-
PDF
- Split View
-
Views
-
Cite
Cite
Christine Wohlfahrt-Veje, Annette Mouritsen, Casper P. Hagen, Jeanette Tinggaard, Mikkel Grunnet Mieritz, Malene Boas, Jørgen Holm Petersen, Niels E. Skakkebæk, Katharina M. Main, Pubertal Onset in Boys and Girls Is Influenced by Pubertal Timing of Both Parents, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 7, 1 July 2016, Pages 2667–2674, https://doi.org/10.1210/jc.2016-1073
Close - Share Icon Share
Epidemiological evidence on maternal and paternal heritability of the wide normal variation within pubertal timing is sparse.
We aimed to estimate the impact of parental pubertal timing on the onset of puberty in boys and girls.
Annual pubertal examinations of healthy children in a longitudinal cohort study. Information on parental timing of puberty (earlier, comparable to, or later compared to peers) and menarche age was retrieved from questionnaires.
A total of 672 girls and 846 boys.
Age at onset of pubic hair (PH2+), breasts (B2+), and menarche in girls; and PH2+, genital stage (G2+), and testis >3 mL with orchidometer (Tvol3+) in boys.
In boys, pubertal onset was significantly associated with pubertal timing of both parents. PH2+ and Tvol3+ were earlier: −11.8 months (95% confidence interval, −16.8, −6.8)/−8.9 (−12.8, −4.9), and −9.5 (−13.9, −5.1)/−7.1 (−10.4, −3.7) if the father/mother, respectively, had early pubertal development compared to late. In girls, menarche was significantly associated with both parents' pubertal timing: −10.5 months (−15.9, −5.1)/−10.1 (−14.3, −6.0) if father/mother had early pubertal development compared to late. For the onset of PH2+ and B2+ in girls, estimates were −7.0 months (−12.6, −1.4) and −4.1 (−10.6, +2.4)/−6.7 (−11.0, −2.5), and −6.7 (−11.0, −2.0) for fathers/mothers, respectively. Maternal age of menarche was significantly associated with the onset of all pubertal milestones except PH2+ in girls.
Maternal as well as paternal pubertal timing was a strong determinant of age at pubertal onset in both girls and boys. Age at breast and pubic hair development in girls, which has declined most during recent years, seemed to be least dependent on heritability.
Maternal as well as paternal pubertal timing was a strong determinant of age at pubertal onset in both girls and boys. Age at breast and pubic hair development in girls, which has declined most during recent years, seemed to be least dependent on heritability.
A large and widely unexplained variation exists within normal pubertal maturation. Early pubertal onset is related to a higher risk of obesity, diabetes, breast cancer, and all-cause mortality (1, 2). Given the recent trend of earlier pubertal maturation, especially in girls, the interest in factors determining pubertal timing is increasing. Timing of puberty is multifactorial. Intrauterine and early infant growth (3–5) as well as environmental compounds (6–8) may be important factors, and genetics undoubtedly plays a major role. According to twin studies, heritability is accountable for 57–61% of the variation in age at menarche (9, 10) and for 91% of the variation in the onset of pubertal growth spurt (11). These high heritability estimates from twin studies are, however, somewhat in contrast to the still limited knowledge of specific regulators of pubertal onset from genetic studies. Genes that affect pubertal timing, eg, MKRN3 (12), KISS1, and KISS1R (13), have been identified, but mutations in these are generally rare. Large genome-wide association studies have drawn attention to single nucleotide polymorphisms affecting pubertal timing. Recently, specific single nucleotide polymorphisms in FSHR and FSHB were found to be strongly associated with pubertal onset (14). But although more than 100 loci have been identified for age at menarche, together these explain only around 3% of the trait variance (15).
A number of studies have shown significant correlations between the age at menarche of mothers and the age at menarche of daughters (16). However, the evidence from epidemiological studies is more sparse concerning earlier pubertal milestones such as age at thelarche (breast development), pubarche (pubic hair development), and gonadarche (genital stage ≥2 or testis size above 3 mL in boys), which are the “gold standard” when clinically evaluating the onset of puberty. The role of paternal heritage is poorly described, except in the clinical conditions of delayed or precocious puberty (17, 18).
Specific knowledge on the heritability is important not only in the clinical setting, but also for researchers when studying environmental factors that may affect pubertal timing. We therefore asked parents of children, who participated in a large prospective birth cohort, to complete a questionnaire about their own pubertal development before the first clinical examination of their children.
We hypothesized that both paternal and maternal pubertal timing was strongly associated with the onset of all secondary sex characteristics in both boys and girls.
Subjects and Methods
In a large prospective mother-child cohort conducted in Copenhagen, Denmark, mothers were recruited between 1997 and 2003 in early pregnancy from three university hospitals. Only Caucasian mothers of Danish origin were included in the cohort. The cohort has previously been described in detail (19).
Between 2006 and 2013, 672 girls and 846 boys attended one to five annual clinical examinations (n = 4128; age range, 4.5–15.1 years). Pubertal stages in girls were evaluated as B1–B5 and PH1–PH5 by inspection according to Tanner (20) as well as palpation of breasts, thus avoiding misclassification of fat tissue as breast development. The onset of puberty was defined as ≥ B2 (B2+/thelarche) and ≥ PH2 (PH2+/pubarche) (21, 22). The girls were asked if they had experienced their first menstrual bleeding since the last examination (and when possible the specific month). In boys, testicular volume was measured by Prader's orchidometer (palpation). Pubertal stages in boys were evaluated by inspection as G1–G5 and PH1–PH5 (23). The onset of puberty in boys was defined as testicular volume >3 mL (Tvol3+/gonadarche), ≥ G2 (G2+/gonadarche) and/or ≥ PH2 (PH2+/pubarche) (24, 25). If testicular volumes of the two testes were not equal, the larger testis measurement was used. Likewise, if a girl had larger breast stage on one side, the larger stage was used. The physicians in the study participated in repetitive workshops to ensure and maintain standardization.
In questionnaires, which were sent to the family 2 weeks before the examination, mothers and (biological) fathers were asked: “Was your own pubertal timing early, average, or late compared to your peers?” Mothers were also asked about their age at menarche.
The study was conducted according to the Helsinki II Declaration and approved by the local ethics committee (KF 01-030/97/ KF 01276357/H-1-2009-074) and the Danish Data Protection Agency (1997-1200-074/2005-41-5545/2010-41-4757). The parents and children gave their written informed consent before examination.
Statistics
We estimated the mean age of onset of puberty and the impact of parental puberty timing by probit analyses using Proc Lifereg (SAS Institute). This allowed taking into account the left, right, and interval-censoring present in the data. In all children, the longitudinal course of pubertal development was evaluated. For most children, we had an age at which they did not have the pubertal marker of interest and a following age at which they did (interval-censored observation). Some of the children had the pubertal marker of interest already at the first examination. The attainment of this marker was therefore known to occur before the age at first examination (left censored observation). Other children did not attain the marker of interest at any of the examinations (observation right censored at last examination). If a precise age of menarche could be obtained, this age was used as noncensored data. The probit analysis allowed estimating the mean as well as the variance of the pubertal age of onset distribution. The latter was used to estimate how much of the variation in the pubertal onset distribution could be explained by parental pubertal timing. The percentages of the variance of onset of pubertal milestones that were explained by parental timing were calculated as 1− (variance for model including information on parents/variance for model without information on parents).
Progression time from the onset of B2 to menarche was estimated using the lowest possible interval and highest possible interval for censoring.
If transient pubertal maturation was observed (eg, breast development appearing at one examination but not at the next), we excluded this observation in the models, hence using age at the previous examination and age when consistent pubertal onset was observed (B2+, n = 33; Tvol3+, n = 1; PH2+, n = 8; G2+, n = 52).
The impact of parents' pubertal timing (categorical variable: being earlier or average vs later than their peers) and of maternal menarche (as a continuous variable, in years) was estimated by including these as covariates (one at the time) in the probit analyses. Estimates are given in months with 95% confidence intervals (CIs). Analyses were also adjusted for the pubertal timing of the other parent (information on both parents in the model). Possible interactions between maternal and paternal pubertal timing were tested, including a product term in the models.
The distribution (cross-tabulation) of mothers' and fathers' pubertal timing was examined with χ2 test.
Results
Mean age at Tvol3+, G2+, and PH2+ for boys and at B2+, PH2+, and menarche in girls is shown in Table 1. Mean age of menarche in girls was significantly earlier compared to mean age of maternal menarche (12.99 years, SD: 1.16; vs 13.17 years, SD: 1.32; P < .001). Mean ages of pubertal milestones (B2+, PH2+, Tvol3+, age of menarche) were not significantly different in children with parental information (n = 1081) vs those without (n = 435; 28.7%) (data not shown).
| Pubertal Milestone . | Mean Age (95% CI), y . | Prediction Intervals (Mean ± 2 SD) . |
|---|---|---|
| Boys | ||
| Gonadarche (Tvol3+) | 11.56 (11.46–11.66) | 9.51–13.61 |
| Gonadarche (G2+) | 11.46 (11.34–11.58) | 8.88–14.06 |
| Pubarche (PH2+) | 11.88 (11.77–12.00) | 9.49–14.29 |
| Girls | ||
| Thelarche (B2+) | 9.95 (9.80–10.08) | 7.54–12.35 |
| Pubarche (PH2+) | 10.99 (10.87–11.12) | 8.64–13.34 |
| Age at menarche, y | 12.99 (12.86–13.12) | 10.68–15.30 |
| Progression timea | 3.30 (3.08–3.52) | 1.16–5.44 |
| Pubertal Milestone . | Mean Age (95% CI), y . | Prediction Intervals (Mean ± 2 SD) . |
|---|---|---|
| Boys | ||
| Gonadarche (Tvol3+) | 11.56 (11.46–11.66) | 9.51–13.61 |
| Gonadarche (G2+) | 11.46 (11.34–11.58) | 8.88–14.06 |
| Pubarche (PH2+) | 11.88 (11.77–12.00) | 9.49–14.29 |
| Girls | ||
| Thelarche (B2+) | 9.95 (9.80–10.08) | 7.54–12.35 |
| Pubarche (PH2+) | 10.99 (10.87–11.12) | 8.64–13.34 |
| Age at menarche, y | 12.99 (12.86–13.12) | 10.68–15.30 |
| Progression timea | 3.30 (3.08–3.52) | 1.16–5.44 |
B2+ to menarche.
| Pubertal Milestone . | Mean Age (95% CI), y . | Prediction Intervals (Mean ± 2 SD) . |
|---|---|---|
| Boys | ||
| Gonadarche (Tvol3+) | 11.56 (11.46–11.66) | 9.51–13.61 |
| Gonadarche (G2+) | 11.46 (11.34–11.58) | 8.88–14.06 |
| Pubarche (PH2+) | 11.88 (11.77–12.00) | 9.49–14.29 |
| Girls | ||
| Thelarche (B2+) | 9.95 (9.80–10.08) | 7.54–12.35 |
| Pubarche (PH2+) | 10.99 (10.87–11.12) | 8.64–13.34 |
| Age at menarche, y | 12.99 (12.86–13.12) | 10.68–15.30 |
| Progression timea | 3.30 (3.08–3.52) | 1.16–5.44 |
| Pubertal Milestone . | Mean Age (95% CI), y . | Prediction Intervals (Mean ± 2 SD) . |
|---|---|---|
| Boys | ||
| Gonadarche (Tvol3+) | 11.56 (11.46–11.66) | 9.51–13.61 |
| Gonadarche (G2+) | 11.46 (11.34–11.58) | 8.88–14.06 |
| Pubarche (PH2+) | 11.88 (11.77–12.00) | 9.49–14.29 |
| Girls | ||
| Thelarche (B2+) | 9.95 (9.80–10.08) | 7.54–12.35 |
| Pubarche (PH2+) | 10.99 (10.87–11.12) | 8.64–13.34 |
| Age at menarche, y | 12.99 (12.86–13.12) | 10.68–15.30 |
| Progression timea | 3.30 (3.08–3.52) | 1.16–5.44 |
B2+ to menarche.
The distribution of parents' pubertal timing as early, average, and late is shown in Supplemental Table 1. The combination of parental pubertal timing was not randomly distributed because children with late maturing fathers more often also had a late maturing mother (χ2, P < .01).
Early (compared to late) pubertal timing in fathers was significantly associated with earlier onset of all pubertal milestones in their sons (Table 2), with the largest estimates for PH2+. Boys with early maturing fathers had pubarche earlier (−11.8 months; 95% CI, −16.8, −6.8) than boys with late maturing fathers and compared to boys with average maturing fathers (−5.4 months; 95% CI, −9.4, −1.3). All associations were also significant for boys with average maturing vs late maturing fathers, but regarding G2+ and Tvol3+, not for boys with early compared to average maturing fathers. In girls, having an early maturing father (compared to late) was associated with significantly earlier age at menarche (−10.5 months; 95% CI, −15.9, −5.1) and earlier age at pubarche (−7.0 months; 95% CI, −12.6, −1.4). In contrast, the association to age at breast development in girls was not significant for early maturing fathers, but we found a significant difference for average vs late maturing fathers. Girls with early maturing fathers did not mature significantly earlier than average maturing fathers (Table 3). The significant associations remained so after adjusting for maternal pubertal timing (data not shown).
Impact of Parental Pubertal Timing on the Onset of Pubarche (PH2+), Gonadarche (Tvol3+), and Tanner Genital Stage 2 (G2+) in Boys
| Boys . | Pubic hair (PH2+), Right/Left/Interval Censored (n = 472/82/275) . | Gonadarche (Tvol3+) Right/Left/Interval-Censored (n = 398/87/322) . | Genital Stage 2 (G2+) Right/Left/Interval Censored (n = 405/108/311) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated Difference . | P Value . | N 829 . | Estimated Difference . | P Value . | N 807 . | Estimated Difference . | P Value . | N 824 . | |
| Fathers' puberty | |||||||||
| Earlya | −11.8 (−16.8, −6.8) | <.001 | 597 | −9.5 (−13.9, −5.1) | <.001 | 595 | −8.6 (−13.9, −3.2) | <.001 | |
| Averageb | −5.4 (−9.4, −1.3) | .010 | −3.1 (−6.6, 0.5) | .090 | −2.9 (−7.3, 1.6) | .203 | 595 | ||
| Latec | 6.4 (2.6, 10.3) | <.001 | 6.4 (3.1, 9.7) | <.001 | 5.7 (1.6, 9.8) | .006 | |||
| Mothers' puberty | |||||||||
| Earlya | −8.9 (−12.8, −4.9) | <.001 | −7.1 (−10.4, −3.7) | <.001 | −6.5 (−10.7, −2.4) | .002 | |||
| Averageb | −1.6 (−5.0, 1.8) | .346 | 663 | 0.0 (−2.9, 2.9) | .990 | 660 | 1.5 (−2.1, 5.1) | .400 | 662 |
| Latec | 7.2 (3.9, 10.6) | <.001 | 7.1 (4.3, 9.9) | <.001 | 8.0 (4.5, 11.5) | <.001 | |||
| Mothers' menarche age | 2.4 (1.4, 3.5) | <.001 | 770 | 2.0 (1.0, 2.9) | <.001 | 743 | 1.6 (0.4, 2.7) | .006 | 767 |
| Boys . | Pubic hair (PH2+), Right/Left/Interval Censored (n = 472/82/275) . | Gonadarche (Tvol3+) Right/Left/Interval-Censored (n = 398/87/322) . | Genital Stage 2 (G2+) Right/Left/Interval Censored (n = 405/108/311) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated Difference . | P Value . | N 829 . | Estimated Difference . | P Value . | N 807 . | Estimated Difference . | P Value . | N 824 . | |
| Fathers' puberty | |||||||||
| Earlya | −11.8 (−16.8, −6.8) | <.001 | 597 | −9.5 (−13.9, −5.1) | <.001 | 595 | −8.6 (−13.9, −3.2) | <.001 | |
| Averageb | −5.4 (−9.4, −1.3) | .010 | −3.1 (−6.6, 0.5) | .090 | −2.9 (−7.3, 1.6) | .203 | 595 | ||
| Latec | 6.4 (2.6, 10.3) | <.001 | 6.4 (3.1, 9.7) | <.001 | 5.7 (1.6, 9.8) | .006 | |||
| Mothers' puberty | |||||||||
| Earlya | −8.9 (−12.8, −4.9) | <.001 | −7.1 (−10.4, −3.7) | <.001 | −6.5 (−10.7, −2.4) | .002 | |||
| Averageb | −1.6 (−5.0, 1.8) | .346 | 663 | 0.0 (−2.9, 2.9) | .990 | 660 | 1.5 (−2.1, 5.1) | .400 | 662 |
| Latec | 7.2 (3.9, 10.6) | <.001 | 7.1 (4.3, 9.9) | <.001 | 8.0 (4.5, 11.5) | <.001 | |||
| Mothers' menarche age | 2.4 (1.4, 3.5) | <.001 | 770 | 2.0 (1.0, 2.9) | <.001 | 743 | 1.6 (0.4, 2.7) | .006 | 767 |
Data are expressed as estimate in months (95% CI). Example: boys with early maturing fathers develop pubic hair 11.8 months earlier than if their fathers were late maturing and 5.4 months earlier than if fathers were average maturing. Boys with late maturing fathers develop pubic hair 6.4 months later than if their father were average maturing. An increase in mothers' menarche age (by 1 year) was associated with 2.2 months later pubic hair development in sons.
Compared to late maturing.
Compared to early maturing.
Compared to average maturing.
Impact of Parental Pubertal Timing on the Onset of Pubarche (PH2+), Gonadarche (Tvol3+), and Tanner Genital Stage 2 (G2+) in Boys
| Boys . | Pubic hair (PH2+), Right/Left/Interval Censored (n = 472/82/275) . | Gonadarche (Tvol3+) Right/Left/Interval-Censored (n = 398/87/322) . | Genital Stage 2 (G2+) Right/Left/Interval Censored (n = 405/108/311) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated Difference . | P Value . | N 829 . | Estimated Difference . | P Value . | N 807 . | Estimated Difference . | P Value . | N 824 . | |
| Fathers' puberty | |||||||||
| Earlya | −11.8 (−16.8, −6.8) | <.001 | 597 | −9.5 (−13.9, −5.1) | <.001 | 595 | −8.6 (−13.9, −3.2) | <.001 | |
| Averageb | −5.4 (−9.4, −1.3) | .010 | −3.1 (−6.6, 0.5) | .090 | −2.9 (−7.3, 1.6) | .203 | 595 | ||
| Latec | 6.4 (2.6, 10.3) | <.001 | 6.4 (3.1, 9.7) | <.001 | 5.7 (1.6, 9.8) | .006 | |||
| Mothers' puberty | |||||||||
| Earlya | −8.9 (−12.8, −4.9) | <.001 | −7.1 (−10.4, −3.7) | <.001 | −6.5 (−10.7, −2.4) | .002 | |||
| Averageb | −1.6 (−5.0, 1.8) | .346 | 663 | 0.0 (−2.9, 2.9) | .990 | 660 | 1.5 (−2.1, 5.1) | .400 | 662 |
| Latec | 7.2 (3.9, 10.6) | <.001 | 7.1 (4.3, 9.9) | <.001 | 8.0 (4.5, 11.5) | <.001 | |||
| Mothers' menarche age | 2.4 (1.4, 3.5) | <.001 | 770 | 2.0 (1.0, 2.9) | <.001 | 743 | 1.6 (0.4, 2.7) | .006 | 767 |
| Boys . | Pubic hair (PH2+), Right/Left/Interval Censored (n = 472/82/275) . | Gonadarche (Tvol3+) Right/Left/Interval-Censored (n = 398/87/322) . | Genital Stage 2 (G2+) Right/Left/Interval Censored (n = 405/108/311) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated Difference . | P Value . | N 829 . | Estimated Difference . | P Value . | N 807 . | Estimated Difference . | P Value . | N 824 . | |
| Fathers' puberty | |||||||||
| Earlya | −11.8 (−16.8, −6.8) | <.001 | 597 | −9.5 (−13.9, −5.1) | <.001 | 595 | −8.6 (−13.9, −3.2) | <.001 | |
| Averageb | −5.4 (−9.4, −1.3) | .010 | −3.1 (−6.6, 0.5) | .090 | −2.9 (−7.3, 1.6) | .203 | 595 | ||
| Latec | 6.4 (2.6, 10.3) | <.001 | 6.4 (3.1, 9.7) | <.001 | 5.7 (1.6, 9.8) | .006 | |||
| Mothers' puberty | |||||||||
| Earlya | −8.9 (−12.8, −4.9) | <.001 | −7.1 (−10.4, −3.7) | <.001 | −6.5 (−10.7, −2.4) | .002 | |||
| Averageb | −1.6 (−5.0, 1.8) | .346 | 663 | 0.0 (−2.9, 2.9) | .990 | 660 | 1.5 (−2.1, 5.1) | .400 | 662 |
| Latec | 7.2 (3.9, 10.6) | <.001 | 7.1 (4.3, 9.9) | <.001 | 8.0 (4.5, 11.5) | <.001 | |||
| Mothers' menarche age | 2.4 (1.4, 3.5) | <.001 | 770 | 2.0 (1.0, 2.9) | <.001 | 743 | 1.6 (0.4, 2.7) | .006 | 767 |
Data are expressed as estimate in months (95% CI). Example: boys with early maturing fathers develop pubic hair 11.8 months earlier than if their fathers were late maturing and 5.4 months earlier than if fathers were average maturing. Boys with late maturing fathers develop pubic hair 6.4 months later than if their father were average maturing. An increase in mothers' menarche age (by 1 year) was associated with 2.2 months later pubic hair development in sons.
Compared to late maturing.
Compared to early maturing.
Compared to average maturing.
Impact of Parental Pubertal Timing on the Onset of Thelarche (B2+), Pubarche (PH2+), and Menarche in Girls
| Girls . | Thelarche (B2+) Right/Left/Interval Censored (n = 156/175/332) . | Pubic Hair (PH2+) Right/Left/Interval Censored (n = 233/115/313) . | Age at Menarche Non/Right/Left/Interval Censored (n = 227/357/0/5) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated Difference . | P Value . | N 663 . | Estimated Difference . | P Value . | N 661 . | Estimated Difference . | P Value . | N 589 . | |
| Fathers' puberty | |||||||||
| Earlya | −4.1 (−10.6, +2.4) | .217 | −7.0 (−12.6, −1.4) | .014 | −10.5 (−15.9, 5.1) | <.001 | |||
| Averageb | 2.1 (−3.3, 7.5) | .400 | 490 | 0.3 (−4.3, 5.0) | .890 | 491 | −4.1 (−8.4, 0.2) | <.060 | 490 |
| Latec | 6.2 (1.5, 11.0) | .011 | 7.4 (3.3, 11.4) | <.001 | 6.4 (2.2, 10.6) | .003 | |||
| Mothers' puberty | |||||||||
| Earlya | −6.7 (−11.4, −2.0) | .005 | −6.7 (−11.0, −2.5) | .002 | −10.1 (−14.3, −6.0) | <.001 | |||
| Averageb | −1.7 (−6.1, 2.7) | .440 | 559 | −2.0 (−5.9, 1.8) | .300 | 560 | −5.7 (−9.2, −2.2) | .002 | 559 |
| Latec | 5.0 (1.7, 8.8) | .011 | 4.7 (1.2, 8.2) | .008 | 4.5 (0.8, 8.2) | .020 | |||
| Mothers' menarche age | 1.8 (0.7, 2.9) | .002 | 615 | 1.0 (−0.0, 2.1) | .061 | 615 | 4.4 (3.1, 5.6) | <.001 | 569 |
| Girls . | Thelarche (B2+) Right/Left/Interval Censored (n = 156/175/332) . | Pubic Hair (PH2+) Right/Left/Interval Censored (n = 233/115/313) . | Age at Menarche Non/Right/Left/Interval Censored (n = 227/357/0/5) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated Difference . | P Value . | N 663 . | Estimated Difference . | P Value . | N 661 . | Estimated Difference . | P Value . | N 589 . | |
| Fathers' puberty | |||||||||
| Earlya | −4.1 (−10.6, +2.4) | .217 | −7.0 (−12.6, −1.4) | .014 | −10.5 (−15.9, 5.1) | <.001 | |||
| Averageb | 2.1 (−3.3, 7.5) | .400 | 490 | 0.3 (−4.3, 5.0) | .890 | 491 | −4.1 (−8.4, 0.2) | <.060 | 490 |
| Latec | 6.2 (1.5, 11.0) | .011 | 7.4 (3.3, 11.4) | <.001 | 6.4 (2.2, 10.6) | .003 | |||
| Mothers' puberty | |||||||||
| Earlya | −6.7 (−11.4, −2.0) | .005 | −6.7 (−11.0, −2.5) | .002 | −10.1 (−14.3, −6.0) | <.001 | |||
| Averageb | −1.7 (−6.1, 2.7) | .440 | 559 | −2.0 (−5.9, 1.8) | .300 | 560 | −5.7 (−9.2, −2.2) | .002 | 559 |
| Latec | 5.0 (1.7, 8.8) | .011 | 4.7 (1.2, 8.2) | .008 | 4.5 (0.8, 8.2) | .020 | |||
| Mothers' menarche age | 1.8 (0.7, 2.9) | .002 | 615 | 1.0 (−0.0, 2.1) | .061 | 615 | 4.4 (3.1, 5.6) | <.001 | 569 |
Data are expressed as estimate in months (95% CI).
Compared to late maturing.
Compared to early maturing.
Compared to average maturing.
Impact of Parental Pubertal Timing on the Onset of Thelarche (B2+), Pubarche (PH2+), and Menarche in Girls
| Girls . | Thelarche (B2+) Right/Left/Interval Censored (n = 156/175/332) . | Pubic Hair (PH2+) Right/Left/Interval Censored (n = 233/115/313) . | Age at Menarche Non/Right/Left/Interval Censored (n = 227/357/0/5) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated Difference . | P Value . | N 663 . | Estimated Difference . | P Value . | N 661 . | Estimated Difference . | P Value . | N 589 . | |
| Fathers' puberty | |||||||||
| Earlya | −4.1 (−10.6, +2.4) | .217 | −7.0 (−12.6, −1.4) | .014 | −10.5 (−15.9, 5.1) | <.001 | |||
| Averageb | 2.1 (−3.3, 7.5) | .400 | 490 | 0.3 (−4.3, 5.0) | .890 | 491 | −4.1 (−8.4, 0.2) | <.060 | 490 |
| Latec | 6.2 (1.5, 11.0) | .011 | 7.4 (3.3, 11.4) | <.001 | 6.4 (2.2, 10.6) | .003 | |||
| Mothers' puberty | |||||||||
| Earlya | −6.7 (−11.4, −2.0) | .005 | −6.7 (−11.0, −2.5) | .002 | −10.1 (−14.3, −6.0) | <.001 | |||
| Averageb | −1.7 (−6.1, 2.7) | .440 | 559 | −2.0 (−5.9, 1.8) | .300 | 560 | −5.7 (−9.2, −2.2) | .002 | 559 |
| Latec | 5.0 (1.7, 8.8) | .011 | 4.7 (1.2, 8.2) | .008 | 4.5 (0.8, 8.2) | .020 | |||
| Mothers' menarche age | 1.8 (0.7, 2.9) | .002 | 615 | 1.0 (−0.0, 2.1) | .061 | 615 | 4.4 (3.1, 5.6) | <.001 | 569 |
| Girls . | Thelarche (B2+) Right/Left/Interval Censored (n = 156/175/332) . | Pubic Hair (PH2+) Right/Left/Interval Censored (n = 233/115/313) . | Age at Menarche Non/Right/Left/Interval Censored (n = 227/357/0/5) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimated Difference . | P Value . | N 663 . | Estimated Difference . | P Value . | N 661 . | Estimated Difference . | P Value . | N 589 . | |
| Fathers' puberty | |||||||||
| Earlya | −4.1 (−10.6, +2.4) | .217 | −7.0 (−12.6, −1.4) | .014 | −10.5 (−15.9, 5.1) | <.001 | |||
| Averageb | 2.1 (−3.3, 7.5) | .400 | 490 | 0.3 (−4.3, 5.0) | .890 | 491 | −4.1 (−8.4, 0.2) | <.060 | 490 |
| Latec | 6.2 (1.5, 11.0) | .011 | 7.4 (3.3, 11.4) | <.001 | 6.4 (2.2, 10.6) | .003 | |||
| Mothers' puberty | |||||||||
| Earlya | −6.7 (−11.4, −2.0) | .005 | −6.7 (−11.0, −2.5) | .002 | −10.1 (−14.3, −6.0) | <.001 | |||
| Averageb | −1.7 (−6.1, 2.7) | .440 | 559 | −2.0 (−5.9, 1.8) | .300 | 560 | −5.7 (−9.2, −2.2) | .002 | 559 |
| Latec | 5.0 (1.7, 8.8) | .011 | 4.7 (1.2, 8.2) | .008 | 4.5 (0.8, 8.2) | .020 | |||
| Mothers' menarche age | 1.8 (0.7, 2.9) | .002 | 615 | 1.0 (−0.0, 2.1) | .061 | 615 | 4.4 (3.1, 5.6) | <.001 | 569 |
Data are expressed as estimate in months (95% CI).
Compared to late maturing.
Compared to early maturing.
Compared to average maturing.
Early and average maternal pubertal timing (compared to late) was significantly associated with earlier onset of all pubertal milestones in both the sons (Table 2) and the daughters (Table 3), with estimated effects of having an early maturing mother compared to having a late maturing mother ranging from −10.1 months, (95% CI, −14.3, −6.0) for menarche in girls to −6.5 months (95% CI, −10.7, −2.4) for G2+ in boys. Children with early maturing mothers did not differ from those with average maturing mothers in age at pubertal onset except for menarche in girls. Adjusting for fathers' pubertal timing did not essentially change estimates (data not shown).
Maternal age of menarche was associated with all pubertal milestones of both boys and girls, except girls' development of pubic hair (Tables 2 and 3). The largest effect estimate was for the association with their daughter's age of menarche: 4.4 months (95% CI, 3.1, 5.6) per year (Table 3).
Parental pubertal timing explained 7.8, 9.3, and 6.0% of the variance in Tvol3+, PH2+, and G2+ in boys (maternal and paternal timing both in the model) and 3.3, 5.9, and 14.5% for B2+, PH2+, and menarche in girls, respectively (for PH2+, maternal and paternal timing was included in the model; for menarche and B2+, paternal timing and maternal menarche was included in the model because maternal menarche in these models explained more variance than maternal timing).
Mean ages of pubertal milestones in subgroups, with combinations of mothers' and fathers' pubertal timing (eg, children with an early father and an early mother), are shown for boys in Figure 1, A—C, and for girls in Figure 2, A–C. Significant interactions between the parents' pubertal timing were found for PH2+ in boys (P = .01) and for menarche in girls (P = .005), because for these milestones, if just one of the parents had matured early, the child would also mature significantly earlier (regardless of the other parent's pubertal timing) (Figures 1C and 2C).
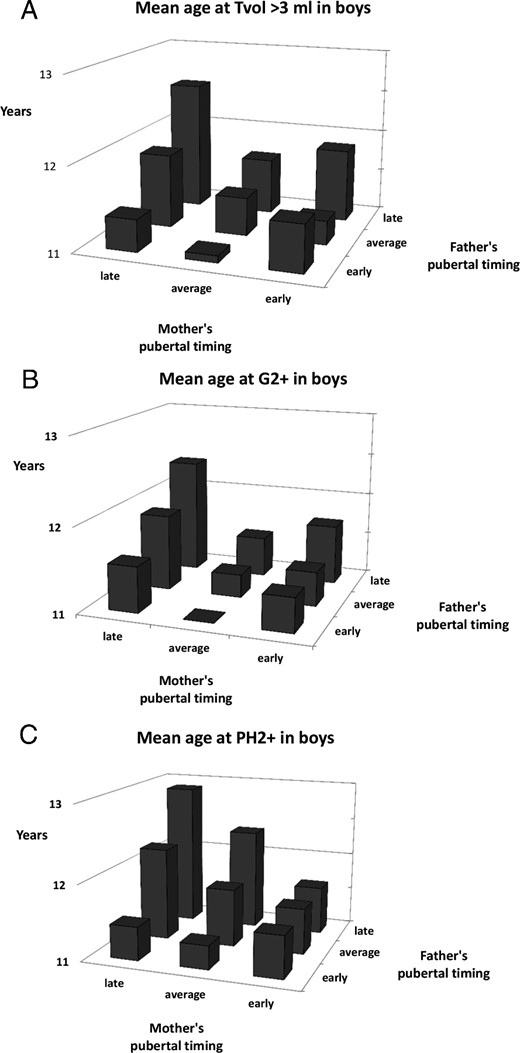
Mean age at onset of pubertal milestones (A, Tvol3+; B, G2+; C, PH2+) for boys with different combinations of parental pubertal timing.
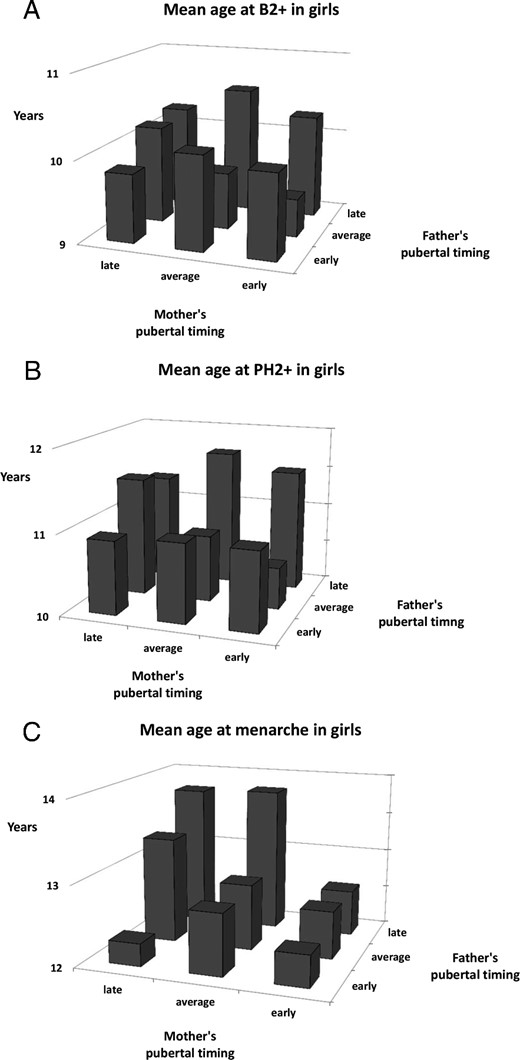
Mean age at onset of pubertal milestones (A, B2+; B, PH2+; C, Menarche) for girls with different combinations of parental pubertal timing.
Discussion
Overall, we found that both the paternal and maternal history of pubertal timing affect the timing of pubertal onset in the sons and daughters. This suggests a significant degree of heritability of the normal variation of pubertal onset, which appears not to be sex-specific. There were subtle differences in the effect of parental timing on individual secondary sex characteristics. In girls, age of menarche appeared to be more strongly correlated to parental pubertal timing than breast and pubic hair development. Although we cannot exclude that these differences are caused by inherent inaccuracies in determining the onset of these early markers of puberty in girls, our findings indicate that nongenetic factors, ie, environmental factors, influence thelarche and pubarche more than age of menarche in girls. Paternal pubertal timing was significantly associated with the onset of pubic hair and age at menarche, but not consistently with breast development in girls. Maternal timing of puberty was associated with all milestones in both boys and girls. When combining maternal and paternal history of pubertal timing, an apparently additive effect could be seen for all pubertal milestones in boys, but only for menarche in girls. Looking into interactions, however, we found that for pubic hair development in boys and for menarche in girls, just having one early maturing parent had significant impact, regardless of the other parent's pubertal timing.
In accordance with other studies (16), we found that maternal age at menarche predicted age at menarche in their daughters. Additionally, maternal age at menarche was related to the onset of breast development in girls as well as to pubic hair development and gonadarche in boys.
More often than expected due to chance, late maturing fathers matched with late maturing mothers. This possible example of assortative mating was adjusted for in our models (adjusting for the other parent by having both in the model), but it did not substantially change the results.
Delayed puberty is more frequent in boys than in girls and is often associated with a history of late pubertal development of their fathers (17). However, we did not find a father-to-son/mother-to-daughter (sex-specific) effect of parental pubertal timing. Pubertal timing of both parents had effects on pubertal onset of both boys and girls. These findings are in line with a large Finnish study of di- and monozygotic twin boys and girls concluding that a large part of the variance in pubertal maturation was explained by genetics and that the genetic effect was not sex dependent (26). Furthermore, recent large genomic analyses found that both recalled age at voice break in boys (27) and Tanner stages in boys and girls (28) shared some genetic etiology with timing of menarche in girls. Also in line with our findings, studies of children with precocious puberty as well as children with constitutional delay of growth found evidence of paternal as well as maternal heritage (17, 29). In addition, our study confirms results from the ALSPAC study in which maternal menarche before 12 years of age was associated with earlier self-reported attainment of B2+, PH2+, and menarche in girls (30). However, this study did not report the association between maternal menarche age and onset of pubertal milestones in the boys (31).
The association between maternal age of menarche and onset of pubic hair in girls was nonsignificant. A possible interpretation of this may be that the female gonadarche (thelarche) pathway and adrenarche (pubarche) pathway differs regarding both physiological and genetic regulation. This is in accordance with a study of genetic correlations evaluating 184 pairs of mono- and dizygotic twin girls. The authors concluded that although gonadarche (breast development and menarche) and adrenarche were to a significant extent heritable and showed high genetic correlations, they were also differently affected by environmental factors and should be regarded as different manifestations and not a unitary physiological factor (32).
We had information from more mothers than fathers, and this could have affected the likelihood of finding significant results. However, the fathers' information was highly associated to age at menarche and onset of pubic hair in girls as well as pubic hair and gonadarche in boys.
To our knowledge, this is the first epidemiological study to investigate the impact of paternal pubertal maturation on the normal variation in pubertal onset in a father's offspring. A reason for this gap of evidence could be that it is often more reliable to ask the mothers. It is easier to recall menarche than any other pubertal sign, and most fathers will not be able to recall when their testes started growing. The question of being early, average, or late compared to peers is usually also simple to answer by memory or looking at school pictures. Approximately half of the mothers in our study categorized themselves as having had average pubertal timing compared to two-thirds of the fathers. This suggests a sex-specific bias, with fathers tending to choose average timing more often, or it reflects the lack of an obvious male milestone like menarche. Because associations of both parents are consistently strong with almost all pubertal milestones in children, we do not believe that this potential recall bias compromises our findings (or that the question we asked the parents was too simple). Furthermore, both age of menarche and timing of maturation (early, average, or late in relationship to peers) have earlier proved to be well remembered by women (33). The parents answered the questionnaires before the clinical examinations of their children, and hence their answers were not likely biased by the results of these. Mean age of pubertal onset was not significantly different in the children with or without data on parental puberty, which indicates that the missing data did not cause systematic bias either.
Some children experienced transient pubertal development (eg, B2, G2, or Tvol3+), which was not observed at the subsequent examination. We cannot rule out that some of these cases were due to interobserver variation, although this was not pronounced in our study (34). It is, however, more likely that these inconsistencies reflect true variations in androgen and estrogen levels that cause intermittent pubertal signs because this is a well-known physiological phenomenon experienced in clinical settings, although only described in a few studies (30, 35, 36).
Mean ages of pubertal onset in this cohort were similar to those from contemporary studies and significantly earlier than from the study performed in 1991–1993 in the same geographic area (37–39), with the exception of mean age of PH2+ in boys that seems unchanged. Our findings confirm the suggested trend toward earlier pubertal onset, especially in girls. Girls in our study also had earlier mean age of menarche than their mothers. This is in line with the hypothesis that the observed trend toward earlier pubertal maturation is not caused by genetics, but is caused most probably by other (environmental) factors (38).
Interestingly, the associations between parental puberty and breast development in girls were generally weaker than associations with menarche and with pubertal milestones in boys. In line with this, a Dutch twin study found that genetic influence on pubertal markers and FSH, LH, T, and estradiol levels at 9 and 12 years was higher for boys than for girls. Heritability had an impact on all hormones except estradiol levels at 9 years in girls, which common environmental factors conversely seemed to influence (40). Although genome-wide association studies have found overlaps between loci associated with menarche and pubertal Tanner stages in both boys and girls (28), these findings could reflect that impact of environment and heritability varies between boys and girls as well as for early and late pubertal markers. Environmental factors such as body composition changes and chemical exposure may play a larger role for an early marker as breast development than for menarche. In line with this hypothesis, the decline of age at breast development was more pronounced than that of age at menarche. Similarly, in boys in whom effect estimates and variance explained by inheritance in general were larger than in girls, the age of pubertal onset has not changed as much over time as it has for breast development in girls.
Conclusions
Both paternal and maternal timing of puberty had a strong influence on the timing of pubertal onset in children, and this was not sex-specific. Mean ages of pubertal onset support earlier findings of a secular trend toward earlier pubertal maturation. This was most pronounced for breast development in girls, for which hereditability appears to have less impact than for menarche. This suggests that other (environmental) factors are responsible for the rapid decline in age at pubertal onset in girls.
Acknowledgments
We are grateful to all the participating families and to all colleagues at the Department of Growth and Reproduction who have been involved in the Copenhagen Mother Child Cohort. We especially thank research nurse Helle Kelkeland for helpful assistance regarding logistics, questionnaires, and child examinations.
Disclosure Summary: The authors have nothing to declare.
Abbreviations
- B2+
onset of B2/thelarche
- CI
confidence interval
- G2+
onset of G2/gonadarche
- PH2+
onset of PH2/pubarche
- Tvol3+
onset of testis volume above 3 mL with orchidometer/gonadarche.



