-
PDF
- Split View
-
Views
-
Cite
Cite
K. Lang, K. Weber, M. Quinkler, A. S. Dietz, H. Wallaschofski, A. Hannemann, N. Friedrichs, L. C. Rump, B. Heinze, C. T. Fuss, I. Quack, H. S. Willenberg, M. Reincke, B. Allolio, S. Hahner, Prevalence of Malignancies in Patients With Primary Aldosteronism, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 4, 1 April 2016, Pages 1656–1663, https://doi.org/10.1210/jc.2015-3405
Close - Share Icon Share
Abstract
Primary aldosteronism (PA) is the most common cause of secondary hypertension. Aldosterone excess can cause DNA damage in vitro and in vivo. Single case reports have indicated a coincidence of PA with renal cell carcinoma and other tumors. However, the prevalence of benign and malignant neoplasms in patients with PA has not yet been studied.
In the multicenter MEPHISTO study, the prevalence of benign and malignant tumors was investigated in 335 patients with confirmed PA. Matched hypertensive subjects from the population-based Study of Health in Pomerania cohort served as controls.
Of the 335 PA patients, 119 (35.5%) had been diagnosed with a tumor at any time, and 30 had two or more neoplasms. Lifetime malignancy occurrence was reported in 9.6% of PA patients compared to 6.0% of hypertensive controls (P = .08). PA patients with a history of malignancy had higher baseline aldosterone levels at diagnosis of PA (P = .009), and a strong association between aldosterone levels and the prevalence of malignancies was observed (P = .03). In total, 157 neoplasms were identified in the PA patients; they were benign in 61% and malignant in 25% of the cases (14% of unknown dignity). Renal cell carcinoma was diagnosed in five patients (13% of all malignancies) and was not reported in controls.
Compared to hypertensive controls, the prevalence of malignancies was positively correlated with aldosterone levels, tended to be higher in PA patients, but did not differ significantly.
Primary aldosteronism (PA) is defined as hypertension caused by endogenous aldosterone excess (1). The most common subtypes of PA, accounting for more than 95% of cases, are the aldosterone-producing adenoma (APA) and idiopathic hyperaldosteronism caused by bilateral adrenal hyperplasia (2–4). PA is known to affect up to 10% of hypertensive patients (2–5) and an even larger fraction of patients with refractory hypertension (6–8). Compared to primary hypertension, it is associated with a higher rate of comorbidities and increased mortality (9). This includes cardio- and cerebrovascular complications (5, 10, 11), renal damage (11–13), as well as metabolic and psychological disorders (11, 14–18). Some of those comorbidities such as diabetes and obesity are known to be independent risk factors for cancer formation (19–21). Recent data from in vitro studies and animal models suggest that high aldosterone levels can cause oxidative stress leading to DNA damage (22–24). This effect has been shown to be independent of blood pressure (22). One recent study found evidence for significantly higher levels of oxidative stress in patients with PA compared to hypertensive control subjects, which could be reversed by adrenalectomy or treatment with mineralocorticoid receptor antagonists (25). Oxidative stress is not only a key factor in the pathogenesis of endothelial dysfunction, inflammation, and metabolic disorders but also plays a major role in the development of cancer (21, 26). Up-regulation of the renin angiotensin system (RAS) has also been postulated as an enzymatic cascade influencing carcinogenesis. Medical suppression of the RAS with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers exerted positive effects on a variety of malignancies, an effect not observed with other antihypertensive agents (27–29). Clinical assessment of angiotensin system inhibition resulted, however, in more conflicting data. Although some analyses indicate a moderate association with an increased cancer incidence (30), several other analyses found insufficient or no association with cancer (31–34). Recent data show a possible influence of aldosterone on tumor development and growth in renal cell carcinomas (RCCs) by aldosterone-mediated up-regulation of the proto-oncogene K-RAS (35). A number of case reports indicate a coincidence of different malignancies, particularly RCC, with PA (36–39). However, a structured analysis regarding the prevalence of neoplasms in PA patients has not yet been performed.
Therefore, we investigated the lifetime prevalence of benign and malignant neoplasms in a well-characterized cohort of PA patients. Data were compared with hypertensive subjects without PA.
Subjects and Methods
The current analysis used the prospective cohort of the German Conn's Registry to identify patients. As a matched control group, hypertensive participants of the Study of Health in Pomerania (SHIP) were selected.
German Conn's Registry
The multicentric national Conn's Registry (www.conn-register.de) was founded in 2006 and started with a retrospective data analysis in five German centers. The aim of the registry was to systematically collect data on diagnostic measures, comorbidities, and the long-term outcome of PA (5, 12). The study protocol was approved by the Ethics Committee of the University of Munich and the local ethics committees of the participating centers (Wuerzburg Ethics Committee permit no. 18/07 and 240/09). All data were entered in a database after pseudonymization, and data protection laws were strictly adhered to. Patients with PA diagnosed since 1990 were included (5).
A prospective follow-up, the MEPHISTO (Multicenter Evaluation of Primary Hyperaldosteronism Diagnostic Testing, Subdifferentiation, Therapy, Outcome and Genetics) study, was started in 2008, and every patient with PA diagnosed since then is followed prospectively in eight centers from different geographical regions in Germany. The diagnostic criteria for the prospective part were defined according to the Endocrine Society's Clinical Practice Guideline 2008 (40). All included patients had both an abnormal aldosterone-to-renin ratio (ARR) and a confirmatory test (saline infusion test, fludrocortisone suppression test, captopril test, or oral salt-loading test with demonstration of elevated excretion of aldosterone and its metabolites in urine). Adjustments of medication before screening and confirmatory tests were performed whenever possible according to the study protocol (41). Subtypes were defined according to imaging, laboratory, and dynamic test results as well as adrenal venous sampling data if performed. Clinical data at the time of diagnosis were taken from patients' charts and records, and patients were asked for comorbidities such as diabetes. At the time of the visit, clinical data were obtained, and standardized laboratory analyses and a structured medical history with special regard to neoplasms and predisposing factors were taken (41). Neoplasms reported by patients were classified and confirmed with clinical notes and charts. Patients with a body mass index (BMI) of 25–29.9 kg/m2 were classified as overweight, and those with a BMI > 30 kg/m2 were classified as obese. Patients underwent regular follow-up visits every 6–12 months, according to the local center's protocol.
The Study of Health in Pomerania (SHIP)
SHIP is a longitudinal population-based study conducted in northeast Germany. Briefly, 4308 men and women from a representative population sample of 7008 subjects participated in baseline examinations between October 1997 and May 2001. All of the subjects were invited to participate in the first follow-up examination, designated as SHIP-1, and 3300 men and women completed these examinations between March 2002 and July 2006. Further details on study design and sampling have been reported previously (42, 43). All participants gave written, informed consent. The study conformed to the principles of the Declaration of Helsinki as reflected by an a priori approval of the Ethics Committee of the Board of Physicians Mecklenburg-West Pomerania at the University of Greifswald.
All SHIP participants underwent standardized medical examinations including blood pressure measurement, blood sampling, and an extensive computer-aided personal interview. Data on sociodemographic characteristics and medical histories were collected. Smoking was classified into smoker, ex-smoker, and nonsmoker status. During the physical examination, standardized measurements of height, weight, waist circumference, and blood pressure were performed. Systolic and diastolic blood pressures were measured three times on the right arm of the seated participant using a digital blood pressure monitor (HEM-705CP; OMRON Corp). For statistical analyses, the mean of the second and third measurements was used. Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or intake of self-reported antihypertensive medication.
All subjects were specifically asked about cancer diagnoses. Moreover, data on medication intake and information regarding previous surgeries were scanned to find additional cancer cases.
Patient selection and data analysis
For the current analysis, we included only PA patients participating in the prospective part of the German Conn's Registry. In September 2011, data from all patients that had completed the initial study visit were extracted from the database, which resulted in a study population of 338 patients (199 males and 139 females).
For the hypertensive control cohort, we used data from SHIP-1 because plasma aldosterone and renin concentrations were not measured in SHIP-0. From 3300 SHIP-1 participants, we selected 1342 hypertensive control subjects without PA who had an ARR within the study-specific reference range (age < 55 years: men, 1.4–14.2, and women, 0.9–20.3; age ≥ 55 years: men, 0.9–22.4, and women, 0.7–25.5). From these 1342 subjects, we excluded those with missing information on cancer history (n = 11), which resulted in a study population of 1331 subjects aged 27–88 years.
Three of the 338 PA patients younger than 27 years had to be excluded due to a lack of an appropriate matching control. A 1:1 matching was performed for the remaining 335 PA patients (198 males and 137 females) with 1331 hypertensive SHIP-1 participants, using the two matching factors sex and age. A maximum age difference of 5 years was allowed between patients and controls. The greedy matching algorithm, as implemented in a SAS macro (SAS Institute Inc.), was used to select controls (44).
Results for continuous variables are expressed as median (with first-third quartile) when non-normally distributed. For categorical variables, we report numbers (proportion). Group differences were tested for statistical significance with Kruskal-Wallis test and χ2 test where appropriate. P values <.05 were considered statistically significant. All statistical analyses were performed with SAS 9.1.3 (SAS Institute Inc) or SPSS, version 22.0 (IBM SPSS Statistics).
Results
Study subjects
The 335 PA patients presented at study visit with a median age of 57 (range, 47–65) years. Median systolic blood pressure (available for 259 subjects) was 133 (range, 124–145) mm Hg, and diastolic blood pressure was 83 (range, 75–91) mm Hg. Biometrical data were available for 325 subjects, and information on smoking status for 286 subjects. Median BMI at visit was 28.1 (range, 25.3–31.4) kg/m2; 39.5% of subjects were classified as overweight and 37.5% as obese. Fifteen percent of PA patients reported being active smokers, and 24% had previously been diagnosed with diabetes. Median serum potassium level at visit was 3.9 (range, 3.5–4.2) mmol/L. Additional information on sex distribution, subtype, and characteristics of PA is given in Figure 1.
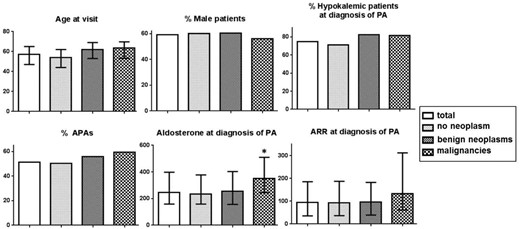
Subgroup characteristics of 216 PA patients without neoplasms, 86 with benign neoplasms, and 32 with malignancies.
Data are expressed as median (first–third quartile) or proportion of total. Serum aldosterone concentrations were included in the analysis for 301 subjects and ARRs were included for 262 of subjects. *, P < .05 (Kruskal-Wallis test).
Lifetime prevalence of neoplasms in PA patients
Of the 335 PA patients, 119 reported a neoplasm at any time in their life, and 30 were diagnosed with multiple neoplasms. In total, 157 neoplasms were reported in our study population (Table 1).
Number and Proportion of Neoplasms and Malignancies in 335 Patients With PA and Malignancies in 335 Age- and Sex-Matched Hypertensive Controls (SHIP Study) Classified According to Etiology
| . | Neoplasms in PA Patients . | Malignancies in PA Patients . | Malignancies in SHIP Controls . | |||
|---|---|---|---|---|---|---|
| n . | Neoplasms, % . | n . | Malignancies, % . | n . | Malignancies, % . | |
| Lung | 4 | 3 | 2 | 5 | 1 | 5 |
| Breast | 5 | 3 | 3 | 8 | 3 | 15 |
| Gut | 7 | 4 | 2 | 5 | 2 | 10 |
| Prostate | 29 | 18 | 6 | 15 | 4 | 20 |
| Endocrine | 50 | 32 | 3 | 8 | 1 | 5 |
| Skin | 15 | 10 | 8 | 21 | 4 | 20 |
| Female reproductive | 16 | 10 | 3 | 8 | 1 | 5 |
| Kidney | 5 | 3 | 5 | 13 | 0 | |
| Brain | 5 | 3 | 1 | 3 | 0 | |
| Hematology | 3 | 2 | 2 | 5 | 1 | 5 |
| Other | 18 | 11 | 4 | 10 | 3 | 15 |
| Total | 157 | 39 | 20 | |||
| . | Neoplasms in PA Patients . | Malignancies in PA Patients . | Malignancies in SHIP Controls . | |||
|---|---|---|---|---|---|---|
| n . | Neoplasms, % . | n . | Malignancies, % . | n . | Malignancies, % . | |
| Lung | 4 | 3 | 2 | 5 | 1 | 5 |
| Breast | 5 | 3 | 3 | 8 | 3 | 15 |
| Gut | 7 | 4 | 2 | 5 | 2 | 10 |
| Prostate | 29 | 18 | 6 | 15 | 4 | 20 |
| Endocrine | 50 | 32 | 3 | 8 | 1 | 5 |
| Skin | 15 | 10 | 8 | 21 | 4 | 20 |
| Female reproductive | 16 | 10 | 3 | 8 | 1 | 5 |
| Kidney | 5 | 3 | 5 | 13 | 0 | |
| Brain | 5 | 3 | 1 | 3 | 0 | |
| Hematology | 3 | 2 | 2 | 5 | 1 | 5 |
| Other | 18 | 11 | 4 | 10 | 3 | 15 |
| Total | 157 | 39 | 20 | |||
Number and Proportion of Neoplasms and Malignancies in 335 Patients With PA and Malignancies in 335 Age- and Sex-Matched Hypertensive Controls (SHIP Study) Classified According to Etiology
| . | Neoplasms in PA Patients . | Malignancies in PA Patients . | Malignancies in SHIP Controls . | |||
|---|---|---|---|---|---|---|
| n . | Neoplasms, % . | n . | Malignancies, % . | n . | Malignancies, % . | |
| Lung | 4 | 3 | 2 | 5 | 1 | 5 |
| Breast | 5 | 3 | 3 | 8 | 3 | 15 |
| Gut | 7 | 4 | 2 | 5 | 2 | 10 |
| Prostate | 29 | 18 | 6 | 15 | 4 | 20 |
| Endocrine | 50 | 32 | 3 | 8 | 1 | 5 |
| Skin | 15 | 10 | 8 | 21 | 4 | 20 |
| Female reproductive | 16 | 10 | 3 | 8 | 1 | 5 |
| Kidney | 5 | 3 | 5 | 13 | 0 | |
| Brain | 5 | 3 | 1 | 3 | 0 | |
| Hematology | 3 | 2 | 2 | 5 | 1 | 5 |
| Other | 18 | 11 | 4 | 10 | 3 | 15 |
| Total | 157 | 39 | 20 | |||
| . | Neoplasms in PA Patients . | Malignancies in PA Patients . | Malignancies in SHIP Controls . | |||
|---|---|---|---|---|---|---|
| n . | Neoplasms, % . | n . | Malignancies, % . | n . | Malignancies, % . | |
| Lung | 4 | 3 | 2 | 5 | 1 | 5 |
| Breast | 5 | 3 | 3 | 8 | 3 | 15 |
| Gut | 7 | 4 | 2 | 5 | 2 | 10 |
| Prostate | 29 | 18 | 6 | 15 | 4 | 20 |
| Endocrine | 50 | 32 | 3 | 8 | 1 | 5 |
| Skin | 15 | 10 | 8 | 21 | 4 | 20 |
| Female reproductive | 16 | 10 | 3 | 8 | 1 | 5 |
| Kidney | 5 | 3 | 5 | 13 | 0 | |
| Brain | 5 | 3 | 1 | 3 | 0 | |
| Hematology | 3 | 2 | 2 | 5 | 1 | 5 |
| Other | 18 | 11 | 4 | 10 | 3 | 15 |
| Total | 157 | 39 | 20 | |||
Malignant neoplasms
Thirty-two PA patients were diagnosed with a malignant tumor, and four had more than one malignancy (in total 39 malignancies were detected) (Table 1). Malignancies were diagnosed at a median age of 56 (range, 44–66) years. Although, compared to the hypertensive controls, there was no relevant difference in predisposing factors, such as BMI or smoking status, the lifetime prevalence for malignancies was higher in the PA group, whereas only a trend toward statistical significance was observed (P = .08) (Table 2). The relative risk of a malignancy in patients with primary hyperaldosteronism relative to matched controls is 1.60 (95% confidence interval, 0.93–2.74). Within the PA patients, those with malignancies in their history were more often but nonsignificantly obese (48% compared to 35%; P = .16) and diabetic (33% compared to 23%; P = .22).
1:1 Matching for Sex and Age of 335 PA Patients With Hypertensive Controls (SHIP study)
| . | PA Patients . | SHIP Controls . | P Value . |
|---|---|---|---|
| BMI, kg/m2a | 28.1 (25.3–31.4) | 28.5 (26.0–31.7) | .27 |
| Smoking, n (%)b | .2 | ||
| Non-smokers | 133 (39.7) | 133 (39.7) | |
| Smokers | 50 (14.9) | 72 (21.5) | |
| Ex-smokers | 103 (30.7) | 130 (38.8) | |
| Subjects with malignancies, n (%) | 32 (9.6) | 20 (6.0) | .08 |
| . | PA Patients . | SHIP Controls . | P Value . |
|---|---|---|---|
| BMI, kg/m2a | 28.1 (25.3–31.4) | 28.5 (26.0–31.7) | .27 |
| Smoking, n (%)b | .2 | ||
| Non-smokers | 133 (39.7) | 133 (39.7) | |
| Smokers | 50 (14.9) | 72 (21.5) | |
| Ex-smokers | 103 (30.7) | 130 (38.8) | |
| Subjects with malignancies, n (%) | 32 (9.6) | 20 (6.0) | .08 |
Data are expressed as number (proportion) or median (1st–3rd quartile). Group differences were tested with χb or Kruskal-Wallis tests.
Due to missing information, data on BMI were only available in 325 patients.
Due to missing information, data on smoking status were only available in 286 patients.
1:1 Matching for Sex and Age of 335 PA Patients With Hypertensive Controls (SHIP study)
| . | PA Patients . | SHIP Controls . | P Value . |
|---|---|---|---|
| BMI, kg/m2a | 28.1 (25.3–31.4) | 28.5 (26.0–31.7) | .27 |
| Smoking, n (%)b | .2 | ||
| Non-smokers | 133 (39.7) | 133 (39.7) | |
| Smokers | 50 (14.9) | 72 (21.5) | |
| Ex-smokers | 103 (30.7) | 130 (38.8) | |
| Subjects with malignancies, n (%) | 32 (9.6) | 20 (6.0) | .08 |
| . | PA Patients . | SHIP Controls . | P Value . |
|---|---|---|---|
| BMI, kg/m2a | 28.1 (25.3–31.4) | 28.5 (26.0–31.7) | .27 |
| Smoking, n (%)b | .2 | ||
| Non-smokers | 133 (39.7) | 133 (39.7) | |
| Smokers | 50 (14.9) | 72 (21.5) | |
| Ex-smokers | 103 (30.7) | 130 (38.8) | |
| Subjects with malignancies, n (%) | 32 (9.6) | 20 (6.0) | .08 |
Data are expressed as number (proportion) or median (1st–3rd quartile). Group differences were tested with χb or Kruskal-Wallis tests.
Due to missing information, data on BMI were only available in 325 patients.
Due to missing information, data on smoking status were only available in 286 patients.
PA patients with a history of malignancies had significantly higher aldosterone levels at diagnosis of PA compared to patients without malignancies (P = .009), whereas this was not observed in the hypertensive controls (Table 3). Accordingly, PA patients with malignancies presented with the lowest renin concentrations and highest ARRs (Figure 1). Evaluating the lifetime prevalence of malignancies according to tertiles of aldosterone levels at initial diagnosis of PA, a significant association between serum aldosterone concentrations and the occurrence of malignant tumors was observed (P = .03) (Figure 2).
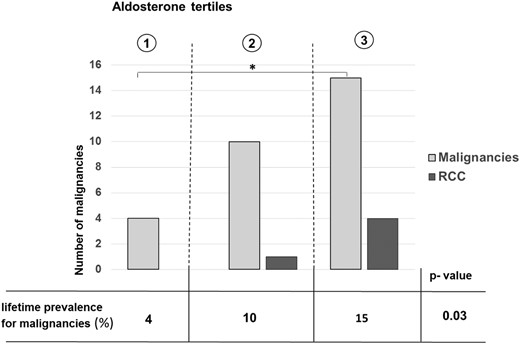
Prevalence of malignancies and renal cell carcinomas by tertiles of aldosterone concentration at initial diagnosis of PA.
*, P < .05 (χ2 test). RCC, renal cell carcinoma.
Median (1st–3rd Quartiles) Serum Aldosterone Concentration at Initial Diagnosis of PA in PA Patients Compared to Median (1st–3rd Quartiles) Plasma Aldosterone Concentration in the Hypertensive Controls
| . | Aldosterone Concentration, ng/L . | P Value . | |
|---|---|---|---|
| Malignancy . | No Malignancy . | ||
| PA patients | 350 (244.5–509.5) (n = 29) | 239.2 (153.4–379.6) (n = 272) | .009 |
| SHIP controls | 56.5 (32.5–71) (n = 20) | 42.0 (28.0–65.0) (n = 315) | .17 |
| . | Aldosterone Concentration, ng/L . | P Value . | |
|---|---|---|---|
| Malignancy . | No Malignancy . | ||
| PA patients | 350 (244.5–509.5) (n = 29) | 239.2 (153.4–379.6) (n = 272) | .009 |
| SHIP controls | 56.5 (32.5–71) (n = 20) | 42.0 (28.0–65.0) (n = 315) | .17 |
Individuals with malignancies in their history were compared to individuals without malignancies in their history. Group differences were tested for statistical significance using the Kruskal-Wallis test.
Median (1st–3rd Quartiles) Serum Aldosterone Concentration at Initial Diagnosis of PA in PA Patients Compared to Median (1st–3rd Quartiles) Plasma Aldosterone Concentration in the Hypertensive Controls
| . | Aldosterone Concentration, ng/L . | P Value . | |
|---|---|---|---|
| Malignancy . | No Malignancy . | ||
| PA patients | 350 (244.5–509.5) (n = 29) | 239.2 (153.4–379.6) (n = 272) | .009 |
| SHIP controls | 56.5 (32.5–71) (n = 20) | 42.0 (28.0–65.0) (n = 315) | .17 |
| . | Aldosterone Concentration, ng/L . | P Value . | |
|---|---|---|---|
| Malignancy . | No Malignancy . | ||
| PA patients | 350 (244.5–509.5) (n = 29) | 239.2 (153.4–379.6) (n = 272) | .009 |
| SHIP controls | 56.5 (32.5–71) (n = 20) | 42.0 (28.0–65.0) (n = 315) | .17 |
Individuals with malignancies in their history were compared to individuals without malignancies in their history. Group differences were tested for statistical significance using the Kruskal-Wallis test.
Only 7.7% (n = 3) of the malignant neoplasms were of endocrine origin, with all of them being thyroid carcinomas. With a median age of 44 (range, 40–69) years, the PA patients with thyroid carcinomas were very young at diagnosis of the malignancy. All three of them were diagnosed with the unilateral subtype of PA and were cured by total thyroidectomy. By contrast, only one patient was diagnosed with a thyroid carcinoma in the hypertensive-matched controls (three in the total 3300 SHIP subjects) (Table 1).
Renal cell carcinoma
Five of the 335 PA patients had been diagnosed with a RCC. None of the matched hypertensive control subjects was diagnosed with a RCC (only seven RCCs were reported in 3300 SHIP subjects) (Table 1).
Four of the five PA patients with RCCs were males, and two were diagnosed with other malignancies, but none reported a high familial incidence of malignancies. Four patients were diagnosed with the unilateral form of PA, and all were hypokalemic at the initial diagnosis of PA. The median age at diagnosis of the RCC was 61 (range, 39–67.5) years. In only one patient was the malignancy discovered during the workup for PA; the RCC had been diagnosed before diagnosis of PA in three patients and after the diagnosis of PA in two patients. None of the tumors was advanced or appeared to be dedifferentiated, and no metastases were found. With regard to histology, three of the tumors were classified as clear cell carcinomas, one as papillary, and one as chromophobe RCC. All patients were cured by surgery. In four of the five patients, renal cysts were found before diagnosis of the malignancy, whereas none of them was diagnosed with end-stage renal disease. Of note, the median aldosterone level of the patients with RCCs was 469 (range, 316–509.5) ng/L at diagnosis of PA and therefore was higher than in any of the other subgroups (see Figure 1 for comparison). Also, the dependency of tumor formation on serum aldosterone levels could be highlighted particularly for those patients (Figure 2).
Discussion
Our study represents the largest analysis so far of tumor prevalence in a well-characterized cohort of patients with primary hyperaldosteronism in comparison to matched hypertensive controls.
The 10-year prevalence for malignancies in Germany was 2.6% in 2006. In our cohort, 9.6% of the PA patients, compared to 6% of the age- and sex-matched hypertensive controls, reported a lifetime event of any malignancy (P = .08). The relative risk of a malignancy in PA patients relative to matched hypertensive controls was 1.60 (95% confidence interval, 0.93–2.74). Although this finding missed statistical significance, there is a trend toward a higher rate of malignancies in this cohort of PA patients. The lack of significance could either be due to the small sample size or suggest a higher incidence of only certain forms of malignancies in PA patients. There was a slightly higher number of obesity and diabetes cases in the PA patients with a history of malignancies. However, the difference regarding the classical predisposing factors such as BMI, diabetes, or smoking status was not significant (19–21). Thus, the trend toward an increased rate of malignancies in PA patients might be an independent finding that is further supported by the observed correlation with aldosterone levels.
Although comorbidities in PA can be found throughout all subtypes, vascular complications seem to be more prevalent in the hypokalemic individuals (5). Hypokalemia on the other hand is associated with the severity of PA and is subsequently more distinct in the unilateral subtype of PA (3). Accordingly, PA patients with malignancies in their history were more often hypokalemic and diagnosed more often with the unilateral subtype. Thus, the quantity of the aldosterone excess expressed by disease severity might have contributed to carcinogenesis. The crucial finding in this context is the significantly higher aldosterone levels at initial diagnosis of PA observed in the subgroup with malignant neoplasms (P = .009) compared to PA patients without malignancies. Intriguingly, a significant increase of lifetime prevalence of malignancies was furthermore found with increasing serum aldosterone levels, suggesting a quantitative aldosterone-related effect on carcinogenesis particularly in PA patients. In contrast, in the hypertensive control subjects, where median aldosterone levels were much lower and within the normal range, no such difference in plasma aldosterone levels was observed.
The three malignant endocrine neoplasms in our PA patients were all thyroid carcinomas. Although they accounted for 7.7% of all malignancies in this study, thyroid carcinomas only accounted for 0.7–1.9% of all malignancies in the German Robert Koch Institute (RKI) national cancer report for the year 2006 (45) and for 5% of malignancies in the matched controls. Although our sample size does not allow drawing a final conclusion, two further studies on thyroid abnormalities in patients with PA also found a relatively high incidence of thyroid papillary carcinoma in those patients (46, 47). Although the cases documented in literature were mostly related to idiopathic hyperaldosteronism, all three of our thyroid carcinoma patients were diagnosed with APA (47). Therefore, formation of thyroid carcinoma does not seem to be related to a distinct subtype of PA.
The fact that hypertension is associated with an increased risk for malignancies could particularly be demonstrated for RCC (48). We identified five patients with RCC in our cohort of 335 PA patients (1.5% of the total cohort). Thus, RCCs account for 13% of all malignancies in our PA patients, whereas no RCC was documented in the SHIP hypertensive control cohort. In the RKI's national cancer reports from 2006 and 2013, RCC accounts for only 3.3–4.4% and 2.5% of all malignancies, respectively (45, 49). Although, due to the small sample size, this finding cannot be regarded as statistically significant, our study leads to the assumption that PA patients are at increased risk of developing RCCs.
The general incidence of RCCs has increased, affecting more men than women. Well-established risk factors are age, obesity, and hypertension, as well as smoking and alcohol intake (50). Age, male sex, and hypokalemia are known to be independent risk factors for a low glomerular filtration rate in PA patients (12). End-stage renal disease and acquired renal cysts are risk factors for developing RCC (50, 51). Although none of our five patients had end-stage renal disease when the RCC was diagnosed, four of the five had a history of renal cysts. Generally, the prevalence of renal cysts is reported to be at least 2-fold higher in patients with PA (up to 44%) compared to hypertensive or normotensive controls and was shown to be higher in hypokalemic individuals and in those with APAs (52–54). Accordingly, in our cohort, all PA patients with RCC were hypokalemic, and four of the five were diagnosed with the unilateral subtype.
One previous study documented a positive correlation between aldosterone levels and renal cysts in patients with PA (54). The pro-oxidative and genotoxic effect of aldosterone in kidney cells in vitro and in vivo in animal studies already led to the assumption that this could be an oncogenic factor for the development of RCC (22, 24). A recent in vitro study demonstrated evidence that aldosterone supports growth and survival of RCC cells through increased expression of the K-RAS4A cellular oncogene. Treatment with the mineralocorticoid antagonist spironolactone reduced K-RAS expression and caused a reduction in tumor cell number (35). In our study, PA patients with RCC in their history showed the highest aldosterone levels at diagnosis of PA, and the association of tumor formation with serum aldosterone levels could be highlighted particularly for RCCs.
In summary, this is the first study describing cancer prevalence in a large well-characterized PA cohort in comparison to an established population-based control cohort. Overall, the rate of malignancies did not differ significantly between groups. However, it was relatively high in the cohort of PA patients and was significantly correlated to baseline aldosterone levels. The association of RCC and high aldosterone is supported by the findings of our study. Generally, our observations in PA patients, in which the RAS is subsequently down-regulated, suggest that it is not the up-regulation of the RAS in general, as postulated by previous studies, but specifically the aldosterone excess that might be a contributing factor in the pathogenesis of malignancies. The observations of our study need to be verified in larger analyses. Furthermore, the question remains unanswered whether putative effects are mediated via the mineralocorticoid receptor and, subsequently, whether patients on long-term mineralocorticoid receptor blockade would benefit less than patients in which the mineralocorticoid excess is removed by surgical treatment of unilateral disease. Our findings suggest that we should consider screening PA patients by ultrasound for cystic kidney lesions to potentially identify individuals at increased risk for RCC.
Strengths and limitations of the study
The strengths of our study are the large cohort of PA patients, the standardized prospective data collection in the context of the German Conn's Registry, the standardized protocols for diagnosis of PA, and the validated large hypertensive control cohort. However, although large for a PA cohort, the sample size remained too small to reliably assess the prevalence of rare malignancies such as RCC. The observations made in our study are in accordance with previous observations. However, the association of hyperaldosteronism with cancer incidence will need to be assessed in more detail in larger multicenter analyses.
Acknowledgments
This work was supported by grants from the Else Kröner-Fresenius-Stiftung (2013_A182 to M.R. and 2013_A213 to S.H.). The study was only feasible due to the support of our PA team and the Endocrine laboratory team.
SHIP is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (Grants 01ZZ9603, 01ZZ0103, and 01ZZ0403), the Ministry of Cultural Affairs and the Social Ministry of the Federal State of Mecklenburg-West Pomerania. This work is also part of the research project Greifswald Approach to Individualized Medicine (GANI MED), which is funded by the Federal Ministry of Education and Research and the Ministry of Cultural Affairs of the Federal State of Mecklenburg-West Pomerania (03IS2061A).
Disclosure Summary: The authors have nothing to disclose.
Deceased August 16, 2015.
Abbreviations
- APA
aldosterone-producing adenoma
- ARR
aldosterone-to-renin ratio
- BMI
body mass index
- PA
primary aldosteronism
- RAS
renin angiotensin system
- RCC
renal cell carcinoma.



