-
PDF
- Split View
-
Views
-
Cite
Cite
Sandrine Andrea Urwyler, Katharina Timper, Wiebke Fenske, Nadia de Mota, Anne Blanchard, Felix Kühn, Nica Frech, Birsen Arici, Jonas Rutishauser, Peter Kopp, Christoph Stettler, Beat Müller, Mira Katan, Catherine Llorens-Cortes, Mirjam Christ-Crain, Plasma Apelin Concentrations in Patients With Polyuria-Polydipsia Syndrome, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 5, 1 May 2016, Pages 1917–1923, https://doi.org/10.1210/jc.2016-1158
Close - Share Icon Share
Abstract
Apelin and arginine vasopressin are antagonists in the regulation of body fluid and osmotic homeostasis. There are no data about apelin levels in patients with polyuria-polydipsia syndrome (PPS).
To investigate plasma apelin levels and plasma apelin to copeptin ratios in patients with PPS and healthy volunteers using copeptin as a surrogate marker for arginine vasopressin.
We included 41 patients with PPS in this post hoc analysis of a prospective study performed in tertiary care hospitals in Switzerland and Germany and 113 healthy volunteers as a control group.
Plasma apelin and copeptin levels were measured in 15 patients with complete central diabetes insipidus (DI), seven patients with complete nephrogenic DI, 19 patients with primary polydipsia (PP), and 113 healthy volunteers.
Plasma apelin levels were highest in patients with complete nephrogenic DI (413 pmol/L; interquartile range, 332–504 pmol/L; P = .01) and lower in patients with PP (190 [172–215] pmol/L; P < .001) or complete central DI (209 [174–241] pmol/L; P = .02) as compared to healthy volunteers (254 [225–311] pmol/L). Plasma apelin to copeptin ratio in patients with PP (53 [38–92] pmol/pmol; P > .9) was similar to healthy volunteers (57 [37–102] pmol/pmol). In contrast, the apelin to copeptin ratio was higher in patients with complete central DI (89 [73–135] pmol/pmol; P = .02) and lower in patients with complete nephrogenic DI (7 [6–10] pmol/pmol; P < .001) compared to healthy volunteers.
In PP, normal plasma apelin to copeptin ratio attests a normal water homeostasis. In contrast, in patients with central or nephrogenic DI, the increased or decreased apelin to copeptin ratio, respectively, reflects a disturbed osmotic and body fluid homeostasis.
Apelin is a neuro-vasoactive peptide (1) derived from preproapelin, which generates a peptide of 36 amino acids and two shorter active peptides: apelin-17 (K17F) and the pyroglutamyl form of apelin-13 (pE13F) (1–3). K17F was found to have the highest affinity for the apelin receptor in humans and rats (4). Apelin and its receptor have been found in the plasma and in various tissues including brain and kidney (5–7). Apelin is involved in various processes such as glucose homeostasis, cell proliferation, angiogenesis, cardiovascular function, and also in the regulation of body fluid homeostasis (7–9).
In the brain, apelin and its receptor are colocalized with arginine vasopressin (AVP) in magnocellular neurons of the supraoptic nucleus and the paraventricular hypothalamic nuclei (5, 10). Due to this close colocalization, an interaction of apelin and AVP has been assumed. In fact, in lactating rats, central injection of K17F inhibited AVP neuron activity and led to a decreased release of AVP into the bloodstream with increased aqueous diuresis (5). This cross-reaction of apelin and AVP has been shown not only in rats but also in humans (11). In healthy volunteers, an increase of plasma osmolality was accompanied by an increase of plasma AVP and a decrease in plasma apelin levels, whereas a decrease in plasma osmolality upon water loading resulted in decreased plasma AVP levels and increased plasma apelin levels (11). These findings suggest that apelin is involved in the maintenance of body fluid homeostasis acting inversely to AVP.
Apelin has also been investigated in pathological states with high AVP secretion and hyponatremia such as the syndrome of inappropriate antidiuretic hormone and heart failure. In these patients, compared to high AVP levels, inappropriately low apelin levels were found, resulting in an imbalance of AVP and apelin with a disturbance in body fluid homeostasis (12).
In addition to the central effect of apelin in the brain, apelin and its receptor are also expressed in the kidney, resulting in a direct diuretic effect by counteracting the antidiuretic effect of AVP action on the AVP receptor type 2 (V2-R) in the collecting ducts (6). This shows that apelin and AVP act both in the brain and the kidney in an opposite way to maintain body fluid and osmotic homeostasis.
In patients with polyuria-polydipsia syndrome (PPS), body fluid and osmotic maintenance are disturbed. We have recently shown that copeptin as a surrogate marker of AVP improves differential diagnosis in patients with PPS (13, 14). So far there exist no data about plasma apelin levels and the plasma apelin to copeptin ratio in patients with PPS.
Subjects and Methods
Setting
This study was a secondary analysis of a prospective multicenter study (ClinicalTrials.gov no. NCT00757276) (14). A complete description has been reported previously (14). The primary endpoint of the original study was to evaluate the diagnostic accuracy of copeptin, mirroring AVP secretion, in patients with different etiologies of PPS. In brief, inclusion criteria were age > 18 years and polyuria of > 40 mL/kg/24 h in the presence of polydipsia. Exclusion criteria were osmotic diuresis due to a known underlying disorder such as diabetes mellitus, hypercalcemia, uncorrected thyroid or adrenal insufficiency, heart failure, or pregnancy. All patients underwent a standardized combined water deprivation and saline infusion test starting at 8 am without prior fluid restriction. Informed consent was obtained from all patients and healthy volunteers.
Patients
For the purpose of this study, we measured apelin and copeptin levels in the remaining blood samples of eight patients with complete central diabetes insipidus (DI), six patients with complete nephrogenic DI, and 14 patients with primary polydipsia (PP). In addition, we prospectively enrolled 13 additional patients with PPS (seven with complete central DI, five with PP, and one with complete nephrogenic DI). The blood samples to determine apelin and copeptin levels were drawn at 8 am before starting the water deprivation test.
Healthy volunteers
We included data of 113 healthy volunteers from both sexes as a reference population. A detailed description of the healthy volunteers has been published previously (12).
Laboratory analysis
Plasma apelin and copeptin levels were determined as previously described (11, 14). In brief, for plasma apelin measurements, blood was sampled into prechilled EDTA-K3 tubes and centrifuged at 1600 × g at 4°C for 15 minutes. Plasma was stored at −80°C until batch analysis. For apelin RIA, plasma samples (0.75 mL) were mixed with 0.05 mL of 1% BSA and 0.25 mL of 3 m HCl and centrifuged at 1600 × g at 4°C for 10 minutes. The supernatants were collected, and their pH was adjusted to 6.5 with 12 m NaOH and 2 m Tris-HCl buffer (pH 7.4). The samples were then mixed with 1 mL of H2O supplemented with 0.1% BSA and loaded onto a Sep-Pak C18 cartridge (Waters Associates), washed with 2 mL of 100% ethanol, and equilibrated with 5 mL of H2O-0.1% BSA. Apelin peptides were eluted with 1.5 mL of 100% ethanol. The recovery rate (mean ± SEM) was 91 ± 3%. The eluates were dried and dissolved in 0.32 mL of RIA buffer. Plasma apelin concentrations were measured by a specific RIA, validated in humans (11), by identification of the molecular forms of apelin present in human plasma in vivo—K17F, pE13F, and to a lesser extent, apelin-36 by combining HPLC analysis with RIA detection. The detection limit of the apelin assay was 6 pmol/L, with an intra-assay variability of 3% and an interassay variability of 5%.
Copeptin was assessed in a single blinded batch by a chemiluminescence sandwich immunoassay (B_R_A_H_M_S CT-proAVP LIA; Thermo Scientific Biomarkers) developed by Morgenthaler et al (15). The detection limit of the copeptin assay was 0.4 pmol/L, with an intra-assay variability of 3.7–2.5% at 2.14–514.4 pmol/L and an interassay variability of 22.9–3.6% at 0.58–902.9 pmol/L (14).
Reference diagnosis
The final diagnosis of PPS was made by two board-certified endocrinologists in the field, blinded to copeptin and apelin levels, according to three diagnostic components: 1) the results of the combined water deprivation/saline infusion test; 2) additional features such as the patient's history and diagnostic findings (eg, cranial magnetic resonance imaging); and 3) treatment response. In case of inconclusive test results, the final diagnosis was based on treatment response (13, 14, 16).
Statistical analyses
Discrete variables are indicated as counts (percentage) and continuous variables as medians (interquartile ranges) unless stated otherwise. The apelin to copeptin ratio was determined by division. We used a hierarchical approach and tested first for any difference between subgroups with the Fisher exact test for categorical variables and Kruskal-Wallis one-way ANOVA for not normally distributed continuous variables; in case of a significant result, we used Dunn's post hoc test for multiple testing to identify the specific group differences.
Testing was two-tailed, and P < .05 was considered statistically significant. For statistical analysis, we used GraphPad Prism version 6.0 for Mac (GraphPad Software) and IBM SPSS Statistics 22.0.
Results
A total of 113 healthy volunteers and 41 patients with PPS were included in this study: 15 patients with complete central DI, 19 patients with PP, and seven patients with complete nephrogenic DI.
The median age of all patients was 48 (interquartile range, 36–56) years, median body mass index (BMI) was 25.6 (22.4–28.9) kg/m2, and 65.9% were female. Median age (47 [35–61] years) and BMI (24.2 [21.8–26] kg/m2) in healthy volunteers were similar compared to patients. A complete description of all baseline characteristics is given in Table 1. Clinical volume status was euvolemic in 80% of the patients with central DI, in 88% of the patients with PP, and in 25% of the patients with nephrogenic DI; none of the patients was hypervolemic. Creatinine levels and heart rate were higher in patients with nephrogenic DI compared to all the other groups. Serum sodium levels and serum osmolality were highest in patients with nephrogenic DI.
| . | Healthy Volunteers . | Central DI . | PP . | Nephrogenic DI . | P Value . |
|---|---|---|---|---|---|
| n | 113 | 15 | 19 | 7 | |
| Age, y | 47 (35–61) | 50 (42–58) | 45 (30–50) | 61 (55–65) | .12 |
| BMI, kg/m2 | 24 (22–26) | 25 (23–29) | 26 (20–29) | 26 (25–28) | .10 |
| Sex, female | 58 (51) | 9 (60) | 13 (68.4) | 5 (71.4) | .43 |
| Ethnicity, Caucasian | n.a. | 15 (100) | 16 (84.2) | 6 (85.7) | .27 |
| Current smoker | n.a. | 7 (46.7) | 4 (21.1) | 3 (42.9) | .23 |
| History of a brain tumor | n.a. | 6 (40) | 1 (5) | 0 (0) | .02 |
| History of trans-sphenoidal surgery | n.a. | 7 (46.7) | 1 (5) | 0 (0) | .02 |
| Systolic blood pressure, mm Hg | 124 (116–134) | 121 (115–137) | 124 (110–131) | 137 (117–140) | .53 |
| Heart rate, bpm | 66 (59–74) | 70 (67–77) | 76 (63–81) | 83 (79–85) | .0005a |
| Clinical volume status: hypo-/eu-/hypervolemic (euvolemic), % | n.a. | 3/12/0 (80) | 2/14/0 (87.5) | 3/1/0 (25) | .039 |
| Hemoglobin, g/L | n.a. | 140 (130–148) | 136 (134–143) | 128 (115–138) | .31 |
| Hematocrit, l/L | 0.41 (0.39–0.43) | 0.39 (0.38–0.44) | 0.40 (0.38–0.43) | 0.37 (0.34–0.4) | .13 |
| Creatinine, μmol/L | 76 (65–84) | 75 (63–85) | 69 (62–74) | 146 (125–266) | <.0001b |
| Albumin, g/L | n.a. | 39 (34–40) | 37 (36–42) | 37 (32–39) | .16 |
| Plasma sodium, mmol/L | 140 (139–141) | 142 (142–144) | 141 (140–142) | 143 (142–146) | <.001c |
| Plasma osmolality, mOsmol/kg H2O | 288 (285–291) | 297 (292–307) | 294 (290–301) | 306 (303–312) | <.001d |
| Diuretics | 0 (0) | 1 (6.7) | 0 (0) | 0 (0) | .54 |
| Lithium | 0 (0) | 0 (0) | 1 (5.3) | 5 (71.4) | <.001 |
| Antiepileptics | 0 (0) | 1 (6.7) | 2 (10.5) | 2 (28.6) | .29 |
| Corticosteroids | 0 (0) | 6 (40) | 2 (10.5) | 0 (0) | .06 |
| Narcotics | 0 (0) | 2 (13.3) | 3 (15.8) | 4 (57.1) | .06 |
| Desmopressin | 0 (0) | 9 (60) | 0 (0) | 0 (0) | <.001 |
| Birth control pill, % of female patients | 0 (0) | 2 (22) | 3 (23) | 0 (0) | <.001 |
| . | Healthy Volunteers . | Central DI . | PP . | Nephrogenic DI . | P Value . |
|---|---|---|---|---|---|
| n | 113 | 15 | 19 | 7 | |
| Age, y | 47 (35–61) | 50 (42–58) | 45 (30–50) | 61 (55–65) | .12 |
| BMI, kg/m2 | 24 (22–26) | 25 (23–29) | 26 (20–29) | 26 (25–28) | .10 |
| Sex, female | 58 (51) | 9 (60) | 13 (68.4) | 5 (71.4) | .43 |
| Ethnicity, Caucasian | n.a. | 15 (100) | 16 (84.2) | 6 (85.7) | .27 |
| Current smoker | n.a. | 7 (46.7) | 4 (21.1) | 3 (42.9) | .23 |
| History of a brain tumor | n.a. | 6 (40) | 1 (5) | 0 (0) | .02 |
| History of trans-sphenoidal surgery | n.a. | 7 (46.7) | 1 (5) | 0 (0) | .02 |
| Systolic blood pressure, mm Hg | 124 (116–134) | 121 (115–137) | 124 (110–131) | 137 (117–140) | .53 |
| Heart rate, bpm | 66 (59–74) | 70 (67–77) | 76 (63–81) | 83 (79–85) | .0005a |
| Clinical volume status: hypo-/eu-/hypervolemic (euvolemic), % | n.a. | 3/12/0 (80) | 2/14/0 (87.5) | 3/1/0 (25) | .039 |
| Hemoglobin, g/L | n.a. | 140 (130–148) | 136 (134–143) | 128 (115–138) | .31 |
| Hematocrit, l/L | 0.41 (0.39–0.43) | 0.39 (0.38–0.44) | 0.40 (0.38–0.43) | 0.37 (0.34–0.4) | .13 |
| Creatinine, μmol/L | 76 (65–84) | 75 (63–85) | 69 (62–74) | 146 (125–266) | <.0001b |
| Albumin, g/L | n.a. | 39 (34–40) | 37 (36–42) | 37 (32–39) | .16 |
| Plasma sodium, mmol/L | 140 (139–141) | 142 (142–144) | 141 (140–142) | 143 (142–146) | <.001c |
| Plasma osmolality, mOsmol/kg H2O | 288 (285–291) | 297 (292–307) | 294 (290–301) | 306 (303–312) | <.001d |
| Diuretics | 0 (0) | 1 (6.7) | 0 (0) | 0 (0) | .54 |
| Lithium | 0 (0) | 0 (0) | 1 (5.3) | 5 (71.4) | <.001 |
| Antiepileptics | 0 (0) | 1 (6.7) | 2 (10.5) | 2 (28.6) | .29 |
| Corticosteroids | 0 (0) | 6 (40) | 2 (10.5) | 0 (0) | .06 |
| Narcotics | 0 (0) | 2 (13.3) | 3 (15.8) | 4 (57.1) | .06 |
| Desmopressin | 0 (0) | 9 (60) | 0 (0) | 0 (0) | <.001 |
| Birth control pill, % of female patients | 0 (0) | 2 (22) | 3 (23) | 0 (0) | <.001 |
Abbreviation: n.a., not applicable. Data are expressed as number (percentage) or median (interquartile range), unless stated otherwise. P values were assessed by Fisher's exact test (categorical variables) and by the Kruskal-Wallis test (continuous variables).
Patients with nephrogenic DI had significantly higher heart rate values compared to healthy volunteers. P value assessed with Dunn's multiple comparison test: P = .004.
Creatinine levels were significantly higher in patients with nephrogenic DI compared to each other group (central DI, PP, and healthy volunteers). P < .001 for each comparison assessed with Dunn's multiple comparison test.
Sodium values in patients with central DI were significantly higher compared to healthy volunteers. P value assessed with Dunn's multiple comparison test: P < .0001.
Osmolality in healthy volunteers was significantly lower compared to all three patient groups. P values assessed with Dunn's multiple comparison test: central DI, P = .0001; PP, P = .0004; nephrogenic DI, P = .004.
| . | Healthy Volunteers . | Central DI . | PP . | Nephrogenic DI . | P Value . |
|---|---|---|---|---|---|
| n | 113 | 15 | 19 | 7 | |
| Age, y | 47 (35–61) | 50 (42–58) | 45 (30–50) | 61 (55–65) | .12 |
| BMI, kg/m2 | 24 (22–26) | 25 (23–29) | 26 (20–29) | 26 (25–28) | .10 |
| Sex, female | 58 (51) | 9 (60) | 13 (68.4) | 5 (71.4) | .43 |
| Ethnicity, Caucasian | n.a. | 15 (100) | 16 (84.2) | 6 (85.7) | .27 |
| Current smoker | n.a. | 7 (46.7) | 4 (21.1) | 3 (42.9) | .23 |
| History of a brain tumor | n.a. | 6 (40) | 1 (5) | 0 (0) | .02 |
| History of trans-sphenoidal surgery | n.a. | 7 (46.7) | 1 (5) | 0 (0) | .02 |
| Systolic blood pressure, mm Hg | 124 (116–134) | 121 (115–137) | 124 (110–131) | 137 (117–140) | .53 |
| Heart rate, bpm | 66 (59–74) | 70 (67–77) | 76 (63–81) | 83 (79–85) | .0005a |
| Clinical volume status: hypo-/eu-/hypervolemic (euvolemic), % | n.a. | 3/12/0 (80) | 2/14/0 (87.5) | 3/1/0 (25) | .039 |
| Hemoglobin, g/L | n.a. | 140 (130–148) | 136 (134–143) | 128 (115–138) | .31 |
| Hematocrit, l/L | 0.41 (0.39–0.43) | 0.39 (0.38–0.44) | 0.40 (0.38–0.43) | 0.37 (0.34–0.4) | .13 |
| Creatinine, μmol/L | 76 (65–84) | 75 (63–85) | 69 (62–74) | 146 (125–266) | <.0001b |
| Albumin, g/L | n.a. | 39 (34–40) | 37 (36–42) | 37 (32–39) | .16 |
| Plasma sodium, mmol/L | 140 (139–141) | 142 (142–144) | 141 (140–142) | 143 (142–146) | <.001c |
| Plasma osmolality, mOsmol/kg H2O | 288 (285–291) | 297 (292–307) | 294 (290–301) | 306 (303–312) | <.001d |
| Diuretics | 0 (0) | 1 (6.7) | 0 (0) | 0 (0) | .54 |
| Lithium | 0 (0) | 0 (0) | 1 (5.3) | 5 (71.4) | <.001 |
| Antiepileptics | 0 (0) | 1 (6.7) | 2 (10.5) | 2 (28.6) | .29 |
| Corticosteroids | 0 (0) | 6 (40) | 2 (10.5) | 0 (0) | .06 |
| Narcotics | 0 (0) | 2 (13.3) | 3 (15.8) | 4 (57.1) | .06 |
| Desmopressin | 0 (0) | 9 (60) | 0 (0) | 0 (0) | <.001 |
| Birth control pill, % of female patients | 0 (0) | 2 (22) | 3 (23) | 0 (0) | <.001 |
| . | Healthy Volunteers . | Central DI . | PP . | Nephrogenic DI . | P Value . |
|---|---|---|---|---|---|
| n | 113 | 15 | 19 | 7 | |
| Age, y | 47 (35–61) | 50 (42–58) | 45 (30–50) | 61 (55–65) | .12 |
| BMI, kg/m2 | 24 (22–26) | 25 (23–29) | 26 (20–29) | 26 (25–28) | .10 |
| Sex, female | 58 (51) | 9 (60) | 13 (68.4) | 5 (71.4) | .43 |
| Ethnicity, Caucasian | n.a. | 15 (100) | 16 (84.2) | 6 (85.7) | .27 |
| Current smoker | n.a. | 7 (46.7) | 4 (21.1) | 3 (42.9) | .23 |
| History of a brain tumor | n.a. | 6 (40) | 1 (5) | 0 (0) | .02 |
| History of trans-sphenoidal surgery | n.a. | 7 (46.7) | 1 (5) | 0 (0) | .02 |
| Systolic blood pressure, mm Hg | 124 (116–134) | 121 (115–137) | 124 (110–131) | 137 (117–140) | .53 |
| Heart rate, bpm | 66 (59–74) | 70 (67–77) | 76 (63–81) | 83 (79–85) | .0005a |
| Clinical volume status: hypo-/eu-/hypervolemic (euvolemic), % | n.a. | 3/12/0 (80) | 2/14/0 (87.5) | 3/1/0 (25) | .039 |
| Hemoglobin, g/L | n.a. | 140 (130–148) | 136 (134–143) | 128 (115–138) | .31 |
| Hematocrit, l/L | 0.41 (0.39–0.43) | 0.39 (0.38–0.44) | 0.40 (0.38–0.43) | 0.37 (0.34–0.4) | .13 |
| Creatinine, μmol/L | 76 (65–84) | 75 (63–85) | 69 (62–74) | 146 (125–266) | <.0001b |
| Albumin, g/L | n.a. | 39 (34–40) | 37 (36–42) | 37 (32–39) | .16 |
| Plasma sodium, mmol/L | 140 (139–141) | 142 (142–144) | 141 (140–142) | 143 (142–146) | <.001c |
| Plasma osmolality, mOsmol/kg H2O | 288 (285–291) | 297 (292–307) | 294 (290–301) | 306 (303–312) | <.001d |
| Diuretics | 0 (0) | 1 (6.7) | 0 (0) | 0 (0) | .54 |
| Lithium | 0 (0) | 0 (0) | 1 (5.3) | 5 (71.4) | <.001 |
| Antiepileptics | 0 (0) | 1 (6.7) | 2 (10.5) | 2 (28.6) | .29 |
| Corticosteroids | 0 (0) | 6 (40) | 2 (10.5) | 0 (0) | .06 |
| Narcotics | 0 (0) | 2 (13.3) | 3 (15.8) | 4 (57.1) | .06 |
| Desmopressin | 0 (0) | 9 (60) | 0 (0) | 0 (0) | <.001 |
| Birth control pill, % of female patients | 0 (0) | 2 (22) | 3 (23) | 0 (0) | <.001 |
Abbreviation: n.a., not applicable. Data are expressed as number (percentage) or median (interquartile range), unless stated otherwise. P values were assessed by Fisher's exact test (categorical variables) and by the Kruskal-Wallis test (continuous variables).
Patients with nephrogenic DI had significantly higher heart rate values compared to healthy volunteers. P value assessed with Dunn's multiple comparison test: P = .004.
Creatinine levels were significantly higher in patients with nephrogenic DI compared to each other group (central DI, PP, and healthy volunteers). P < .001 for each comparison assessed with Dunn's multiple comparison test.
Sodium values in patients with central DI were significantly higher compared to healthy volunteers. P value assessed with Dunn's multiple comparison test: P < .0001.
Osmolality in healthy volunteers was significantly lower compared to all three patient groups. P values assessed with Dunn's multiple comparison test: central DI, P = .0001; PP, P = .0004; nephrogenic DI, P = .004.
Basal plasma copeptin and apelin levels as well as apelin to copeptin ratio of all four groups (healthy volunteers, complete central DI, PP, and complete nephrogenic DI) are shown in Table 2.
Plasma Apelin and Copeptin Levels in Healthy Volunteers and Patients With the PPS
| . | Healthy Volunteers . | Complete cDI . | PP . | Complete nDI . | P Value . |
|---|---|---|---|---|---|
| n | 113 | 15 | 19 | 7 | |
| Plasma copeptin, pmol/L | 4.1 (2.6–6.9)a | 1.9 (1.9–2.5) | 3.9 (2.2–4.7) | 56.7 (39.7–70) | <.001c |
| Plasma apelin, pmol/L | 254 (225–311) | 209 (174–241) | 190 (172–215) | 413 (332–504) | <.001d |
| Plasma apelin/copeptin, pmol/pmol | 57 (37–102)b | 89 (73–135) | 53 (38–92) | 7 (6–10) | <.001e |
| . | Healthy Volunteers . | Complete cDI . | PP . | Complete nDI . | P Value . |
|---|---|---|---|---|---|
| n | 113 | 15 | 19 | 7 | |
| Plasma copeptin, pmol/L | 4.1 (2.6–6.9)a | 1.9 (1.9–2.5) | 3.9 (2.2–4.7) | 56.7 (39.7–70) | <.001c |
| Plasma apelin, pmol/L | 254 (225–311) | 209 (174–241) | 190 (172–215) | 413 (332–504) | <.001d |
| Plasma apelin/copeptin, pmol/pmol | 57 (37–102)b | 89 (73–135) | 53 (38–92) | 7 (6–10) | <.001e |
Abbreviations: cDI, central DI; nDI, nephrogenic DI; hV, healthy volunteers. Data are expressed as median (interquartile range). P values are according to the Kruskal-Wallis test.
In one patient, the copeptin value is lacking.
According to the lacking copeptin value, the apelin to copeptin ratio is also missing in one person.
P values assessed with Dunn's multiple comparison test for plasma copeptin: cDI vs hV, P < .001; PP vs hV, p = ns; nDI vs hV, P < .001; cDI vs PP, p = ns; cDI vs nDI, P < .001; PP vs nDI, P < .001.
P values assessed with Dunn's multiple comparison test for plasma apelin: cDI vs hV, P = .02; PP vs hV, P < .001; nDI vs hV, P = .01; cDI vs PP, P = ns; cDI vs nDI, P < .001; PP vs nDI, P < .001.
P values assessed with Dunn's multiple comparison test for plasma apelin to copeptin ratio: cDI vs hV, P = .02; PP vs hV, P = ns; nDI vs hV, P < .001; cDI vs PP, P = ns; cDI vs nDI, P < .001; PP vs nDI, P = .003.
Plasma Apelin and Copeptin Levels in Healthy Volunteers and Patients With the PPS
| . | Healthy Volunteers . | Complete cDI . | PP . | Complete nDI . | P Value . |
|---|---|---|---|---|---|
| n | 113 | 15 | 19 | 7 | |
| Plasma copeptin, pmol/L | 4.1 (2.6–6.9)a | 1.9 (1.9–2.5) | 3.9 (2.2–4.7) | 56.7 (39.7–70) | <.001c |
| Plasma apelin, pmol/L | 254 (225–311) | 209 (174–241) | 190 (172–215) | 413 (332–504) | <.001d |
| Plasma apelin/copeptin, pmol/pmol | 57 (37–102)b | 89 (73–135) | 53 (38–92) | 7 (6–10) | <.001e |
| . | Healthy Volunteers . | Complete cDI . | PP . | Complete nDI . | P Value . |
|---|---|---|---|---|---|
| n | 113 | 15 | 19 | 7 | |
| Plasma copeptin, pmol/L | 4.1 (2.6–6.9)a | 1.9 (1.9–2.5) | 3.9 (2.2–4.7) | 56.7 (39.7–70) | <.001c |
| Plasma apelin, pmol/L | 254 (225–311) | 209 (174–241) | 190 (172–215) | 413 (332–504) | <.001d |
| Plasma apelin/copeptin, pmol/pmol | 57 (37–102)b | 89 (73–135) | 53 (38–92) | 7 (6–10) | <.001e |
Abbreviations: cDI, central DI; nDI, nephrogenic DI; hV, healthy volunteers. Data are expressed as median (interquartile range). P values are according to the Kruskal-Wallis test.
In one patient, the copeptin value is lacking.
According to the lacking copeptin value, the apelin to copeptin ratio is also missing in one person.
P values assessed with Dunn's multiple comparison test for plasma copeptin: cDI vs hV, P < .001; PP vs hV, p = ns; nDI vs hV, P < .001; cDI vs PP, p = ns; cDI vs nDI, P < .001; PP vs nDI, P < .001.
P values assessed with Dunn's multiple comparison test for plasma apelin: cDI vs hV, P = .02; PP vs hV, P < .001; nDI vs hV, P = .01; cDI vs PP, P = ns; cDI vs nDI, P < .001; PP vs nDI, P < .001.
P values assessed with Dunn's multiple comparison test for plasma apelin to copeptin ratio: cDI vs hV, P = .02; PP vs hV, P = ns; nDI vs hV, P < .001; cDI vs PP, P = ns; cDI vs nDI, P < .001; PP vs nDI, P = .003.
Apelin levels in all three patient groups significantly differed from apelin levels in healthy volunteers (254 [225–311] pmol/L). Specifically, patients with complete nephrogenic DI had higher apelin levels (413 [332–504] pmol/L; P = .01); and patients with complete central DI (209 [174–241] pmol/L; P = .02) and PP (190 [172–215] pmol/L; P = .0001) had lower apelin levels compared to healthy volunteers (Figure 1A).
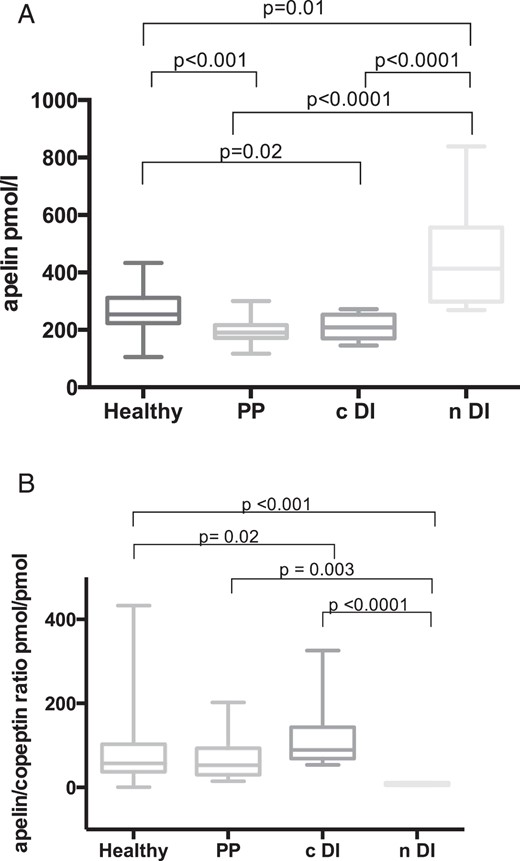
A, Plasma apelin concentrations (pmol/L) in healthy volunteers (n = 113) and in patients with central DI (n = 15), PP (n = 19), and nephrogenic DI (n = 7). Multiple comparisons were assessed with Dunn's multiple comparison test. B, Plasma apelin to copeptin ratios (pmol/pmol) in healthy volunteers (n = 112) and in patients with central DI (n = 15), PP (n = 19), and nephrogenic DI (n = 7). Multiple comparisons were assessed with Dunn's multiple comparison test. cDI, central DI; nDI, nephrogenic DI.
Median copeptin levels were: in healthy volunteers, 4.1 (2.6–6.9) pmol/L; in patients with complete central DI, 1.9 [1.9–2.5] pmol/L; in patients with PP, 3.9 [2.2–4.7] pmol/L; and were highest in patients with nephrogenic DI, 56.7 (39.7–70) pmol/L (Table 2).
The plasma apelin to copeptin ratio in patients with PP (53 [38–92] pmol/pmol) was similar to healthy volunteers (57 [37–102] pmol/pmol). In contrast, the plasma apelin to copeptin ratio in patients with complete central DI (89 [73–135] pmol/pmol; P = .02) and in patients with complete nephrogenic DI (7 [6–10] pmol/pmol; P < .001) was significantly lower compared to healthy volunteers (Figure 1B and Table 2).
Discussion
The main finding of our study is that apelin levels in patients with PPS differ significantly from healthy volunteers. Specifically, apelin levels are highest in patients with complete nephrogenic DI and lower in patients with PP or complete central DI as compared to healthy volunteers.
The colocalization of AVP and apelin in hypothalamic magnocellular neurons and the presence of their receptors on these neurons suggest an interaction between AVP and apelin (7). Accordingly, previous studies showed an interaction of AVP and apelin both in rodents and humans. In states known to be associated with elevated AVP release (eg, dehydration or lactating rats), the central injection of the endogenous peptide K17F inhibited the phasic electrical activity of AVP neurons, leading to reduced AVP release in the blood circulation and increased aqueous diuresis in rodents (5, 10). In addition to this diuretic effect at a central level, there is also an effect of apelin at the kidney level because apelin receptors are present in the collecting ducts of the kidney. K17F acts on these receptors and herein inhibits AVP-induced cAMP production occurring via V2-Rs. This leads to an increased aqueous diuresis by decreasing aquaporin-2 insertion in the apical membrane (6). Thus, the diuretic effect of apelin is not only due to a central effect by inhibiting AVP release in the blood circulation but also due to a direct renal action of apelin on collecting ducts, by counteracting the antidiuretic effect of AVP occurring via V2-R.
In healthy volunteers, increasing plasma osmolality due to hypertonic saline infusion was accompanied by a parallel, linear increase in plasma AVP concentration and by a decrease in plasma apelin concentration. In contrast, water loading led to a decrease in plasma osmolality and AVP concentrations and to an increase in apelin concentrations (11). Thus, apelin together with AVP is involved in the regulation of body fluid homeostasis in both rodents and humans (11).
In contrast, in patients with an abnormal body fluid homeostasis due to the syndrome of inappropriate antidiuretic hormone, plasma apelin levels show a parallel increase together with AVP. Specifically, apelin levels were shown to be increased compared to healthy controls; however, the increase of apelin levels was not able to compensate for the elevated AVP levels, arguing for an inappropriate response of apelin in these patients contributing to a disturbed body fluid homeostasis (12).
Similarly, and again in contrast to healthy volunteers, also in patients with central or nephrogenic DI, apelin and AVP/copeptin decrease or increase in parallel to each other. We assume that these parallel changes in apelin and AVP/copeptin level occurred to compensate for the failure of AVP in order to re-establish a balance between the antidiuretic effect of AVP and the diuretic effect of apelin. However, apelin fails to fully compensate, resulting in a disturbed body fluid homeostasis.
In patients with PPS, different pathologies result in increased diuresis. Patients with central DI suffer from AVP deficiency mirrored by low plasma copeptin levels. In parallel, we found lower plasma apelin levels in patients with central DI compared to healthy volunteers. However, the plasma apelin to copeptin ratio of 89 (73–135) pmol/pmol in patients with central DI is higher compared to healthy volunteers (57 [37–102] pmol/pmol). Possibly, the decrease in plasma apelin levels of patients with central DI is insufficient as compared to the decrease of AVP/copeptin to re-establish a balance between the antidiuretic effect of AVP and the diuretic effect of apelin, resulting in polyuria.
In patients with nephrogenic DI, we observed higher apelin levels as compared to the healthy volunteers. Patients with nephrogenic DI are known to have a renal resistance to AVP and have elevated plasma AVP and copeptin levels (16). Again, we hypothesize that this parallel increase of apelin and copeptin is an effort of the apelin/vasopressin-system to re-establish the water balance mediated by the two hormones. However, there is a renal resistance to AVP together with an increase in apelin levels, which results in polyuria. According to published data, apelin seems to increase as estimated glomerular filtration rate increases (17), suggesting that a higher renal clearance is associated with higher apelin levels. Based on these data we would expect rather lower apelin levels in patients with impaired kidney function; we therefore do not assume that elevated apelin levels in patients with nephrogenic DI are caused by the slightly impaired kidney function.
The pathophysiological mechanism of PP is not based on a deficiency or resistance of AVP but rather results from an excessive fluid intake over an extended period of time (16). Patients with PP had slightly but significantly lower plasma apelin levels compared to healthy volunteers. However, plasma copeptin levels and the plasma apelin to copeptin ratio were similar in comparison to healthy volunteers. The “normal” apelin to copeptin ratio in PP attests for balanced water homeostasis, whereas plasma apelin to copeptin ratio in central or nephrogenic DI is increased or decreased compared to healthy volunteers, reflecting a disturbed water homeostasis. Another interesting explanation is that apelin itself might also be involved directly in the pathogenesis of PP. Apelin immunoreactive cell bodies and fibers were found in rats in the hypothalamus, more precisely in the anteroventral third ventricle region (in the subfornical organ, the vascular organ of the lamina terminalis, and the median preoptic nucleus) (18). These structures are involved in the control of drinking behavior (19, 20). Intracerebroventricular injection of apelin-13 resulted in a significantly decreased fluid intake in adult water-deprived rats with free access to water (10). Low apelin levels in patients with PP could therefore be the reason for the altered drinking behavior leading to an inadequate fluid intake in these patients. However, at present this is rather speculative, and further studies are needed to investigate the underlying mechanisms.
The following limitations of this study have to be taken into account. First, this is a proof of concept study, where we investigated plasma apelin levels in patients with complete central or complete nephrogenic DI and PP, whereas patients with partial central or partial nephrogenic DI were not analyzed. Second, it is rather a small sample size, and further studies with larger patient numbers are needed to confirm our results. Third, the small sample size did not allow conducting subgroup analysis in terms of age or sex, both known to influence apelin levels. Nevertheless, taking into consideration, that we provide for the first time data about plasma apelin levels and the apelin to copeptin ratio in patients with PPS, we see our results as interesting and noteworthy in terms of understanding pathophysiological mechanisms of apelin, and AVP in dysregulated body fluid homeostasis such as in patients with PPS.
Conclusion
In conclusion, this study describes for the first time plasma apelin levels in patients with PPS. We show that apelin levels are highest in patients with nephrogenic DI and lower in patients with PP or complete central DI compared to healthy subjects. In contrast to healthy volunteers, where an inverse action of AVP/copeptin and apelin is known, this study shows that in patients with central and nephrogenic DI, AVP/copeptin and apelin decrease or increase, respectively, in parallel with each other.
Thus, in patients with PP, the apelin to copeptin ratio is similar compared to healthy volunteers, suggesting a normal water homeostasis, whereas, in patients with complete central or nephrogenic DI, the increased or decreased apelin to copeptin ratio, respectively, reflects a disturbed osmotic and body fluid homeostasis. However, the physiological relevance and interpretation of this ratio needs further studies in a larger population of patients.
Acknowledgments
We thank the staff from all participating endocrine departments for their support of the study. We thank the central laboratory at University Hospital Basel.
This work was investigator initiated. It was supported by the Swiss National Science Foundation with Grant PP00P3–12346 (to M.C.-C.), by the University of Basel with Grants Nachwuchsförderung 2008 and 2015 (to K.T. and S.A.U.), and by the Institut National de la Sante et de la Recherche Medicale and the College de France. Thermo Scientific Biomarkers, Hennigsdorf, Germany, manufacturer/developer of copeptin assays, performed the study copeptin testing using reagents supplied gratis. No funding source had any other involvement in the collection, analysis, or interpretation of the data or the decision to approve publication of the finished manuscript.
ClinicalTrials.gov no. NCT00757276.
Disclosure Summary: The authors have nothing to disclose.
Equally contributing first authors;
equally contributing last authors.
Abbreviations
- AVP
arginine vasopressin
- BMI
body mass index
- DI
diabetes insipidus
- PP
primary polydipsia
- PPS
polyuria-polydipsia syndrome
- V2-R
AVP receptor type 2.



