-
PDF
- Split View
-
Views
-
Cite
Cite
Dongbin Ahn, Jin Ho Sohn, Jae Han Jeon, Hypothyroidism Following Hemithyroidectomy: Incidence, Risk Factors, and Clinical Characteristics, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 4, 1 April 2016, Pages 1429–1436, https://doi.org/10.1210/jc.2015-3997
Close - Share Icon Share
Abstract
Postoperative thyroid hormone replacement for hypothyroidism following hemithyroidectomy is usually administered without fully understanding the clinical characteristics of the condition.
We aimed to evaluate the clinical characteristics of hypothyroidism following hemithyroidectomy, along with its actual incidence and risk factors.
We conducted a retrospective cohort study involving 405 patients undergoing hemithyroidectomy between 2004 and 2011. The mean follow-up duration was 56.4 mo.
Standardized routine measurement of thyroid function at regular intervals, along with strict criteria for thyroid hormone replacement, was introduced.
The development and clinical course of hypothyroidism following hemithyroidectomy including spontaneous normalization of thyroid function or the need for treatment were evaluated.
Hypothyroidism developed in 226 patients (55.8%) after hemithyroidectomy. A preoperative TSH level ≥ 2 mIU/L (odds ratio [OR], 5.517; 95% confidence interval [CI], 3.540–8.598; P < .001), and the coexistence of Hashimoto's thyroiditis (OR, 1.996; 95% CI, 1.107–3.601; P = .022) were found to be independent risk factors for the development of hypothyroidism. Among 222 subclinical hypothyroidism cases, 149 (67.1%) exhibited spontaneous recovery; subgroup analysis of these patients suggested that age ≥ 46 y (OR, 2.395; 95% CI, 1.266–4.533; P = .007) and preoperative TSH level ≥ 2.6 mIU/L (OR, 2.444; 95% CI, 1.330–4.492; P = .004) were independent risk factors for unrecovered subclinical hypothyroidism following hemithyroidectomy.
The use of thyroid hormone replacement for subclinical hypothyroidism that develops after hemithyroidectomy should be carefully considered with close followup, while considering the likelihood of the spontaneous return to euthyroidism.
The development of hypothyroidism following hemithyroidectomy is a known complication of thyroid surgery. It reportedly develops in 5.6–48.9% of patients who undergo hemithyroidectomy, and this value varies according to the follow-up period and follow-up strategy or the definition of hypothyroidism used in the study (1–5). Given that the incidences of well-known complications of hemithyroidectomy, including laryngeal nerve injury, postoperative bleeding, and wound infection, are approximately 1%, it seems that postoperative hypothyroidism may represent the most common complication of hemithyroidectomy. However, its clinical significance has been underestimated given that it is not an acute complication; moreover, thyroid hormone replacement therapy—used for the management of hypothyroidism—has definite long-term adverse cardiovascular and skeletal outcomes, particularly increased heart rate and left ventricular mass, atrial fibrillation, and osteoporosis (6, 7).
Most studies have primarily focused on the incidence of and risk factors for hypothyroidism development, and used a relatively short follow-up period. However, the measurement of thyroid function at a relatively early stage after hemithyroidectomy, without any further followup, may increase the likelihood of detecting only a transient compensating TSH elevation; in such cases, the true incidence and risk factors would remain unclear (3, 8). In addition, as the primary end point of the studies was the development of hypothyroidism alone, thyroid hormone replacement therapy was imprudently used without standard criteria, even in cases of subclinical hypothyroidism; considerations of the natural clinical course of the condition and efforts to develop an appropriate treatment policy of postoperative hypothyroidism were lacking in previous studies. In fact, when hypothyroidism developed, the patients would immediately receive thyroid hormone replacement, which would mask the probability of the natural recovery of the hypothyroidism (3, 8, 9).
To establish an appropriate follow-up strategy in patients who underwent hemithyroidectomy, we consider it paramount to not only identify the actual incidence and predictors of hypothyroidism development following hemithyroidectomy, but to also understand its natural clinical course. Therefore, in the present study, we aimed to determine the clinical characteristics of hypothyroidism that developed after hemithyroidectomy by using our standardized follow-up protocol, and to assess its actual incidence and risk factors. This information would be important in ensuring appropriate patient care and surveillance after hemithyroidectomy.
Materials and Methods
Patients
Permission for this study was granted from the investigation review board of our institution. From August 2004 to February 2011, a total of 464 patients underwent hemithyroidectomy for various indications at our institution. Among these patients, we excluded those who were not available for a preoperative thyroid function study, including antithyroid antibody measurements (antithyroglobulin antibody [anti-Tg] and antithyroid peroxidase antibody [anti-TPO]; n = 4), those who did not undergo regular measurement of postoperative thyroid function in compliance with our follow-up protocol (n = 20), those with a preoperative history of thyroid hormone replacement or antithyroid medication (n = 22), those who received empirical TSH suppression after hemithyroidectomy (n = 11), or those who were not followed up for at least 12 months (n = 2). Finally, 405 patients were included in this study, and their baseline characteristics are presented in Table 1. The mean preoperative TSH level was 2.41 mIU/L (range, 0.1–9.6 mIU/L) and the mean follow-up duration was 56.4 months (range, 12–105 mo).
| Characteristic . | Patients, n (n = 405) . |
|---|---|
| Age, y | 47.4 (18–86) |
| Sex, men:women | 60:345 |
| Preoperative thyroid function examination | |
| TSH level (mIU/L) | 2.41 (0.1–9.6) |
| Antithyroglobulin antibody positivity (> 35 IU/mL) | 339 (83.7%) |
| Antiperoxidase antibody positivity (> 60 IU/mL) | 81 (20.0%) |
| Final histology | |
| Malignant | 377 (93.1%) |
| Papillary thyroid carcinoma | 373 |
| Follicular thyroid carcinoma | 4 |
| Benign | 28 (6.9%) |
| Follicular adenoma | 8 |
| Nodular hyperplasia | 12 |
| Other benign histology | 8 |
| Coexistence of Hashimoto's thyroiditis | 93 (23.0%) |
| Characteristic . | Patients, n (n = 405) . |
|---|---|
| Age, y | 47.4 (18–86) |
| Sex, men:women | 60:345 |
| Preoperative thyroid function examination | |
| TSH level (mIU/L) | 2.41 (0.1–9.6) |
| Antithyroglobulin antibody positivity (> 35 IU/mL) | 339 (83.7%) |
| Antiperoxidase antibody positivity (> 60 IU/mL) | 81 (20.0%) |
| Final histology | |
| Malignant | 377 (93.1%) |
| Papillary thyroid carcinoma | 373 |
| Follicular thyroid carcinoma | 4 |
| Benign | 28 (6.9%) |
| Follicular adenoma | 8 |
| Nodular hyperplasia | 12 |
| Other benign histology | 8 |
| Coexistence of Hashimoto's thyroiditis | 93 (23.0%) |
| Characteristic . | Patients, n (n = 405) . |
|---|---|
| Age, y | 47.4 (18–86) |
| Sex, men:women | 60:345 |
| Preoperative thyroid function examination | |
| TSH level (mIU/L) | 2.41 (0.1–9.6) |
| Antithyroglobulin antibody positivity (> 35 IU/mL) | 339 (83.7%) |
| Antiperoxidase antibody positivity (> 60 IU/mL) | 81 (20.0%) |
| Final histology | |
| Malignant | 377 (93.1%) |
| Papillary thyroid carcinoma | 373 |
| Follicular thyroid carcinoma | 4 |
| Benign | 28 (6.9%) |
| Follicular adenoma | 8 |
| Nodular hyperplasia | 12 |
| Other benign histology | 8 |
| Coexistence of Hashimoto's thyroiditis | 93 (23.0%) |
| Characteristic . | Patients, n (n = 405) . |
|---|---|
| Age, y | 47.4 (18–86) |
| Sex, men:women | 60:345 |
| Preoperative thyroid function examination | |
| TSH level (mIU/L) | 2.41 (0.1–9.6) |
| Antithyroglobulin antibody positivity (> 35 IU/mL) | 339 (83.7%) |
| Antiperoxidase antibody positivity (> 60 IU/mL) | 81 (20.0%) |
| Final histology | |
| Malignant | 377 (93.1%) |
| Papillary thyroid carcinoma | 373 |
| Follicular thyroid carcinoma | 4 |
| Benign | 28 (6.9%) |
| Follicular adenoma | 8 |
| Nodular hyperplasia | 12 |
| Other benign histology | 8 |
| Coexistence of Hashimoto's thyroiditis | 93 (23.0%) |
Definition of thyroid function status
In accordance with the scientific review and guidelines for subclinical thyroid disorders published in 2004 and standard practice in our institution's laboratory, the reference range of TSH, free l-thyroxine (T4), and triiodo-l-thyronine (T3) concentration was defined as 0.45–4.5, 0.8–2.0, and 0.6–1.9 ng/mL, respectively (10, 11). Euthyroidism was defined as the presence of normal levels of serum TSH, free T4, and T3. Subclinical hypothyroidism was defined as an elevation in serum TSH levels beyond the upper limit of the reference range, with normal free T4 levels (12). Overt hypothyroidism was defined as an increase in serum TSH levels above the reference range and a decrease in free T4 levels below the reference range (13, 14). Subclinical hyperthyroidism was defined as a decrease in serum TSH levels below the reference range, with normal serum free T4 and T3 concentrations (15). Overt hyperthyroidism was defined as a decrease in serum TSH levels below the reference range, with increases in free T4 and T3 levels above their respective reference ranges (11).
Among subclinical hypothyroidism cases, recovered subclinical hypothyroidism was defined as spontaneous normalization of TSH levels without any treatment during the follow-up period, returning to a euthyroid state. Unrecovered subclinical hypothyroidism was defined as subclinical hypothyroidism that did not recover spontaneously, required treatment, or progressed to overt hypothyroidism during the follow-up period.
Follow-up assessments of thyroid function and management of hypothyroidism
The first measurement of postoperative thyroid function was performed within 1–3 months after surgery. If it was found to be normal, follow-up measurements of thyroid function were performed every 6 months. If subclinical hypothyroidism was identified at any time during the follow-up period, thyroid function measurement was performed 3 months after that point and was repeated every 3–6 months without any treatment, as long as the TSH level remained between 4.5 and 10 mIU/L and the patient did not show any signs/symptoms. In patients with subclinical hypothyroidism, if the TSH level increased to > 10 mIU/L or if progression to overt hypothyroidism was noted, levothyroxine replacement was initiated. In addition, if their signs/symptoms, especially fatigue, weight gain, and facial edema, which were routinely checked and graded using the Common Terminology Criteria for Adverse Events on every follow-up visit, were identified as grade 2 or higher, levothyroxine replacement was initiated. However, we never recommended levothyroxine replacement in patients with euthyroidism. In cases where overt hypothyroidism was diagnosed during followup, levothyroxine replacement was initiated immediately. If spontaneous recovery of subclinical hypothyroidism was observed during the follow-up period, thyroid function measurement was resumed at 6-months intervals (see online appendix).
Assessment parameters and statistical analyses
We evaluated the baseline patient characteristics, including age, sex, preoperative TSH/free T4/T3 levels, positivity for antithyroid antibodies, final histological diagnosis after hemithyroidectomy, and coexistence of Hashimoto's thyroiditis. Positive anti-Tg and anti-TPO were defined as anti-Tg and anti-TPO levels > 35 and > 60 IU/mL, respectively. A diagnosis of Hashimoto's thyroiditis was determined solely on the basis of the histological results after surgery, because histological findings of Hashimoto's thyroiditis, namely destruction of the thyroid tissue by infiltrating lymphocytes, might be associated with decreased thyroid function rather than laboratory findings of Hashimoto's thyroiditis.
During the follow-up period, the development of hypothyroidism, type of hypothyroidism, TSH level at diagnosis, interval between surgery and the development of hypothyroidism, spontaneous recovery from hypothyroidism, interval between the development of hypothyroidism and recovery, and the use of thyroid hormone substitution were recorded.
χ2 test for categorical data and independent Student t test for continuous data were performed to evaluate the association of the clinicopathological characteristics with the development of hypothyroidism. The cut-off value of continuous data, such as age and preoperative TSH level, for predicting the development of overall hypothyroidism and unrecovered subclinical hypothyroidism was estimated by using receiver operating characteristic curves. Binary logistic regression was performed to evaluate the influence of each variable, and variables with a P < .3 for the risk of hypothyroidism development in the univariate analysis were subsequently included in multivariate analysis. The results of binary logistic regression are presented as the odds ratio (OR) with 95% confidence interval (CI) and P-value. Statistical significance was defined as P < .05 and all P-values were two sided.
Results
Development of hypothyroidism following hemithyroidectomy
During the mean follow-up duration of 56.4 months, postoperative hypothyroidism was observed in 226 of 405 patients, with an overall incidence of 55.8%. Of these patients, 222 developed subclinical hypothyroidism and four developed overt hypothyroidism that required immediate levothyroxine replacement. For the 222 patients with postoperative subclinical hypothyroidism, the mean TSH level at diagnosis was 8.6 mIU/L (range, 4.6–90.4 mIU/L). In 179 patients with postoperative euthyroidism, the mean TSH level measured at the last followup was higher than the preoperative TSH level (2.6 vs1.6 mIU/L), and the difference was significant (P < .001).
The interval between surgery and the development of hypothyroidism is illustrated in Figure 1. The mean time internal between these stages was 4.3 months (range, 1–65 mo). Hypothyroidism was diagnosed in 191 patients (84.5%) within 3 months after surgery and in 206 patients (91.2%) within 9 months after the surgery; only 20 patients (8.8%) were diagnosed with hypothyroidism more than 9 months after the surgery.
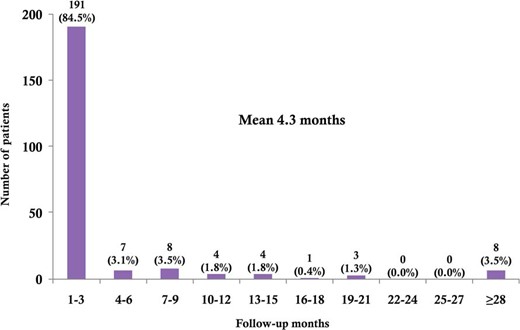
The interval between surgery and the development of hypothyroidism.
Risk factors for hypothyroidism after hemithyroidectomy
The association between the clinicopathological characteristics and the development of hypothyroidism after hemithyroidectomy is shown in Table 2. Female sex, TSH at least 2.0 mIU/L, positivity for anti-TPO, and coexistence of Hashimoto's thyroiditis were found to be significantly associated with the development of hypothyroidism in the univariate analysis. In multivariate analysis with adjustment, TSH at least 2.0 mIU/L (OR, 5.517; 95% CI, 3.540–8.598; P < .001) and the coexistence of Hashimoto's thyroiditis (OR, 1.996; 95% CI, 1.107–3.601; P = .022) were confirmed as independent risk factors for the development of hypothyroidism (Table 3). Although female sex and positivity for anti-TPO were found to be significant in the univariate analysis, their influence on the development of hypothyroidism was not found to be significant after adjustment for others factors in the multivariate analysis.
Association Between Clinicopathological Characteristics and Developments of Hypothyroidism After Hemithyroidectomy
| Characteristic . | Euthyroid (n = 179) . | Hypothyroidism (n = 226) . | P-Value . |
|---|---|---|---|
| Age, y | 47.2 (18–86) | 47.5 (19–76) | .847 |
| Sex | |||
| Male | 36 (20.1%) | 24 (10.6%) | .008 |
| Female | 143 (79.9%) | 202 (89.4%) | |
| Preoperative TSH level, mIU/L | |||
| Mean | 1.64 (0.2–6.3) | 3.01 (0.1–9.6) | <.001 |
| < 2.0 | 127 (70.9%) | 64 (28.3%) | <.001 |
| ≥ 2.0 | 52 (29.1%) | 162 (71.7%) | |
| Positivity for antithyroid antibody | |||
| Antithyroglobulin antibody | 149 (83.2%) | 190 (84.1%) | .822 |
| Antiperoxidase antibody | 28 (15.6%) | 54 (23.5%) | .047 |
| Final histology | |||
| Malignant | 167 (93.3%) | 210 (92.9%) | .882 |
| Benign | 12 (6.7%) | 16 (7.1%) | |
| Coexistence of Hashimoto's thyroiditis | |||
| Present | 26 (14.5%) | 67 (29.6%) | <.001 |
| Absent | 153 (85.5%) | 159 (70.4%) |
| Characteristic . | Euthyroid (n = 179) . | Hypothyroidism (n = 226) . | P-Value . |
|---|---|---|---|
| Age, y | 47.2 (18–86) | 47.5 (19–76) | .847 |
| Sex | |||
| Male | 36 (20.1%) | 24 (10.6%) | .008 |
| Female | 143 (79.9%) | 202 (89.4%) | |
| Preoperative TSH level, mIU/L | |||
| Mean | 1.64 (0.2–6.3) | 3.01 (0.1–9.6) | <.001 |
| < 2.0 | 127 (70.9%) | 64 (28.3%) | <.001 |
| ≥ 2.0 | 52 (29.1%) | 162 (71.7%) | |
| Positivity for antithyroid antibody | |||
| Antithyroglobulin antibody | 149 (83.2%) | 190 (84.1%) | .822 |
| Antiperoxidase antibody | 28 (15.6%) | 54 (23.5%) | .047 |
| Final histology | |||
| Malignant | 167 (93.3%) | 210 (92.9%) | .882 |
| Benign | 12 (6.7%) | 16 (7.1%) | |
| Coexistence of Hashimoto's thyroiditis | |||
| Present | 26 (14.5%) | 67 (29.6%) | <.001 |
| Absent | 153 (85.5%) | 159 (70.4%) |
Association Between Clinicopathological Characteristics and Developments of Hypothyroidism After Hemithyroidectomy
| Characteristic . | Euthyroid (n = 179) . | Hypothyroidism (n = 226) . | P-Value . |
|---|---|---|---|
| Age, y | 47.2 (18–86) | 47.5 (19–76) | .847 |
| Sex | |||
| Male | 36 (20.1%) | 24 (10.6%) | .008 |
| Female | 143 (79.9%) | 202 (89.4%) | |
| Preoperative TSH level, mIU/L | |||
| Mean | 1.64 (0.2–6.3) | 3.01 (0.1–9.6) | <.001 |
| < 2.0 | 127 (70.9%) | 64 (28.3%) | <.001 |
| ≥ 2.0 | 52 (29.1%) | 162 (71.7%) | |
| Positivity for antithyroid antibody | |||
| Antithyroglobulin antibody | 149 (83.2%) | 190 (84.1%) | .822 |
| Antiperoxidase antibody | 28 (15.6%) | 54 (23.5%) | .047 |
| Final histology | |||
| Malignant | 167 (93.3%) | 210 (92.9%) | .882 |
| Benign | 12 (6.7%) | 16 (7.1%) | |
| Coexistence of Hashimoto's thyroiditis | |||
| Present | 26 (14.5%) | 67 (29.6%) | <.001 |
| Absent | 153 (85.5%) | 159 (70.4%) |
| Characteristic . | Euthyroid (n = 179) . | Hypothyroidism (n = 226) . | P-Value . |
|---|---|---|---|
| Age, y | 47.2 (18–86) | 47.5 (19–76) | .847 |
| Sex | |||
| Male | 36 (20.1%) | 24 (10.6%) | .008 |
| Female | 143 (79.9%) | 202 (89.4%) | |
| Preoperative TSH level, mIU/L | |||
| Mean | 1.64 (0.2–6.3) | 3.01 (0.1–9.6) | <.001 |
| < 2.0 | 127 (70.9%) | 64 (28.3%) | <.001 |
| ≥ 2.0 | 52 (29.1%) | 162 (71.7%) | |
| Positivity for antithyroid antibody | |||
| Antithyroglobulin antibody | 149 (83.2%) | 190 (84.1%) | .822 |
| Antiperoxidase antibody | 28 (15.6%) | 54 (23.5%) | .047 |
| Final histology | |||
| Malignant | 167 (93.3%) | 210 (92.9%) | .882 |
| Benign | 12 (6.7%) | 16 (7.1%) | |
| Coexistence of Hashimoto's thyroiditis | |||
| Present | 26 (14.5%) | 67 (29.6%) | <.001 |
| Absent | 153 (85.5%) | 159 (70.4%) |
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-Value . | OR . | 95% CI . | P-Value . | |
| Female sex | 2.119 | 1.211–3.706 | .008 | 1.551 | 0.824–2.920 | .174 |
| Preoperative TSH level ≥ 2.0 mIU/L | 6.182 | 4.008–9.536 | <.001 | 5.517 | 3.540–8.598 | <.001 |
| Positivity for antiperoxidase antibody | 1.669 | 1.004–2.774 | .048 | 1.043 | 0.569–1.913 | .891 |
| Coexistence of Hashimoto's thyroiditis | 2.480 | 1.498–4.106 | <.001 | 1.996 | 1.107–3.601 | .022 |
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-Value . | OR . | 95% CI . | P-Value . | |
| Female sex | 2.119 | 1.211–3.706 | .008 | 1.551 | 0.824–2.920 | .174 |
| Preoperative TSH level ≥ 2.0 mIU/L | 6.182 | 4.008–9.536 | <.001 | 5.517 | 3.540–8.598 | <.001 |
| Positivity for antiperoxidase antibody | 1.669 | 1.004–2.774 | .048 | 1.043 | 0.569–1.913 | .891 |
| Coexistence of Hashimoto's thyroiditis | 2.480 | 1.498–4.106 | <.001 | 1.996 | 1.107–3.601 | .022 |
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-Value . | OR . | 95% CI . | P-Value . | |
| Female sex | 2.119 | 1.211–3.706 | .008 | 1.551 | 0.824–2.920 | .174 |
| Preoperative TSH level ≥ 2.0 mIU/L | 6.182 | 4.008–9.536 | <.001 | 5.517 | 3.540–8.598 | <.001 |
| Positivity for antiperoxidase antibody | 1.669 | 1.004–2.774 | .048 | 1.043 | 0.569–1.913 | .891 |
| Coexistence of Hashimoto's thyroiditis | 2.480 | 1.498–4.106 | <.001 | 1.996 | 1.107–3.601 | .022 |
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-Value . | OR . | 95% CI . | P-Value . | |
| Female sex | 2.119 | 1.211–3.706 | .008 | 1.551 | 0.824–2.920 | .174 |
| Preoperative TSH level ≥ 2.0 mIU/L | 6.182 | 4.008–9.536 | <.001 | 5.517 | 3.540–8.598 | <.001 |
| Positivity for antiperoxidase antibody | 1.669 | 1.004–2.774 | .048 | 1.043 | 0.569–1.913 | .891 |
| Coexistence of Hashimoto's thyroiditis | 2.480 | 1.498–4.106 | <.001 | 1.996 | 1.107–3.601 | .022 |
Clinical course and treatment of subclinical hypothyroidism
Of 222 patients with subclinical hypothyroidism who were followed up for 57.7 months (range, 12–105 mo), only 7 (3.2%) exhibited progression to overt hypothyroidism; all of these patients received levothyroxine replacement. Subclinical hypothyroidism persisted during the follow-up period in 66 patients (29.7%), and 47 of these patients (21.2%) required levothyroxine replacement due to further elevations of the TSH level beyond 10 mIU/L or because of the presence of subjective symptoms; the remaining 19 patients (8.5%) with persistent subclinical hypothyroidism exhibited a constant TSH level between 4.5 and10 mIU/L, and hence only underwent regular follow-up measurements of thyroid function without any treatment. Spontaneous recovery of subclinical hypothyroidism was observed in 149 patients (67.1%) during the follow-up period, who eventually achieved a euthyroid state. The mean time for recovery was 14.0 months (range, 1–55 mo) since the diagnosis of hypothyroidism.
Overall incidence of hypothyroidism after hemithyroidectomy that required treatment
Of 405 patients, a total of 58 (14.3%) required levothyroxine replacement, including four patients with overt hypothyroidism at the time of the initial diagnosis, seven patients with subclinical hypothyroidism at the initial stage that progressed to over hypothyroidism during the follow-up period, and 47 patients with subclinical hypothyroidism who exhibited TSH level greater than 10 mIU/L or subjective symptoms. The remaining 347 patients (85.7%) did not require levothyroxine replacement.
Comparison between recovered and unrecovered subclinical hypothyroidism
We divided the 222 patients with subclinical hypothyroidism into the recovered (n = 149) and unrecovered (n = 73) groups and performed subgroup analysis. The association between the clinicopathological characteristics and the development of recovered or unrecovered subclinical hypothyroidism after hemithyroidectomy is shown in Table 4. In univariate analysis, age at least 46 years and preoperative TSH level at least 2.6 mIU/L were significantly associated with unrecovered subclinical hypothyroidism, whereas positivity for anti-TPO and coexistence of Hashimoto's thyroiditis were not significantly associated with unrecovered subclinical hypothyroidism. Female sex, which is a risk factor for the development of overall hypothyroidism, was also found to be a risk factor for unrecovered subclinical hypothyroidism. The multivariate analysis suggested that age at least 45 years (OR, 2.395; 95% CI, 1.266–4.533; P = .007) and preoperative TSH level at least 2.6 mIU/L (OR, 2.444; 95% CI, 1.330–4.492; P = .004) were independent risk factors for unrecovered subclinical hypothyroidism after hemithyroidectomy (Table 5). Although female sex and malignant histology were also considered to be risk factors for unrecovered subclinical hypothyroidism, the significance was borderline (P = .067 and P = .062, respectively).
| . | Recovered Subclinical Hypothyroidism (n = 149) . | Unrecovered Subclinical Hypothyroidism (n = 73) . | P-Value . |
|---|---|---|---|
| Age, y | |||
| Mean | 46.2 (19–76) | 49.4 (21–75) | .051 |
| < 46 | 72 (48.3%) | 23 (31.5%) | .017 |
| ≥ 46 | 77 (51.7%) | 50 (68.5%) | |
| Sex | |||
| Male | 22 (14.8%) | 2 (2.7%) | .007 |
| Female | 127 (85.2%) | 71 (97.3%) | |
| Preoperative TSH level, mIU/L | |||
| Mean | 2.80 (0.1–9.5) | 3.40 (0.1–9.6) | .037 |
| < 2.6 | 86 (57.7%) | 26 (35.6%) | .002 |
| ≥ 2.6 | 63 (42.3%) | 47 (64.4%) | |
| Positivity for antithyroid antibody | |||
| Antithyroglobulin antibody | 124 (83.2%) | 62 (84.9%) | .745 |
| Antiperoxidase antibody | 33 (22.1%) | 18 (24.7%) | .676 |
| Final histology | |||
| Malignant | 136 (91.3%) | 71 (97.3%) | .152 |
| Benign | 13 (8.7%) | 2 (2.7%) | |
| Coexistence of Hashimoto's thyroiditis | |||
| Present | 39 (26.2%) | 26 (35.6%) | .146 |
| Absent | 110 (73.8%) | 47 (64.4%) |
| . | Recovered Subclinical Hypothyroidism (n = 149) . | Unrecovered Subclinical Hypothyroidism (n = 73) . | P-Value . |
|---|---|---|---|
| Age, y | |||
| Mean | 46.2 (19–76) | 49.4 (21–75) | .051 |
| < 46 | 72 (48.3%) | 23 (31.5%) | .017 |
| ≥ 46 | 77 (51.7%) | 50 (68.5%) | |
| Sex | |||
| Male | 22 (14.8%) | 2 (2.7%) | .007 |
| Female | 127 (85.2%) | 71 (97.3%) | |
| Preoperative TSH level, mIU/L | |||
| Mean | 2.80 (0.1–9.5) | 3.40 (0.1–9.6) | .037 |
| < 2.6 | 86 (57.7%) | 26 (35.6%) | .002 |
| ≥ 2.6 | 63 (42.3%) | 47 (64.4%) | |
| Positivity for antithyroid antibody | |||
| Antithyroglobulin antibody | 124 (83.2%) | 62 (84.9%) | .745 |
| Antiperoxidase antibody | 33 (22.1%) | 18 (24.7%) | .676 |
| Final histology | |||
| Malignant | 136 (91.3%) | 71 (97.3%) | .152 |
| Benign | 13 (8.7%) | 2 (2.7%) | |
| Coexistence of Hashimoto's thyroiditis | |||
| Present | 39 (26.2%) | 26 (35.6%) | .146 |
| Absent | 110 (73.8%) | 47 (64.4%) |
| . | Recovered Subclinical Hypothyroidism (n = 149) . | Unrecovered Subclinical Hypothyroidism (n = 73) . | P-Value . |
|---|---|---|---|
| Age, y | |||
| Mean | 46.2 (19–76) | 49.4 (21–75) | .051 |
| < 46 | 72 (48.3%) | 23 (31.5%) | .017 |
| ≥ 46 | 77 (51.7%) | 50 (68.5%) | |
| Sex | |||
| Male | 22 (14.8%) | 2 (2.7%) | .007 |
| Female | 127 (85.2%) | 71 (97.3%) | |
| Preoperative TSH level, mIU/L | |||
| Mean | 2.80 (0.1–9.5) | 3.40 (0.1–9.6) | .037 |
| < 2.6 | 86 (57.7%) | 26 (35.6%) | .002 |
| ≥ 2.6 | 63 (42.3%) | 47 (64.4%) | |
| Positivity for antithyroid antibody | |||
| Antithyroglobulin antibody | 124 (83.2%) | 62 (84.9%) | .745 |
| Antiperoxidase antibody | 33 (22.1%) | 18 (24.7%) | .676 |
| Final histology | |||
| Malignant | 136 (91.3%) | 71 (97.3%) | .152 |
| Benign | 13 (8.7%) | 2 (2.7%) | |
| Coexistence of Hashimoto's thyroiditis | |||
| Present | 39 (26.2%) | 26 (35.6%) | .146 |
| Absent | 110 (73.8%) | 47 (64.4%) |
| . | Recovered Subclinical Hypothyroidism (n = 149) . | Unrecovered Subclinical Hypothyroidism (n = 73) . | P-Value . |
|---|---|---|---|
| Age, y | |||
| Mean | 46.2 (19–76) | 49.4 (21–75) | .051 |
| < 46 | 72 (48.3%) | 23 (31.5%) | .017 |
| ≥ 46 | 77 (51.7%) | 50 (68.5%) | |
| Sex | |||
| Male | 22 (14.8%) | 2 (2.7%) | .007 |
| Female | 127 (85.2%) | 71 (97.3%) | |
| Preoperative TSH level, mIU/L | |||
| Mean | 2.80 (0.1–9.5) | 3.40 (0.1–9.6) | .037 |
| < 2.6 | 86 (57.7%) | 26 (35.6%) | .002 |
| ≥ 2.6 | 63 (42.3%) | 47 (64.4%) | |
| Positivity for antithyroid antibody | |||
| Antithyroglobulin antibody | 124 (83.2%) | 62 (84.9%) | .745 |
| Antiperoxidase antibody | 33 (22.1%) | 18 (24.7%) | .676 |
| Final histology | |||
| Malignant | 136 (91.3%) | 71 (97.3%) | .152 |
| Benign | 13 (8.7%) | 2 (2.7%) | |
| Coexistence of Hashimoto's thyroiditis | |||
| Present | 39 (26.2%) | 26 (35.6%) | .146 |
| Absent | 110 (73.8%) | 47 (64.4%) |
Univariate and Multivariate Analysis for the Development of Unrecovered Subclinical Hypothyroidism
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-Value . | OR . | 95% CI . | P-Value . | |
| Female sex | 6.150 | 1.405–26.917 | .016 | 4.108 | 0.905–18.636 | .067 |
| Age ≥ 46 y | 2.033 | 1.128–3.664 | .018 | 2.395 | 1.266–4.533 | .007 |
| Preoperative TSH level ≥ 2.6 mIU/L | 2.468 | 1.383–4.402 | .002 | 2.444 | 1.330–4.492 | .004 |
| Malignant histology | 3.393 | 0.745–15.455 | .114 | 4.559 | 0.926–22.440 | .062 |
| Coexistence of Hashimoto's thyroiditis | 1.560 | 0.854–2.850 | .148 | 1.675 | 0.869–3.240 | .123 |
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-Value . | OR . | 95% CI . | P-Value . | |
| Female sex | 6.150 | 1.405–26.917 | .016 | 4.108 | 0.905–18.636 | .067 |
| Age ≥ 46 y | 2.033 | 1.128–3.664 | .018 | 2.395 | 1.266–4.533 | .007 |
| Preoperative TSH level ≥ 2.6 mIU/L | 2.468 | 1.383–4.402 | .002 | 2.444 | 1.330–4.492 | .004 |
| Malignant histology | 3.393 | 0.745–15.455 | .114 | 4.559 | 0.926–22.440 | .062 |
| Coexistence of Hashimoto's thyroiditis | 1.560 | 0.854–2.850 | .148 | 1.675 | 0.869–3.240 | .123 |
Univariate and Multivariate Analysis for the Development of Unrecovered Subclinical Hypothyroidism
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-Value . | OR . | 95% CI . | P-Value . | |
| Female sex | 6.150 | 1.405–26.917 | .016 | 4.108 | 0.905–18.636 | .067 |
| Age ≥ 46 y | 2.033 | 1.128–3.664 | .018 | 2.395 | 1.266–4.533 | .007 |
| Preoperative TSH level ≥ 2.6 mIU/L | 2.468 | 1.383–4.402 | .002 | 2.444 | 1.330–4.492 | .004 |
| Malignant histology | 3.393 | 0.745–15.455 | .114 | 4.559 | 0.926–22.440 | .062 |
| Coexistence of Hashimoto's thyroiditis | 1.560 | 0.854–2.850 | .148 | 1.675 | 0.869–3.240 | .123 |
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-Value . | OR . | 95% CI . | P-Value . | |
| Female sex | 6.150 | 1.405–26.917 | .016 | 4.108 | 0.905–18.636 | .067 |
| Age ≥ 46 y | 2.033 | 1.128–3.664 | .018 | 2.395 | 1.266–4.533 | .007 |
| Preoperative TSH level ≥ 2.6 mIU/L | 2.468 | 1.383–4.402 | .002 | 2.444 | 1.330–4.492 | .004 |
| Malignant histology | 3.393 | 0.745–15.455 | .114 | 4.559 | 0.926–22.440 | .062 |
| Coexistence of Hashimoto's thyroiditis | 1.560 | 0.854–2.850 | .148 | 1.675 | 0.869–3.240 | .123 |
Discussion
Our study showed that the overall incidence of hypothyroidism following hemithyroidectomy was 55.8% (226/405), which was relatively higher than that reported in previous studies (5.6–48.9%) (1, 2, 4, 8, 9). The higher incidence noted in the present study may be due to the prospective detection of biochemical hypothyroidism by using the standardized follow-up protocol after surgery, which included the regular measurement of thyroid function even in patients without any signs/symptoms of hypothyroidism. In addition, the longer mean follow-up duration (56.4 mo) of the present study, compared with previous studies, may have also contributed to the higher incidence of hypothyroidism (8, 16). In fact, the mean interval between surgery and diagnosis of hypothyroidism was only 4.3 months, and the trends in the incidence over time suggested that hypothyroidism developed during the early postoperative periods in most cases; 84.5% of patients (191/226) were diagnosed within 3 months, and 91.2% of patients (206/226) were diagnosed within 9 months. This result suggests that thyroid function should be followed up for at least 9 months in patients who have undergone hemithyroidectomy to ensure that at least 90% of cases of postoperative hypothyroidism are detected. However, 8.8% of patients (20/226) developed hypothyroidism even beyond 9 months after surgery, which cannot be ignored; hence, further followup of thyroid function is required in selective patients, particularly in those who have risk factors for hypothyroidism.
In the present study, preoperative TSH level at least 2.0 mIU/L and coexistence of Hashimoto's thyroiditis diagnosed in histological examination after surgery were confirmed to be independent risk factors for the development of hypothyroidism following hemithyroidectomy, with approximately 5.5- and 2-fold higher risks, respectively. This result is consistent with that of several previous studies, which suggested that higher preoperative TSH level and lymphocytic infiltration of the thyroid gland are associated with hypothyroidism development (1, 3, 8, 9). In addition to the results of previous studies, we determined the specific cut-off value of the preoperative TSH level that increases the risk of hypothyroidism after hemithyroidectomy by using receiver operating characteristic curves. Given that the preoperative TSH level was confirmed as the most powerful risk factor for hypothyroidism, the determination of such a specific preoperative TSH cutoff level—rather than the consideration of a “higher TSH level”—can be very useful in preoperative patient counseling regarding the risks of postoperative hypothyroidism and in selecting patients who require long-term followup of postoperative thyroid function.
Traditionally, the hypothyroidism that develops after hemithyroidectomy has been incorrectly assumed to be permanent; hence, most patients who developed hypothyroidism after the surgery received thyroid hormone replacement therapy without any consideration for the natural recovery, even in cases of subclinical hypothyroidism with mild TSH level elevation (3, 8). In addition, once thyroid hormone replacement is initiated, it is unlikely that it will be discontinued to assess whether the remaining thyroid is functioning adequately to maintain the euthyroid state (8). The adverse effects of thyroid hormone replacement therapy have recently become a major issue in the management of subclinical thyroid disorders, and its negative influence on the cardiovascular and skeletal system was found to be greater than expected (6, 7, 10, 11, 17). Several clinical trials, population-based studies, and comprehensive scientific reviews in the 2000s have suggested that routine thyroid hormone replacement is not recommended for cases of subclinical hypothyroidism with TSH level less than 10 mIU/L and without signs/symptoms, due to the lack of clinical benefits and concerns regarding the adverse effects of thyroid hormone replacement (10, 11, 15, 17, 18). In the present study, we rigorously followed up with and managed the patients who underwent hemithyroidectomy according to this consensus, and showed that this consensus is even applicable to patients with postoperative hypothyroidism following hemithyroidectomy as well as to the general population. In fact, we observed that the subclinical hypothyroidism in approximately two thirds of the cases with TSH levels between 4.5 and 10 mIU/L was transient, and showed a spontaneous return to the euthyroid state without any intervention during the follow-up period. In addition, the normalization of thyroid function occurred at a mean duration of 14 months from the diagnosis of subclinical hypothyroidism, which is comparable to the duration of 18 months reported by Díez et al (19) in general patients with subclinical hypothyroidism. These results suggest that the pituitary-thyroid axis undergoes a similar adaptation process regardless of whether the hypothyroidism is caused by the abrupt surgical loss of thyroid tissue. Therefore, the application of thyroid hormone replacement for subclinical hypothyroidism that develops after hemithyroidectomy should be determined in a more prudent manner as in patients who do not undergo surgery because subclinical hypothyroidism at early postoperative stage may only represent a transient state involving compensatory TSH level increases rather than a true state of hypothyroidism (3).
In the present study, we did not use levothyroxine supplementation to suppress TSH, even for patients with thyroid carcinoma. Although some studies reported that an elevated TSH level is associated with a higher incidence of thyroid carcinoma and advanced-stage tumors, there is currently no high-quality evidence of TSH suppression therapy decreasing the incidence of recurrence of differentiated thyroid carcinoma in low-risk patients suggested for lobectomy or hemithyroidectomy (20–24). In fact, among a total of 377 patients in the present study with differentiated thyroid carcinomas, the disease-free survival at the 5-year followup was not different between the 167 patients with normal postoperative TSH levels and the 210 patients with elevated postoperative TSH levels (95.2 vs 94.8%; P = .852), even though this was not included as an assessment parameter in our study. Recently, the 2015 American Thyroid Association guidelines also stated that there is little evidence to guide TSH targets or the use of thyroid hormone in low-risk patients who have undergone lobectomy (23). Therefore, we believe that the application of levothyroxine supplementation to suppress TSH should be determined in a more prudent manner until more studies are performed in this area to help guide the management of patients undergoing thyroid-conserving surgery.
To identify the risk factors for a true state of hypothyroidism that is not likely to recover or that requires treatment, after excluding transient cases with compensatory TSH level increase, we performed a subgroup analysis of patients with recovered and unrecovered subclinical hypothyroidism. Univariate and multivariate analyses suggested that age at least 46 years and preoperative TSH level at least 2.6 mIU/L were independent risk factors for the development of unrecovered subclinical hypothyroidism (approximately 2.4-fold higher risk with each parameter); moreover, these risk factors differed from those associated with the development of hypothyroidism alone, indicating that a more specifically tailored follow-up strategy may be required for each patient undergoing hemithyroidectomy, by separating the risks of developing hypothyroidism alone and the risk of developing persistent hypothyroidism or hypothyroidism requiring treatment. In addition, the finding of a TSH level of at least 2.6 mIU/L as a risk factor for unrecovered subclinical hypothyroidism is consistent with the upper reference limits of 2.5 mIU/L suggested by the National Academy of Clinical Biochemists and several population-based studies wherein greater than 95% of rigorously screened normal euthyroid volunteers had serum TSH levels between 0.4 and 2.5 mIU/L. This finding suggests the reliability of our study (15, 16, 25, 26).
Interestingly, among 149 patients who achieved the euthyroid state after they were diagnosed with subclinical hypothyroidism, 15 patients (10.1%) showed the redevelopment of subclinical hypothyroidism. Among these, seven patients ultimately required levothyroxine replacement during the follow-up period. However, we did not include this aspect our analysis, because the mean time interval between surgery and the redevelopment of subclinical hypothyroidism was 46.3 months. Considering that the mean interval between surgery and the development of initial hypothyroidism was only 4.3 months and that the compensation process of the pituitary-thyroid axis to normalize thyroid function was completed in a mean of 14 and 18 months in the present and previous study, respectively, the redevelopment of subclinical hypothyroidism at a mean time intervals of 46 months was not regarded as subclinical hypothyroidism caused by surgery alone (19).
In conclusion, the present study, with a relatively long-term surveillance period for thyroid function, showed that the overall incidence of hypothyroidism after hemithyroidectomy was much higher than expected, but thyroid hormone replacement was required in only 14.3% of hemithyroidectomy cases. To ensure that at least 90% of cases of postoperative hypothyroidism are detected, we recommend that regular thyroid function measurements should be performed for a minimum of 9 months after surgery in patients undergoing hemithyroidectomy. In addition, further followup of thyroid function is required in selective patients, particularly those with preoperative TSH at least 2 mIU/L and coexistence of Hashimoto's thyroiditis. However, the application of levothyroxine replacement for subclinical hypothyroidism following hemithyroidectomy should be determined in a more prudent manner, as thyroid function can spontaneously normalize in approximately two thirds of patients, particularly in those age less than 46 years and with preoperative TSH level less than 2.6 mIU/L.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- anti-Tg
antithyroglobulin antibody
- anti-TPO
antithyroid peroxidase antibody
- CI
confidence interval
- OR
odds ratio.



