-
PDF
- Split View
-
Views
-
Cite
Cite
Anand K. Annamalai, Krishnaswamy Sampathkumar, Shubhada Kane, Nitin S. Shetty, Suyash Kulkarni, Venkatesh Rangarajan, Nilendu Purandare, Prathamesh S. Pai, Ankit D. Mahuvakar, Radhakrishnan Shanthi, Govindarajulu Suriyakumar, Vipla Puri, Subramaniam Aram, Chandrasekhar Gopalakrishnan, Mathirajan Chelian, K. G. Srinivasan, Anthony J. Gill, Mark Gurnell, Roderick Clifton-Bligh, Needle(s) in the Haystack—Synchronous Multifocal Tumor-Induced Osteomalacia, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 2, 1 February 2016, Pages 390–393, https://doi.org/10.1210/jc.2015-3854
Close - Share Icon Share
A 49-year-old man was referred to our endocrine unit in 2012 with a 15-year history of widespread incapacitating bone and joint pain, which was now causing marked insomnia. Physical examination revealed proximal muscle weakness, waddling gait, kyphosis, and a right metacarpal swelling. Previous biochemical investigations had revealed a low serum 25-hydroxyvitamin D of 16 ng/mL (reference range [RR], 30–100), elevated serum alkaline phosphatase (391 U/L; RR, 53–128), normal serum corrected calcium (9 mg/dL; RR, 8.5–10.5), and a low serum phosphorous level (1.8 mg/dL; RR, 2.5–5). He had previously undergone extensive radiological and rheumatological investigations, which were “inconclusive.” Plain x-ray studies had revealed multiple rib and vertebral fractures. Magnetic resonance imaging of the spine showed diffuse sclerosis of the vertebral column and pelvic bones. A Tc-99m-methylene diphosphonate (MDP) bone scan performed in 2000 had shown increased uptake over the ribs bilaterally (Figure 1A), which was initially reported as suspicious for metastases. However, extensive cross-sectional imaging with computed tomography (CT) and magnetic resonance imaging failed to identify an underlying primary lesion. Before the current presentation, he was treated with multiple analgesics, calcium, phosphorous, and calcitriol preparations. However, none of these resulted in any improvement in his symptoms.
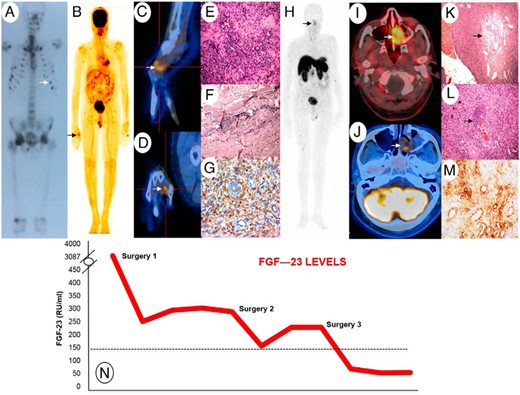
A, Tc-99m-MDP bone scan showing increased tracer uptake at multiple sites. B, Whole-body 18F-FDG-PET scan revealing FDG avid soft tissue thickening over the radial and dorsal aspect of the head of the right second metacarpal (SUVmax = 2.5). C and D, Fused 18F-FDG-PET and CT of the right hand in sagittal (C) and coronal (D) planes. E, Histological examination of metacarpal lesion showing diffuse proliferation of bland tumor cells in a patternless arrangement around vessels (F) with bluish smudgy matrix. G, Vimentin positivity on immunohistochemistry. H, Whole-body Ga-68-DOTANOC-PET scan showing avid tracer uptake within left nasal cavity lesion (2.9 × 2.0 cm;, SUVmax, 3.9). I, Fused Ga-68 DOTANOC PET and CT scan head. J, 18F-FDG-PET SPECT CT head axial imaging demonstrates the left nasal cavity lesion with a low SUV uptake (SUV max = 2.1). K, Histological examination of nasal lesion showing bland tumor cells with fibromyxoid stroma, hemangiopericytomatous pattern of vasculature (arrow). L, Smudgy matrix (arrow). M, Immunohistochemistry shows strong positive staining for somatostatin receptor subtype 2 A (SSTR2A). N, Graph depicting the trends in FGF-23 levels over time.
Because there were no other systemic/localizing features, we considered the possibility of a primary metabolic bone disorder. A repeat phosphorus level was persistently low (1.86 mg/dL), with an inappropriate 24-hour urinary phosphorus of 745 mg/d (RR < 1000), and the percentage tubular reabsorption of phosphate off supplements was 0.68 (RR > 0.8). 25-Hydroxyvitamin D (32 ng/mL) and PTH (49 pg/mL; RR, 14–72) levels were normal, but 1,25-dihydroxyvitamin D was inappropriately low (3.5 pg/mL; RR, 19.6–54.3). In view of this, plasma C-terminal fibroblast growth factor 23 (FGF-23) level was measured (Immutopics), and was grossly elevated at 3088 RU/mL (RR, 0–150). A whole-body 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) scan showed focal tracer uptake on the radial and dorsal aspect of the head of the right second metacarpal (maximum standardized uptake value [SUVmax] = 2.5) (Figure 1, B–D). Histological analyses of the surgically resected metacarpal lesion revealed a phosphaturic mesenchymal cell tumor, mixed connective tissue variant with negative FGF-23, and somatostatin receptor subtype 2A immunostaining (Figure 1, E–G). Despite the latter, FGF-23 levels declined rapidly to 246 RU/mL on day 6 postoperatively, with progressive symptom relief, confirming a diagnosis of tumor-induced osteomalacia (TIO).
In view of persisting (albeit considerably improved) pain and mild hypophosphatemia (2.4 mg/dL), a repeat 18F-FDG-PET scan was performed in 2013 and showed low-grade residual uptake over the head of the right second metacarpal. After careful discussion with the patient, a ray amputation of the index finger was performed, with histology confirming complete excision of the residual tumor. After the second operation, FGF-23 levels dropped to 152 RU/mL, but the hypophosphatemia persisted. His pain improved, although he described ongoing mild symptoms. Several months later, the patient reported an increase in the intensity of his bone pain. He had persistent hypophosphatemia with rising FGF-23 levels (224.5 RU/mL). In 2015, a whole-body 68Ga-DOTANOC-PET scan was performed, which revealed no uptake at the site of the previous surgery. However, a left nasal cavity lesion (2.9 × 2.0 cm) (Figure 1, H and I) with avid tracer uptake (SUVmax, 3.79) was seen. Retrospectively, this lesion could be seen on the 18F-FDG-PET, although at that time the relatively low level of tracer uptake was not felt to be significant. However, axial reconstruction clearly shows the lesion (Figure 1J). Further review of the more historical imaging suggested that this was also probably visible on the original Tc-99m-MDP bone scan (Supplemental Figure 1, A and B). Transnasal endoscopic resection of the nasal lesion revealed a predominant, phosphaturic mesenchymal cell tumor (Figure 1, K and L) with negative FGF-23 immunostaining but positive somatostatin receptor subtype 2A immunostaining (Figure 1M), suggestive of a multifocal TIO. Postoperatively, FGF-23 levels returned to normal within 48 hours of surgery (64.6 RU/mL), and they currently remain within the RR (50.3 RU/mL) at 3 months, with normalization of phosphorous levels (4.2 mg/dL) and, for the first time, complete resolution of his symptoms.
Tumors that secrete phosphaturic factors/phosphatonins (eg, FGF-23) are usually benign and are the predominant cause of TIO or oncogenic osteomalacia. As in our patient, the resulting hypophosphatemia can cause debilitating pain. Complete resection of a unifocal tumor typically leads to full resolution of symptoms. However, localization of these tumors is often difficult due to their small size and diverse locations (limb extremities to the head, including soft tissues, bones, sinuses, brain). Systematic physical examination, combined with cross-sectional and functional imaging is essential for localization. Among functional scans, some studies have shown somatostatin receptor ligand-based imaging [eg, 111Indium-Octreotide single photon emission CT (SPECT) (1) 99Tc-HYNIC-TOC SPECT (2), and Gallium-based 68Ga-DOTA-TATE PET (2–5)] to be better for identifying phosphaturic mesenchymal cell tumor in comparison to other imaging modalities such as 18F-FDG-PET. In a large case series, Chong et al (1) showed that among 19 pathologically confirmed TIO subjects, 18 were octreo-SPECT scan-positive (sensitivity, 0.95; specificity, 0.64; positive predictive value, 0.82; and negative predictive value, 0.88), and 14 of 16 confirmed TIO subjects were FDG-PET-positive (sensitivity, 0.88; specificity, 0.36; positive predictive value, 0.62; and negative predictive value, 0.50). In addition, in a small number of subjects, 68Ga-DOTA-TATE/DOTANOC PET has localized TIOs that were not previously identified with 18F-FDG-PET (2) and 111Indium-Octreotide SPECT imaging (3). In our case, the intense brain uptake of 18F-FDG-PET obscured the lesion in the nasal cavity (Figure 1B), and it was only on retrospective review of axial imaging that the lesion was noted (Figure 1J).
Occasionally, even with the best efforts to localize these tumors, they may remain elusive (“a needle in the haystack”). In this scenario, a combined imaging approach with structural and nuclear imaging affords the best opportunity for localization. Moreover, in the setting of multiple suspicious lesions on initial imaging, or with a possible multifocal TIO similar to our case, an ideal initial approach retrospectively could have included biopsy of both the lesions and/or selective venous sampling of FGF-23 around the sites of the lesions. Selective venous sampling has been shown to help in tumor localization by using an FGF-23 venous concentration ratio between the venous drainage of the tumor bed in comparison to the general circulation (6). In this way, selective venous sampling can differentiate between a culprit lesion and other incidental lesions, thereby avoiding unnecessary surgery (6).
Previous reports have always noted complete normalization of hypophosphatemia and FGF-23 levels with successful surgery. In our case, biochemical normalization did not happen, despite complete surgical excision of the metacarpal lesion after the second surgery. Although multifocality is extremely rare and has only previously been described in four patients (7–10), persisting symptoms and failure of FGF-23 levels to normalize after complete excision of the metacarpal lesion prompted a detailed reevaluation of the case and the adoption of more sensitive imaging in the form of Ga-68 DOTANOC PET. It is likely that if the latter had been used as the preferred imaging modality at the time of his referral to our service, then a structured approach as outlined above, combining selective venous sampling and biopsy, would potentially have led to earlier identification of the multifocality, although still requiring two separate surgical procedures.
This image highlights the importance of a multimodal, perseverant approach toward the investigation and treatment of patients with persistent unexplained hypophosphatemia and chronic bone pain, and it serves as an important reminder of possible multifocal disease in unresolved cases of TIO.
Acknowledgments
Authors' Contributions: All of the authors were involved in the clinical care of the patient and contributed to the writing of the manuscript.
M.G. is supported by the NIHR Cambridge Biomedical Research Centre.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Abbreviations
- CT
computed tomography
- FDG-PET
fluorodeoxyglucose positron emission tomography
- FGF-23
fibroblast growth factor 23
- MDP
methylene diphosphonate
- RR
reference range
- SPECT
single photon emission CT
- SUVmax
maximum standardized uptake value
- TIO
tumor-induced osteomalacia.



