-
PDF
- Split View
-
Views
-
Cite
Cite
Joakim Crona, Olov Norlén, Pantelis Antonodimitrakis, Staffan Welin, Peter Stålberg, Barbro Eriksson, Multiple and Secondary Hormone Secretion in Patients With Metastatic Pancreatic Neuroendocrine Tumours, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 2, 1 February 2016, Pages 445–452, https://doi.org/10.1210/jc.2015-2436
Close - Share Icon Share
Abstract
As a group, neuroendocrine tumors (NETs) secrete many different peptide hormones, yet heretofore each NET patient is typically thought to produce at most one hormone that causes a distinct hormonal syndrome. A minority of patients have multiple hormones at diagnosis and may also develop secondary hormone secretion at a later stage.
The objectives of the study were to determine the frequency and to describe the impact of multiple and secondary hormone secretion in sporadic gasteroenteropancreatic NET patients.
This was a retrospective analysis of patients (n = 972) with gasteroenteropancreatic NET treated at Uppsala University Hospital, Uppsala, Sweden. Patients with the secretion of multiple hormones at diagnosis and/or those developing secondary hormone secretion during the disease course were identified and studied in further detail.
In pancreatic NETs (PNETs), a total of 19 of 323 patients (6%) had secretion of multiple hormones at diagnosis, and 14 of 323 (4%) had secondary changes during the disease course. These phenomena occurred exclusively in patients with an advanced disease stage, and secondary hormones were detected in a close time span with progressive disease. Patients with secondary insulin hypersecretion had increased morbidity as well as reduced survival (P < .002). In contrast, multiple and secondary hormone secretion was rarely seen in NETs of the small intestine with 0 and 1 of 603 cases, respectively.
Diversity of PNET hormone secretion either at diagnosis or during the disease course occurred in a minority of patients (9.3%). These phenomena had a major impact on patient outcome both through increased morbidity and mortality. Our results support that patients with metastatic PNETs should be monitored for clinical symptoms of secondary hormone secretion during the disease course.
Pronounced interpatient heterogeneity characterizes the natural histories of patients with neuroendocrine tumors (NETs) including pancreatic NETs (1–3). This clinical variability reflects the diverse biology of NETs that show wide spectra of differentiation, proliferation, and invasiveness. A major determinant of the clinical course is attributed to the increased production and secretion of neuropeptides and amines that cause distinct clinical syndromes (2, 4). Pancreatic NETs (PNETs) originate from neuroendocrine islet cells and may secrete a variety of different neuropeptides (5, 6). Severe morbidity and mortality is attributed to the PNET hormone secretion with the severity of the associated symptoms dependent on the type and amount of the specific peptide (7). Whereas secretion of pancreatic polypeptide (PP) results in no detectable symptoms, hypersecretion of insulin and/or vasoactive intestinal polypeptide (VIP) show variable responses to therapy and may be potentially lethal (7).
Excess of a single symptomatic hormone or absence of elevated hormones are the two most common scenarios observed in sporadic NET patients (8). In contrast, concomitant secretion of multiple peptides at diagnosis can be more frequently observed in patients with multiple endocrine neoplasias and is attributed to the coexistence of tumors from separate origins (8, 9). Concurrent secretion of multiple hormones at diagnosis as well as development of secondary hormone secretion have been described to occur in a minority of sporadic cases with functional PNETs (10–14). Therefore, recent guidelines favor regular biochemical screening in these patients (11–13, 15). This is contrary to nonfunctioning PNETs in which hormone screening is recommended only with the emergence of new symptoms (16). The objective of the current study was to describe the frequency and impact of multiple and secondary hormone secretion in a large cohort of gasteroenteropancreatic NETs.
Materials and Methods
This was a retrospective analysis that included patients with pathology-confirmed small intestinal (SI) NETs and PNETs treated at the Departments of Endocrine Oncology and Endocrine Surgery, Uppsala University Hospital (Uppsala, Sweden). Data had been collected between 1985 and 2013 for PNETs and between 1985 and 2010 for SINETs (17). All patients had provided written informed consent to participate in the studies. Ethical approval had been obtained from the regional ethical review board in Uppsala (number 2011/375 and 2012/160).
To ensure high-quality longitudinal data, only patients of Swedish citizenship were included. Patients diagnosed with endocrine neoplasia susceptibility syndromes multiple endocrine neoplasia 1 and von Hippel Lindau were excluded. There were a total of 926 patients who met these criteria including 603 small intestinal, 293 pancreatic, 15 duodenal, and 10 gastric NETs as well as five cases that were classified by the pathology report as well differentiated gasteroenteropancreatic NETs of foregut origin. Pancreatic, duodenal, gastric, and NETs originating from the upper gastrointestinal tract will be denoted as PNET in the following sections if not mentioned otherwise. The clinical characteristics of the PNET cohort are summarized in Table 1.
| n . | Subtype, Pan/Duo/Gastric . | Female/ Male . | Age at Diagnosis, y, Median (Range) . | Follow-Up, mo, Median . | Metastatic, n . | No Hormones, Single Hormone, or Multiple Hormones at Diagnosis . | ENETS Grade 1/2/3 . |
|---|---|---|---|---|---|---|---|
| 323 | 293/15/10 | 150/173 | 58 (15–86) | 70 | 247 | 126/139/38 | 56/124/27 |
| n . | Subtype, Pan/Duo/Gastric . | Female/ Male . | Age at Diagnosis, y, Median (Range) . | Follow-Up, mo, Median . | Metastatic, n . | No Hormones, Single Hormone, or Multiple Hormones at Diagnosis . | ENETS Grade 1/2/3 . |
|---|---|---|---|---|---|---|---|
| 323 | 293/15/10 | 150/173 | 58 (15–86) | 70 | 247 | 126/139/38 | 56/124/27 |
Abbreviation: Duo, duodenal; ENETS, European Neuroendocrine Tumor Society; Pan, pancreatic. This is an overview of the studied PNET cohort. Hormonal status was determined at diagnosis.
| n . | Subtype, Pan/Duo/Gastric . | Female/ Male . | Age at Diagnosis, y, Median (Range) . | Follow-Up, mo, Median . | Metastatic, n . | No Hormones, Single Hormone, or Multiple Hormones at Diagnosis . | ENETS Grade 1/2/3 . |
|---|---|---|---|---|---|---|---|
| 323 | 293/15/10 | 150/173 | 58 (15–86) | 70 | 247 | 126/139/38 | 56/124/27 |
| n . | Subtype, Pan/Duo/Gastric . | Female/ Male . | Age at Diagnosis, y, Median (Range) . | Follow-Up, mo, Median . | Metastatic, n . | No Hormones, Single Hormone, or Multiple Hormones at Diagnosis . | ENETS Grade 1/2/3 . |
|---|---|---|---|---|---|---|---|
| 323 | 293/15/10 | 150/173 | 58 (15–86) | 70 | 247 | 126/139/38 | 56/124/27 |
Abbreviation: Duo, duodenal; ENETS, European Neuroendocrine Tumor Society; Pan, pancreatic. This is an overview of the studied PNET cohort. Hormonal status was determined at diagnosis.
Clinical annotation of PNETs
Age at diagnosis was considered as the time of radiological diagnosis. Ki67 values had been determined according to World Health Organization (WHO) (18) criteria and were extracted together with available immunohistochemistry analyses from pathology reports. Metastatic disease was established if intraoperative, pathological, or radiological investigation suggested the presence of NET in either lymph nodes or distant organs.
Hormonal assays and screening protocols
This study analyzed data that had been collected over 4 decades of clinical work-up. We extracted data from the laboratory system; secondary information available in patient records were not considered. Only measurements performed by the Department of Clinical Chemistry, Uppsala University Hospital (Uppsala, Sweden) were taken into account. For comparison between different assays of the same hormone, values were converted to relative units to the upper reference limit.
Routine procedures at our department state that at the time of diagnosis, prior to surgical resection and/or initiation of somatostatin analog (SSA) treatment, patients with PNETs should be screened for calcitonin, c-peptide, gastrin, glucagon, insulin, PP, proinsulin, VIP, and 24-hour urinary U-5-hydroxyindoleacetic acid (U-5-HIAA) as well as chromogranin A. ACTH was analyzed only if there were clinical signs of Cushing's syndrome. PTH-related peptide was evaluated only in patients with hypercalcemia. Neuroendocrine tumors of the small intestine were evaluated only for secretion of chromogranin A and 24-hour urinary U-5-HIAA; other peptide hormones were examined in the presence of suggestive symptoms.
Classification of elevated hormones and hormonal syndromes
We classified patients as having tumor secretion of none, a single, or multiple hormones at diagnosis. Furthermore, patients were considered as functioning or nonfunctioning. The criteria for these classifications are presented in detail in Supplemental Table 1. In summary, patients were considered as without elevated hormones only when calcitonin, c-peptide, gastrin, glucagon, insulin, proinsulin, and VIP as well as 24-hour urinary U-5-HIAA were within reference intervals in SSA-naïve patients. Classification of hormone secretion was given only when two independent measurements were 3 times the upper reference limit together with the absence of confounding factors. These included diabetes mellitus or severe obesity (body mass index > 35 kg/m2) in patients with hyperinsulinism, PPI, H2 blockade, or gastric surgery in patients with hypergastrinemia. In addition, ectopic Cushing's syndrome was diagnosed after confirming elevated plasma ACTH and 24-hour urinary cortisol together with no suppression of cortisol and ACTH after a 1-mg overnight dexamethasone suppression test (19). Secondary hormone secretion was classified only when the particular hormone was below upper reference limit at diagnosis. Patients were disqualified from this classification if baseline hormone measurements had not been performed (20).
Functional hormonal syndrome caused by secretion of insulin was classified according to The Endocrine Society guidelines (20). Functional hormonal syndromes caused by secretion of ACTH, calcitonin, gastrin, glucagon, serotonin, and/or VIP were classified according to European Neuroendocrine Tumor Society criteria (Supplemental Table 1) (15). Nonfunctioning status was defined as the absence of hormonal symptoms.
Radiological assessment
Six of the patients with secondary hormone secretion had available radiology scans that were evaluable according to Response Evaluation Criteria in Solid Tumors 1.1. Briefly, responses on computed tomography/magnetic resonance imaging were rated as complete response, with a disappearance of all lesions or partial response if the sum of the longest diameter of the index lesions decreased 30% or more (21). Progressive disease was established with an increase of 20% or more. Stable disease was less than 30% decrease and less than less than 20% increase in the diameter of the index lesions.
Statistical analysis
Because only zero and one SI-NET patient had multiple hormones at diagnosis and secondary secretion, respectively, these patients were excluded from the statistical comparisons. Patients with PNETs were grouped based on none, single, or multiple hormone secretion at diagnosis as well as the absence or presence of secondary hormone secretion during the disease course. Univariate analyses were performed using Mann-Whitney U, Kruskal-Wallis, and χ2 tests as appropriate. Kaplan-Meier curves and log-rank tests were executed to evaluate the impact on survival. Calculations were performed using SPSS 22.0 (IBM).
Results
Multiple hormone secretion at diagnosis and development of secondary hormone secretion during the disease course were observed in 19 and 14 of 323 patients with PNETs, respectively. In addition, there was one (0.2%, 1 of 603) NET of small intestinal origin, which showed secondary hormone secretion. There were no cases of multiple hormone secretion in SI-NETs (0 of 603). These differences between the subgroups were statistically significant for secondary hormone secretion (χ2 test, P < .001) and multiple hormone secretion (χ2 test, P < .001).
Secretion of multiple hormones at diagnosis
In PNETs, multiple hormone secretion could be detected in 19 of 323 patients (6%). There were 11 patients with elevated gastrin, nine with calcitonin, eight with glucagon, six with insulin/proinsulin, five with VIP, two with serotonin, and one with elevated ACTH. Contrary to tumors with none or secretion of a single hormone, which occurred both in patients with localized and advanced stage, multiple hormone secretion was observed exclusively in those with metastatic disease (χ2 test P < .001, Table 2). There was a trend toward shorter survival in those with multiple hormone secretion (median 56 mo, 95% confidence interval [CI] 15–97) compared with those with single-hormone secretion (median 143 mo, 95% CI 83-203, log rank test P = .124, Figure 1A). In fact, the survival curve of patients with multiple hormones overlapped that of those with no hormone secretion who had a median survival of 81 months (95% CI 48–115). Exclusion of patients without metastatic disease resulted in a tighter estimation with a median survival of 68 months (95% CI 46–90) in those without hormone secretion. In patients with multiple secretion two had WHO grade 1 and eleven had WHO grade 2 tumors. These proportions were different from those with none or a single-hormone secretion, which showed higher degrees of WHO grades 1 and 3 (χ2 test, P = .039). A slightly larger proportion of patients with multiple hormones at diagnosis developed secondary hormone secretion; 16% (3 of 19) compared with those without 5% (7 of 148) or with a single elevated hormone 3% (4 of 127, χ2 test P = .054).
| Factor . | Elevated Hormones at Diagnosis . | P Value . | ||
|---|---|---|---|---|
| None (n = 151) . | Single (n = 134) . | Multiple (n = 19) . | ||
| Age, y | 58 (25–85) | 59 (16–86) | 59 (15–77) | .771 |
| Ki67 | 7 (1–90) | 5 (1–60) | 5 (1–20) | .105 |
| Dead, no/yes | 59/92 | 63/71 | 12/7 | .351 |
| Gender, F/M | 74/77 | 58/75 | 10/9 | .578 |
| Pancreas, duodenum, gastric | 136/4/8 | 122/9/1 | 18/1/0 | .085 |
| WHO grade 1/2/3 | 25/70/19 | 27/36/8 | 2/11/0 | .039 |
| Metastasis, no/yes | 19/129 | 41/89 | 0/19 | <.001 |
| Secondary hormone production, no/yes | 141/7 | 123/4 | 16/3 | .054 |
| Survival, median (95% CI) | 81 (48–115) | 143 (83–203) | 56 (15–97) | .057 |
| Survival, single vs multiple hormones | .124 | |||
| Survival with localized stage excluded, median (95% CI) | 68 (46–90) | 89 (65–113) | 56 (15–97) | .13 |
| Factor . | Elevated Hormones at Diagnosis . | P Value . | ||
|---|---|---|---|---|
| None (n = 151) . | Single (n = 134) . | Multiple (n = 19) . | ||
| Age, y | 58 (25–85) | 59 (16–86) | 59 (15–77) | .771 |
| Ki67 | 7 (1–90) | 5 (1–60) | 5 (1–20) | .105 |
| Dead, no/yes | 59/92 | 63/71 | 12/7 | .351 |
| Gender, F/M | 74/77 | 58/75 | 10/9 | .578 |
| Pancreas, duodenum, gastric | 136/4/8 | 122/9/1 | 18/1/0 | .085 |
| WHO grade 1/2/3 | 25/70/19 | 27/36/8 | 2/11/0 | .039 |
| Metastasis, no/yes | 19/129 | 41/89 | 0/19 | <.001 |
| Secondary hormone production, no/yes | 141/7 | 123/4 | 16/3 | .054 |
| Survival, median (95% CI) | 81 (48–115) | 143 (83–203) | 56 (15–97) | .057 |
| Survival, single vs multiple hormones | .124 | |||
| Survival with localized stage excluded, median (95% CI) | 68 (46–90) | 89 (65–113) | 56 (15–97) | .13 |
Abbreviations: F, female; M, male. The table gives a statistical evaluation of hormone status at diagnosis. P values < .05 are indicated with bold text.
| Factor . | Elevated Hormones at Diagnosis . | P Value . | ||
|---|---|---|---|---|
| None (n = 151) . | Single (n = 134) . | Multiple (n = 19) . | ||
| Age, y | 58 (25–85) | 59 (16–86) | 59 (15–77) | .771 |
| Ki67 | 7 (1–90) | 5 (1–60) | 5 (1–20) | .105 |
| Dead, no/yes | 59/92 | 63/71 | 12/7 | .351 |
| Gender, F/M | 74/77 | 58/75 | 10/9 | .578 |
| Pancreas, duodenum, gastric | 136/4/8 | 122/9/1 | 18/1/0 | .085 |
| WHO grade 1/2/3 | 25/70/19 | 27/36/8 | 2/11/0 | .039 |
| Metastasis, no/yes | 19/129 | 41/89 | 0/19 | <.001 |
| Secondary hormone production, no/yes | 141/7 | 123/4 | 16/3 | .054 |
| Survival, median (95% CI) | 81 (48–115) | 143 (83–203) | 56 (15–97) | .057 |
| Survival, single vs multiple hormones | .124 | |||
| Survival with localized stage excluded, median (95% CI) | 68 (46–90) | 89 (65–113) | 56 (15–97) | .13 |
| Factor . | Elevated Hormones at Diagnosis . | P Value . | ||
|---|---|---|---|---|
| None (n = 151) . | Single (n = 134) . | Multiple (n = 19) . | ||
| Age, y | 58 (25–85) | 59 (16–86) | 59 (15–77) | .771 |
| Ki67 | 7 (1–90) | 5 (1–60) | 5 (1–20) | .105 |
| Dead, no/yes | 59/92 | 63/71 | 12/7 | .351 |
| Gender, F/M | 74/77 | 58/75 | 10/9 | .578 |
| Pancreas, duodenum, gastric | 136/4/8 | 122/9/1 | 18/1/0 | .085 |
| WHO grade 1/2/3 | 25/70/19 | 27/36/8 | 2/11/0 | .039 |
| Metastasis, no/yes | 19/129 | 41/89 | 0/19 | <.001 |
| Secondary hormone production, no/yes | 141/7 | 123/4 | 16/3 | .054 |
| Survival, median (95% CI) | 81 (48–115) | 143 (83–203) | 56 (15–97) | .057 |
| Survival, single vs multiple hormones | .124 | |||
| Survival with localized stage excluded, median (95% CI) | 68 (46–90) | 89 (65–113) | 56 (15–97) | .13 |
Abbreviations: F, female; M, male. The table gives a statistical evaluation of hormone status at diagnosis. P values < .05 are indicated with bold text.
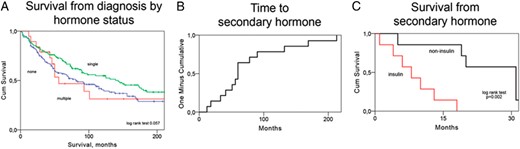
A–C, Kaplan-Meier curves showing survival from diagnosis stratified by hormone secretion pattern (A), time elapsed until secondary hormone secretion (B), and survival from secondary hormone secretion stratified into patients with insulinoma and noninsulinoma (C).
Secondary hormone secretion
In PNETs, there were 4.3% of patients (14 of 323) who developed secondary hormone secretion. Clinical characteristics of these patients are presented in Table 3 and Figure 2 and in the supplemental information. In three additional patients, hormone secretion and changes in hormone related symptoms was detected during the disease course but without baseline measurements to confirm secondary secretion. These patients were excluded from the numerical presentations but were included as case reports (supplemental information).
| Number . | Age . | Gender . | Ki67 . | Biochemistry at Diagnosis . | Hormone Symptoms at Diagnosis . | Secondary Hormone Production . | Time From Diagnosis to Second Hormone . | Time From Second Hormone to Death . | Progression From Second Hormone, mo . | Survival, Months . | Treatment Prior to Conversion . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | M | VIP | VIPoma | Calcitonin | 132 | 20 | Dead, 152 | SSA, Stz + 5FU | ||
| 2 | 75 | F | 12 | PP | 0 | Glucagon, inulin | 90 | 4 | Dead, 94 | Platinum, SSA, Inf, Tmz, Emb, Eve | |
| 3 | 76 | M | 6 | PP | 0 | Insulin, proinsulin | 41 | 18 | Dead, 60 | Tmz, SSA, Stz + 5FU | |
| 4 | 74 | M | 9 | 0 | 0 | Insulin | 18 | 2 | −3 | Dead, 20 | Tmz |
| 5 | 56 | F | Glucagon, proinsulin | Glucagonoma | Insulin | 213 | 20 | Dead, 233 | Strz + 5FU, Tmz, 177Lu-DOTA-octreotate | ||
| 6 | 74 | M | 1 → 40 | 0 | 0 | Insulin, glucagon | 47 | 6 | 0 | Dead, 52 | Stz + 5FU, platinum, |
| 7 | 57 | M | 3 → 1 | 0 | 0 | Glucagon | 12 | 68 | Dead, 81 | ||
| 8 | 56 | F | 10 | PP, proinsulin | 0 | Insulin | 51 | 10 | 0 | Dead, 61 | Stz + Doxo Stz + 5FU, Inf, Im, Tmz |
| 9 | 44 | M | 30 | Gastrin | Gastrinoma | ACTH | 169 | 5 | Dead, 173 | ||
| 10 | 62 | F | 4 | Glucagon, PP, calcitonin, VIP | VIPoma | Insulin | 22 | 13 | 1 | Dead, 46 | Stz + 5FU, Eve |
| 11 | 57 | M | 4 → 17 | 0 | 0 | ACTH | 56 | 19 | 1 | Dead, 76 | Stz + 5FU, Platinum, Tmz + Cap |
| 12 | 44 | M | 3 | VIP | VIPoma | Glucagon, gastrin | 60 | 31 | 0 | Dead, 90 | Stz + 5FU, Tmz |
| 13 | 17 | F | Insulin, pro-insulin, gastrin | Insulinoma | ACTH | 42 | 31 | Dead, 73 | — | ||
| 14 | 49 | M | 0 | 0 | Gastrin | 60 | 31 | Dead, 71 | SSA + Inf, Stz + 5FU, Pac + Doxo, Platinum |
| Number . | Age . | Gender . | Ki67 . | Biochemistry at Diagnosis . | Hormone Symptoms at Diagnosis . | Secondary Hormone Production . | Time From Diagnosis to Second Hormone . | Time From Second Hormone to Death . | Progression From Second Hormone, mo . | Survival, Months . | Treatment Prior to Conversion . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | M | VIP | VIPoma | Calcitonin | 132 | 20 | Dead, 152 | SSA, Stz + 5FU | ||
| 2 | 75 | F | 12 | PP | 0 | Glucagon, inulin | 90 | 4 | Dead, 94 | Platinum, SSA, Inf, Tmz, Emb, Eve | |
| 3 | 76 | M | 6 | PP | 0 | Insulin, proinsulin | 41 | 18 | Dead, 60 | Tmz, SSA, Stz + 5FU | |
| 4 | 74 | M | 9 | 0 | 0 | Insulin | 18 | 2 | −3 | Dead, 20 | Tmz |
| 5 | 56 | F | Glucagon, proinsulin | Glucagonoma | Insulin | 213 | 20 | Dead, 233 | Strz + 5FU, Tmz, 177Lu-DOTA-octreotate | ||
| 6 | 74 | M | 1 → 40 | 0 | 0 | Insulin, glucagon | 47 | 6 | 0 | Dead, 52 | Stz + 5FU, platinum, |
| 7 | 57 | M | 3 → 1 | 0 | 0 | Glucagon | 12 | 68 | Dead, 81 | ||
| 8 | 56 | F | 10 | PP, proinsulin | 0 | Insulin | 51 | 10 | 0 | Dead, 61 | Stz + Doxo Stz + 5FU, Inf, Im, Tmz |
| 9 | 44 | M | 30 | Gastrin | Gastrinoma | ACTH | 169 | 5 | Dead, 173 | ||
| 10 | 62 | F | 4 | Glucagon, PP, calcitonin, VIP | VIPoma | Insulin | 22 | 13 | 1 | Dead, 46 | Stz + 5FU, Eve |
| 11 | 57 | M | 4 → 17 | 0 | 0 | ACTH | 56 | 19 | 1 | Dead, 76 | Stz + 5FU, Platinum, Tmz + Cap |
| 12 | 44 | M | 3 | VIP | VIPoma | Glucagon, gastrin | 60 | 31 | 0 | Dead, 90 | Stz + 5FU, Tmz |
| 13 | 17 | F | Insulin, pro-insulin, gastrin | Insulinoma | ACTH | 42 | 31 | Dead, 73 | — | ||
| 14 | 49 | M | 0 | 0 | Gastrin | 60 | 31 | Dead, 71 | SSA + Inf, Stz + 5FU, Pac + Doxo, Platinum |
Abbreviations: Cap, capecitabine; D, duodenal; Doxo, doxorubicin; Eve, everolimus; F, female; Inf, interferon; M, male; P, pancreatic; SSA, somatostatin receptor analog; Stz, streptozocin; Tmz, temozolomide. The table shows clinical characteristics of patients with secondary hormone secretion. Age was determined as the age at radiological diagnosis. The arrow described the patients with Ki67 values analyzed before and after secondary hormone production.
| Number . | Age . | Gender . | Ki67 . | Biochemistry at Diagnosis . | Hormone Symptoms at Diagnosis . | Secondary Hormone Production . | Time From Diagnosis to Second Hormone . | Time From Second Hormone to Death . | Progression From Second Hormone, mo . | Survival, Months . | Treatment Prior to Conversion . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | M | VIP | VIPoma | Calcitonin | 132 | 20 | Dead, 152 | SSA, Stz + 5FU | ||
| 2 | 75 | F | 12 | PP | 0 | Glucagon, inulin | 90 | 4 | Dead, 94 | Platinum, SSA, Inf, Tmz, Emb, Eve | |
| 3 | 76 | M | 6 | PP | 0 | Insulin, proinsulin | 41 | 18 | Dead, 60 | Tmz, SSA, Stz + 5FU | |
| 4 | 74 | M | 9 | 0 | 0 | Insulin | 18 | 2 | −3 | Dead, 20 | Tmz |
| 5 | 56 | F | Glucagon, proinsulin | Glucagonoma | Insulin | 213 | 20 | Dead, 233 | Strz + 5FU, Tmz, 177Lu-DOTA-octreotate | ||
| 6 | 74 | M | 1 → 40 | 0 | 0 | Insulin, glucagon | 47 | 6 | 0 | Dead, 52 | Stz + 5FU, platinum, |
| 7 | 57 | M | 3 → 1 | 0 | 0 | Glucagon | 12 | 68 | Dead, 81 | ||
| 8 | 56 | F | 10 | PP, proinsulin | 0 | Insulin | 51 | 10 | 0 | Dead, 61 | Stz + Doxo Stz + 5FU, Inf, Im, Tmz |
| 9 | 44 | M | 30 | Gastrin | Gastrinoma | ACTH | 169 | 5 | Dead, 173 | ||
| 10 | 62 | F | 4 | Glucagon, PP, calcitonin, VIP | VIPoma | Insulin | 22 | 13 | 1 | Dead, 46 | Stz + 5FU, Eve |
| 11 | 57 | M | 4 → 17 | 0 | 0 | ACTH | 56 | 19 | 1 | Dead, 76 | Stz + 5FU, Platinum, Tmz + Cap |
| 12 | 44 | M | 3 | VIP | VIPoma | Glucagon, gastrin | 60 | 31 | 0 | Dead, 90 | Stz + 5FU, Tmz |
| 13 | 17 | F | Insulin, pro-insulin, gastrin | Insulinoma | ACTH | 42 | 31 | Dead, 73 | — | ||
| 14 | 49 | M | 0 | 0 | Gastrin | 60 | 31 | Dead, 71 | SSA + Inf, Stz + 5FU, Pac + Doxo, Platinum |
| Number . | Age . | Gender . | Ki67 . | Biochemistry at Diagnosis . | Hormone Symptoms at Diagnosis . | Secondary Hormone Production . | Time From Diagnosis to Second Hormone . | Time From Second Hormone to Death . | Progression From Second Hormone, mo . | Survival, Months . | Treatment Prior to Conversion . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | M | VIP | VIPoma | Calcitonin | 132 | 20 | Dead, 152 | SSA, Stz + 5FU | ||
| 2 | 75 | F | 12 | PP | 0 | Glucagon, inulin | 90 | 4 | Dead, 94 | Platinum, SSA, Inf, Tmz, Emb, Eve | |
| 3 | 76 | M | 6 | PP | 0 | Insulin, proinsulin | 41 | 18 | Dead, 60 | Tmz, SSA, Stz + 5FU | |
| 4 | 74 | M | 9 | 0 | 0 | Insulin | 18 | 2 | −3 | Dead, 20 | Tmz |
| 5 | 56 | F | Glucagon, proinsulin | Glucagonoma | Insulin | 213 | 20 | Dead, 233 | Strz + 5FU, Tmz, 177Lu-DOTA-octreotate | ||
| 6 | 74 | M | 1 → 40 | 0 | 0 | Insulin, glucagon | 47 | 6 | 0 | Dead, 52 | Stz + 5FU, platinum, |
| 7 | 57 | M | 3 → 1 | 0 | 0 | Glucagon | 12 | 68 | Dead, 81 | ||
| 8 | 56 | F | 10 | PP, proinsulin | 0 | Insulin | 51 | 10 | 0 | Dead, 61 | Stz + Doxo Stz + 5FU, Inf, Im, Tmz |
| 9 | 44 | M | 30 | Gastrin | Gastrinoma | ACTH | 169 | 5 | Dead, 173 | ||
| 10 | 62 | F | 4 | Glucagon, PP, calcitonin, VIP | VIPoma | Insulin | 22 | 13 | 1 | Dead, 46 | Stz + 5FU, Eve |
| 11 | 57 | M | 4 → 17 | 0 | 0 | ACTH | 56 | 19 | 1 | Dead, 76 | Stz + 5FU, Platinum, Tmz + Cap |
| 12 | 44 | M | 3 | VIP | VIPoma | Glucagon, gastrin | 60 | 31 | 0 | Dead, 90 | Stz + 5FU, Tmz |
| 13 | 17 | F | Insulin, pro-insulin, gastrin | Insulinoma | ACTH | 42 | 31 | Dead, 73 | — | ||
| 14 | 49 | M | 0 | 0 | Gastrin | 60 | 31 | Dead, 71 | SSA + Inf, Stz + 5FU, Pac + Doxo, Platinum |
Abbreviations: Cap, capecitabine; D, duodenal; Doxo, doxorubicin; Eve, everolimus; F, female; Inf, interferon; M, male; P, pancreatic; SSA, somatostatin receptor analog; Stz, streptozocin; Tmz, temozolomide. The table shows clinical characteristics of patients with secondary hormone secretion. Age was determined as the age at radiological diagnosis. The arrow described the patients with Ki67 values analyzed before and after secondary hormone production.
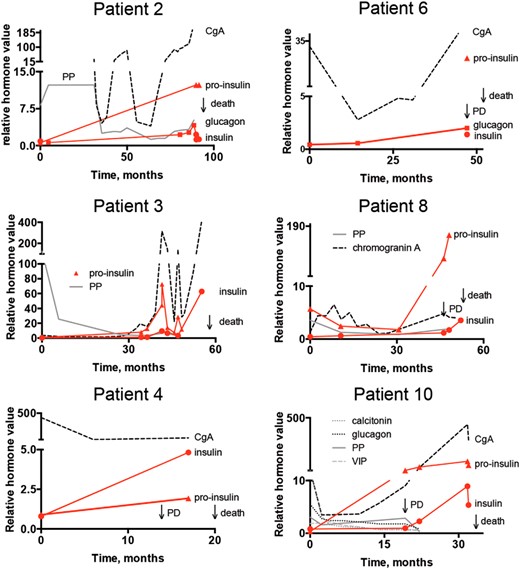
X and Y plots showing the disease course from the first hormone value in six of the patients with secondary insulin secretion and simultaneous development of insulinoma. Progressive disease (PD) was noted near secondary hormone secretion.
The following hormones appeared as secondary: insulin (seven cases), glucagon (four cases), ACTH (three cases), gastrin (two cases), and calcitonin (one case). Three patients had secretion of multiple secondary hormones: insulin and glucagon (patient 2), insulin and glucagon (patient 6), and glucagon and gastrin (patient 12). Median time from the diagnosis of PNET to secondary hormone secretion was 53.5 months (range 12–213 mo, Figure 1B), and the median survival from detection of secondary secretion was 18.5 months (range 2–68 mo). Six of these patients had radiology scans that were evaluable using Response Evaluation Criteria in Solid Tumors criteria. This analysis revealed that all patients had progressive disease within a 3-month time span from diagnosis of secondary hormone secretion. Three patients had Ki67 analyzed before and after secondary hormone. Two of these had increases in Ki67, one of whom progressed from WHO grade 2 to grade 3. We observed that the survival durations from detection of secondary hormone secretion were shorter in those with secondary insulin secretion (median 10 mo, range 2–20 mo, Figure 1C) compared with those with other secondary hormones (median 31 mo, range 5–68 mo, log rank test P = .002). Patients with secondary hormone secretion (median 81 mo, 95% CI 66–96) showed a trend toward worse survival compared with those without secondary hormone secretion (median 115 mo, 95% CI 82–148, log rank test P = .059, Table 4).
| . | Secondary . | No Secondary . | P Value . | Tests . |
|---|---|---|---|---|
| Age at diagnosis, median, y (range) | 57 (17–76) | 59 (16–86) | .823 | Mann-Whitney |
| Ki67 | 9.5 (3–30) | 6 (1–90) | .237 | Mann-Whitney |
| Dead (yes/no) | 14/0 | 162/135 | .001 | χ2 |
| Gender (female/male) | 5/9 | 135/161 | .527 | χ2 |
| Tumour subtype pancreas/duodenum/gastric | 14/0/0 | 271/13/8 | .582 | χ2 |
| Hormone production none/single/multi | 7/4/3 | 141/123/16 | .054 | χ2 |
| WHO grade 1/2/3 | 0/8/2 | 54/112/24 | .139 | χ2 |
| Metastatic, no/yes | 0/14 | 62/229 | .053 | χ2 |
| Survival from diagnosis, median (95% CI) | 81 (66–96) | 115 (82–148) | .059 | Log rank |
| Insulin | Noninsulin | |||
| Survival from secondary hormone secretion, median (95% CI) | 10 (3–13) | 31 (22–39) | .002 | Log rank |
| . | Secondary . | No Secondary . | P Value . | Tests . |
|---|---|---|---|---|
| Age at diagnosis, median, y (range) | 57 (17–76) | 59 (16–86) | .823 | Mann-Whitney |
| Ki67 | 9.5 (3–30) | 6 (1–90) | .237 | Mann-Whitney |
| Dead (yes/no) | 14/0 | 162/135 | .001 | χ2 |
| Gender (female/male) | 5/9 | 135/161 | .527 | χ2 |
| Tumour subtype pancreas/duodenum/gastric | 14/0/0 | 271/13/8 | .582 | χ2 |
| Hormone production none/single/multi | 7/4/3 | 141/123/16 | .054 | χ2 |
| WHO grade 1/2/3 | 0/8/2 | 54/112/24 | .139 | χ2 |
| Metastatic, no/yes | 0/14 | 62/229 | .053 | χ2 |
| Survival from diagnosis, median (95% CI) | 81 (66–96) | 115 (82–148) | .059 | Log rank |
| Insulin | Noninsulin | |||
| Survival from secondary hormone secretion, median (95% CI) | 10 (3–13) | 31 (22–39) | .002 | Log rank |
The table shows the correlation between clinical parameters and hormone conversion. P values < .05 are indicated with bold text.
| . | Secondary . | No Secondary . | P Value . | Tests . |
|---|---|---|---|---|
| Age at diagnosis, median, y (range) | 57 (17–76) | 59 (16–86) | .823 | Mann-Whitney |
| Ki67 | 9.5 (3–30) | 6 (1–90) | .237 | Mann-Whitney |
| Dead (yes/no) | 14/0 | 162/135 | .001 | χ2 |
| Gender (female/male) | 5/9 | 135/161 | .527 | χ2 |
| Tumour subtype pancreas/duodenum/gastric | 14/0/0 | 271/13/8 | .582 | χ2 |
| Hormone production none/single/multi | 7/4/3 | 141/123/16 | .054 | χ2 |
| WHO grade 1/2/3 | 0/8/2 | 54/112/24 | .139 | χ2 |
| Metastatic, no/yes | 0/14 | 62/229 | .053 | χ2 |
| Survival from diagnosis, median (95% CI) | 81 (66–96) | 115 (82–148) | .059 | Log rank |
| Insulin | Noninsulin | |||
| Survival from secondary hormone secretion, median (95% CI) | 10 (3–13) | 31 (22–39) | .002 | Log rank |
| . | Secondary . | No Secondary . | P Value . | Tests . |
|---|---|---|---|---|
| Age at diagnosis, median, y (range) | 57 (17–76) | 59 (16–86) | .823 | Mann-Whitney |
| Ki67 | 9.5 (3–30) | 6 (1–90) | .237 | Mann-Whitney |
| Dead (yes/no) | 14/0 | 162/135 | .001 | χ2 |
| Gender (female/male) | 5/9 | 135/161 | .527 | χ2 |
| Tumour subtype pancreas/duodenum/gastric | 14/0/0 | 271/13/8 | .582 | χ2 |
| Hormone production none/single/multi | 7/4/3 | 141/123/16 | .054 | χ2 |
| WHO grade 1/2/3 | 0/8/2 | 54/112/24 | .139 | χ2 |
| Metastatic, no/yes | 0/14 | 62/229 | .053 | χ2 |
| Survival from diagnosis, median (95% CI) | 81 (66–96) | 115 (82–148) | .059 | Log rank |
| Insulin | Noninsulin | |||
| Survival from secondary hormone secretion, median (95% CI) | 10 (3–13) | 31 (22–39) | .002 | Log rank |
The table shows the correlation between clinical parameters and hormone conversion. P values < .05 are indicated with bold text.
Related symptoms of patients with secondary hormone secretion
All patients experienced symptoms due to their secondary hormone secretion. Those with insulin conversion presented with symptoms due to hypoglycemia: most commonly syncope, tremor, nausea and weight gain. These symptoms resulted in increased morbidity that ultimately required hospitalization for palliative care (Figure 2). Several therapeutic lines were attempted, chemotherapy if not given before, 177Lu-DOTA-octreotate, everolimus, and sunitinib but with short-lasting responses.
Four patients had secondary glucagon secretion: two of whom experienced hyperglycemia and another patient had severe weight loss. Three patients had secondary ACTH secretion and were diagnosed with the ectopic Cushing's syndrome. In two of the cases that were refractory to medical treatment, bilateral adrenalectomy was performed in an attempt to achieve symptom control (22). One patient died due to overt hormonal symptoms and progressive disease upon conversion from a nonfunctional status to ectopic Cushing's syndrome. Two patients with secondary gastrin secretion experienced diarrhea, vomiting, and/or dyspepsia that was relieved by proton pump inhibitor medication.
Discussion
In this retrospective study, we observed that close to 1 of 10 PNET patients experienced either multiple or secondary hormone secretion during their disease course. Notably, these phenomena occurred in the context of metastatic disease. Secondary hormone secretion was closely associated with radiological progression, and those with secondary insulin had a worse survival. This may suggest a potential use of these hormonal patterns as prognostic biomarkers.
We investigated the hormonal profile and associated symptoms during the disease course of close to 1000 patients with gasteroenteropancreatic NETs. Whereas there were no cases with multiple hormone secretion in SI-NETs (0 of 603), a total of 6% of PNET patients (19 of 323) had multiple hormonal secretion at the time of diagnosis. Secondary hormone secretion was discovered in one patient with SI-NET (1 of 603) and in 4% of PNETs (14 of 323). These figures overlap the proportions of patients with these phenotypes described in previous retrospective and prospective studies (11, 13, 14).
We hypothesized that complex hormone secretion could serve as a marker of tumor behavior; presence of multiple hormone secretion could indicate a unique phenotype equal to that of nonfunctioning and functioning PNETs (23). Similarly, the dynamics of the hormone secretion during the disease course might reflect changes in tumor biology. We found that multiple and secondary hormone secretion occurred exclusively in patients with advanced stage. Although Ki67 was no different in patients with multiple hormone secretion, there was a trend toward decreased survival compared with those with secretion of a single hormone. The survival curves of patients with multiple hormones at diagnosis seemed to overlap with those without hormone secretion. However, because of the low sample size, these findings should be interpreted with caution.
de Mestier et al (14) recently described patients with symptomatic secondary hormone secretion and suggested a correlation with tumor dedifferentiation. Our descriptive analysis confirms that secondary hormone secretion had a profound impact on both patient morbidity and mortality. Secondary insulin secretion had the most aggressive phenotype with a median survival of 10 months from conversion. In line with de Mestier et al (14), we show that secondary hormone secretion may correlate with increases in Ki67. Furthermore, all analyzed patients had progressive disease within a close time span from detection of secondary hormone secretion. These findings highlight that hormonal plasticity both at the diagnosis and during the disease course should be recognized and further investigated as potential biomarkers in metastatic PNET patients (12–14, 24).
The molecular basis underlying production and secretion of different hormones in PNETs is poorly studied, although pancreatic islet cells have an inherent capability of hormonal plasticity (25). Reprogramming of the hormone secretion in δ-, α-, and β-cells has been shown to occur both spontaneously and after experimental manipulation in persons with diabetes (26–28). PNETs often display immunostaining for multiple peptide hormones, and in those with genetic endocrine tumor syndromes, multiple hormone secretion can also be attributed to simultaneous expansion of multiple different tumor cell clones (29–31). In sporadic PNETs a scenario with clonal evolution in which molecular changes underlie both multiple and secondary hormone secretion may be considered. The co-occurrence with tumor dedifferentiation and progression in combination with previous findings showing genetic heterogeneity in PNETs provides a rationale to further investigate this theory (14, 32–34).
The findings presented in this paper were based on a retrospective review that stretched over several decades at a single tertiary referral center. Hormonal screening was not performed on a regular basis and did not include the complete spectrum of pancreatic islet cell peptides. The results may therefore deviate from a prospectively studied multicenter cohort, and it is possible that the frequency of secondary hormone secretion causing minor or no clinical symptoms was underestimated. This is especially true for SI-NET patients in whom hormonal screening was performed only in those with exceptional symptoms deviating from the classical carcinoid syndrome. To reduce false-positive findings, we used strict classifications for hormone secretion. Still, to properly classify secretion of hormones as due to tumor and not other physiological reasons and to connect hormone secretion to potentially related clinical symptoms can be difficult, especially when encountering scenarios with multiple secretion.
In conclusion, we have demonstrated that multiple and secondary hormone secretion occurs in a minor proportion (9.3%) of PNET patients and were restricted to patients with metastatic disease. Secondary hormone secretion was associated with disease progression as well as increased morbidity and mortality. Our results support that patients with metastatic PNETs should be monitored for the appearance of clinical symptoms of secondary hormone secretion during the disease course, whereas those with SI-NETs should be investigated in the presence of exceptional symptoms.
Acknowledgments
We thank all patients who participated in this study.
Tobias Åkerström contributed with excellent revisions of the manuscript.
This work was supported by grants from Lions Cancerforskningsfond i Uppsala, Svenska Endokrinologföreningen (to J.C.) and the Nordic Neuroendocrine Tumor Group (to J.C.).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- CI
confidence interval
- NET
neuroendocrine tumor
- PNET
pancreatic NET
- PP
pancreatic polypeptide
- SI
small intestinal
- SSA
somatostatin analog
- U-5-HIAA
U-5-hydroxyindoleacetic acid
- VIP
vasoactive intestinal polypeptide
- WHO
World Health Organization.



