-
PDF
- Split View
-
Views
-
Cite
Cite
S. Cannavo, M. Ragonese, S. Puglisi, P. D. Romeo, M. L. Torre, A. Alibrandi, C. Scaroni, G. Occhi, F. Ceccato, D. Regazzo, E. De Menis, P. Sartorato, G. Arnaldi, L. Trementino, F. Trimarchi, F. Ferrau, Acromegaly Is More Severe in Patients With AHR or AIP Gene Variants Living in Highly Polluted Areas, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 4, 1 April 2016, Pages 1872–1879, https://doi.org/10.1210/jc.2015-4191
Close - Share Icon Share
Abstract
An increased prevalence of acromegaly was found some years ago in a highly polluted area in North-Eastern Sicily, where high concentration of nonmethane hydrocarbons, volatile organic compounds, and cadmium was found. Aryl hydrocarbon receptor (AHR) pathway has a key role in detoxification of these compounds and in tumorigenesis.
We correlated the occurrence of AHR and/or AHR-interacting protein (AIP) gene variants with acromegaly severity according to pollution exposition.
This was an observational, perspective study conducted over 7 years in four Italian referral centers for pituitary diseases in which 210 patients with acromegaly were enrolled between 2008 and 2015.
Genetic screening of patients for AHR and AIP variants.
Clinical, biochemical, and radiological data of patients with and without AIP and/or AHR gene variants, living in polluted (high-risk for health, [HR]) or nonpolluted (NP) areas of five Italian regions were evaluated and compared.
Among the 23 patients from HR areas, nine showed AHR or AIP variants. Mean IGF-I levels and pituitary tumor diameter were significantly higher in these nine patients (HR/VAR+) than in the other 14 (HR/VAR−) and in the 187 from NP areas (44 NP/VAR+). Somatostatin analogs significantly decreased mean GH and IGF-I levels in patients from NP areas and in HR/VAR− (GH P < .05; IGF-I times the upper limit of normal P < .01) but not in HR/VAR+ group.
Genetic variants potentially inducing functional abnormalities of the aryl hydrocarbon receptor (AHR) pathway are associated with a more severe acromegaly, increased pituitary tumor size, and somatostatin analog resistance in patients living in HR areas.
Acromegaly is a rare endocrine disease caused by a GH-secreting pituitary tumor, which occurs in less than 125 individuals per one million inhabitants (1, 2). Increased prevalence of acromegaly was demonstrated by some of us in a district of the province of Messina (Sicily, Italy), identified as high-risk for health (HR) area by the Italian government on the basis of high atmospheric concentrations of nonmethane hydrocarbons and volatile organic compounds (3). The prevalence of acromegaly in this polluted area (where 47 554 peoples are living) reached 210 cases per million inhabitants (cpm) and the relative risk of developing this disease was 8-fold higher, in comparison with a reference area, in the same province of Messina, characterized by low industrial density (3). Subsequent unpublished investigations in the polluted area suggest that the current prevalence is 330 cpm.
Recent preliminary in vitro studies showed that long-term incubation with some endocrine disruptors, eg, phenol and bis-(2-ethylhexyl)-phthalate, increases energy content and proliferation of adenomatous and normal pituitary cells, in rats as well as in humans (4). In addition, the long-term benzene exposition increased GH synthesis in GH3 cells, and this effect was associated with decreased aryl-hydrocarbon interacting protein (AIP) and increased aryl-hydrocarbon receptor (AHR) expression (5). In the same study, exposition to benzene also increased the expression of the somatostatin receptor subtype 2 but decreased ZAC1 expression, effect in accordance with a potential impairment of the sensitivity to somatostatin analogs (SSa) (5). Moreover, a very recent study demonstrated that polychlorobiphenyls could affect apoptosis in mouse pituitary gland cells through an AHR or thyroid receptor–dependent mechanism (6).
The AHR pathway has a key role in cellular detoxification mechanisms and several studies suggest that it is implicated in tumorigenesis (7, 8). In contrast, inactivating mutations of the AIP gene, encoding for a cochaperone cytoplasmic protein involved in the regulation of AHR activity and in many other intracellular interactions, have been found in 40% of patients with isolated familial somatotropinomas. These mutations are also found in up to 13% of cases with sporadic GH-secreting pituitary macroadenomas diagnosed under 30 years of age and in up to 4% of cases with sporadic acromegaly regardless of tumor size and age of disease onset (9–12).
Recently, some of us showed that the AHR gene rs2066853 polymorphism is significantly more frequent in acromegalic patients, compared with controls, and is associated with increased disease aggressiveness as well as with increased prevalence of some secondary tumors, eg, those of the thyroid or the bladder (13). Moreover, some authors demonstrated an increased plasma concentration of organ-halogenated compounds, such as polyclorobyphenils and other substances with estrogen-like activity, in domestic acromegalic cats, thus suggesting an impairment of the xenobiotic metabolizing enzymatic system (14).
This study aimed to correlate the occurrence of AHR and/or AIP genes variants in acromegalic patients with disease severity and/or with the response to SSa treatment according to pollution exposition. For this purpose, we collected clinical, biochemical, and imaging data and performed germline AHR and AIP genotyping of a cohort of acromegalic patients who had lived for a long time before diagnosis, and in almost all cases even after, in HR or nonpolluted (NP) areas of five Italian regions (Veneto Region, Marche Region, Apulia, Calabria Region, and Sicily).
Materials and Methods
Subjects
We enrolled 210 consecutive acromegalic patients (79 men; age 47.0 ± 1.0 y; range, 26–88 y), recruited at the Endocrinology Units of the University Hospitals of Messina (Sicily), Padua (Veneto Region), and Ancona (Marche Region), and at the Internal Medicine Unit of the General Hospital of Montebelluna (Treviso, Veneto Region) between 2008 and 2015. Inclusion criteria were: 1) acromegaly diagnosed after the age of 20 years, 2) active disease, 3) availability of information concerning birth place and permanent address during the 20 years before diagnosis, and 4) consent for DNA sampling and genetic studies. Exclusion criteria were: 1) foreign nationality, 2) relocation during the 20 years before diagnosis, and 3) previous specific treatments for acromegaly (surgery, SSa, dopamine agonists, pegvisomant, radiotherapy). Acromegaly was diagnosed according to the current diagnostic guidelines (15).
Twenty-three of 210 patients had lived for at least 20 years before diagnosis in HR areas, reported in the list of the areas of national interest for environmental risk (SIN) identified by the Department of Environment of the Italian Government (www.minambiente.it) on the basis of data collected by the Regional Agencies for Environment Protection (ARPAs).
All patients gave their informed consent and the study was approved by the Ethical Committee of the University Hospital of Messina.
Hormonal assays
Serum GH and IGF-I levels were assayed by commercial methods in each referral center. Serum IGF-I levels were presented as times upper limit of normal (×ULN) to normalize hormone levels in patients with different ages. In each case, IGF-I ×ULN values were calculated as mean of three determinations collected in three different days over 2 weeks. Serum IGF-I ×ULN was calculated on the basis of the age-specific reference range routinely used in each Endocrine Unit participating in the study. Serum GH and IGF-I values were measured in all patients at diagnosis, and after first-line SSa treatment (octreotide long-acting release, 20 mg im or lanreotide, 90 mg autogel sc every 28 d for 6 mo) in 160 cases. In this study, patients were considered resistant to SSa when serum IGF-I levels did not decrease below 1.3 ×ULN and serum GH concentrations were greater than 2.5 μg/L at the end of treatment.
Genotype analyses
We evaluated all the exons and paraexonic introns of AIP gene, and the exon 10 of the AHR gene searching for polymorphisms rs2066853 and rs4986826. The sequencing was restricted to exon 10 of the AHR gene according to the results of our previous study (13). Genomic DNA extraction and genetic analyses were carried out as previously described (13, 16). Primers and annealing temperatures used in the PCR reactions are listed in Supplemental Table 1.
Statistical analysis
Values are expressed as mean ± SE and categorical variables as number and percentage. Clinical characteristics of patients (sex, age at diagnosis, presence of a micro- or macroadenoma, resistance to SSa treatment) were compared using the Pearson's χ2 test. The nonparametric Mann-Whitney U test was used to compare the adenoma diameter, serum levels of GH, and IGF-I ×ULN at diagnosis and at the last visit, because these variables did not present the normal distribution as verified by Kolmogorov-Smirnov test. P values were considered significant at a level of <.05. Statistical analysis was performed using StatSoft version 11.0 (StataCorp).
Results
Clinical and biochemical characteristics and tumor size of the 210 patients with acromegaly are summarized in Table 1. Overall, 187 patients were from NP and 23 from HR areas. Age at diagnosis (47.2 ± 1.0 vs 45.3 ± 3.1 y) and sex distribution (35.8 vs 52.2% of males) were not significantly different between the two groups, as well as mean serum GH (19.2 ± 3.3 vs 11.7 ± 3.6 μg/L) and IGF-I levels at presentation (2.8 ± 0.1 vs 2.8 ± 0.2 ×ULN), prevalence of macroadenomas (69.0 vs 65.2%), and mean tumor diameter (16.4 ± 1.2 vs 20.1 ± 5.5 mm). Responsiveness to SSa treatment was evaluated in 142/187 and 18/23 cases, respectively. The percentages of patients who normalized IGF-I levels (56.3 vs 38.8%) or decreased baseline GH levels more than 50% (77.4 vs 38.8%), and the percentage of GH reduction (68.4 ± 2.7 vs 62.3 ± 9.6%) were not significantly different between the two groups.
Clinical and Biochemical Characteristics of Acromegalic Patients Stratified According to the Areas Where They Had Lived During the 20 Years Before Diagnosis
| Characteristic . | Overall . | NP Areas . | HR Areas . | P . |
|---|---|---|---|---|
| Patients, n | 210 | 187 | 23 | |
| Male sex, n | 79 | 67 | 12 | NS |
| Age at diagnosis, y | 47.0 ± 1.0 | 47.2 ± 1.0 | 45.3 ± 3.1 | NS |
| Mean GH levels at diagnosis, μg/L | 18.6 ± 3.0 | 19.2 ± 3.3 | 11.7 ± 3.6 | NS |
| Mean IGF-I levels at diagnosis, ×ULN | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.2 | NS |
| Mean pituitary tumor diameter, mm | 17.1 ± 1.2 | 16.4 ± 1.2 | 20.1 ± 5.5 | NS |
| Macroadenomas, % | 68.6 | 69.0 | 65.2 | NS |
| AIP-mutated patients | 7 (3.3%) | 5 (2.7%) | 2 (8.7%) | NS |
| FIPA | 5 (2.4%) | 4 (2.1%) | 1 (4.3%) | NS |
| Characteristic . | Overall . | NP Areas . | HR Areas . | P . |
|---|---|---|---|---|
| Patients, n | 210 | 187 | 23 | |
| Male sex, n | 79 | 67 | 12 | NS |
| Age at diagnosis, y | 47.0 ± 1.0 | 47.2 ± 1.0 | 45.3 ± 3.1 | NS |
| Mean GH levels at diagnosis, μg/L | 18.6 ± 3.0 | 19.2 ± 3.3 | 11.7 ± 3.6 | NS |
| Mean IGF-I levels at diagnosis, ×ULN | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.2 | NS |
| Mean pituitary tumor diameter, mm | 17.1 ± 1.2 | 16.4 ± 1.2 | 20.1 ± 5.5 | NS |
| Macroadenomas, % | 68.6 | 69.0 | 65.2 | NS |
| AIP-mutated patients | 7 (3.3%) | 5 (2.7%) | 2 (8.7%) | NS |
| FIPA | 5 (2.4%) | 4 (2.1%) | 1 (4.3%) | NS |
Clinical and Biochemical Characteristics of Acromegalic Patients Stratified According to the Areas Where They Had Lived During the 20 Years Before Diagnosis
| Characteristic . | Overall . | NP Areas . | HR Areas . | P . |
|---|---|---|---|---|
| Patients, n | 210 | 187 | 23 | |
| Male sex, n | 79 | 67 | 12 | NS |
| Age at diagnosis, y | 47.0 ± 1.0 | 47.2 ± 1.0 | 45.3 ± 3.1 | NS |
| Mean GH levels at diagnosis, μg/L | 18.6 ± 3.0 | 19.2 ± 3.3 | 11.7 ± 3.6 | NS |
| Mean IGF-I levels at diagnosis, ×ULN | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.2 | NS |
| Mean pituitary tumor diameter, mm | 17.1 ± 1.2 | 16.4 ± 1.2 | 20.1 ± 5.5 | NS |
| Macroadenomas, % | 68.6 | 69.0 | 65.2 | NS |
| AIP-mutated patients | 7 (3.3%) | 5 (2.7%) | 2 (8.7%) | NS |
| FIPA | 5 (2.4%) | 4 (2.1%) | 1 (4.3%) | NS |
| Characteristic . | Overall . | NP Areas . | HR Areas . | P . |
|---|---|---|---|---|
| Patients, n | 210 | 187 | 23 | |
| Male sex, n | 79 | 67 | 12 | NS |
| Age at diagnosis, y | 47.0 ± 1.0 | 47.2 ± 1.0 | 45.3 ± 3.1 | NS |
| Mean GH levels at diagnosis, μg/L | 18.6 ± 3.0 | 19.2 ± 3.3 | 11.7 ± 3.6 | NS |
| Mean IGF-I levels at diagnosis, ×ULN | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.2 | NS |
| Mean pituitary tumor diameter, mm | 17.1 ± 1.2 | 16.4 ± 1.2 | 20.1 ± 5.5 | NS |
| Macroadenomas, % | 68.6 | 69.0 | 65.2 | NS |
| AIP-mutated patients | 7 (3.3%) | 5 (2.7%) | 2 (8.7%) | NS |
| FIPA | 5 (2.4%) | 4 (2.1%) | 1 (4.3%) | NS |
AIP genotyping
Heterozygous variants of AIP gene were detected in 7 of 210 patients (AIP+, 3.3%), two familial and five sporadic cases. We found a p.R304Q mutation (c.911G>A) or a p.R304* nonsense mutation (c.910C>T) in three and one patients, respectively, and a nucleotide substitution in the donor splice site of intron 3 (IVS3+1 G>A) in one patient, and a p.R16H change (c.47G>A) in the remaining two cases. Two patients were from HR areas of the Marche Region (p.R304Q) and Veneto Region (p.R304*), respectively. At diagnosis, microadenomas were detected in the two cases from HR and in 1 from NP areas, whereas macroadenomas were present in the other ones (Table 2). Among six patients treated with SSa for at least 6 months, four (66.7%) decreased baseline GH levels more than 50% and normalized IGF-I levels. In these six patients, mean GH and IGF-I values were lower at last visit than at diagnosis (GH 14.22 ± 8.9 vs 3.8 ± 0.9 ng/mL; P = not significant [NS]; IGF-I, 3.1 ± 0.5 vs 1.8 ± 0.3 ×ULN; P = NS). The percentages of cases that normalized IGF-I levels (66.7 vs 53.8%) or decreased baseline GH levels more than 50% (66.7 vs 73.4%) and the percentage of GH reduction (41.3 ± 15.1 vs 68.0 ± 2.6%) were not significantly lower in the AIP+ than in the AIP− patients. Mean IGF-I levels at last visit were also not significantly different between the two groups (1.8 ± 0.3 vs 2.6 ± 1.1 ×ULN). Resistance to treatment was demonstrated in one patient from HR areas and in one of four cases from NP areas. Overall, AIP mutations were found in 8.7% of patients from HR areas and in 2.7% of the other cases (P = NS).
Clinical and Biochemical Characteristics of Patients With AIP Gene Variants
| Case No. . | HR Area . | Sex, M/F . | Agea . | Adenoma, m/M . | GH, μg/La . | IGF-I, ×ULNa . | AIP Variant . | FIPA . | AHR Variant . | SSa, S/R . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | M | 32 | m | 4.2 | 4.1 | p.R304* | Yes | R | |
| 2 | Yes | F | 53 | m | 4.4 | 3.1 | p.R304Q | No | S | |
| 3 | No | F | 26 | m | 1.1 | 1.1 | p.R16H | Yes | rs2066853b | NA |
| 4 | No | F | 62 | M | 4.4 | 1.9 | p.R304Q | No | R | |
| 5 | No | M | 50 | M | 58 | 5.1 | p.R16H | No | S | |
| 6 | No | F | 67 | M | 13.3 | 3.4 | p.R304Q | No | S | |
| 7 | No | F | 28 | M | 35 | 3.3 | IVS3 + 1G>A | No | S |
| Case No. . | HR Area . | Sex, M/F . | Agea . | Adenoma, m/M . | GH, μg/La . | IGF-I, ×ULNa . | AIP Variant . | FIPA . | AHR Variant . | SSa, S/R . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | M | 32 | m | 4.2 | 4.1 | p.R304* | Yes | R | |
| 2 | Yes | F | 53 | m | 4.4 | 3.1 | p.R304Q | No | S | |
| 3 | No | F | 26 | m | 1.1 | 1.1 | p.R16H | Yes | rs2066853b | NA |
| 4 | No | F | 62 | M | 4.4 | 1.9 | p.R304Q | No | R | |
| 5 | No | M | 50 | M | 58 | 5.1 | p.R16H | No | S | |
| 6 | No | F | 67 | M | 13.3 | 3.4 | p.R304Q | No | S | |
| 7 | No | F | 28 | M | 35 | 3.3 | IVS3 + 1G>A | No | S |
Abbreviations: m/M: micro- or macroadenoma; NA, not applicable; R, resistant; S, sensible.
At diagnosis.
rs2066853 heterozygous AHR gene variant.
Clinical and Biochemical Characteristics of Patients With AIP Gene Variants
| Case No. . | HR Area . | Sex, M/F . | Agea . | Adenoma, m/M . | GH, μg/La . | IGF-I, ×ULNa . | AIP Variant . | FIPA . | AHR Variant . | SSa, S/R . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | M | 32 | m | 4.2 | 4.1 | p.R304* | Yes | R | |
| 2 | Yes | F | 53 | m | 4.4 | 3.1 | p.R304Q | No | S | |
| 3 | No | F | 26 | m | 1.1 | 1.1 | p.R16H | Yes | rs2066853b | NA |
| 4 | No | F | 62 | M | 4.4 | 1.9 | p.R304Q | No | R | |
| 5 | No | M | 50 | M | 58 | 5.1 | p.R16H | No | S | |
| 6 | No | F | 67 | M | 13.3 | 3.4 | p.R304Q | No | S | |
| 7 | No | F | 28 | M | 35 | 3.3 | IVS3 + 1G>A | No | S |
| Case No. . | HR Area . | Sex, M/F . | Agea . | Adenoma, m/M . | GH, μg/La . | IGF-I, ×ULNa . | AIP Variant . | FIPA . | AHR Variant . | SSa, S/R . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | M | 32 | m | 4.2 | 4.1 | p.R304* | Yes | R | |
| 2 | Yes | F | 53 | m | 4.4 | 3.1 | p.R304Q | No | S | |
| 3 | No | F | 26 | m | 1.1 | 1.1 | p.R16H | Yes | rs2066853b | NA |
| 4 | No | F | 62 | M | 4.4 | 1.9 | p.R304Q | No | R | |
| 5 | No | M | 50 | M | 58 | 5.1 | p.R16H | No | S | |
| 6 | No | F | 67 | M | 13.3 | 3.4 | p.R304Q | No | S | |
| 7 | No | F | 28 | M | 35 | 3.3 | IVS3 + 1G>A | No | S |
Abbreviations: m/M: micro- or macroadenoma; NA, not applicable; R, resistant; S, sensible.
At diagnosis.
rs2066853 heterozygous AHR gene variant.
AHR genotyping
Overall, rs2066853 (c.1661G>A) AHR polymorphism was found in homozygosis in one patient and in heterozygosis in 46 cases (AHR+, 22.4%). Moreover, heterozygous rs4986826 (c.1708G>A) AHR change was detected in six cases with rs2066853 AHR polymorphism (2.9%), one in homozygosis and five in heterozygosis. Other variants of the AHR gene were not found. Among the patients with AIP mutations, rs2066853 polymorphism was detected in only one woman with the p.R16H variant (Table 2). In AHR+ patients, the percentage of males (42.5 vs 36.2%), mean age (46.3 ± 2.4 vs 47.0 ± 1.1 y), GH values (24.0 ± 9.4 vs 17.1 ± 2.9 ng/mL), IGF-I levels (2.7 ± 0.2 vs 2.8 ± 0.1 ×ULN), prevalence of macroadenomas (70.2 vs 68.0%), and mean tumor diameter (17.3 ± 2.1 vs 16.9 ± 1.4 mm) at diagnosis were not significantly different than in the AHR− ones (Table 3). In addition, SSa treatment significantly decreased GH and IGF-I levels in both groups of patients (AHR+P < .05 and P < 0.0001; AHR−P < .0001 and P < 0.0001, respectively). SSa induced normalization of IGF-I levels in 47.5 and 56.6%, and reduced baseline GH levels more than 50% in 75.0% of AHR+ and in 72.5% of AHR− patients, respectively (P = NS). The percentage of GH reduction (68.3 ± 5.4 vs 67.9 ± 2.9%) and mean IGF-I levels at last visit (1.5 ± 0.1 vs 1.4 ± 0.1 ×ULN) were similar between the two groups of patients.
Clinical and Biochemical Characteristics of Acromegalic Patients, Overall and Stratified on the basis of the Occurrence of rs2066853 AHR Polymorphism
| Characteristics . | Overall . | AHR+ . | AHR− . | P . |
|---|---|---|---|---|
| Patients, n | 210 | 47 | 163 | |
| Male sex, n | 79 | 20 | 59 | NS |
| Age at diagnosis, y | 47.0 ± 1.0 | 46.3 ± 2.4 | 47.0 ± 1.1 | NS |
| Mean GH levels at diagnosis, μg/L | 18.6 ± 3.0 | 24.0 ± 9.4 | 17.1 ± 2.9 | NS |
| Mean IGF-I levels at diagnosis, ×ULN | 2.8 ± 0.1 | 2.7 ± 0.2 | 2.8 ± 0.1 | NS |
| Mean pituitary tumor diameter, mm | 17.1 ± 1.2 | 17.3 ± 2.1 | 16.9 ± 1.4 | NS |
| Macroadenomas, % | 68.6 | 70.2 | 68.0 | NS |
| AIP-mutated patients | 7 (3.3%) | 1 (2.1%) | 6 (3.7%) | NS |
| FIPA | 5 (2.4%) | 1 (2.1%) | 4 (2.4%) | NS |
| Characteristics . | Overall . | AHR+ . | AHR− . | P . |
|---|---|---|---|---|
| Patients, n | 210 | 47 | 163 | |
| Male sex, n | 79 | 20 | 59 | NS |
| Age at diagnosis, y | 47.0 ± 1.0 | 46.3 ± 2.4 | 47.0 ± 1.1 | NS |
| Mean GH levels at diagnosis, μg/L | 18.6 ± 3.0 | 24.0 ± 9.4 | 17.1 ± 2.9 | NS |
| Mean IGF-I levels at diagnosis, ×ULN | 2.8 ± 0.1 | 2.7 ± 0.2 | 2.8 ± 0.1 | NS |
| Mean pituitary tumor diameter, mm | 17.1 ± 1.2 | 17.3 ± 2.1 | 16.9 ± 1.4 | NS |
| Macroadenomas, % | 68.6 | 70.2 | 68.0 | NS |
| AIP-mutated patients | 7 (3.3%) | 1 (2.1%) | 6 (3.7%) | NS |
| FIPA | 5 (2.4%) | 1 (2.1%) | 4 (2.4%) | NS |
Clinical and Biochemical Characteristics of Acromegalic Patients, Overall and Stratified on the basis of the Occurrence of rs2066853 AHR Polymorphism
| Characteristics . | Overall . | AHR+ . | AHR− . | P . |
|---|---|---|---|---|
| Patients, n | 210 | 47 | 163 | |
| Male sex, n | 79 | 20 | 59 | NS |
| Age at diagnosis, y | 47.0 ± 1.0 | 46.3 ± 2.4 | 47.0 ± 1.1 | NS |
| Mean GH levels at diagnosis, μg/L | 18.6 ± 3.0 | 24.0 ± 9.4 | 17.1 ± 2.9 | NS |
| Mean IGF-I levels at diagnosis, ×ULN | 2.8 ± 0.1 | 2.7 ± 0.2 | 2.8 ± 0.1 | NS |
| Mean pituitary tumor diameter, mm | 17.1 ± 1.2 | 17.3 ± 2.1 | 16.9 ± 1.4 | NS |
| Macroadenomas, % | 68.6 | 70.2 | 68.0 | NS |
| AIP-mutated patients | 7 (3.3%) | 1 (2.1%) | 6 (3.7%) | NS |
| FIPA | 5 (2.4%) | 1 (2.1%) | 4 (2.4%) | NS |
| Characteristics . | Overall . | AHR+ . | AHR− . | P . |
|---|---|---|---|---|
| Patients, n | 210 | 47 | 163 | |
| Male sex, n | 79 | 20 | 59 | NS |
| Age at diagnosis, y | 47.0 ± 1.0 | 46.3 ± 2.4 | 47.0 ± 1.1 | NS |
| Mean GH levels at diagnosis, μg/L | 18.6 ± 3.0 | 24.0 ± 9.4 | 17.1 ± 2.9 | NS |
| Mean IGF-I levels at diagnosis, ×ULN | 2.8 ± 0.1 | 2.7 ± 0.2 | 2.8 ± 0.1 | NS |
| Mean pituitary tumor diameter, mm | 17.1 ± 1.2 | 17.3 ± 2.1 | 16.9 ± 1.4 | NS |
| Macroadenomas, % | 68.6 | 70.2 | 68.0 | NS |
| AIP-mutated patients | 7 (3.3%) | 1 (2.1%) | 6 (3.7%) | NS |
| FIPA | 5 (2.4%) | 1 (2.1%) | 4 (2.4%) | NS |
Genetic changes and pollution exposition
Among the 23 patients from HR areas, seven showed AHR polymorphisms (30.4%) and two were found with AIP mutations (8.7%). Mean IGF-I levels and pituitary tumor diameter were significantly higher in these nine patients (HR/VAR+) than in the other 14 (HR/VAR−) (Table 4). On the contrary, mean GH levels were lower, although not statistically significantly, in the former group than in the latter one (Table 4). Mean age at diagnosis was not significantly lower in the HR/VAR+ than in the HR/VAR− group (41.0 ± 6.2 vs 47.5 ± 3.6 yrs, P = NS). Gender and macroadenoma prevalence were not different between the two groups. HR/VAR+ patients showed significantly higher IGF-I levels and tumor diameter at diagnosis also in comparison with those from NP areas, regardless of whether they were carriers of AHR and/or AIP variants (NP/VAR+) or not (NP/VAR−) (Table 4). Treatment with SSa normalized IGF-I levels in six of 11 HR/VAR− patients but in only one of seven HR/VAR+ cases (χ2 2.9; P = NS) (Figure 1A). The prevalence of patients who decreased baseline GH levels more than 50% was 14.2% in HR/VAR+ and 54.5% in the other HR group (P = NS) (Figure 1A). SSa significantly decreased mean GH and IGF-I levels in HR/VAR− (GH: 3.4 ± 1.7 vs 15.5 ± 5.5 ng/mL, P < .05; IGF-I: 1.4 ± 0.4 vs 2.5 ± 0.2 ×ULN, P < .01) but not in HR/VAR+ (GH: 2.1 ± 0.8 vs 5.8 ± 1.9 ng/mL, P = NS; IGF-I: 3.2 ± 0.9 vs 4.3 ± 0.1 ×ULN, P = NS) group (Figure 1B). In NP/VAR+ and NP/VAR− groups, 56.4 and 56.3% normalized IGF-I levels and 71.7 and 79.6% of patients decreased GH levels more than 50%, respectively. In both groups, mean GH and IGF-I levels were significantly lower during treatment than at baseline (Figure 1A). SSa treatment normalized both IGF-I and GH levels in none of the HR/VAR+ patients and in 54.5% of HR/VAR−, but in 25.6 and 50.5% of NP/VAR+ and NP/VAR− groups, respectively (P = NS) (Figure 1A).
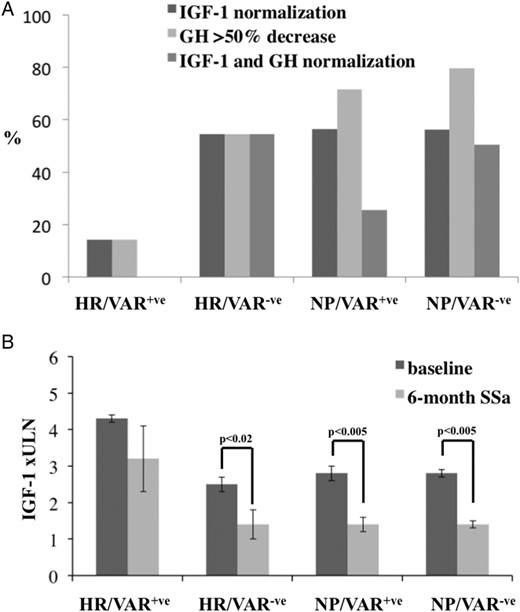
A, the percentage of patients who normalized IGF-I levels or reduced GH concentrations >50% or normalized both IGF-I and GH levels, stratified on the basis of HR or NP areas where they lived for ≥20 y before diagnosis and on the basis of the occurrence (VAR+) or not (VAR−) of AHR and/or AIP variants. B, the differences of IGF-I levels, expressed as ×ULN at baseline and after 6-mo treatment in the four groups of patients.
Clinical and Biochemical Characteristics of Acromegalic Patients Stratified According to the Presence of AHR and/or AIP Variants (VAR+) or not (VAR−), and to the Area Where They Had Lived Before Diagnosis
| Characteristic . | HR/VAR+ . | HR/VAR− . | P . | NP/VAR+ . | NP/VAR− . | P . |
|---|---|---|---|---|---|---|
| Cases, n | 9 | 14 | NS | 44 | 143 | NS |
| Male sex, n | 5 | 7 | NS | 17 | 50 | NS |
| Age at diagnosis, y | 41.0 ± 6.2 | 47.5 ± 3.6 | NS | 48.9 ± 2.5 | 46.6 ± 1.1 | NS |
| Mean GH levels at diagnosis, μg/L | 8.0 ± 3.4 | 15.4 ± 5.5 | NS | 16.4 ± 3.3 | 14.7 ± 1.8 | NS |
| Mean IGF-I levels at diagnosis, ×ULN | 4.3 ± 0.1 | 2.5 ± 0.2 | <.001 | 2.8 ± 0.2 | 2.8 ± 0.1 | NS |
| Mean pituitary tumor diameter, mm | 24.2 ± 3.7 | 12.1 ± 3.3 | <.02 | 16.4 ± 2.5 | 15.9 ± 1.3 | NS |
| Macroadenomas, n | 6 | 9 | NS | 31 | 98 | NS |
| FIPA | 0 | 1 | NS | 1 | 3 | NS |
| Characteristic . | HR/VAR+ . | HR/VAR− . | P . | NP/VAR+ . | NP/VAR− . | P . |
|---|---|---|---|---|---|---|
| Cases, n | 9 | 14 | NS | 44 | 143 | NS |
| Male sex, n | 5 | 7 | NS | 17 | 50 | NS |
| Age at diagnosis, y | 41.0 ± 6.2 | 47.5 ± 3.6 | NS | 48.9 ± 2.5 | 46.6 ± 1.1 | NS |
| Mean GH levels at diagnosis, μg/L | 8.0 ± 3.4 | 15.4 ± 5.5 | NS | 16.4 ± 3.3 | 14.7 ± 1.8 | NS |
| Mean IGF-I levels at diagnosis, ×ULN | 4.3 ± 0.1 | 2.5 ± 0.2 | <.001 | 2.8 ± 0.2 | 2.8 ± 0.1 | NS |
| Mean pituitary tumor diameter, mm | 24.2 ± 3.7 | 12.1 ± 3.3 | <.02 | 16.4 ± 2.5 | 15.9 ± 1.3 | NS |
| Macroadenomas, n | 6 | 9 | NS | 31 | 98 | NS |
| FIPA | 0 | 1 | NS | 1 | 3 | NS |
Clinical and Biochemical Characteristics of Acromegalic Patients Stratified According to the Presence of AHR and/or AIP Variants (VAR+) or not (VAR−), and to the Area Where They Had Lived Before Diagnosis
| Characteristic . | HR/VAR+ . | HR/VAR− . | P . | NP/VAR+ . | NP/VAR− . | P . |
|---|---|---|---|---|---|---|
| Cases, n | 9 | 14 | NS | 44 | 143 | NS |
| Male sex, n | 5 | 7 | NS | 17 | 50 | NS |
| Age at diagnosis, y | 41.0 ± 6.2 | 47.5 ± 3.6 | NS | 48.9 ± 2.5 | 46.6 ± 1.1 | NS |
| Mean GH levels at diagnosis, μg/L | 8.0 ± 3.4 | 15.4 ± 5.5 | NS | 16.4 ± 3.3 | 14.7 ± 1.8 | NS |
| Mean IGF-I levels at diagnosis, ×ULN | 4.3 ± 0.1 | 2.5 ± 0.2 | <.001 | 2.8 ± 0.2 | 2.8 ± 0.1 | NS |
| Mean pituitary tumor diameter, mm | 24.2 ± 3.7 | 12.1 ± 3.3 | <.02 | 16.4 ± 2.5 | 15.9 ± 1.3 | NS |
| Macroadenomas, n | 6 | 9 | NS | 31 | 98 | NS |
| FIPA | 0 | 1 | NS | 1 | 3 | NS |
| Characteristic . | HR/VAR+ . | HR/VAR− . | P . | NP/VAR+ . | NP/VAR− . | P . |
|---|---|---|---|---|---|---|
| Cases, n | 9 | 14 | NS | 44 | 143 | NS |
| Male sex, n | 5 | 7 | NS | 17 | 50 | NS |
| Age at diagnosis, y | 41.0 ± 6.2 | 47.5 ± 3.6 | NS | 48.9 ± 2.5 | 46.6 ± 1.1 | NS |
| Mean GH levels at diagnosis, μg/L | 8.0 ± 3.4 | 15.4 ± 5.5 | NS | 16.4 ± 3.3 | 14.7 ± 1.8 | NS |
| Mean IGF-I levels at diagnosis, ×ULN | 4.3 ± 0.1 | 2.5 ± 0.2 | <.001 | 2.8 ± 0.2 | 2.8 ± 0.1 | NS |
| Mean pituitary tumor diameter, mm | 24.2 ± 3.7 | 12.1 ± 3.3 | <.02 | 16.4 ± 2.5 | 15.9 ± 1.3 | NS |
| Macroadenomas, n | 6 | 9 | NS | 31 | 98 | NS |
| FIPA | 0 | 1 | NS | 1 | 3 | NS |
Discussion
The present study suggests that acromegaly is more biochemically severe and resistant to SSa treatment in patients from HR areas, if they also carry specific AHR and/or AIP gene variants. Indeed, patients in HR/VAR+ group showed higher IGF-I levels and bigger GH-secreting pituitary adenomas than the other ones, and treatment with SSa normalized IGF-I levels in only 14% of cases, in contrast with 54–56% of patients in the other groups. Accordingly, SSa halved GH values in 54–80% of cases in HR/VAR−, NP/VAR+, and NP/VAR− groups, but only in 14% of those in the HR/VAR+ one, and significantly reduced GH and IGF-I levels in the formers but not in the latter. Moreover, SSa treatment normalized both IGF-I and GH levels in none of the patients HR/VAR+ but 25–54% of the patients of the other groups.
The AHR is a transcription factor belonging to the basic helix-loop-helix/Per/ARNT/Sim family and is the only one that is activated by a ligand (7). It is stimulated by several natural compounds that are present in food such as indoles and flavonoids, or by tryptophan derivatives, arachidonic acid metabolites, and other endogenous products. The most potent AHR ligand known so far is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) but more than 400 exogenous compounds act as AHR ligands, most of which are environmental endocrine disruptors, including a variety of polycyclic aromatic hydrocarbons (PAHs), with benzo[a]pyrene as prototype, and planar polychlorinated biphenyls (17).
It is generally accepted that the metabolic responses to environmental pollutants can be the direct consequence of AHR activation. Moreover, it is worth considering also that AHR interacts with other receptor pathways such as the estrogen receptor one, which means that compounds with estrogen-like activity could indirectly affect AHR function (18). In the cytosol, the unbound receptor forms a complex with AIP, the cochaperone protein p23, and two heat shock protein 90 molecules. Ligand binding results in nuclear translocation of AHR, dissociation from the chaperone proteins, heterodimerization with a nuclear translocator (ARNT), and subsequent binding to xenobiotic-responsive elements, leading to transactivation of several genes encoding phase I and II xenobiotic-metabolizing enzymes, such as cytochrome P450s (CYP1A1, CYP1A2, and CYP1B1) (7). In animal models, activation of the AHR pathway by PAHs leads to their detoxification and excretion and, at the same time, to their metabolic activation to genotoxic compounds. In contrast, TCDD is metabolically inert and its binding to AHR does not lead to the production of genotoxic metabolites. These effects could seem apparently paradoxical, given that TCDD is a well-known tumor promoter whereas PAHs lose carcinogenic activity in AHR-knockout animals (7). However, it is worth noting that other noncanonical effects consequent to the activation of the AHR pathway can play an important role in tumor promotion. Some studies have recently suggested that the AHR pathway is involved in the transition from a benign to a malignant tumor, as also demonstrated in vitro by the higher nuclear AHR expression in invasive than in noninvasive tumor cells or by increased tumor invasion associated with AHR down-regulation (19, 20). Recently, AHR protein overexpression was demonstrated in differentiated thyroid cancer of both acromegalic and nonacromegalic patients, especially in association with the BRAF V600E mutation (21). It has been demonstrated that several AHR xenobiotic ligands induce the activation of the MAPK/ERK1/2-signaling pathway, as observed in presence of BRAF mutations (21). Nevertheless, the effects of AHR activation are tissue- and species specific and the molecular mechanisms of AHR-dependent carcinogenesis are still largely unknown.
Several AHR gene polymorphisms have been detected in humans, the majority in exon 10. Some of these genetic changes were associated with an altered induction of CYP1A1 or CYP1A2 activity, in response to specific ligands (22). In a previous study, some of us found only two polymorphisms of exon 10, rs2066853 and rs4986826, in acromegalic patients (13). The rs2066853 variant, consisting in a G>A substitution and causing an arginine to lysine replacement in the transactivating domain, was found in a quarter of cases and was associated with higher IGF-I values and tumor invasiveness of cavernous sinus (13). In a recent study, the occurrence of this polymorphism and the rs2158041 variant was associated with a significant risk of developing gliomas and with a higher level of PAH-DNA adducts in glioma tissue, which correlated with tumor grade (22). In our previous study, the rs4986826 polymorphism was extremely rare and was always associated with the rs2066853 change (23). In the present study, according to our previous findings, the prevalence of rs2066853 and rs4986826 among acromegalic patients was much higher (22.4 and 2.9%, respectively) than what was reported in the ExAC database for the European population (9.8 and 0.2%, respectively). Nevertheless, these AHR variants per se did not associate with acromegaly severity or pituitary tumor size, whereas cavernous sinus invasiveness was not investigated because imaging data were available only in a minority of cases. However, it is noteworthy that many of the patients with the rs2066853 variant enrolled onto our previous study were from an HR area of the province of Messina, whereas in this new study the majority (84.7%) were from NP areas.
Mutations of AIP gene were found in seven of our 210 patients (3.3%). Of them, two were familial isolated pituitary adenomas (FIPA) and two were from HR areas. In two patients we detected a p.R16H change, whose pathogenic role is still debated (24). Some AIP gene mutations cause low-penetrance pituitary tumor predisposition and are associated with younger age at diagnosis, higher serum GH and IGF-I values, larger size of pituitary tumors, and resistance to SSa treatment (25). Nevertheless, the pathogenic mechanism by which AIP mutations cause pituitary tumor predisposition is still unclear. A recent study demonstrated that AIP deficiency leads to elevated cAMP concentrations through defective inhibitory G protein α subunits (Gα)i-2 and Gαi-3, which normally inhibit cAMP synthesis. The same authors showed by immunostaining that AIP deficiency is associated with a reduction in Gαi-2 protein expression in human and mouse GH-secreting pituitary adenomas, thus indicating defective Gαi signaling in these tumors (26). The AIP exerts several intracellular regulatory activities, but it is also required for a correct cytosolic AHR expression. Several studies showed that AIP gene mutations could alter the function of the AHR pathway (27, 28). Among twenty different interactions described for AIP, the AHR-cAMP-phosphodiesterase pathway seems to be a very attractive candidate for the involvement in pituitary tumorigenesis (7). However, there is considerable controversy regarding the effects of AIP on AHR function. Some studies reported that AIP could enhance the transcription activity and expression levels of AHR, whereas others demonstrated the opposite (28). Some authors also suggested that AIP protects AHR from ubiquitin-dependent degradation through the proteasome, and low levels or loss of AIP correlates with low expression of AHR (29–31). Therefore, the AHR down-regulation could be involved in pathophysiology of AIP mutated and/or aggressive somatotropinomas (31).
It could be argued that the presence of two patients with AIP gene mutations in the HR/VAR+ group has influenced the results of this study. In our opinion, however, this is not the case. Indeed, these AIP+ patients showed microadenomas and slightly increased GH values, and SSa sensitivity was demonstrated in one of the two cases. The other five AIP+ patients (three cases with well-known mutations associated with pituitary tumor predisposition) were from NP areas, and at least two of them showed high or very high serum GH and IGF-I levels, three a macroadenoma, but only 25% SSa resistance.
Mean GH values were significantly lower in the HR/VAR+ group than in the other ones, despite that mean IGF-I values were significantly higher, suggesting an increased GH bioactivity. Other studies are necessary to confirm this evidence and to clarify the mechanism causing it.
In Italy, 44 SINs were identified in 2014, one of which was in Veneto Region, one in Calabria Region, two were in Marche Region, four in Apulia, and the other four in Sicily. Among our patients, 12 were from 2 SINs of Sicily, seven were from the SIN of Veneto Region, two from 2 SINs of Marche Region, one from a SIN of Apulia, and one from the SIN of Calabria Region. In these areas, ARPAs have reported increased concentration of nonmethane hydrocarbons, volatile organic compounds, or heavy metals. The presence of asbestos was reported only in the SIN of Apulia. Detail of the most relevant pollutants found in the SINs where our patients lived, are reported in Supplemental Table 2. Recently, an increased concentration of cadmium was found in the urine of adolescents from one of the Sicilian SINs where 11 of our patients are living (32).
With regard to the results of our study, we cannot exclude a synergistic effect on clinical expression of somatotropinomas of AHR/AIP genetic variants and environmental endocrine disruptors acting via non-AHR pathways. On the other hand, this study has some limitations: 1) we could not provide epidemiological data on acromegaly prevalence in the polluted or nonpolluted areas because of the study's design; 2) we enrolled a relatively low number of patients from HR areas, but it must be considered that acromegaly is a very rare disease, the polluted areas represent less than 2% of the country's surface, and the inclusion/exclusion criteria of the study were strict (eg, permanent address for at least 20 y before diagnosis); 3) we did not evaluate tumor shrinkage but only the biochemical response after 6 months of SSa treatment.
In conclusion, we found that genetic variants potentially inducing functional abnormalities of the AHR pathway are associated with a more severe disease, increased pituitary tumor size, and resistance to SSa treatment in patients living in highly polluted areas. Moreover, we confirm that approximately one quarter of acromegalic patients are carriers of the AHR rs2066853 polymorphism, and 3% of them are carriers of AIP gene mutations, both in a sporadic or familial setting. Additional studies evaluating AHR expression in pituitary tumors of acromegalic patients from polluted and nonpolluted areas and with or without AHR/AIP genetic variants would contribute to clarifying the role of the AHR pathway in this peculiar clinical context.
Acknowledgments
This work was supported by a grant of the Ministry of Education, University and Research of the Italian Government (PRIN 2010/2011, cod- DI1112000360001).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- AHR
aryl hydrocarbon receptor
- AIP
aryl-hydrocarbon interacting protein
- ARPA
Regional Agency for Environment Protection
- cpm
cases per million inhabitants
- FIPA
familial isolated pituitary adenomas
- HR
high-risk for health
- NP
nonpolluted
- NS
not significant
- PAH
polycyclic aromatic hydrocarbon
- SIN
area of national interest for environmental risk
- SSa
somatostatin analog
- TCDD
the 2,3,7,8-tetrachlorodibenzo-p-dioxin
- ×ULN
times upper limit of normal
- VAR
variant.



