes is more frequent than monogenic forms of diabetes.
The incidence rates of neonatal diabetes vary according to the criteria of neonatal diabetes and across the populations, with an overall estimated incidence of less than 2/100 000/year (5, 6, 31). In our study, the overall incidence including all different forms of diabetes diagnosed during the first 12 months after birth was 4.4/100,000/year between the years 1980 and 2014. This rate indicates that diabetes in this age group is rare when compared to the total incidence 62.5/100 000/year of type 1 diabetes in Finland among children under the age of 15 years (15, 32). The incidence of neonatal diabetes, diabetes diagnosed during the first 6 months of life, was 1.5/100 000/year live births, which is very close to overall incidence reported in previous studies (13). Only 13% had the transient form of the disease. All cases of monogenic diabetes were diagnosed at younger than 9 months.
The Finnish population has experienced a “bottleneck phenomenon” in the 15th–16th centuries when Northern and Eastern Finland was populated. As a consequence, more than 35 recessive monogenic diseases are enriched in Eastern Finland. However, we did not find any founder mutations in patients with neonatal diabetes, in contrast to congenital hyperinsulinism in which founder mutations explain most the cases (17, 18). Nearly all disease-causing mutations were sporadic and scattered in single families. Only two families shared the same mutation, the most common mutation R201H in the KCNJ11 gene (33).
The genetic risk for type 1 diabetes can be estimated by HLA class II genotyping that reflects approximately one-half of the genetic component of type 1 diabetes (34). Our study shows that protective or low-risk HLA genotypes were more common in patients diagnosed during the first months of life, whereas the higher risk genotypes became more frequent among patients who were diagnosed with diabetes at the end of the first year (Figure 2). Similarly, positivity for beta cell autoimmunity increased with age (Figure 3). The only mutation-positive patient who was islet antibody positive at the time of diagnosis was a patient with a STAT3 mutation (23). This observation suggests that autoantibody positivity is a rare phenomenon in monogenic diabetes. However, about 40% of the patients diagnosed during the first 6 months tested positive for two or more autoantibodies. In an earlier study, only around 3% of newborn infants of nondiabetic Finnish mothers had at least one autoantibody detectable in cord blood (35). Autoantibody levels decreased during the follow-up, and all subjects were autoantibody-negative by the age of 15 months suggesting that autoantibodies represented transplacentally transferred maternal autoantibodies. Thus, the autoantibodies observed in the patients diagnosed before the age of 6 months in our study likely represent de novo synthesized autoantibodies.
All disease-causing variants were detected in patients diagnosed with diabetes before the age of 9 months. Mutations in the INS gene were the most frequent and explained about one-fourth of all cases. All cases with INS mutations were diagnosed before the age of 9 months. Gene variants in K-ATP channel genes accounted for 36% of the monogenic cases, which is less than reported in other Caucasian populations (30). Single mutations in GCK (G261 and novel del 212) (22), RFX6, and in FOXP3 (30, 36) as well as the previously published STAT3 mutation (23) associated with diabetes in sporadic families. In addition, targeted sequencing revealed several gene variants (Supplemental Table 3) whose significance is difficult to assess but they may have some effect on glucose metabolism by affecting either insulin sensitivity, insulin secretion, or both.
Homozygous mutations in RFX6 (regulatory factor 6) gene are a rare cause of syndrome presenting with neonatal diabetes, intestinal atresias, and hepatobiliary abnormalities (37). Until today, eight patients with neonatal-onset diabetes share these clinical characteristics and two patients have been diagnosed with diabetes later in the childhood (at the ages of 3 and 6 years) (38). Our patient with the homozygous RFX6 mutation shared this previously described phenotype with diabetes diagnosed during the first day of life (37). Detection of the same mutation as heterozygous in one patient (Supplemental Table 3) led us to investigate the significance of this variant in METSIM cohort (Supplemental Table 4). Our results showed that the risk of diabetes was not increased in the group of heterozygous carriers of this variant. Therefore, it is unlikely that heterozygous RFX6 mutation alone has a major effect on glucose metabolism, although it may confer increased risk for diabetes.
Case 21, now a 20-year-old male with neonatal-onset diabetes and a hemizygous missense mutation R337Q in the FOXP3 gene, had a very similar phenotype as the patients described earlier with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome caused by FOXP3 mutations (30, 36). His two older brothers with the same syndrome without diabetes died at the age of 6 months and 19 years. The patient has been treated successfully with hematopoietic stem cell transplantation. In this case, as in the case of the RFX6 mutation, the syndromic features could be the clue to the correct diagnosis.
In all patients with K-ATP channel gene mutations, diabetes appeared in an isolated form and before the age of 6 months. The most common PNDM causing mutation R201H of KCNJ11 (14) was found in a family with two affected members, a mother and her son (Table 1, family 3), who were both diagnosed with diabetes during the first day after birth. The mother (case 4) was treated with insulin for 28 years before the mutation was detected. She was switched to SU treatment, but in spite of some recovery of insulin secretion and increase in C-peptide level, the maximal dose of glibenclamide resulted only in a partial treatment effect, and an additional low-dose insulin treatment was needed. Her son (case 3), case 5 with the same mutation, and case 2 with the mutation Q52L were successfully switched to oral medication with a low-dose SU therapy. Previous studies have shown that the effect of SUs on glucose control declines with increasing duration of diabetes (39, 40), and therefore, an early genetic diagnosis is important to initiate SU treatment without any delay. Unlike the previously described phenotype associated with the R50Q missense mutation (26) our patient (case 1) remitted after 7 months of insulin therapy, and has been normoglycemic ever since.
The patient carrying a novel missense mutation T588I in ABCC8 (case 6) had hyperglycemia after birth, but developed a remission during the neonatal period. Three of her family members (mother, mother's father, and mother's brother) who all carried this missense variant have been diagnosed with diabetes in adulthood, and have been successfully treated with oral medication (metformin and sitagliptin). Also the patient (case 7) with R825W in ABCC8 had a transient form of diabetes. Previous studies have shown that in ABCC8-associated diabetes, transient forms of neonatal diabetes are more frequent than PNDM but it may, however, relapse around puberty.
In case 18, neonatal hyperglycemia (up to 11.3 mmol/liter), the positive family history of gestational diabetes and adult-onset diabetes led to suspicion of GCK mutation in this family. A previously unpublished deletion of codon 212 in GCK was detected, which was consequently also found in five family members in three consecutive generations who were previously diagnosed with either diabetes or gestational diabetes.
Of the patients diagnosed with diabetes during the first 6 months, 40% were mutation-negative in targeted next-generation sequencing of 104 diabetes-associated genes. This result is in accordance with the findings of previous genetic studies of neonatal diabetes (13). Currently unknown genes causing neonatal diabetes are likely to be identified either by exome or genome sequencing.
In conclusion, 14 different mutations in 22 Finnish children aged 0–9 months and born between years 1980–2015 were associated with monogenic diabetes. Most variants located in KCNJ11, ABCC8, or INS genes. One novel heterozygous gene deletion in GCK, and one previously unpublished homozygous deletion in RFX6 were also detected. In our study, the monogenic etiology of diabetes was most common in the patients diagnosed before 6 months, with the exception of few patients with INS gene mutations. At the end of the first year of life, the diagnosis of autoimmune type 1 diabetes associated with moderately or strongly increased risk HLA genotypes and islet autoantibody positivity was more likely. Our results emphasize the importance of genetic testing for all patients diagnosed with diabetes before the age of 6 months because type 1 diabetes is very rare in this age group. After this age, type 1 diabetes is more common and genetic testing should be targeted to those patients without islet antibodies.
Acknowledgments
This work was funded by the Finnish Diabetes Research Foundation (H.H.), the Maud Kuistila Foundation (H.H.), the Finnish Cultural Foundation, North Savo Regional Fund (H.H.), and a VTR grant from the Kuopio University Hospital (H.H.).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- HLA
human leukocyte antigen
- Kir6.2
potassium inward rectifier
- METSIM
Metabolic Syndrome In Men
- NDM
neonatal diabetes
- PNDM
permanent neonatal diabetes
- SU
sulfonylurea.



